1. Introduction
Hesperidinase is an enzyme mainly produced by filamentous fungi of the genera
Aspergillus and
Penicillium [
1,
2]. The genus
Penicillium has been isolated from various environments, such as soil, air, or marine, and is known to produce enzymes and bioactive compounds [
3]. An example is oxidases obtained from various
Penicillium species, namely,
Penicillium adametzii and
Penicillium funiculosum, as well as from
Aspergillus niger, and these compounds have been purified, assessed, and applied in amperometric glucose biosensors [
4].
Hesperidinase exhibits both α-L-rhamnosidase and β-D-glucosidase activities, catalyzing the hydrolysis of hesperidin to hesperetin, rhamnose, and glucose in a deglycosylation reaction [
5,
6]. In addition to this deglycosylation reaction, hesperidinase can also catalyze the hydrolysis of rutin to quercetin, rhamnose, and glucose, with isoquercetin as an intermediate compound in the reaction [
2,
5]. These reactions may enhance the bioactivity of hesperetin and quercetin aglycones by increasing their bioavailability [
7]. One problem is the low solubility of quercetin. Holzhausen et al. [
8] encapsulated quercetin into liposomes from an aqueous solution using meglumine as solubility enhancer and stabilizer for quercetin. The β-D-glucosidase activity of hesperidinase is not specific to the two previously described reactions and can also be involved in the deglycosylation of other glucose-containing compounds, namely, anthocyanins [
7].
Polyphenols are secondary plant metabolites with one or more phenol groups in their structure [
9,
10]. They contribute to the color and flavor of plants, defending them against pathogens, radiation, and toxins [
11]. Initially considered antinutritional, nowadays, they have the potential to impact human disease risk by modulating biological pathways, which has been supported by in vitro and in vivo studies [
12]. Divided into four classes—phenolic acids, flavonoids, tannins, and stilbenes—flavonoids can be further categorized into flavonols, anthocyanidins, flavan-3-ols, isoflavonoids, flavanones, and flavones [
9]. Primary sources include coffee, tea, citrus fruits, red fruits, and fruit juices, with polyphenol intake influenced by food type and frequency. Fruit juices, especially 100% juice, contribute the anthocyanins, flavonols, and flavanones [
9].
Differences in polyphenol bioavailability between whole fruits and juices are ascribed to processing and fiber presence [
9]. Natural bioflavonoids, especially in glycosylated form, are predominant and offer health benefits, being rich in citrus fruits, apples, and red fruits [
5,
13,
14,
15]. The glycosylation or deglycosylation of bioflavonoids may enhance their solubility, stability, and pharmacological properties. Studies over the past two decades have delved into absorption and metabolism, highlighting the impact of sugar moieties on various levels. Glycosylated flavonoids are primarily absorbed through sugar group hydrolysis [
11]. Absorption occurs in the colon, where enzymes and microflora facilitate hydrolysis before absorption. Conjugation processes, such as methylation and glucuronidation, promote elimination through bile and urine. The nature of the sugar affects absorption, with glucosides being absorbed more rapidly than other glycosides like rhamnosides. The absence of the enzyme α-L-rhamnosidase in the human body makes the bioavailability of rhamnose containing flavonoids dependent on deglycosylation by the intestinal microbiota [
16].
Hesperidin, a glycosylated flavanone, consists of hesperitin aglycone linked to glucose and rhamnose, forming rutinose [
17]. Hesperidin and hesperitin, classified as bioflavonoids, are noteworthy for their antioxidant and anti-inflammatory properties. Inflammation and oxidative stress are major contributors to diseases like cancer and cardiovascular diseases [
18]. Hesperidin and hesperitin exhibit anti-inflammatory effects by inhibiting the NF-kB signaling pathway and blocking the production of pro-inflammatory mediators and enzymes [
19]. The antioxidant and anti-inflammatory capabilities of hesperidin and hesperitin translate into other health properties, including anticarcinogenic, antidiabetic, and cardioprotective effects [
17,
20].
Hesperidin’s bioavailability and efficacy depend on factors such as food form, processing, storage, individual characteristics, and compound structure [
21]. Hesperidin, with lower bioavailability than hesperetin, is rapidly excreted due to the rhamnose group [
6]. Therefore, improving hesperidin to hesperetin is essential for enhanced bioavailability and bioactivity [
1]. Glycosylated flavanones, like hesperidin, resist stomach and small intestine enzymes, reaching the colon intact, where intestinal microbiota assimilate them. Hesperidin molecules break, releasing hesperetin, which enters the bloodstream [
21]. Though intestinal microbiota contributes to increased bioavailability, it varies among individuals and may not suffice for significant health benefits. Therefore, pre-ingestion conversion of hesperidin to hesperetin, facilitated by hesperidinase, is essential for improved bioproducts.
Hesperidinase finds application in the food industry to improve the sensory properties of certain drinks, particularly by reducing the bitter taste of naringin in citrus juices [
5,
6] or to prevent the formation of hesperidin crystals in citrus products [
2]. Nevertheless, its use is limited due to instability, low recovery and reuse rates in soluble form, and the failure of its application in certain operating conditions [
6]. In this context, enzymatic immobilization emerges as a solution. Enzyme encapsulation is a frequently employed tool to enhance the stability of enzymes, thereby increasing their activity and allowing for reuse [
5,
22]. Maintaining the stability of free enzymes during reactions poses a challenging task; hence, immobilized enzymes, capable of maintaining consistent efficiency over several uses, have become a preferred choice [
22]. This choice has led to robust developments and rapid progress in the field. Various enzyme immobilization techniques include adsorption, covalent bonding, affinity immobilization, and encapsulation, along with different support materials such as alginate, chitosan, collagen, gelatin, cellulose, pectin, and inorganic materials like silica [
18]. In addition to being cost-effective, an ideal matrix or support must possess inert characteristics, stability, regenerability, and the ability to enhance the specificity and activity of the enzyme, reduce product inhibition, and prevent external contamination [
22].
The immobilization of enzymes or proteins within solid-state host materials allows them to retain their functionality while often exhibiting enhanced thermal, storage, and operational stability. Additionally, immobilized enzymes are easier to separate from reaction products and can be reused, making them more efficient than their soluble counterparts. Among the various immobilization techniques, sol–gel-derived silica glasses are widely used due to their high porosity, optical transparency, chemical stability, and ease of preparation [
23]. A key benefit of entrapping enzymes within sol–gel matrices is the reduction in enzyme leaching during biocatalytic processes, which is advantageous both economically and environmentally. This feature has long been recognized as a significant advantage of the sol–gel approach [
23]. However, certain challenges exist in the sol–gel immobilization process. One issue is gel shrinkage during condensation and drying, which may lead to enzyme denaturation [
23].
Analytical methods reveal certain limitations, such as the potential loss of activity during immobilization, interactions between the matrix and nutrients, and challenges in large-scale implementation.
One key advantage of this technique is the preservation and enhancement of antioxidant and anti-inflammatory properties, which contribute to the nutritional and functional quality of the juice. To evaluate these benefits, robust analytical methods are employed to quantify bioactivity and assess the efficacy of the sol-gel approach.
Hesperidin is a bioflavonoid present in citrus fruits like in oranges. Therefore, juice bioprocessing to enhance bioflavonoid bioavailability allows these compounds to circulate and exert biological activity, yielding functional products. In fact, the food industry searches for value-added products, and the primary goal of this work was to obtain bioactive compounds with increased biological activity to enhance the nutraceutical properties of flavonoids-rich drinks like orange, apple, and red fruit juices. Considering the high consumption of these juices and consumer interest in their health benefits, obtaining value-added products becomes essential. To attain that goal, the second aim was the production of hesperidinase using a microbial-based approach, followed by the encapsulation of the biosynthesized enzyme in sol–gel matrices. The third goal was the evaluation of the biological activity of the bioprocessed juices.
2. Materials and Methods
2.1. Substrates
The substrates 4-nitrophenyl α-L-rhamnopyranoside (p-NPR), 4-nitrophenyl β-D-glucopyranoside (p-NPG), and hesperidin were from Sigma-Aldrich (St. Louis, MO, USA).
The following juices, packaged (Tetra Pak
®, Pully, Switzerland), were used: 100% squeezed orange juice; 100% apple juice [apple (99.5%) and lemon (0.5%) juices based on concentrates] [
19]; red fruit juice [red grape juice (12%), apple (9%), raspberry (6%), blackcurrant (5%), strawberry (4%), and cranberry (4%) based on concentrates, with a fruit content of at least 40%] [
24].
2.2. Other Chemicals
Other chemicals used in this work were from different brands, namely, 4-nitrophenol, TMOS (tetramethoxysilane) from Fluka Analytical (Darmstadt, Germany), sodium acetate, 1,1-Diphenyl-2-hydroxy-3,5-dinitrobenzoic acid, ethanol, gallic acid, glycerol, sodium acetate trihydrate, and sodium hydroxide were from Merck (Darmstadt, Germany); acetic acid glacial, ferric chloride hexahydrate, Folin–Ciocalteu reagent, hydrochloric acid, 36.5–38%, potassium-sodium tartrate tetrahydrate, sodium carbonate, sodium hydrogen carbonate, magnesium sulfate heptahydrate, sodium nitrate were from Scharlau (Barcelona, Spain). Picrylhydrazyl free radical was from Tokyo Chemical Industry Co. (Tokyo, Japan), Boereveldseweg (Zwijndrecht, Belgium), and Trolox®, 97% from Acros Organics (Geel, Belgium), Janssen-Pharmaceuticalaan (Geel, Belgium), from Merck (Darmstadt, Germany); bovine serum albumin (BSA) (≥96%), DPPH (2,2-diphenyl-1-picrylhydrazyl), D-Glucose, polyacrylic acid, L-rhamnose monohydrate from Sigma-Aldrich (St. Louis, MO, USA); yeast extract, PDA (potato dextrose agar) from Biokar Diagnostics; Triton X-100 from Fisher BioReagents (Pittsburgh, PA, USA), Trolox Acros Organics.
2.3. Analytical Methods
The reducing sugar content was evaluated by the 3,5-dinitrosalicylic acid (DNS) method described by Miller [
25] and Nunes et al. [
26] employing a microscale approach to accomplish microassays in 96-well microtiter plates. The reduced 3-amino,5-nitrosalicylic acid was detected by measuring absorbance at 575 nm [
27]. A glucose calibration curve was done for reducing sugar quantification.
The protein content in the medium was evaluated using the Bradford method using a micromethod [
28] for faster multiple sample processing. In this adapted method, 50 µL of Bradford reagent (Coomassie Brilliant Blue dye) was added to 100 µL of the sample in a microplate (Thermo Scientific Nunc
TM 96-well microplates, Thermo Scientific, Essex, UK). The reaction developed for 5 min, and absorbance was read at 595 nm in a microplate reader (BMG LABTACH Fluostar Omega, Multi-user Software package including Reader Control and MARS Data Analysis Software, FDA 21 CFR part 11 compliant, Biogen Cientifica S.L., Madrid, Spain). The calibration curve for protein quantification was obtained using BSA standard solutions.
The Folin–Ciocalteu method is widely used for quantifying the total phenolic or polyphenolic content in a sample. This method relies on the Folin–Ciocalteu reagent, which comprises a mixture of phosphotungstic acid and phosphomolybdic acid. In an alkaline medium, the reagent reacts with phenolic compounds, leading to its reduction and the formation of blue-colored chromogens. The intensity of the blue coloration is directly proportional to the extent of reduction and, consequently, to the total phenolic content in the sample [
29], which is detected spectrophotometrically at 760 nm. The calibration curve for total phenolic compound quantification was obtained using gallic acid standard solutions.
The DPPH (2,2-Diphenyl-1-Picrylhydrazyl) method allows for evaluating the antioxidant capacity of a sample. In the presence of antioxidants in the sample, the DPPH radical is reduced, changing from a purple color to a pale yellow; this color change is faster the higher the antioxidant content [
30]. The DPPH assay lasted 30 min, with monitoring of the reaction over time, namely, every 10 min. The time at which the samples changed color was also recorded—a change that, the faster it occurs, may be indicative of the greater antioxidant potential. After 30 min, the concentration of DPPH in solution was measured, wherein the higher it is, the lower the antioxidant potential of the sample.
The calibration curve for antioxidant activity quantification was obtained using Trolox standard solutions. The antioxidant activity was assessed by the scavenging percentage of the DPPH radical calculated according to Equation (1):
Equation (1)—DPPH radical scavenging activity, where
A0 is the time at 0 min absorbance, and
At is the absorbance at a given time,
t min, adapted from [
31].
The FRAP (Ferric Reducing Antioxidant Power) method, or the determination of antioxidant activity by the iron reduction method, allows the antioxidant capacity of compounds in a sample to be evaluated. In the presence of antioxidants in the sample, at low pH, the Fe
3+-TPTZ (ferric-tripyridyltriazine) complex is reduced to Fe
2+-TPTZ (ferrous-tripyridyltriazine), gaining an intense blue color [
32]. The color acquisition is as far as higher the antioxidant content. The calibration curve for antioxidant activity quantification was obtained using Trolox standard solutions.
The anti-inflammatory activity was evaluated based on its capacity to inhibit albumin thermal denaturation, as described by Mizushima and Kobayashi [
33]. The chosen method was an adaptation of the one illustrated by Chandra et al. [
34].
A 1% BSA solution (25 mL) in distilled water was prepared. For juice samples analysis, 1 mL of the BSA solution was added to 200 µL of the sample in an Eppendorf tube. This mixture was then incubated at 37 °C for 15 min, followed by a water bath at 70 °C for 5 min. Absorbances were read at 660 nm against a blank. The control assay was conducted accordingly with 1 mL of the albumin solution, and the absorbance was read against distilled water. Subsequently, the anti-inflammatory activity was assessed by the inhibition percentage of protein denaturation calculated through Equation (2).
Equation (2)—Inhibition of protein denaturation, where
A0 is the control assay absorbance, and
A1 is the sample absorbance [
34].
The addition of specific substrates, namely, 4-nitrophenyl β-D-glucopyranoside (
p-NPG) and 4-nitrophenyl
α-L-rhamnopyranoside (
p-NPR), to a medium containing the enzymes
β-D-glucosidase and α-L-rhamnosidase, respectively, makes it possible to evaluate their presence and activity through the quantification of 4-nitrophenol released throughout the enzymatic assay, as described by Rajal et al. [
35] and Romero et al. [
36]. 4-nitrophenol is a product of both enzymatic reactions, and carrying out an assay with known concentrations of this compound allows the creation of a calibration curve whose equation is then used to quantify the
p-nitrophenol released in the respective reactions, allowing for the evaluation of the presence and activity of enzymes.
2.4. Experimental Methods
2.4.1. Preparation of Culture Media, Spore Collection, and Fungal Growth
The fungus Penicillium sp. used in the production of the enzyme, hesperidinase, was obtained from soil. It is a microorganism considered safe (GRAS), being part of the collection of non-pathogenic fungi of the Faculty of Pharmacy of the University of Lisbon.
The production of hesperidinase from the active spores of the fungus Penicillium sp. was carried out in a liquid medium containing rhamnose as an inducer. Previously, the spores were developed on solid PDA (potato dextrose agar). This solid medium was prepared in a 1 L flask by adding 39 g of PDA to 1 L of double-distilled water. Once solubilized, the medium was placed in the autoclave for 30 min at 121 °C and after cooling to around 60 °C, it was distributed in a laminar flow chamber over Petri dishes. After solidification, the plates were stored at 4 °C.
The liquid culture medium, for enzyme production from the spores, contained 10 g of yeast extract, 1.8 g of sodium nitrate, 1.3 g of potassium phosphate, 0.76 g of polyacrylic acid, 0.076 g of magnesium sulfate, and 12.5 g of sucrose, for a final volume of 250 mL. These solutions were autoclaved at 121 °C for 20 min. A total of 0.025 g of rhamnose was dissolved in double-distilled water, for a final volume of 50 mL. After the two solutions were added to each other, achieving the final culture medium in a total volume of 300 mL.
To collect Penicillium sp. spores, grown in solid PDA culture medium, a Triton X-100 solution was initially prepared by adding 3 mL of 100% (v/v) Triton X-100 to 300 mL of distilled water, autoclaved at 121 °C for 20 min. The spore collection procedure consisted of adding a total of 100 mL of Triton X-100 solution to the plate containing the spores fungus, repeated 4 times with 25 mL each. After each addition of Triton X-100, the supernatant was collected. The spores were stored at 4 °C.
Once the spores were collected, the previously prepared liquid culture medium was added to the flask, and the final mixture was incubated at 30 °C for approximately three weeks until the fungus reached the desired growth. On days 0 (immediately before adding the spores to the medium), 3, 7, 14, and 21, aliquots of 2 mL of the medium were collected from previously sterilized Eppendorf tubes to evaluate the evolution of fungal growth and quantification of reducing sugars and proteins in the medium. The fungus growth was also followed daily by visualization. The analyses were carried out immediately after acquisition and repeated after 21 days when the growth assay finished. Similar results were obtained.
Once the growth of the fungus ended, the culture medium was collected by filtration (0.45 mm) and stored at 4 °C for future assays.
2.4.2. Enzyme Immobilization
The immobilization was achieved via encapsulation of the enzymes in hydrogels, namely sol–gel with the precursor TMOS (tetramethoxysilane) [
23]. The sol–gel encapsulation method followed three steps. The first consisted of the preparation of the sol solution through hydrolysis of the precursor, followed by condensation and polycondensation [
32]. Suspension of the precursor in a solution of acidic pH resulted in its hydrolysis with the formation of silanol groups (Si-OH) [
37]. Subsequently, gel formation occurred when a buffer solution with the enzyme was added, which resulted in a change in pH [
23]. The hydrolyzed precursor was activated by the addition of the basic pH buffer and condensation reactions between the silanol groups began, resulting in the formation of siloxane (Si-O-Si) polymers [
37]. Finally, aging occurred, which entailed the drying process when the solvent was removed simultaneously with continuous condensation reactions [
23]. As the network grew with time and temperature, the viscosity of the liquid increased exponentially until the xerogel was formed [
37], resulting in the formation of a matrix in which the enzyme was encapsulated [
37].
2.4.3. Enzymatic Assays
p-NPG and
p-NPR are specific substrates of the enzymes
β-D-glucosidase and
α-L-rhamnosidase, respectively, and one of the products of both enzymatic reactions is
p-nitrophenol, which can be detected and quantified by the
p-nitrophenol method. Solutions of 1 mM
p-NPG and 1 mM
p-NPR were prepared in distilled water [
6].
To evaluate the
β-D-glucosidase and
α-L-rhamnosidase activities, 2 mL of culture medium was centrifuged at 10,000 rpm for 10 min. First, 4.5 mL of p-NPG was added to the second 4.5 mL of
p-NPR and the third 4.5 mL of 0.02 M sodium acetate buffer (control). Then, the 3 flasks were placed in a shaker at 45 °C and 150 rpm, and 0.5 mL of centrifuged medium was added to each of them, with aliquots of 250 µL, at 0, 10, 20, 30, 40, 50, 60, 90, 120, 150, and 180 min, according to [
5]. After collecting the samples at all times, 750 µL of the NaOH solution was added to each Eppendorf and analyzed using the
p-nitrophenol method [
6]. These assays were carried out at least in triplicate.
The assays with encapsulated enzyme were carried out in a ratio of 1:10 [1 g, lens):10 (specific substrates in acetate buffer solution or juice, mL] at 45 °C and 200 rpm. The samples were collected at 0, 15, 30, 45, 60, 90, and 120 min. After collecting the samples, 750 µL of the NaOH solution was added to each Eppendorf, and they were analyzed using the p-nitrophenol method.
In the case of different juices, which contain complex substrates, such as hesperidin, rutin, and anthocyanins, the activity of the enzyme was evaluated through the quantification of reducing sugars (rhamnose and glucose) released in the hydrolysis reactions. These sugars can be detected and quantified using the DNS method.
For free enzyme, in each tube were added 4.5 mL of orange juice, 4.5 mL of apple juice, and 4.5 mL of red fruit juice. These assays were carried out at least in triplicate. Then, the flasks were placed on a shaker at 45 °C and 150 rpm. Half of the flasks, containing each juice, served as controls, with 0.5 mL of 20 mM sodium acetate buffer. To the other tubes, 0.5 mL of enzymatic extract was added. Aliquots of 250 µL were collected from each vial, at 0, 15, 30, 60, 90, 120, 180, 300 min, and 24 h. The collected samples were analyzed using the DNS method and also using the Bradford method, for times 0, 300, and 1440 min. The Bradford method allowed for the evaluation of whether the amount of protein remained throughout the assays.
For the encapsulated enzyme, the performed assays for the immobilized enzyme were identical to those performed for the free enzyme, except that the agitation rate was 200 rpm, and we adjusted the volumes of each juice for the number of lenses. The only changes were in terms of the sampling times at which the samples were collected, in this case at 0, 60, 120, 180, and 1440 min.
The collected samples were analyzed using the DNS method and Bradford method. This method made it possible to evaluate whether there was a loss of protein (enzyme) from the lenses throughout the test.
2.4.4. Operational Stability Evaluation
After encapsulation of the enzyme, the operational stability was evaluated using the specific substrates,
p-NPR and
p-NPG, and juices (orange, apple, and red fruits). This assessment was achieved by performing several enzymatic tests, quantifying
p-nitrophenol and reducing sugars released throughout them, using the
p-nitrophenol and DNS methods, respectively. The residual activity of bioimmobilizates was evaluated [
6,
23]. For this evaluation, it was necessary to calculate the activities of the enzymes using the following formula:
where Δ
C is the difference in concentration of the compound released as a result of the enzymatic reaction in a given time interval Δ
t [
6,
23].
The residual activity was then obtained as follows:
where
A1 and
A2 are the activities calculated at the times
t1 and
t2.
2.5. Statistical Analysis
All data assays were repeated at least 3 times. The representativeness of the data was presented by the mean ± standard deviation. Statistical analyses were performed using IBM SPSS Statistics, version 24.0 55, considering a significance level, α, of 0.05.
3. Results and Discussion
3.1. Hesperidinase Production
Hesperidinase is an enzyme of significant interest to the beverage industry and other sectors due to its capacity to enhance the functional and nutraceutical properties of value-added products. By catalyzing the hydrolysis of natural polyphenols, hesperidinase facilitates the production of their deglycosylated forms. These derivatives retain their nutraceutical properties while exhibiting improved bioavailability, allowing for more efficient absorption by the body and thereby enhancing their functional benefits.
To produce this enzyme of interest, capable of hydrolyzing natural polyphenols such as hesperidin, rutin, and anthocyanins present in orange, apple, and red fruit juices, respectively, the fungus Penicillium sp. was used. This microorganism is abundantly found in nature, particularly in soil environments, offering an economical, safe, and sustainable source for enzyme production. Previous studies conducted in our laboratory have confirmed the ability of the filamentous fungus to effectively produce hesperidinase.
3.1.1. Growth of Penicillium sp.
For the production of hesperidinase from
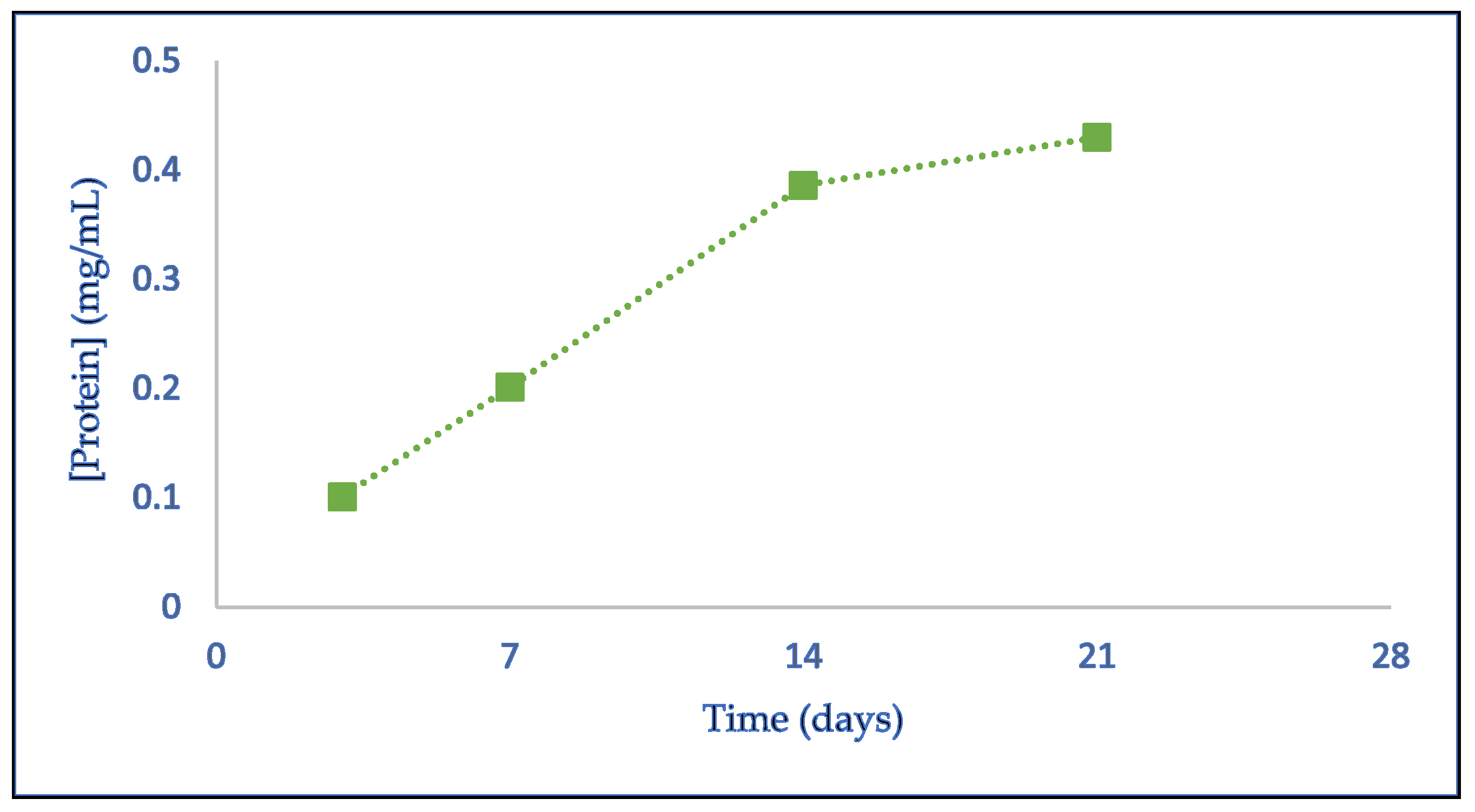
Penicillium sp., a liquid culture medium was prepared using rhamnose as a specific inducer for the enzyme synthesis. The fungal spores, which were initially produced on solid PDA medium, were harvested and introduced into the liquid medium. The cultures were incubated at 30 °C for approximately three weeks (≅21 days). The growth of the fungus was monitored through photographic images and by collecting aliquots on the 3rd, 7th, 14th, and 21st days of incubation. An initial aliquot of the culture medium was also collected before spore inoculation. The collected aliquots were centrifuged and filtered, and the supernatant was analyzed for reducing sugar content, protein concentration, and hesperidinase activity. The protein concentrations obtained on different days of enzyme production are presented in
Figure 1.
The results presented in
Figure 1 demonstrate a progressive increase in protein content within the culture medium over time, as expected. Between the 3rd and 14th days of growth, the increase in protein concentration was linear, with the most pronounced rise occurring between the 7th and 14th days. From the 14th to the 21st day, while an increase in protein concentration was still observed, the rate of increase was lower, indicating a stabilization in protein production.
Different
Penicillium sp. have been used in the production of enzymes and bioactive compounds. An example is the
Penicillium sp. FSDE15 used in the production of xylanases, as well as converting lignocellulosic biomass into value-added products, particularly in saccharification and XOS production [
38].
Penicillium sp. FSDE15 has also been used in the production of cellulases using agroindustrial wastes, such as wheat bran, which are a promising alternative to reduce the cost of these enzymes and facilitate their use in various industrial processes [
39].
3.1.2. Hesperidinase Activity
The production of the enzyme of interest, hesperidinase, was assessed through specific enzymatic assays designed to measure its activity. These assays utilized substrates selective for the activity of the hesperidinase subunits, namely,
α-L-rhamnosidase and
β-D-glucosidase, and their substrates,
p-nitrophenyl-α-L-rhamnopyranoside (
p-NPR) and
p-nitrophenyl-β-D-glucopyranoside (
p-NPG), respectively, produce
p-nitrophenol as the reaction product in the presence of the corresponding enzyme activities. The evaluation was conducted using the extract obtained after the growth of
Penicillium sp., and the results of the enzymatic assays with these specific substrates are presented in
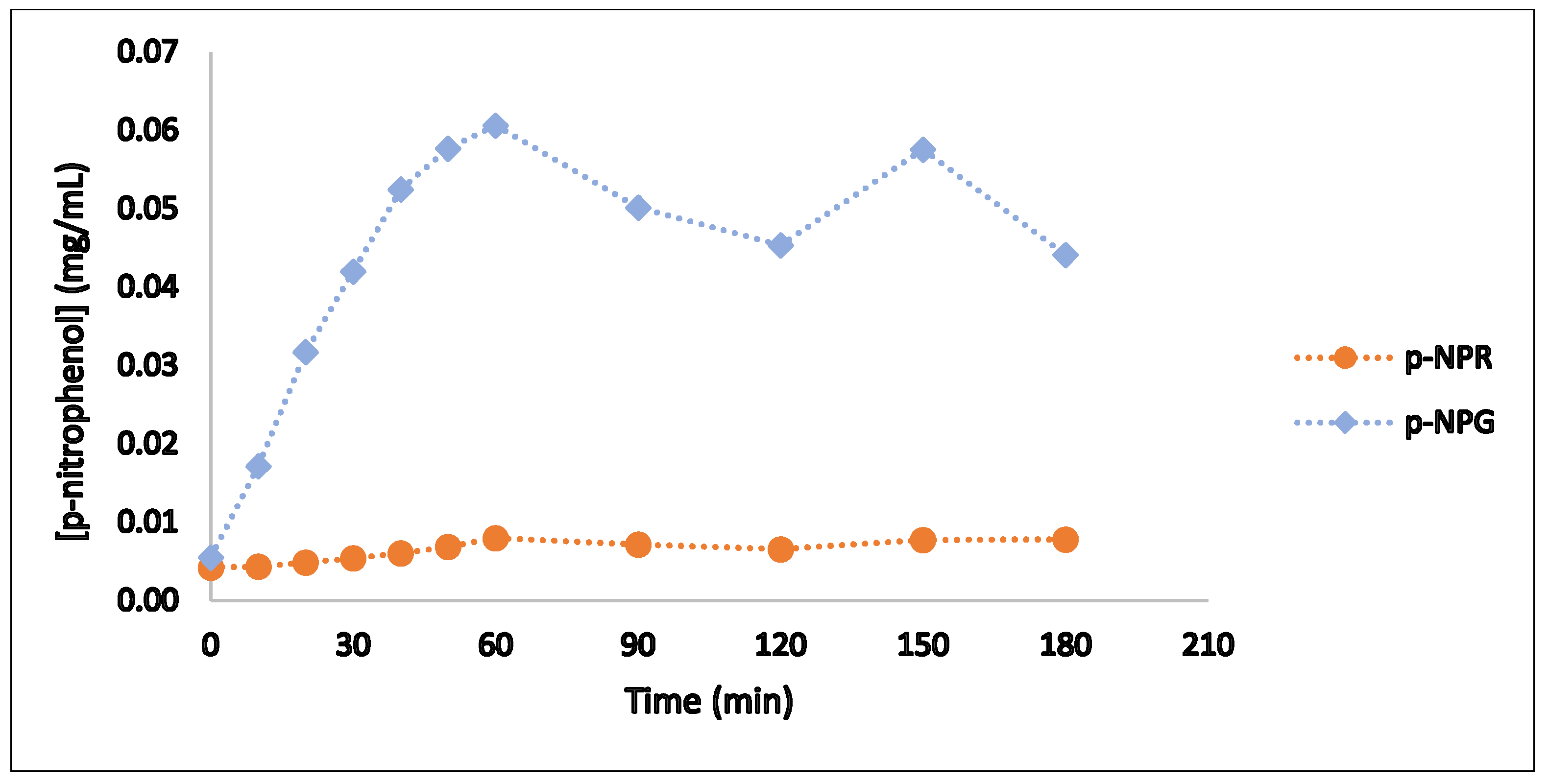
Figure 2.
The results indicate that both subunits of hesperidinase exhibited enzymatic activity, confirming the successful production of this enzyme by
Penicillium sp. The maximum production of
p-nitrophenol was observed after one hour of reaction for both hesperidinase subunits. During the first 60 min of the bioreaction, the formation of the reaction product increased progressively, with a more pronounced increase for β-D-glucosidase compared to α-L-rhamnosidase. In the final stages of the assay, the production of
p-nitrophenol by α-L-rhamnosidase stabilized, indicating a plateau in its hydrolytic activity. These findings suggest that the overall hydrolytic activity on glycoside substrates may be limited by the α-L-rhamnosidase subunit [
1]. These results are consistent with previous findings obtained in our laboratory using
Aspergillus niger.
3.2. Immobilization of Hesperidinase: Activity and Stability
Once the hesperidinase was produced, the next step was the immobilization by sol-gel encapsulation method and its subsequent application in the juices’ bioprocessing to improve their biologic activity. The activity of the bioencapsulates was evaluated, in a similar way to that carried out for the free enzymatic extract of hesperidinase, using the use of specific substrates p-NPR and p-NPG for the evaluation of the activities of the subunits, α-L-rhamnosidase and β-D-glucosidase.
As one of the main objectives of enzyme immobilization is their reuse and recycling, from a circular economy perspective, the ability of encapsulated hesperidinase was evaluated using the specific substrates and fruit juices as an example of food matrix applications. Four consecutive batches were carried out.
Figure 3 presents the results of the activity of the sol–gel-encapsulated hesperidinase.
Figure 3A, indicates that the enzyme maintained its activity after immobilization, even increasing
α-L-rhamnosidase activity up to 45 min of assay. The highest amount of p-nitrophenol released for this subunit was verified at 45 min. For
β-D-glucosidase, the highest p-nitrophenol value (0.059 mg/mL) was obtained at 2 h of the assay (0.059 immobilized and 0.061 free mg/mL). In the first 20 min, a high activity of α-L-rhamnosidase was observed compared to the activity of β-D-glucosidase. Then, the high initial activity of the
α-L-rhamnosidase subunit contributed to a better conversion of polyphenols.
The activity of
α-L-rhamnosidase decreased in the following second and third reutilizations of the sol–gel biocatalyst. The peak of p-nitrophenol released by
β-D-glucosidase was reached at 120 min, in the three reutilizations, while for α-L-rhamnosidase, the maximum value was recorded at 90 min in the fourth use (
Figure 3). The fourth use was the one in which the enzyme expressed the second highest activity, which shows a renewal of the hydrolytic capacity, possibly due to better access of substrates to the active center of the enzyme. To check whether there was a loss of enzyme from the lenses during the second, third and fourth uses, the protein content in solution was evaluated at the end of each one. The results allow us to conclude that there was no measurable loss of enzyme from the lenses during the three assays. The data also show the advantages of the type of immobilization. The encapsulation of hesperidinase in sol–gel thus allowed the formation of a matrix to which the enzyme was steadily encapsulated. However, the decrease in activity could be due to the fact that most of the enzyme’s active centers were occupied at the end of the first test, leaving few available for other uses. To evaluate the operational stability of the bioimmobilizates after carrying out the enzymatic assays with specific substrates, their residual activity was determined throughout the various uses of the lenses based on the value of the concentration of p-nitrophenol released after 120 min.
Figure 4 shows the results obtained for the residual activities of immobilized assets with the specific substrate
p-NPG.
The results show a high decrease in the residual activity of the enzyme with β-D-glucosidase activity, from the first to the last use, in the order of 70%.
In the work of Furtado et al. [
6], hesperidinase was immobilized in calcium alginate (2%), k-carrageenan (2%), and chitosan (2%) beads. The relation between the bioprocessing conditions and hesperidinase stability was studied. A residual activity of 193% was obtained with immobilized hesperidinase compared to the soluble form, and a half-life of 770 min was attained with hesperidinase encapsulated in calcium alginate beads.
3.3. Operational Stability
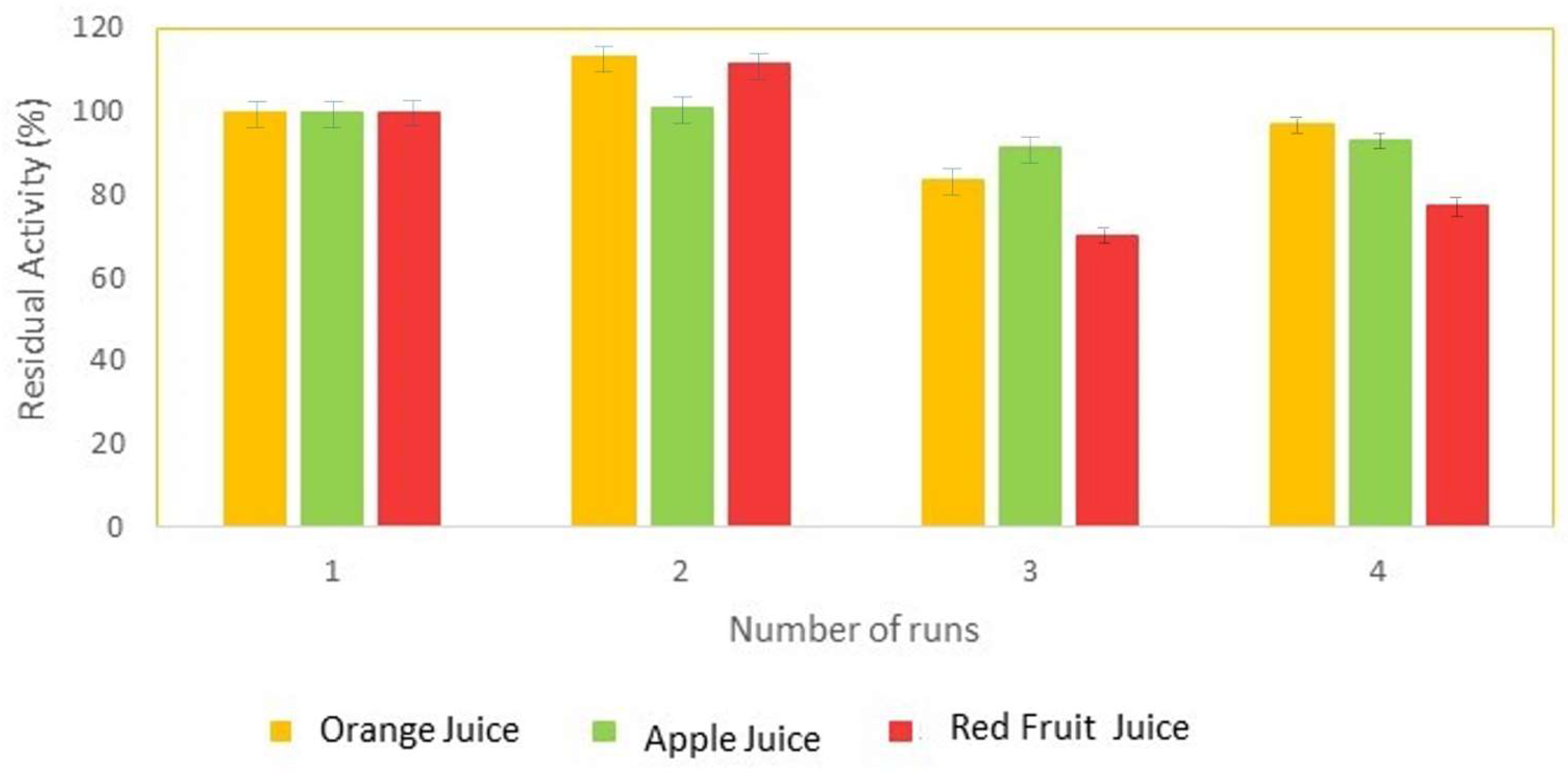
The operational stability of the sol–gel bioencapsulates of hesperidinase was evaluated in the orange, apple, and red fruit juices. The residual activity was evaluated based on the concentration of reducing sugars released after 60 min of assay for each reuse.
Figure 5 shows the results obtained for the residual activities of hesperidinase, with the complex substrates, in the three juices.
The results obtained allow us to observe an increase in the residual activity for all juices, in the second reuse, registering the largest increase for orange juice at 13.4%, and the smallest for apple juice at 0.8%. After three uses, residual activity drops for all juices, with red fruit juice registering the lowest value, showing a decrease of 30%. Finally, in the fourth and final use of the lenses, the residual activity of the bioimmobilizates increased again for all juices, being approximately 97% for orange juice, 93% for apple juice, and 77% for red fruit juice.
It is known that sugars play a role in the stabilization of proteins, particularly enzymes, although there is no single unified theory that explains how this stabilization is achieved [
2]. Sugars can slow down the chemical/physical degradation of proteins by forming hydrogen bonds with them, reducing their local or global mobility for example [
2]. Therefore, the maintenance of the residual activity of bioimmobilized substances observed in
Figure 5 and above all the increases in values between the third and fourth uses may be the result of the stabilization of the encapsulated hesperidinase, either by the sugars present in the juices or by those produced as a result of the bioconversion.
Furtado et al. [
6], to produce hesperitin from hesperidin, used commercial hesperidinase immobilized in calcium alginate beads (2%). In this work, the authors found a decrease in residual activity of 19% and on the third and fourth days to around 3% [
6].
These performances are indicative that the immobilization of enzymes by encapsulation in sol–gel is a more efficient method that assures greater stability of hesperidinase than encapsulation in calcium alginate beads [
6].
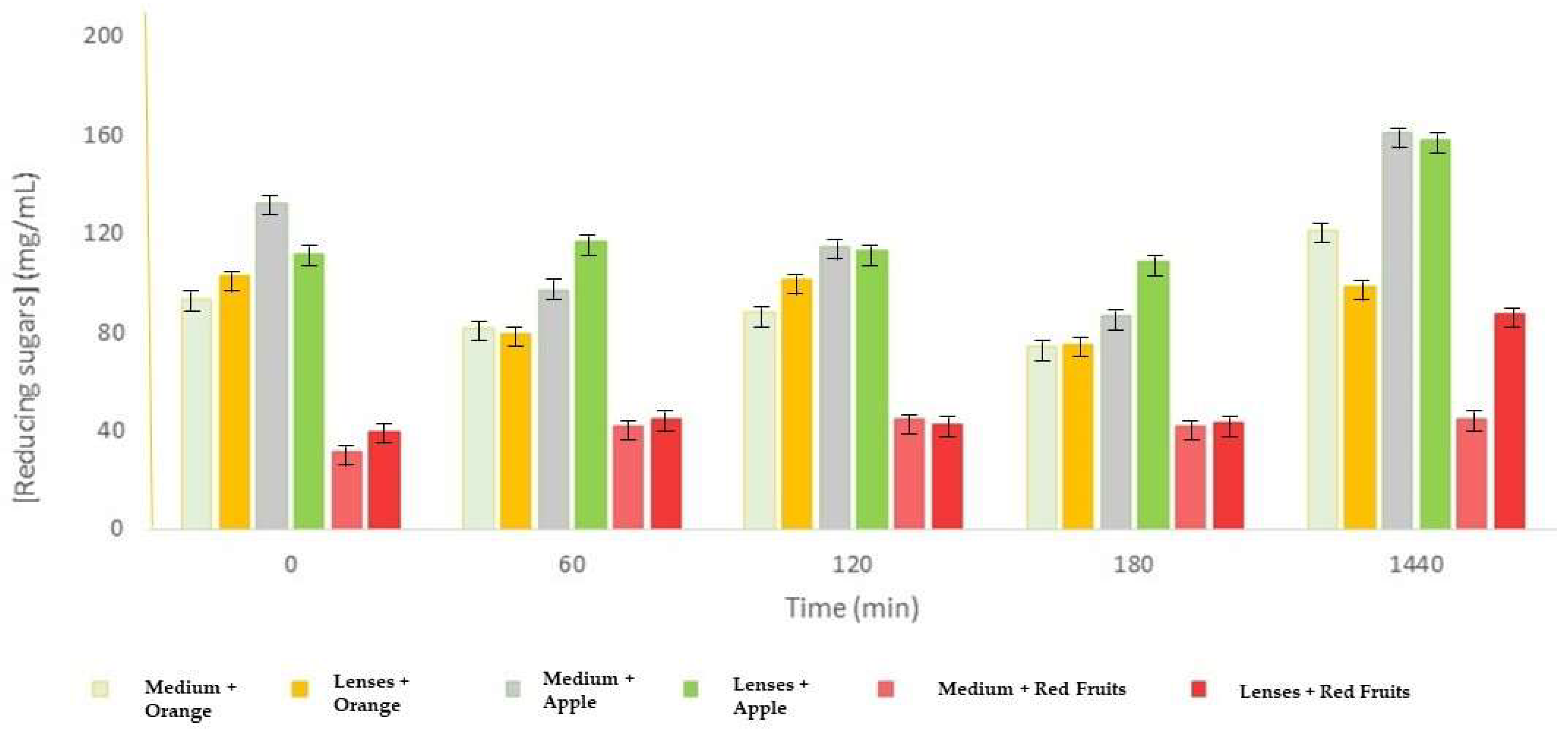
Considering the objective of this study and the analysis of the obtained results, in general, the concentrations are comparable for both forms of the enzyme, with the case of orange juice after 24 h, where the free enzyme exhibited higher values than the immobilized enzyme (
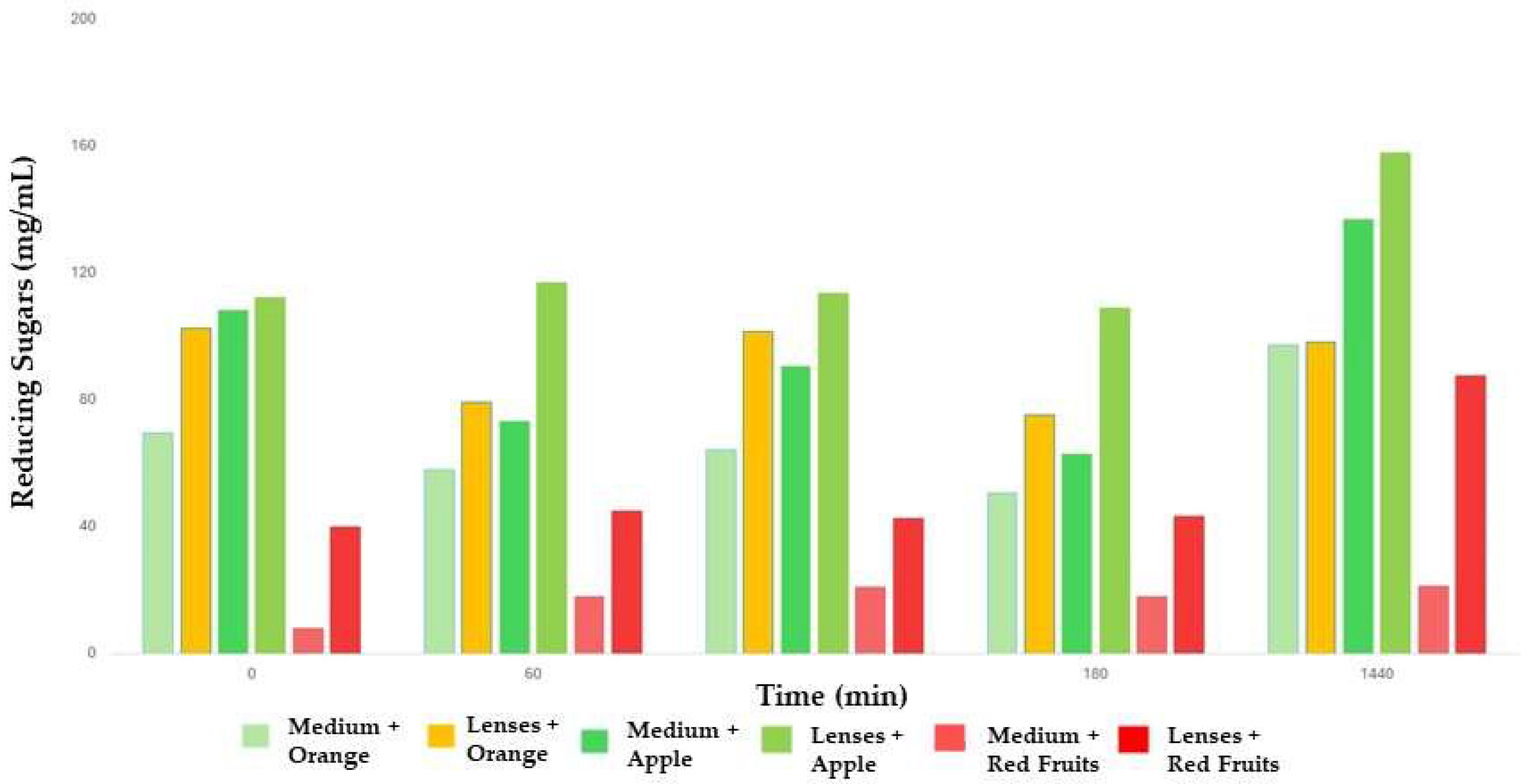
Figure 6). Therefore, for a better estimate of the real values, that is, taking into account only the sugars resulting from the hydrolysis catalyzed by hesperidinase, from the values observed for the free enzyme, the last concentration recorded for the medium during the growth of the fungus, in this case on the 21st day of growth (23.93 mg/mL), was when the fungus practically reached its maximum size. In the case of the immobilized enzyme, the extract was incorporated with the sol solution before the formation of the lenses, that is, before its solidification, meaning that there was no liquid extract in the solution with the juices when carrying out the tests.
The concentration value for the immobilized enzyme was higher for all measurement times. These results show the advantages of immobilizing hesperidinase in a matrix to which it is stably attached, being protected from inactivation and denaturation. In this way, its action at the level of complex substrates, with their hydrolysis to the compounds of interest, is higher in its immobilized form, registering higher concentrations of reducing sugars, which are the products of this hydrolysis. These concentrations are notably higher after 24 h of testing, particularly in apple and red fruit juices (
Figure 7).
3.4. Polyphenols Evaluation
To quantify the polyphenol compounds presented in the three juices, the Folin–Ciocalteu method was used. The juice with the highest content of phenolic compounds was orange (0.630 g/L), followed by red fruit (0.217 g/L) and apple (0.107 g/L). Polyphenols are strongly associated with the various nutraceutical properties of the foods, namely, their antioxidant and anti-inflammatory activities. Apple juice showed the lowest value. In the technological process of converting apples into juice, around 50 to 90% of the polyphenols with health benefits are lost, meaning that most clarified apple juices for sale to the public contain only small amounts of them [
15].
After the bioprocessing of the juices with the sol–gel encapsulates of hesperidinase, an increase of 18% in total polyphenols in red fruit juice (0.256 g/L) was observed, while in orange (0.531 g/L) and apple (0.076 g/L) juices, decreases of 14.7% and 29%, respectively, were detected. The results observed for orange and apple juices can be explained by the chemical characteristics of the polyphenols themselves. These are unstable and susceptible to degradation, or reactions with factors such as oxygen, temperature, and light, during the processing and storage of the products in which they are present [
11].
The increase in red fruit juice can be explained by the fact that the main flavonoids in these juices are anthocyanins. Most of them contain only glucose in their structure [
15], unlike rutin and hesperidin from apple and orange juices, respectively, which contain the rutinose group, which is composed of glucose and rhamnose. Thus, while for rutin and hesperidin, hesperidinase has to express its two activities,
α-L-rhamnosidase and
β-D-glucosidase, to be able to remove the two sugars, for most anthocyanins, it only has to express the
β-D-glucosidase activity to remove glucose. Therefore, and taking into account that
β-D-glucosidase showed higher activity in the assays with specific substrates, it is easier and faster to convert anthocyanins into anthocyanidins than to convert rutin into quercetin and hesperidin into hesperitin. The subsequent increase, in solution, of these anthocyanidins may therefore be responsible for the increase observed.
3.5. Antioxidant Activity
Two methods were used to study the antioxidant activity of juice samples: DPPH and FRAP—the iron reduction method. These are two indirect methods of measuring the antioxidant activity of a sample, as they only estimate its reducing power. Both assays consist of using radicals/complexes as probes that, in the presence of antioxidant compounds, are reduced, changing color. This color change is, in turn, measured spectrophotometrically.
For the FRAP method, and since the analog used is Trolox, an antioxidant compound, the results are expressed in Trolox equivalents (ET), and the higher the concentration obtained, the greater the reducing power of the sample and, consequently, the greater the antioxidant potential [
12].
3.5.1. DPPH
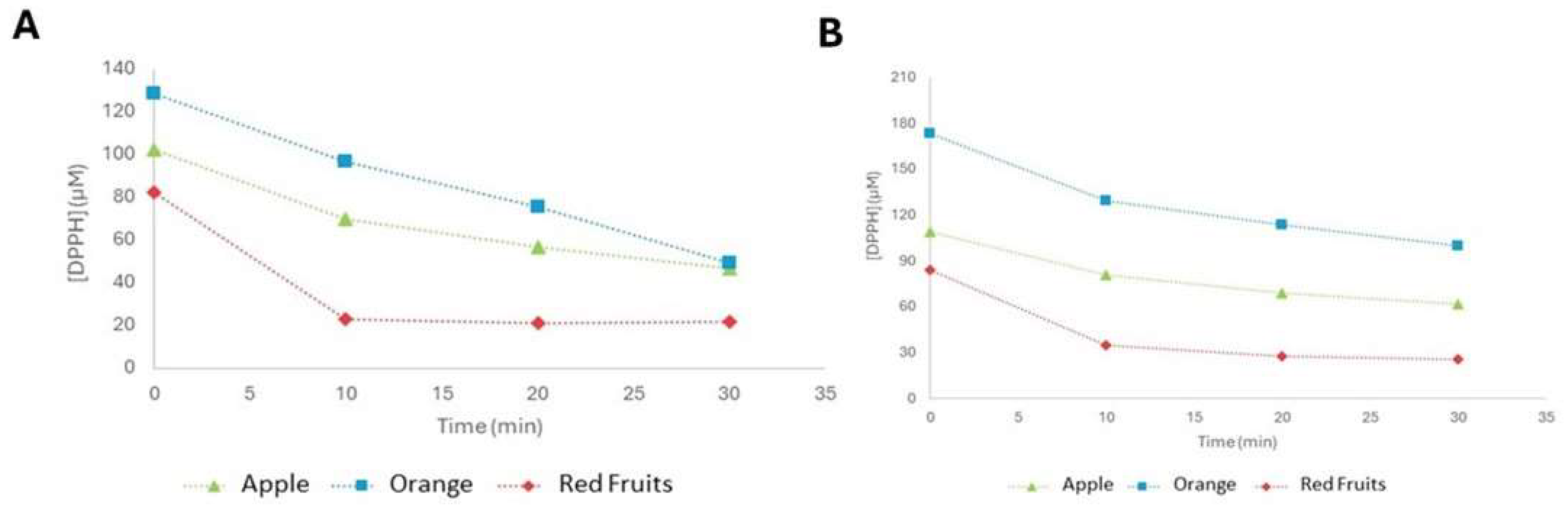
In
Figure 8, it is possible to see the evolution of DPPH concentration over the 30 min of testing for the three juices. Throughout the experiment, all exhibited a decrease in DPPH concentration, namely, 49.33, 46.50, and 21.59 µM for orange, apple, and red fruit juices, respectively, demonstrating the antioxidant activity of all tested juices.
According to the values obtained, it is possible to state that red fruit juice is the one with the highest antioxidant potential. It is also important to note that, although the speed with which the color changes in the samples occur may be indicative of their antioxidant capacity, this is not always proportional to this capacity. The juice that registered the fastest color change, just after the addition of the DPPH agent, was orange, which, on the other hand, was the juice that had the lowest antioxidant potential. However, it is possible to verify that the speed with which the samples changed color is proportional to the concentrations of polyphenols obtained for the respective packaged juices. Orange juice, with the highest polyphenol content, recorded the fastest color change, followed by red fruit juice and, finally, apple juice. Even so, despite this correlation being verified, it is also possible to observe that a higher concentration of polyphenols does not necessarily mean greater antioxidant activity.
After the bioprocessing of the three juices with sol–gel encapsulates of hesperidinase, a decrease in the concentration of DPPH was observed during the 30 min testing period for all juices, indicating that they presented antioxidant activity values (
Figure 8A) of 100, 61.78, and 25.84 µM of DPPH for orange, apple, and red fruit juices, respectively.
As for juices, the results show that the bioprocessed red fruit juice is the one with the greatest antioxidant potential, followed by apple and, finally, orange (
Figure 8B). However, there was a reduction in this potential compared to the former. This decrease was more pronounced in orange juice, for which there was a 103% increase in the concentration of DPPH. In the case of apple juice, this increase was 33%, and in the case of red fruits, it was around 20%.
The degradation of polyphenols leads to changes in their structures which, in turn, lead to a decrease in their biological activities [
11]. These results may indicate that the polyphenols present in orange juice contribute more to its antioxidant activity than those present in apple juice. On the other hand, the increase in the concentration of phenolic compounds, recorded for red fruit juice after bioprocessing, may have contributed to the fact that it experienced the smallest decrease in antioxidant activity.
3.5.2. FRAP
The concentrations of Trolox equivalents (ET) obtained for the orange, apple, and red fruit juices were 11.52, 2.89, and 15.04 mM, respectively. The obtained values confirm that the juice with the highest antioxidant activity was the red fruit juice, as obtained with DPPH.
Odriozola-Serrano et al. [
40] also found low antioxidant activity with the lowest values (0.119 mmol of Trolox/L) in apple juice analyzed by the FRAP assay when compared to orange juice.
Antioxidant activity, determined by DPPH and FRAP by Esposto et al. [
41], was positively correlated with the total phenolic compounds in commercial pomegranate juices.
3.6. Anti-Inflammatory Activity
Inflammation is the body’s response to injuries, infections, and lesions caused by toxic allergens, physical trauma, or microbial agents and is characterized by consequent heat, redness, swelling, and pain [
13,
15]. It is a process activated by the production and release of pro-inflammatory mediators, such as ROS and cytokines.
The denaturation of proteins, in turn, has been correlated with the occurrence of the inflammatory response, which can act as antigens and promote the appearance of autoimmune diseases, such as rheumatoid arthritis, and inflammatory diseases [
5,
13,
15]. Therefore, natural agents that can prevent the denaturation of proteins constitute an important step in the development of new anti-inflammatory medicines, since existing and commonly used medicines often have adverse effects, particularly on the gastric mucosa, causing irritations that culminate in the appearance of ulcers [
13,
15].
To evaluate the anti-inflammatory activity, the albumin denaturation inhibition method was used, which evaluates the ability of a sample to inhibit the thermal denaturation of the protein when it is subjected to high temperatures. An increase in the absorbance of the samples to the control therefore indicates a stabilization of the protein, ergo, an inhibition of its heat-induced denaturation.
The percentages of inhibition of albumin denaturation were calculated according to the formula for the three packaged juices, in which the control was the 2% albumin solution that was subject to the same treatment as the samples.
The inhibition of albumin denaturation (%) with orange and red fruit juices was 697 and 514, respectively, while apple juice did not show any denaturation. The results show that, compared to the denaturation of the control, orange and red fruit juices displayed a high rate of inhibition of albumin denaturation, especially the first, indicating high antialbumin potential. These values are in line with the polyphenol content recorded for the juices. Orange juice recorded the highest concentration of polyphenols, followed by red fruit and apple juice. The results therefore show a direct correlation between the content of phenolic compounds and the anti-inflammatory activity of the juices. In the case of apple juice, despite having a concentration of polyphenols, this was not reflected in its anti-inflammatory activity, similar to what happened with its antioxidant activity, which was shown to be one of the lowest by the DPPH method and the lowest using the FRAP method.
The inhibition of albumin denaturation (%) for bioprocessed orange, apple, and red fruit juices, was 2665, 16.8, and 1700, respectively (
Table 1). As for packaged juices, the results show that orange juice was the one with the greatest anti-inflammatory activity, followed by red fruit juice and, finally, apple juice. For all juices, there was an increase in this potential compared to the non-bioprocessed. The most pronounced of all occurred in apple juice, for which there was a high increase in the capacity to inhibit the thermal denaturation of albumin. This is a significant increase when taking into account the incapacity of inhibition recorded for the juice. In the case of orange juice, this increase was around 283%, and in the case of red fruits, it was 231%.
These results are therefore in line with the goals of this work, as there was an increase in anti-inflammatory activity in all bioprocessed juices. Furthermore, taking into account the decreases observed in the polyphenol contents of orange and apple juices, possibly resulting from the degradation of these compounds, it would be expected that decreases in the anti-inflammatory activities of the same juices would be observed, similar to what happened with antioxidant activities; however, this was not the case. Therefore, the results may indicate that the polyphenols present in orange and apple juices contribute more to their anti-inflammatory activity than to their antioxidant activity. On the other hand, those present in red fruit juice seem to contribute more to its antioxidant activity than to its anti-inflammatory activity, since it was for this juice that the smallest decrease in antioxidant activity and the smallest increase in anti-inflammatory activity were recorded.
3.7. Bioprocessing of Juices Using the Sol–Gel Technique
The sol–gel technique has emerged as a promising approach in juice bioprocessing, particularly for immobilizing enzymes and bioactive compounds that enhance juice quality, stability, and bioavailability of nutrients. Free enzymes often suffer from instability and loss of activity. The sol–gel matrix provides a protective microenvironment, maintaining enzyme activity over multiple processing cycles, enhancing enzyme stability, allowing for enzyme reuse, reducing production costs, preventing contamination of the final juice product by free enzymes, and facilitating continuous processing in bioreactors. The enhancement of nutritional and functional properties is another advantage of this bioprocessing. The integration of sol–gel technology in juice processing aligns with sustainable bioprocessing goals, as it reduces chemical additives used for stabilization and clarification; extends enzyme lifespan, lowering waste generation; and enhances juice quality naturally, reducing the need for synthetic preservatives. Overall, the sol–gel method offers a multifunctional approach in juice bioprocessing, providing advantages in enzyme immobilization, bioactive encapsulation, and juice clarification. As research progresses, further innovations in sol–gel materials could enhance industrial applications, making juice production more efficient, cost-effective, and nutritionally superior.