Vibrational Spectroscopies for Investigating Structural and Biochemical Modifications Induced in Hard Dental Tissues by Femtosecond Laser Ablation: A Brief Review
Abstract
:1. Introduction
2. Representative Results of Applications of Vibrational Spectroscopies to Monitor Changes Occurring in Dentin and Enamel after Laser Ablation
2.1. Representative Results of FT-IR Spectroscopy Investigations
2.2. Representative Results of Raman Spectroscopy Investigations
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vogel, A.; Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, S.; Qayyum, H.; Rafaqat, Z.; Qadir, A.; Fayyaz, S.; Khan, A.; Jabeen, H.; Muhammad, N.; Khan, M.A. Laser as an innovative tool, its implications and advances in dentistry: A systematic review. J. Photochem. Photobiol. 2022, 12, 100148. [Google Scholar] [CrossRef]
- Ionin, A.A.; Kudryashov, S.I.; Samokhin, A.A. Material surface ablation produced by ultrashort laser pulses. Phys. Uspekhi 2017, 60, 149–160. [Google Scholar] [CrossRef]
- Walsh, L.J. The current status of laser applications in dentistry. Aust. Dent. J. 2003, 48, 146–155. [Google Scholar] [CrossRef] [PubMed]
- George, R. Laser in dentistry-review. Int. J. Dent. Clin. 2009, 1, 13–19. [Google Scholar]
- Lagunov, V.L.; Rybachuk, M.; Itthagarun, A.; Walsh, L.J.; George, R. Modification of dental enamel, dentin by an ultra-fast femtosecond laser irradiation: A systematic review. Opt. Laser Technol. 2022, 155, 108439. [Google Scholar] [CrossRef]
- He, L.H.; Swain, M.V. Understanding the mechanical behavior of human enamel from its structural and compositional characteristics. J. Mech. Behav. Biomed. Mater. 2008, 1, 18–29. [Google Scholar] [CrossRef]
- Gómez de Ferraris, M.E.; Campos Muñoz, A. Histología, Embriología e Ingeniería Tisular Bucodental. In Editorial Médica, 3rd ed.; Panamericana: Madrid, Spain, 2009; pp. 256–331. [Google Scholar]
- Beniash, E.; Stifler, C.A.; Sun, C.-Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Pupa, U.P.; Gilbert, A. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef]
- Murata, M.; Akazawa, T.; Mitsugi, M.; Arafat, M.; Um, I.W.; Minamida, Y.; Kim, K.W.; Kim, Y.K.; Sun, Y.; Qin, C. Autograft of dentin materials for bone regeneration. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; IntechOpen: London, UK, 2013; pp. 391–403. [Google Scholar]
- Hikov, T.; Pecheva, E.; Montgomery, P.; Antoni, F.; Leong-Hoi, A.; Petrov, T. Precise femtosecond laser ablation of dental hard tissue: Preliminary investigation on adequate laser parameters. J. Phys. Conf. Ser. 2017, 794, 012036. [Google Scholar] [CrossRef]
- Petrov, T.; Pecheva, E.; Walmsley, A.D.; Dimov, S. Femtosecond laser ablation of dentin and enamel for fast and more precise dental cavity preparation. Mater. Sci. Eng. C 2018, 90, 433–438. [Google Scholar] [CrossRef]
- Rapp, L.; Madden, S.; Brand, J.; Walsh, L.J.; Spallek, H.; Zuaiter, O.; Habeb, A.; Hirst, T.R.; Rode, A.V. Femtosecond laser dentistry for precise and efficient cavity preparation in teeth. Biomed. Opt. Express. 2022, 13, 4559–4571. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Bertrand, C.; Vilar, R. Femtosecond laser ablation of enamel. J. Biomed. Opt. 2016, 21, 065005. [Google Scholar] [CrossRef] [PubMed]
- Rapp, L.; Madden, S.; Brand, J.; Maximova, K.; Walsh, L.J.; Spallek, H.; Zuaiter, O.; Habeb, A.; Hirst, T.R.; Rode, A.V. Investigation of laser wavelength effect on the ablation of enamel and dentin using femtosecond laser pulses. Sci. Rep. 2023, 13, 20156. [Google Scholar] [CrossRef] [PubMed]
- Sabel, N.; Karlsson, A.; Sjölin, L. XRMA analysis and X-ray diffraction analysis of dental enamel from human permanent teeth exposed to hydrogen peroxide of varying pH. J. Clin. Exp. Dent. 2019, 11, e512–e520. [Google Scholar] [CrossRef]
- Alves, S.; Oliveira, V.; Vilar, R. Femtosecond laser ablation of dentin. J. Phys. D Appl. Phys. 2012, 45, 24540. [Google Scholar] [CrossRef]
- Shah, D.; Roychowdhury, T.; Bahr, S.; Dietrich, P.; Meyer, M.; Thißen, A.; Linford, M.R. Human tooth, by near-ambient pressure x-ray photoelectron spectroscopy. Surf. Sci. Spectra 2019, 26, 014016. [Google Scholar] [CrossRef]
- Loganathan, S.; Santhanakrishnan, S.; Bathe, R.; Arunachalam, M. Prediction of femtosecond laser ablation parameter on human teeth using chemical compositional analysis. Procedia Manuf. 2019, 34, 379–384. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Sun, Y.C.; Wang, Y.; Lü, P.J. Femtosecond laser for cavity preparation in enamel and dentin: Ablation efficiency related factors. Sci. Rep. 2016, 6, 20950. [Google Scholar] [CrossRef]
- Cassimiro-Silva, P.F.; Rego Filho, F.M.G.; Santos Afonso de Melo, L.; Costa Dias, T.J.; Cruz Falcão, C.; Queiroz de Melo Monteiro, G.; Gomes, A.S.L. Effects of femtosecond laser irradiation on the microshear bond strength of sound and demineralized dentin. J. Laser Appl. 2019, 31, 012002. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y. Proanthocyanidins’ efficacy in stabilizing dentin collagen against enzymatic degradation: MALDI-TOF and FTIR analyses. J. Dent. 2013, 4, 535–542. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Liu, Y.W.; Wang, Y. A Fourier transform infrared spectroscopy analysis of carious dentin from transparent zone to normal zone. Caries Res. 2014, 48, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ruiz, A.J.; de Dios Teruel-Fernández, J.; Alcolea-Rubio, L.A.; Hernández-Fernández, A.; Martínez-Beneyt, Y.; Gispert-Guirado, F. Structural differences in enamel and dentin in human, bovine, porcine, and ovine teeth. Ann. Anat. 2018, 218, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Lada, A. Analysis of dentistry cements using FTIR spectroscopy. Sci. Technol. Innov. 2020, 11, 33–39. [Google Scholar] [CrossRef]
- France, C.A.M.; Sugiyama, N.; Aguayo, E. Establishing a preservation index for bone, dentin, and enamel bioapatite mineral using ATR-FTIR. J. Archaeol. Sci. Rep. 2020, 33, 10255. [Google Scholar] [CrossRef]
- Bistey, T.; Nagy, I.P.; Simò, A.; Hegedus, C. In vitro FT-IR study of the effects of hydrogen peroxide on superficial tooth enamel. J. Dent. 2007, 35, 325–330. [Google Scholar] [CrossRef]
- Diez, C.; Rojo, M.Á.; Martín-Gil, J.; Martín-Ramos, P.; Garrosa, M.; Córdoba-Diaz, D. Infrared Spectroscopic Analysis of the Inorganic Components from Teeth Exposed to Psychotherapeutic Drugs. Minerals 2022, 12, 28. [Google Scholar] [CrossRef]
- Douchy, L.; Gauthier, R.; Abouelleil-Sayeda, H.; Colon, P.; Grosgogeat, B.; Bosco, J. The effect of therapeutic radiation on dental enamel and dentin: A systematic review. Dent. Mater. 2022, 38, e181–e201. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Buylov, N.; Nesterov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Kuyumchyan, S.; Vongsvivut, J. A Study of the Effects of Medical Dental Laser and Diamond Drill on Dentin Tissue during Dental Restoration Based on Spectral Imaging and Multivariate Analysis of Synchrotron FTIR Microspectroscopy Data. Photonics 2023, 10, 881. [Google Scholar] [CrossRef]
- Anwar Alebrahim, M.; Krafft, C.; Popp, J. Raman imaging to study structural and chemical features of the dentin enamel junction. IOP Conf. Ser. Mater. Sci. Eng. 2015, 92, 012014. [Google Scholar] [CrossRef]
- Gaeta, G.; Riccio, R.; De Rosa, A.; Camerlingo, C.; Lepore, M. A comparative study on dentine/resin interface of lased and unlased tooth cavities by micro-Raman spectroscopy. J. Oral. Laser Appl. 2003, 3, 243–249. [Google Scholar]
- Camerlingo, C.; Lepore, M.; Gaeta, G.M.; Riccio, R. Raman spectroscopy on dentin/resin interface of lased and unlased dental samples. In Lasers in Dentistry IX; Rechmann, P., Fried, D., Hennig, T., Eds.; SPIE: St. Bellingham, WA, USA, 2003; Volume 4950, pp. 64–72. [Google Scholar]
- Camerlingo, C.; Lepore, M.; Gaeta, G.M.; Riccio, R.; Riccio, C.; De Rosa, A.; De Rosa, M. Er-YAG laser treatments on dentine surface: Micro-Raman spectroscopy and SEM analysis. J. Dent. 2004, 32, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Gaeta, G.M.; Riccio, R.; Rosso, F.; Muscariello, L.; Lepore, M. Micro-Raman Spectroscopy and E-SEM analysis of hybrid layer at the dentin/resin interface of three different composite restorative resins. In Lasers in Dentistry XII; Rechmann, P., Fried, D., Eds.; SPIE: St. Bellingham, WA, USA, 2006. [Google Scholar]
- Alves, S.; Vilar, R.; Oliveira, V.; Cangueiro, L.; Almeida, A. Femtosecond laser ablation of dentin. In Proceedings of the ICALEO 2012—31st International Congress on Applications of Lasers and Electro-Optics, Anaheim, CA, USA, 23–27 September 2012; pp. 882–888. [Google Scholar]
- Le, Q.T.; Bertrand, C.; Vilar, R. Structural modifications induced in dentin by femtosecond laser. J. Biomed. Opt. 2016, 21, 125007. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, S.; Santhanakrishnan, S.; Bathe, R.; Arunachalam, M. FTIR and Raman as a noninvasive probe for predicting the femtosecond laser ablation profile on heterogeneous human teeth. J. Mech. Behav. Biomed. Mater. 2021, 115, 104256. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.F.; Li, L.; Devlin, H.; Liu, Z.; Whitehead, D.; Wang, Z.B.; Wang, W.; Jiao, J. Ti:sapphire femtosecond laser interaction with human dental dentine. Surf. Eng. 2011, 27, 749–753. [Google Scholar] [CrossRef]
- Weinlaender, M.; Beumer, J.; Kenney, E.B.; Moy, P.K.; Adar, F. Raman microprobe investigation of the calcium phosphate phases of three commercially available plasma-flame-sprayed hydroxyapatite-coated dental implants. J. Mater. Sci. Mater. Med. 1992, 3, 397–401. [Google Scholar] [CrossRef]
- Miro, S.; Costantini, J.M.; Bardeau, J.F.; Chateigner, D.; Studer, F.; Balanzat, E. Raman spectroscopy study of damage induced in fluorapatite by swift heavy ion irradiations. J. Raman Spectrosc. 2011, 42, 2036–2041. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Alamara, K.; Saber-Samandari, S.; Gross, K.A. Micro-Raman spectroscopy shows how the coating process affects the characteristics of hydroxylapatite. Acta Biomater. 2013, 9, 9538–9546. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Biomolecular and bioanalytical applications of infrared spectroscopy: A review. Anal. Chim. Acta 2020, 1133, 150e177. [Google Scholar] [CrossRef]
- d’Apuzzo, F.; Nucci, L.; Delfino, I.; Portaccio, M.; Minervini, G.; Isola, G.; Serino, I.; Camerlingo, C.; Lepore, M. Application of Vibrational Spectroscopies in the Qualitative Analysis of Gingival Crevicular Fluid and Periodontal Ligament duringOrthodontic Tooth Movement. J. Clin. Med. 2021, 10, 1405. [Google Scholar] [CrossRef]
- Faramarzi, B.; Moggio, M.; Diano, N.; Portaccio, M.; Lepore, M. A Brief Review of FT-IR Spectroscopy Studies of Sphingolipids in Human Cells. Biophysica 2023, 3, 158–180. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22. [Google Scholar] [CrossRef]
- Errico, S.; Moggio, M.; Diano, N.; Portaccio, M.; Lepore, M. Different experimental approaches for Fourier-Transform infrared spectroscopy applications in biology and biotechnology: A selected choice of representative results. Biotechnol. Appl. Biochem. 2023, 70, 937–961. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Arends, J. Raman spectroscopy in dental research: A short review of recent studies. Adv. Dent. Res. 1997, 11, 539–547. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy in cell biology and microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Otel, I. Overall Review on Recent Applications of Raman Spectroscopy Technique in Dentistry. Quantum Beam Sci. 2023, 7, 5. [Google Scholar] [CrossRef]
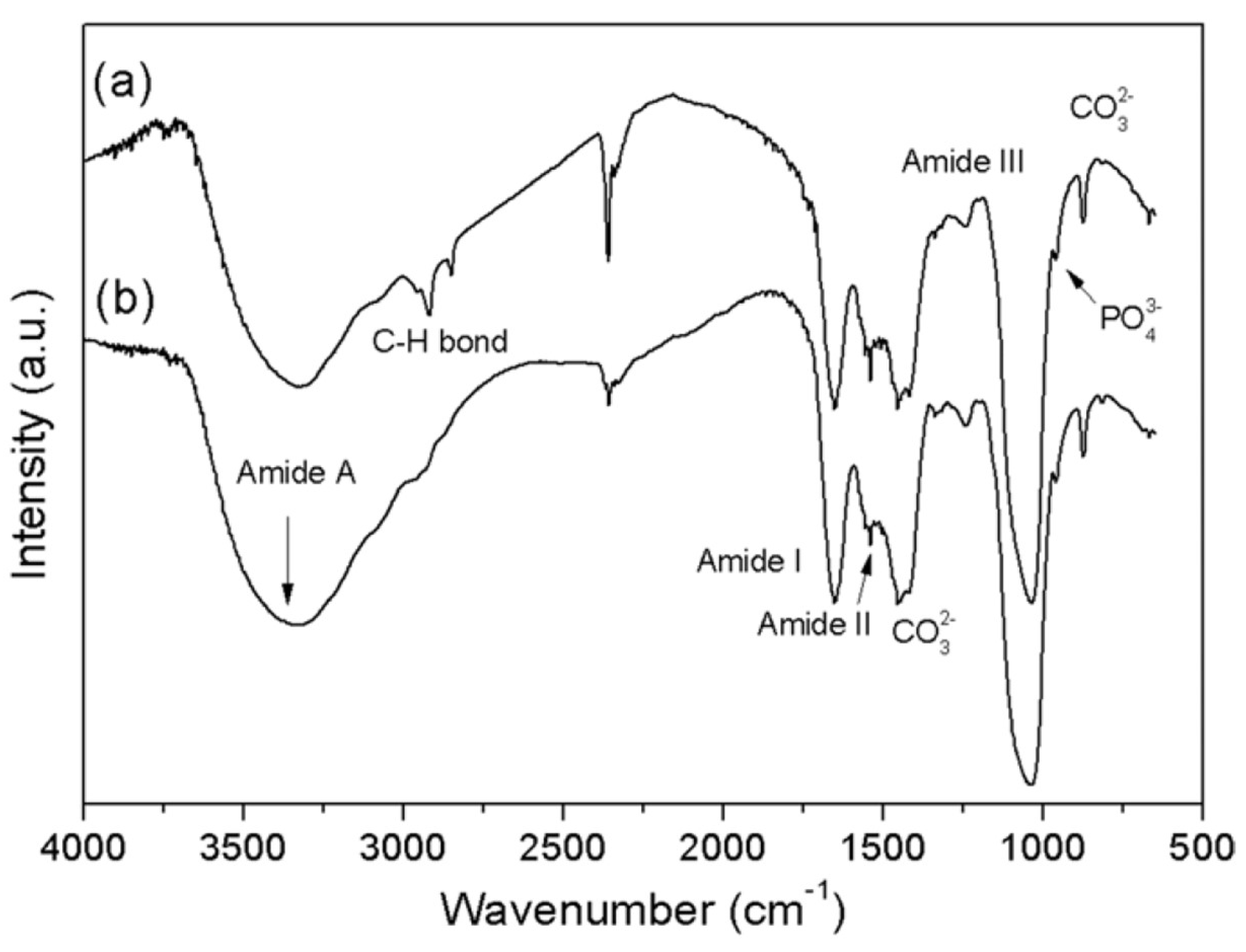
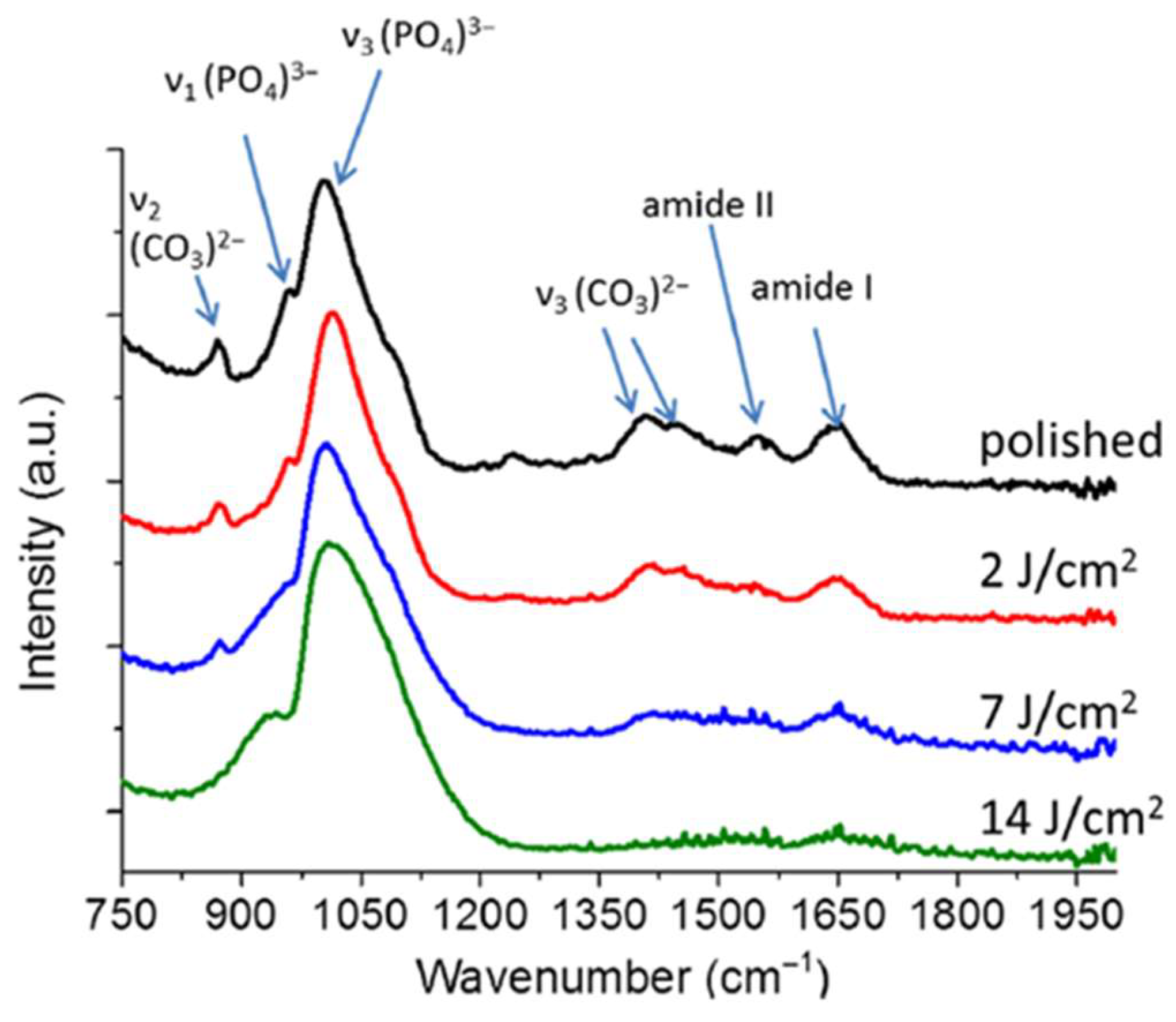
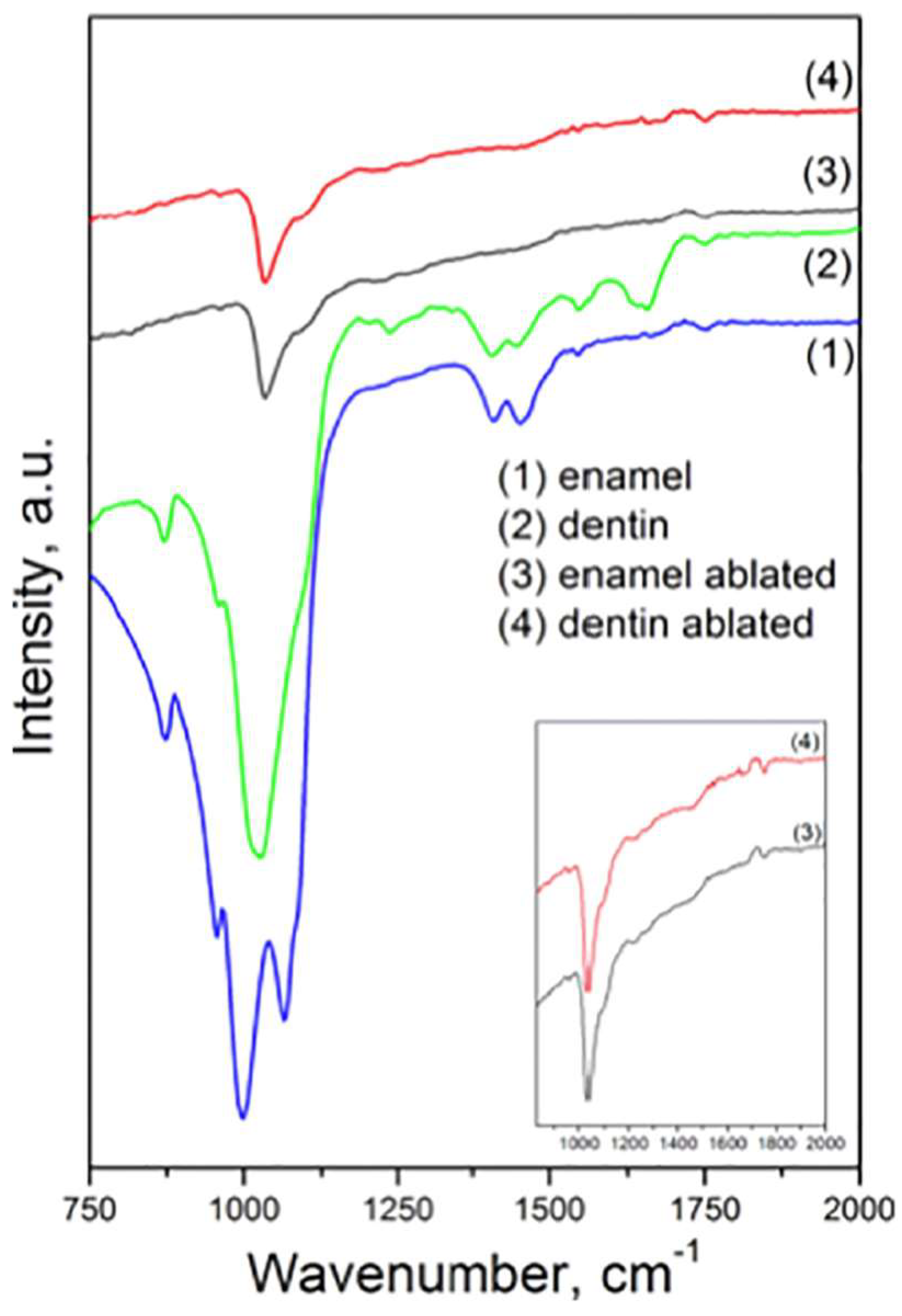
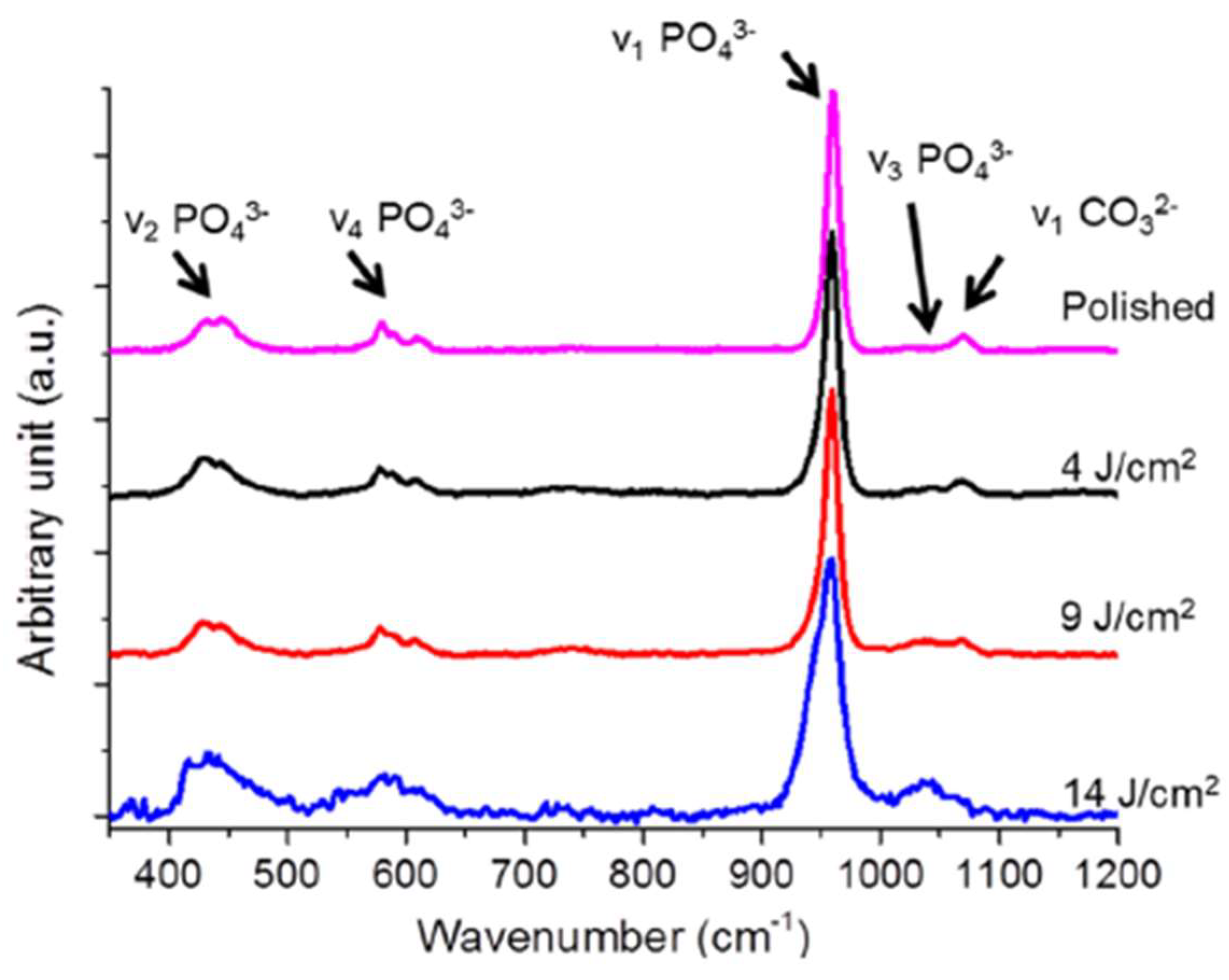
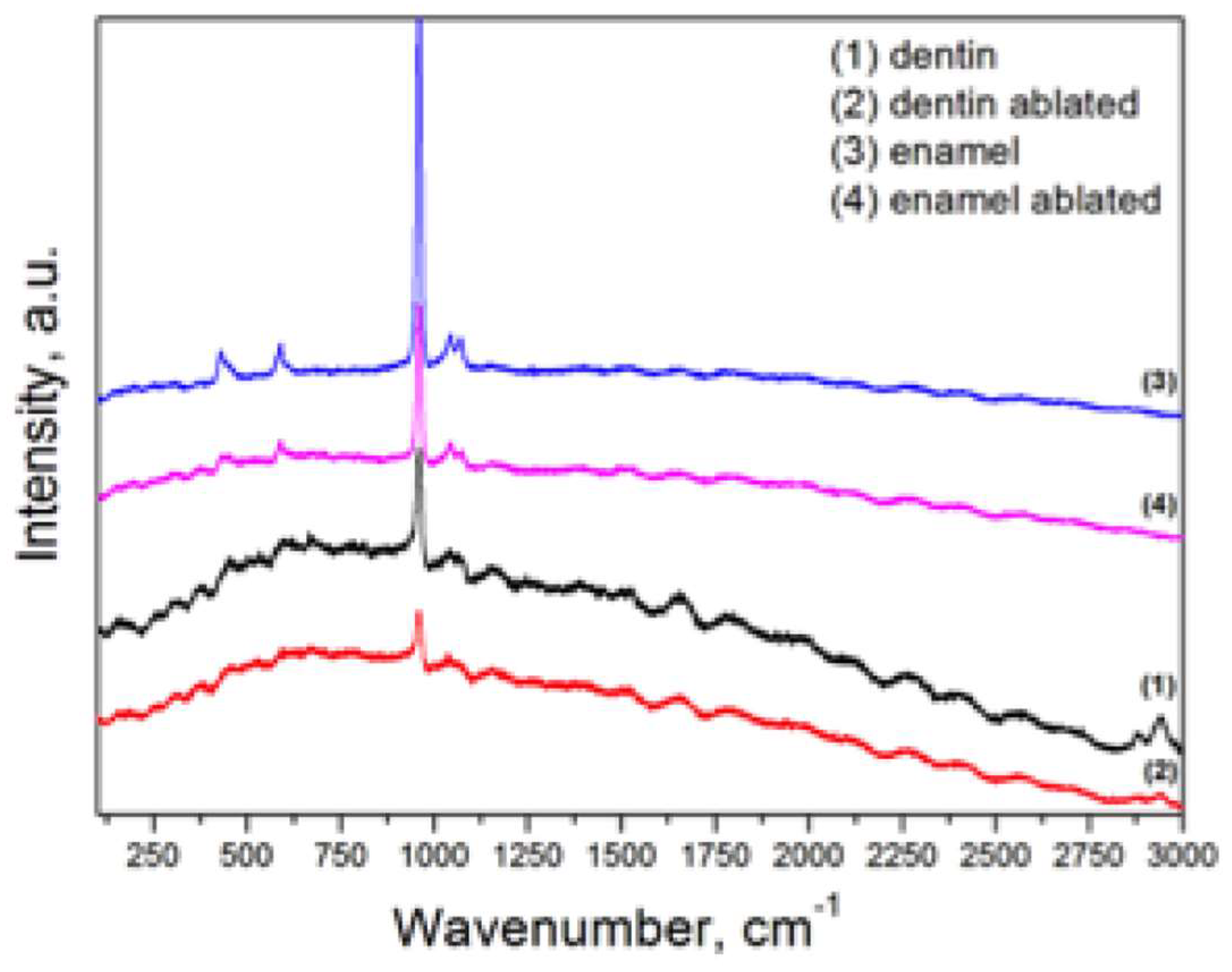

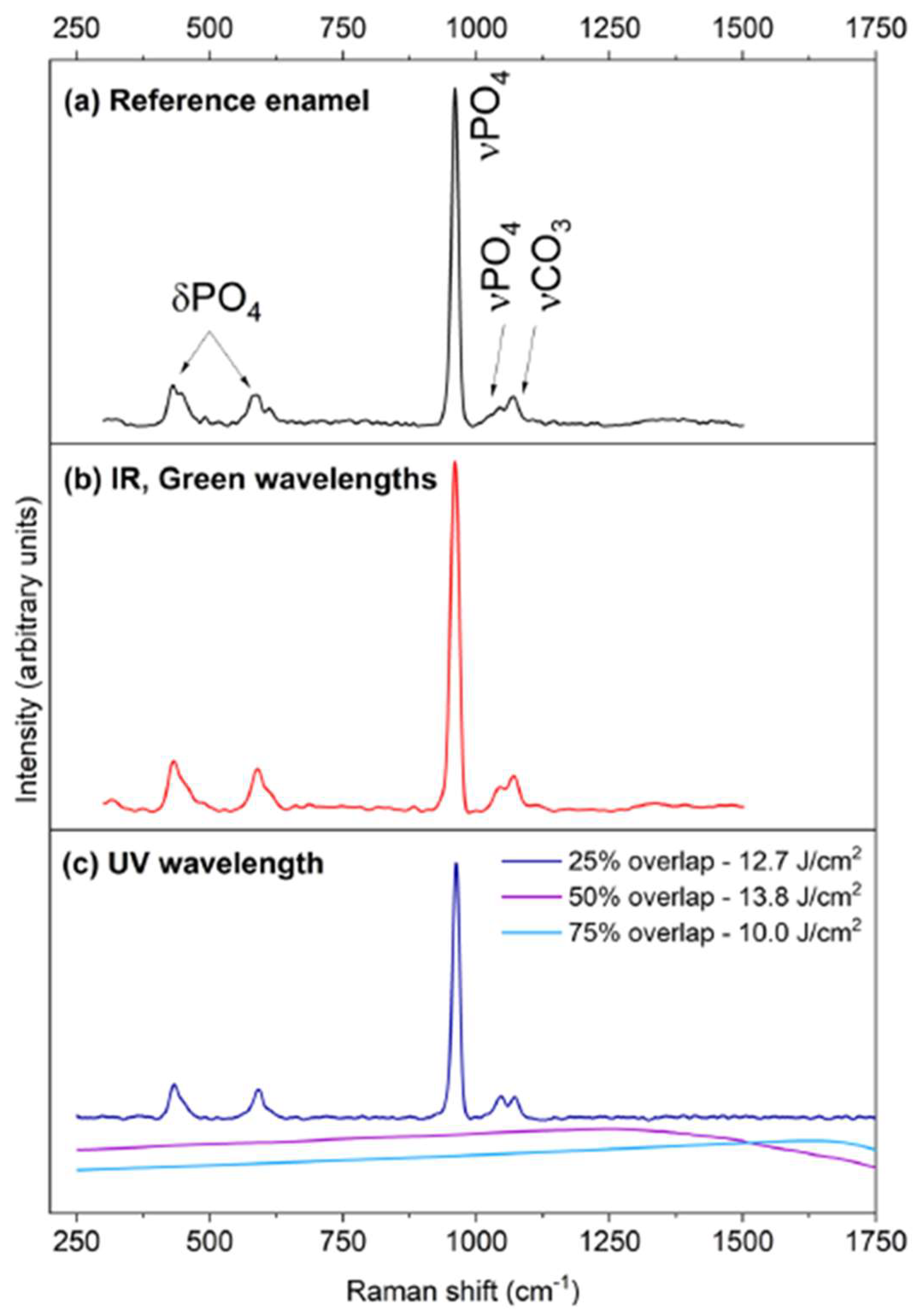
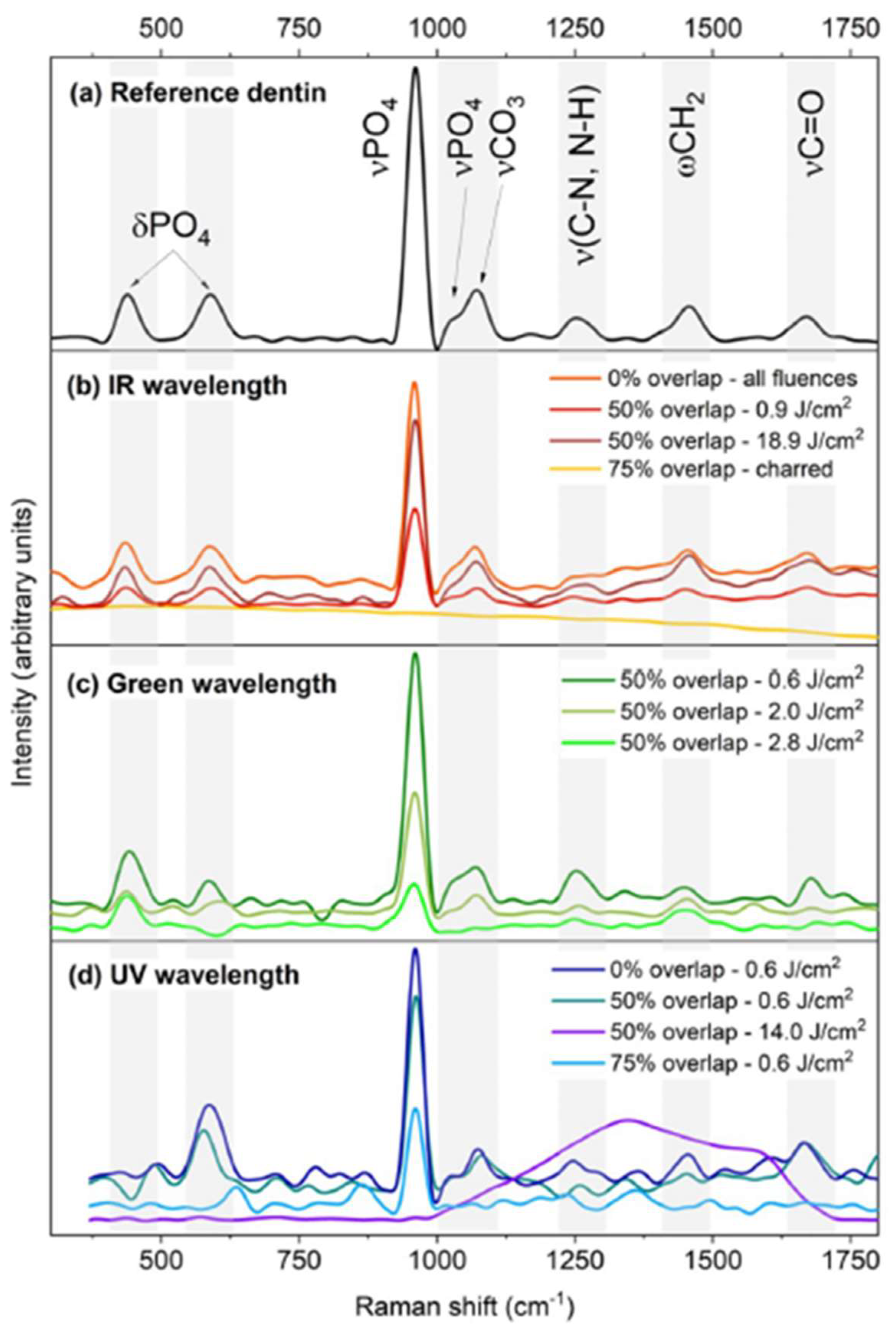
| Dentin | Enamel | |||
|---|---|---|---|---|
| Peak Position (cm−1) [17,36] | Peak Position (cm−1) [14] | Peak Position (cm−1) [12] | Peak Position (cm−1) [12] | Assignments |
| 3320 | ν OH− ν N-H (Amide A) of collagen | |||
| 2920 2850 | ν C-H of organic compounds and contaminants | |||
| 1750 | ν C=O | |||
| 1647 | 1680–1600 | 1650 | absent | ν C=O (Amide I) of collagen |
| 1541 | 1580–1510 | 1550 | 1550 | ν C-N and d N-H (Amide II) of collagen |
| 1456 | 1450 | ν3 CO3−2 substituted in A-type OH− | ||
| 1450 | 1450 | ν3 C-O | ||
| 1437 | 1415 | ν3 CO3−2 substituted in B-type PO43− | ||
| 1410 | 1410 | d C-O | ||
| 1227 | 1237 | absent | Amide III of collagen | |
| 1045 | 1005 | 1066 | ν1 PO43− of HA | |
| Vibrational Technique | Collection Geometry | Accumulated Scans | Spectral Range and Resolution (cm−1) | Investigated Spatial Area | Refs. |
|---|---|---|---|---|---|
| FT-IR | Transmission | 256 | 4000–650; 4 | n. a. | [17] |
| ATR | n. a. | 2000–700; n. a. | n. a. | [37] | |
| ATR | n. a. | 4000–400; 8 | n. a. | [12] | |
| Raman | Acquisition time (s) | Wavenumber shift range and spectral resolution (cm−1) | Laser spot diameter (cm−1) | ||
| Back scattering | n. a. | 700–1000; n. a. | n. a. | [39] | |
| Back scattering (a) | n. a. | 300–1200; 1 | n. a. | [37] | |
| Back scattering (b) | n. a. | 100–3000; 1 | n. a. | [11] | |
| Back scattering (c) | 20 | 300–1800; n. a. | n. a. | [13,15] |
| Dentin | Enamel | |||||
|---|---|---|---|---|---|---|
| Peak Position (cm−1) [39] | Peak Position (cm−1) [11] | Peak Position (cm−1) [13,15] | Peak Position (cm−1) [14] | Peak Position (cm−1) [11] | Peak Position (cm−1) [13,15] | Assignments |
| 430 | 434.83 ± 0.33 | 430 | 427.5 ± 0.4 | δs PO4 | ||
| 444.5 ± 1.6 | δs PO4 | |||||
| 470 | ν2 PO43− of HA | |||||
| 590 | 590 | 584.72 ± 0.4 | δas PO4 | |||
| 614.3 ± 0.7 | δas PO4 | |||||
| 960 | 960 | 960 | 960 | 959.96 ± 0.02 | νs PO43− of HA | |
| 1045 | 1024.55 ± 1.63 | 1025 | 1045 | 1041.4 ± 2.1 | νas PO43− of HA | |
| 1070 | 1070.3 ± 0.8 | C-O bond in CO3 | ||||
| 1247 | 1240.7 ± 0.1 | Amide III | ||||
| 1450 | 1455.44 ± 0.70 | CH2 wagging | ||||
| 1520 | Amide II | |||||
| 1660 | 1665.92 ± 0.75 | Amide I | ||||
| 1780 | ν C=O of carbonyl groups | |||||
| 2882 | ν CH of organic group | |||||
| 2942 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portaccio, M.; Delfino, I.; Gaeta, G.M.; Romeo, U.; Lepore, M. Vibrational Spectroscopies for Investigating Structural and Biochemical Modifications Induced in Hard Dental Tissues by Femtosecond Laser Ablation: A Brief Review. Compounds 2024, 4, 587-603. https://doi.org/10.3390/compounds4040036
Portaccio M, Delfino I, Gaeta GM, Romeo U, Lepore M. Vibrational Spectroscopies for Investigating Structural and Biochemical Modifications Induced in Hard Dental Tissues by Femtosecond Laser Ablation: A Brief Review. Compounds. 2024; 4(4):587-603. https://doi.org/10.3390/compounds4040036
Chicago/Turabian StylePortaccio, Marianna, Ines Delfino, Giovanni Maria Gaeta, Umberto Romeo, and Maria Lepore. 2024. "Vibrational Spectroscopies for Investigating Structural and Biochemical Modifications Induced in Hard Dental Tissues by Femtosecond Laser Ablation: A Brief Review" Compounds 4, no. 4: 587-603. https://doi.org/10.3390/compounds4040036
APA StylePortaccio, M., Delfino, I., Gaeta, G. M., Romeo, U., & Lepore, M. (2024). Vibrational Spectroscopies for Investigating Structural and Biochemical Modifications Induced in Hard Dental Tissues by Femtosecond Laser Ablation: A Brief Review. Compounds, 4(4), 587-603. https://doi.org/10.3390/compounds4040036









