The Solution Combustion Synthesis of ZnO Nanoparticles Using Allium schoenoprasum (Chives) as a Green Fuel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Extract
2.3. ZnO NPs Synthesis
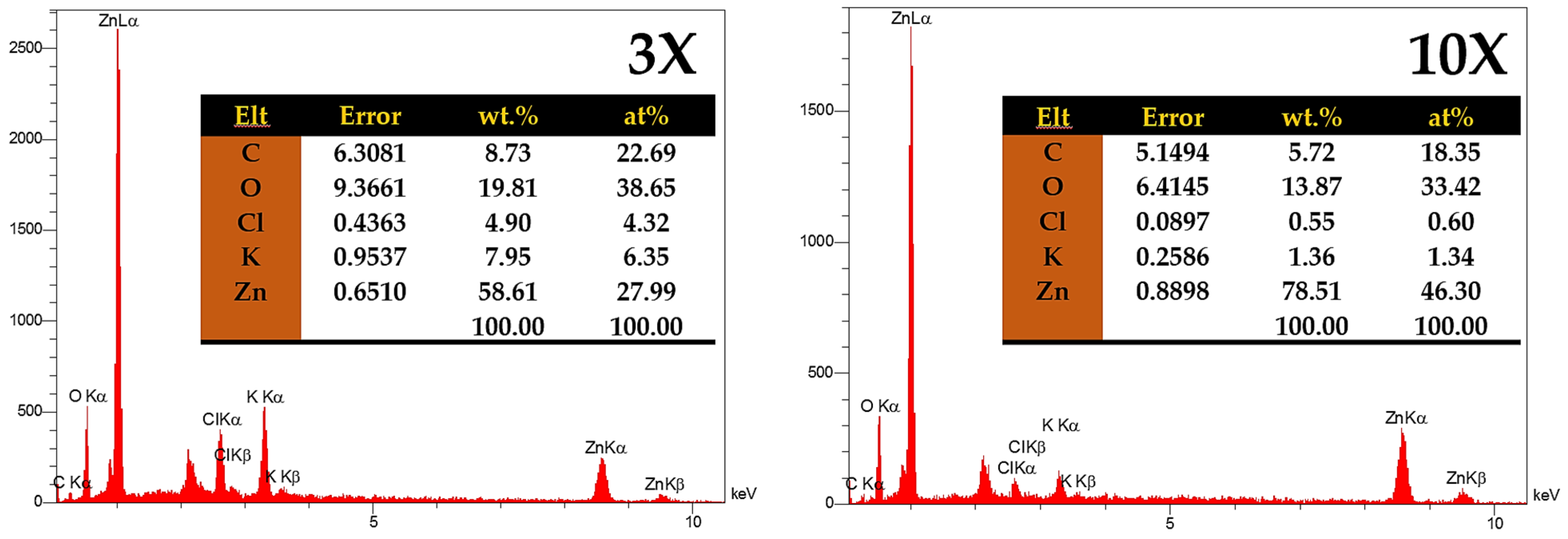
2.4. Characterization of ZnO
3. Results
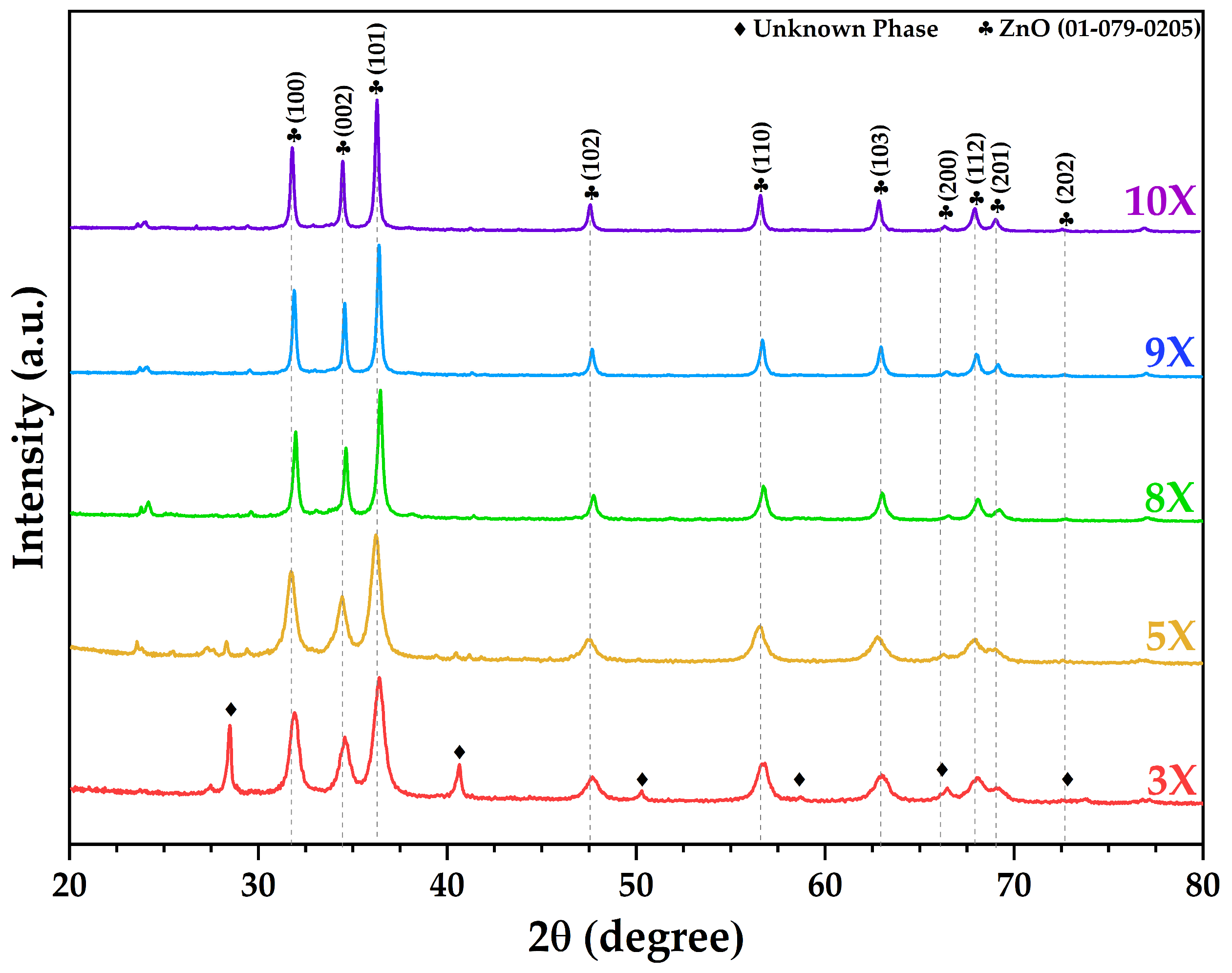
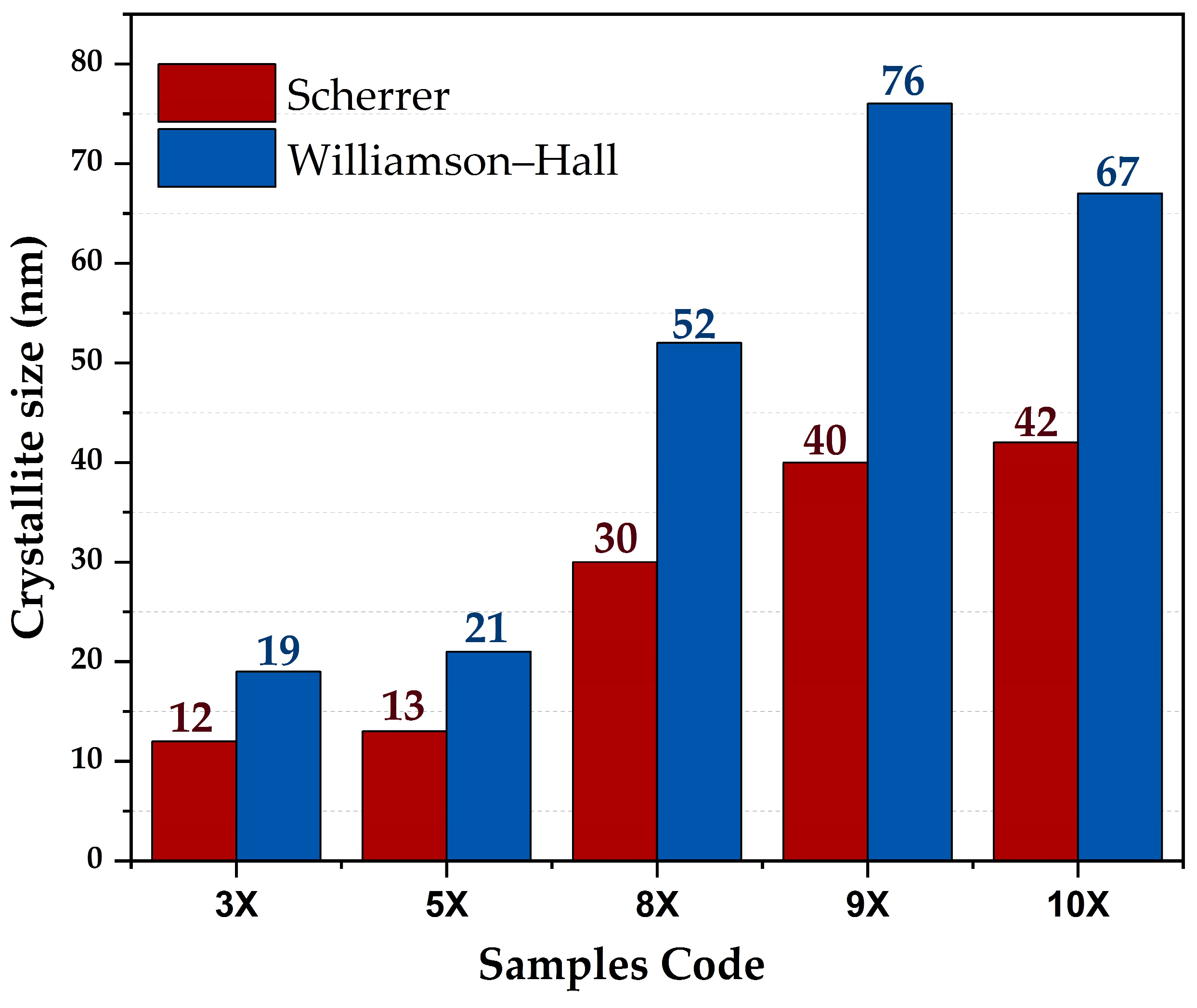
3.1. XRD
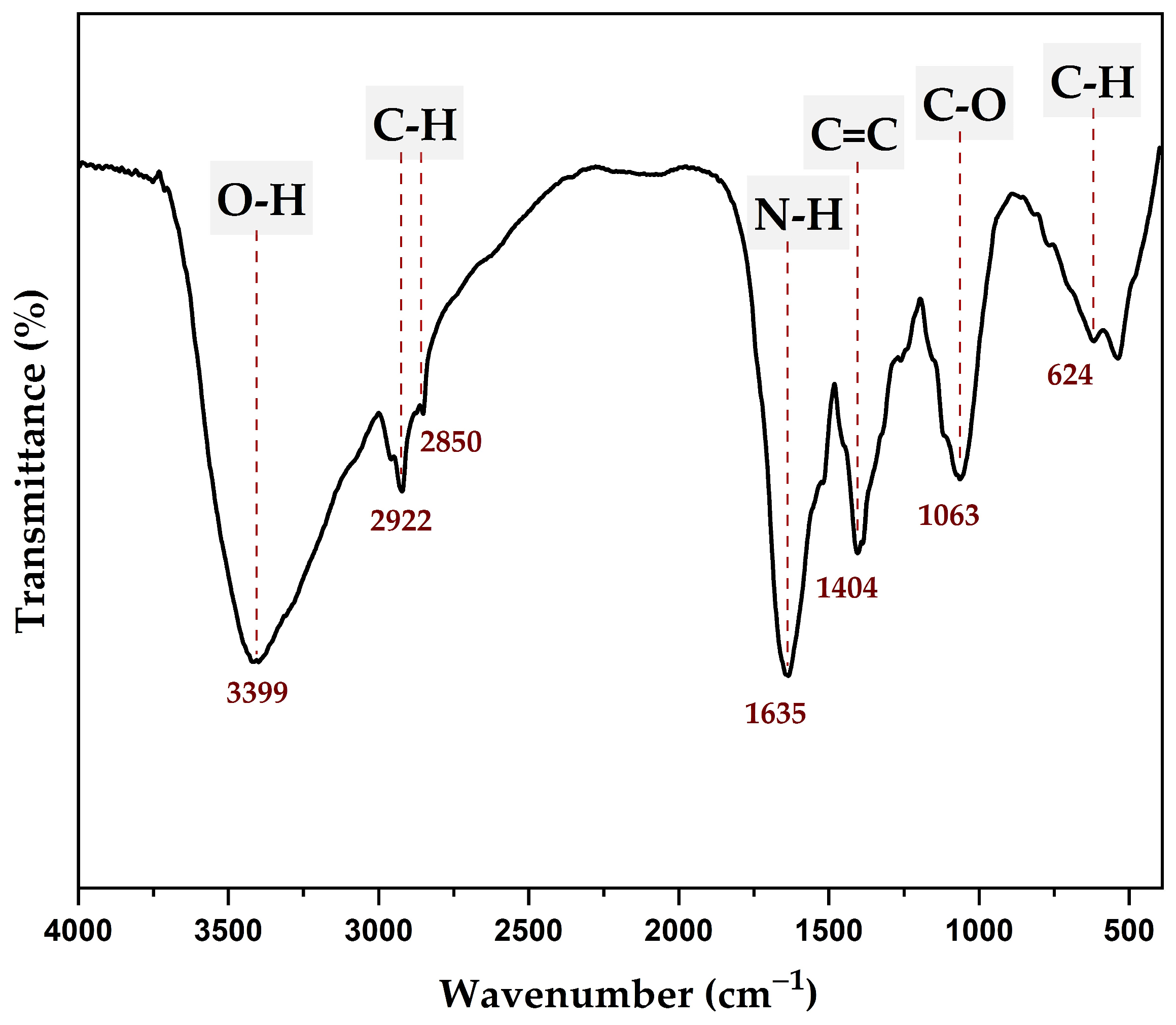
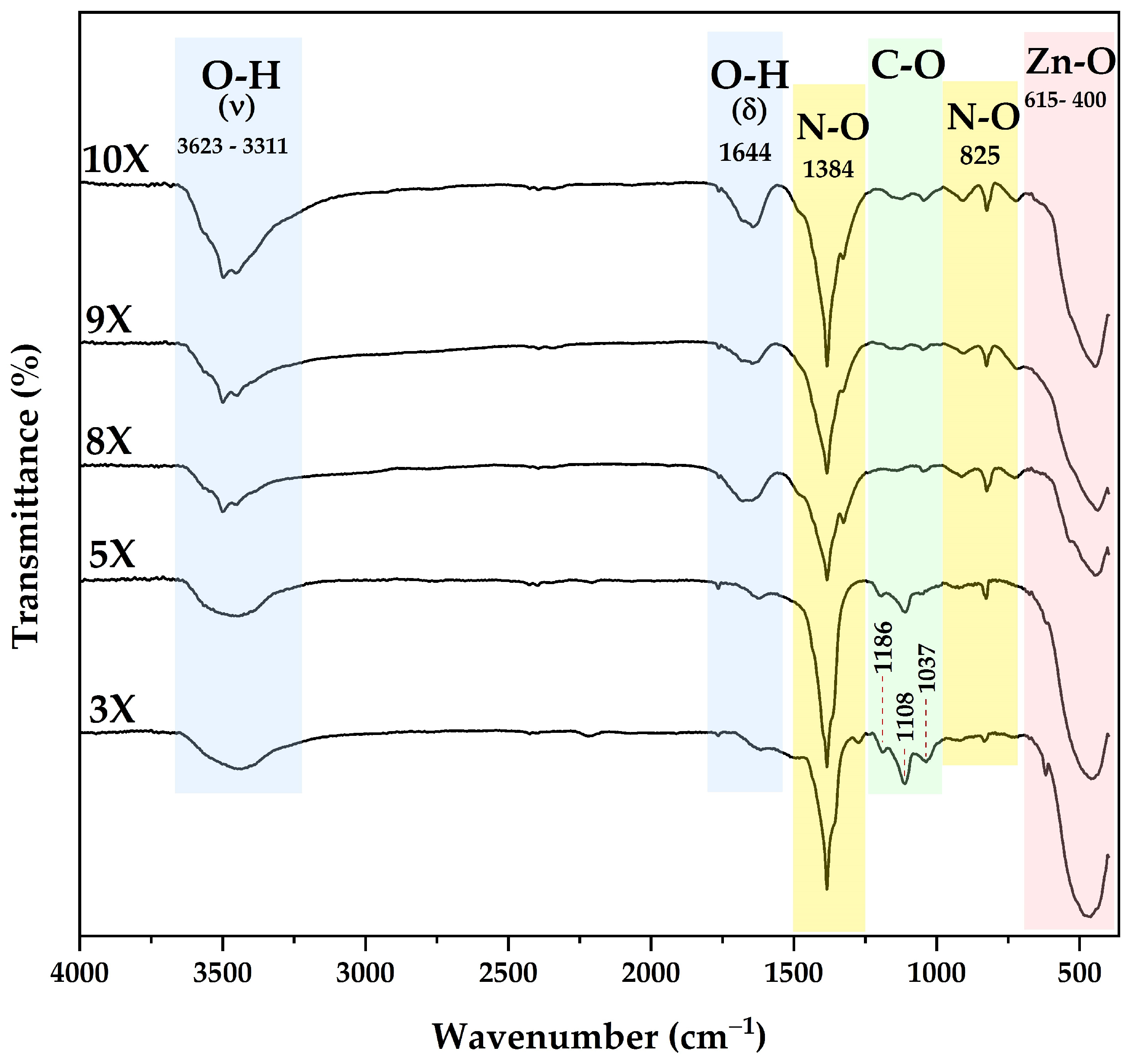
3.2. FTIR
3.3. FESEM
- The heat of the combustion reaction;
- The amount of gas released during the reaction;
- The type and number of functional groups in the fuel.
3.4. Zeta-Potential
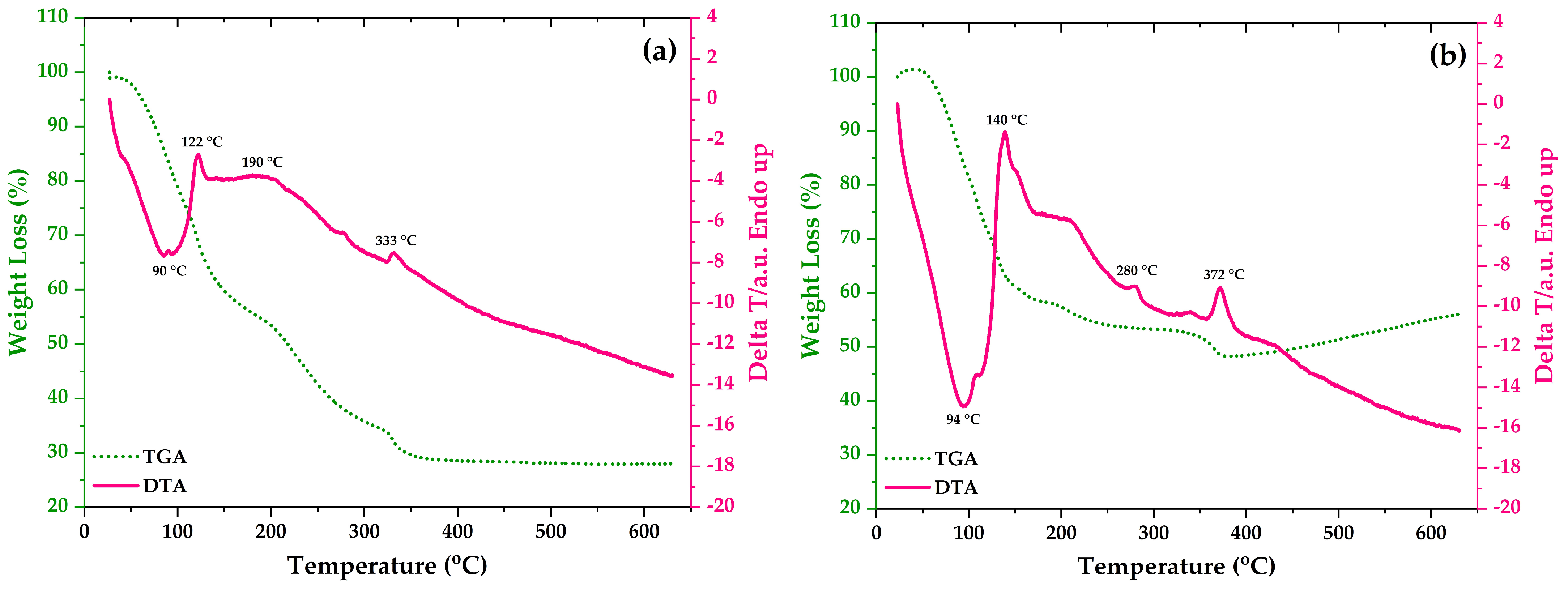
3.5. TG-DTA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed Riyas, Z.; Gayathri, R.; Prabhu, M.R.; Velsankar, K.; Sudhahar, S. Green synthesis and biomedical behavior of Mg-doped ZnO nanoparticle using leaf extract of Ficus religiosa. Ceram. Int. 2022, 48, 24619–24628. [Google Scholar] [CrossRef]
- MalligArjuna Rao, S.; Kotteeswaran, S.; Visagamani, A.M. Green synthesis of zinc oxide nanoparticles from camellia sinensis: Organic dye degradation and antibacterial activity. Inorg. Chem. Commun. 2021, 134, 108956. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Güngör, A.A.; Selvi, İ. Synthesis of nanoparticles by green synthesis method. Int. J. Innov. Res. Rev. 2017, 1, 6–9. [Google Scholar]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Kumar, M.P.; Naika, H.R.; Nagabhushana, H.; Sharma, S.C. Chironji mediated facile green synthesis of ZnO nanoparticles and their photoluminescence, photodegradative, antimicrobial and antioxidant activities. Mater. Sci. Semicond. Process. 2015, 40, 759–765. [Google Scholar] [CrossRef]
- Umar, A.; Sabrina, V.; Yulizar, Y. Synthesis of ZnO nanoparticles using Sapindus rarak DC fruit pericarp extract for rhodamine B photodegradation. Inorg. Chem. Commun. 2022, 141, 109593. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Puttaraju, T.D.; Manjunatha, M.; Maruti, G.; Srinivasan, V.; Haseen Buvabi, S.; Bharathi, T.R. Anthelmintic and antibacterial studies of zinc oxide NPs: Synthesized using dragon fruit juice as novel fuel. Mater. Today Proc. 2021, 47, 4652–4656. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohamad, R.; Mahdavi Shahri, M. Green Microwave-Assisted Combustion Synthesis of Zinc Oxide Nanoparticles with Citrullus colocynthis (L.) Schrad: Characterization and Biomedical Applications. Molecules 2017, 22, 301. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef]
- Hameed, H.; Waheed, A.; Sharif, M.S.; Saleem, M.; Afreen, A.; Tariq, M.; Kamal, A.; Al-onazi, W.A.; Al Farraj, D.A.; Ahmad, S.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles from Green Algae and Their Assessment in Various Biological Applications. Micromachines 2023, 14, 928. [Google Scholar] [CrossRef]
- Iosin, A.; Raba, D.N.; Moldovan, C.; Popa, V.M.; Dumbrava, D.G. The influence of freezing on the content of Vitamin C, chlorophylls and carotenoids in chives (Allium schoenoprasum L.). Sci. Tech. Bull. 2017, 14, 49–52. [Google Scholar]
- Singh, V.; Chauhan, G.; Krishan, P.; Shri, R. Allium schoenoprasum L.: A review of phytochemistry, pharmacology and future directions. Nat. Prod. Res. 2018, 32, 2202–2216. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Mansour, A.T.; Abdelwahab, A.M.; Alprol, A.E. Metal oxide nanoparticles’ green synthesis by plants: Prospects in phyto-and bioremediation and photocatalytic degradation of organic pollutants. Processes 2023, 11, 3356. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant Extract as Reducing Agent in Synthesis of Metallic Nanoparticles: A Review. Adv. Mater. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Goodarzi, V.; Zamani, H.; Bajuli, L.; Moradshahi, A. Evaluation of antioxidant potential and reduction capacity of some plant extracts in silver nanoparticles’ synthesis. Mol. Biol. Res. Commun. 2014, 3, 165–174. [Google Scholar]
- Yilmaz, C.; Gökmen, V. Chlorophyll. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 37–41. [Google Scholar]
- Qiu, N.W.; Jiang, D.C.; Wang, X.S.; Wang, B.S.; Zhou, F. Advances in the members and biosynthesis of chlorophyll family. Photosynthetica 2019, 57, 974–984. [Google Scholar] [CrossRef]
- Paulus, D.; Barbieri, L.; Febraio, A.M.d.O.; Becker, D. Growth and quality of chives in hydroponic system with concentrations of magnesium sulfate. Hortic. Bras. 2022, 40, 311–320. [Google Scholar] [CrossRef]
- Novitskaya, E.; Kelly, J.P.; Bhaduri, S.; Graeve, O.A. A review of solution combustion synthesis: An analysis of parameters controlling powder characteristics. Int. Mater. Rev. 2021, 66, 188–214. [Google Scholar] [CrossRef]
- Rani, S.; Bansal, K.; Rani, N.; Ilyas, M.T.; Singh, G.; Singh, S. Facile solution combustion based synthesis of V2O5 nanocrystals and size-strain study by XRD analysis. AIP Conf. Proc. 2021, 2352, 040024. [Google Scholar]
- Islam, M.R.; Obaid, J.E.; Saiduzzaman, M.; Nishat, S.S.; Debnath, T.; Kabir, A. Effect of Al doping on the structural and optical properties of CuO nanoparticles prepared by solution combustion method: Experiment and DFT investigation. J. Phys. Chem. Solids 2020, 147, 109646. [Google Scholar] [CrossRef]
- Vahdat Vasei, H.; Masoudpanah, S.M.; Habibollahzadeh, M. Different morphologies of ZnO via solution combustion synthesis: The role of fuel. Mater. Res. Bull. 2020, 125, 110784. [Google Scholar] [CrossRef]
- Srinatha, N.; Dinesh Kumar, V.; Nair, K.G.M.; Angadi, B. The effect of fuel and fuel-oxidizer combinations on ZnO nanoparticles synthesized by solution combustion technique. Adv. Powder Technol. 2015, 26, 1355–1363. [Google Scholar] [CrossRef]
- Lutukurthi, D.N.V.V.K.; Dutta, S.; Behara, D.K. Effect of ignition temperature and fuel amount on photocatalytic activity of solution combustion synthesized ZnO. Ceram. Int. 2020, 46, 22419–22428. [Google Scholar] [CrossRef]
- Rasouli, S.; Moeen, S.J. Combustion synthesis of Co-doped zinc oxide nanoparticles using mixture of citric acid–glycine fuels. J. Alloys Compd. 2011, 509, 1915–1919. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Mohammed, A.K.; Sumaila, A. Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods at different acquisition geometries for determination of crystallite size in nano-scale materials. Mater. Charact. 2013, 85, 111–123. [Google Scholar] [CrossRef]
- Rahman, M. Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Tepe, B.; Sökmen, M.; Akpulat, H.A.; Sokmen, A. In vitro antioxidant activities of the methanol extracts of five Allium species from Turkey. Food Chem. 2005, 92, 89–92. [Google Scholar] [CrossRef]
- Baghalian, K.; Ziai, S.; Naghavi, M.R.; Naghdi Badi, H.; Khalighi, A. Evaluation of allicin content and botanical traits in Iranian garlic (Allium satimm L.) ecotypes. Sci. Hortic. 2005, 103, 155–166. [Google Scholar] [CrossRef]
- Dhivya, A.; Yadav, R. An Eco-approach synthesis of undoped and Mn doped ZnO nano-photocatalyst for prompt decoloration of methylene blue dye. Mater. Today Proc. 2022, 48, 494–501. [Google Scholar] [CrossRef]
- Nga, N.K.; Thuy Chau, N.T.; Viet, P.H. Preparation and characterization of a chitosan/MgO composite for the effective removal of reactive blue 19 dye from aqueous solution. J. Sci. Adv. Mater. Devices 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Stajner, D.; Canadanović-Brunet, J.; Pavlović, A. Allium schoenoprasum L., as a natural antioxidant. Phytother. Res. 2004, 18, 522–524. [Google Scholar] [CrossRef]
- Nga, N.K.; Chinh, H.D.; Hong, P.T.T.; Huy, T.Q. Facile Preparation of Chitosan Films for High Performance Removal of Reactive Blue 19 Dye from Aqueous Solution. J. Polym. Environ. 2017, 25, 146–155. [Google Scholar] [CrossRef]
- Khadiran, N.F.; Hussein, M.Z.; Ahmad, R.; Khadiran, T.; Zainal, Z.; Kadir, W.R.W.A.; Hashim, S.S. Preparation and properties of zinc layered hydroxide with nitrate and phosphate as the counter anion, a novel control release fertilizer formulation. J. Porous Mater. 2021, 28, 1797–1811. [Google Scholar] [CrossRef]
- Wypych, F.; Guadalupe Carbajal Arízaga, G.; Ferreira da Costa Gardolinski, J.E. Intercalation and functionalization of zinc hydroxide nitrate with mono- and dicarboxylic acids. J. Colloid Interface Sci. 2005, 283, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chuo, S.C.; Nasir, H.M.; Mohd-Setapar, S.H.; Mohamed, S.F.; Ahmad, A.; Wani, W.A.; Muddassir, M.; Alarifi, A. A Glimpse into the Extraction Methods of Active Compounds from Plants. Crit. Rev. Anal. Chem. 2022, 52, 667–696. [Google Scholar] [CrossRef] [PubMed]
- Idham, Z.; Nasir, H.; Yunus, M.; Lee, N.; Wong, L.; Hassan, H.; Setapar, S. Optimisation of supercritical CO2 extraction of red colour from roselle (Hibiscus sabdariffa Linn.) calyces. Chem. Eng. Trans. 2017, 56, 871–876. [Google Scholar]
- Vasei, H.V.; Masoudpanah, S.M.; Adeli, M.; Aboutalebi, M.R. Facile synthesis of ZnO nanosheets as ultraviolet photocatalyst. J. Sol-Gel Sci. Technol. 2019, 89, 594–601. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, D.; Feng, T.; Shi, J. Fabrication, structural, and spectroscopic investigation of Tb-doped Lu3Al5O12 phosphor. J. Mater. Res. 2005, 20, 2934–2939. [Google Scholar] [CrossRef]
- Leonardi, S.G. Two-Dimensional Zinc Oxide Nanostructures for Gas Sensor Applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Tabrizi Hafez Moghaddas, S.M.; Elahi, B.; Darroudi, M.; Javanbakht, V. Green synthesis of hexagonal-shaped zinc oxide nanosheets using mucilage from flaxseed for removal of methylene blue from aqueous solution. J. Mol. Liq. 2019, 296, 111834. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Raşa, M.; Philipse, A.P. Evidence for a macroscopic electric field in the sedimentation profiles of charged colloids. Nature 2004, 429, 857–860. [Google Scholar] [CrossRef]
- Assad, N.; Abbas, A.; ur Rehman, M.F.; Naeem-ul-Hassan, M. Photo-catalytic and biological applications of phyto-functionalized zinc oxide nanoparticles synthesized using a polar extract of Equisetum diffusum D. RSC Adv. 2024, 14, 22344–22358. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Guerrero, A.; Romero, A. Efficient and Sustainable Synthesis of Zinc Salt-Dependent Polycrystal Zinc Oxide Nanoparticles: Comprehensive Assessment of Physicochemical and Functional Properties. Appl. Sci. 2024, 14, 1815. [Google Scholar] [CrossRef]
- Abebe, B.; Tsegaye, D.; Sori, C.; Ravikumar, C.R.; Murthy, H.C.A. Synthesis of optically enriched cobalt-doped zinc oxide nanocomposites: Reduction of methylene blue dye. Opt. Mater. 2023, 142, 114072. [Google Scholar] [CrossRef]
- Vahdat Vasei, H.; Masoudpanah, S.M.; Sarmadi, A.; Komeili Birjandi, B. Effect of sulfate group-containing fuels on the morphology of ZnO powders prepared by solution combustion synthesis. J. Mater. Res. Technol. 2020, 9, 11876–11883. [Google Scholar] [CrossRef]
- Vasei, H.V.; Masoudpanah, S.M.; Adeli, M.; Aboutalebi, M.R. Solution combustion synthesis of ZnO powders using CTAB as fuel. Ceram. Int. 2018, 44, 7741–7745. [Google Scholar] [CrossRef]




| Amino Acids | Value per gram |
| Tryptophan | 0.037 g |
| Threonine | 0.128 g |
| Isoleucine | 0.139 g |
| Leucine | 0.195 g |
| Lysine | 0.163 g |
| Methionine | 0.036 g |
| Phenylalanine | 0.105 g |
| Tyrosine | 0.095 g |
| Valine | 0.145 g |
| Arginine | 0.237 g |
| Histidine | 0.057 g |
| Aspartic acid | 0.303 g |
| Glutamic acid | 0.677 g |
| Glycine | 0.162 g |
| Proline | 0.216 g |
| Serine | 0.148 g |
| Minerals | Value per gram |
| Ca (Calcium) | 92 mg |
| Fe (Iron) | 1.6 mg |
| Mg (Magnesium) | 42 mg |
| P (Phosphorus) | 58 mg |
| K (Potassium) | 296 mg |
| Na (Sodium) | 3 mg |
| Zn (Zinc) | 0.56 mg |
| Cu (Copper) | 1.157 mg |
| Mn (Manganese) | 0.373 mg |
| Se (Selenium) | 0.9 μg |
| Lipids | Value per gram |
| Total saturated fatty acids | 0.146 g |
| Total monounsaturated fatty acids | 0.095 g |
| Total polyunsaturated fatty acids | 0.267 g |
| Phytosterols | 9 mg |
| Sample Code | 3X | 5X | 8X | 9X | 10X |
|---|---|---|---|---|---|
| Zinc nitrate (g) | 2.835 | 4.72 | 7.56 | 8.50 | 9.45 |
| Chives extract (g) | 1 | 1 | 1 | 1 | 1 |
| Fuel Type | F/O Ratio | Crystallite Size (nm) | Calculation Method | Particle Morphology | Particle Size (nm) | References | |
|---|---|---|---|---|---|---|---|
| PVP (C6H9NO)n | 0.5 | 36 | Rietveld refinement | Pyramid shape | >1000 | [28] | |
| 0.75 | 22 | Pyramid + Cubic | 353 | ||||
| 1 | 19 | Pyramid + Cubic | 353 | ||||
| 1.5 | 18 | Hexagonal | 114 | ||||
| L-Valine (C5H11NO2) | 0.7 | 31.7 | Williamson–Hall | - | - | [29] | |
| 1 | 29.5 | Spherical shape | 15–50 | ||||
| 2 | 16.2 | - | - | ||||
| Glutamine (C5H10N2O3) | 0.7 | 23.8 | Williamson–Hall | - | - | [29] | |
| 1 | 21.2 | Spherical shape + Nano plate | 14–26 | ||||
| 2 | 19.4 | - | - | ||||
| Mixture of Citric acid(C6H8O7) + glycine(C2H5NO2) | F/O = 1 | C75G25 * | 37 | Scherer | Semi-spherical | - | [31] |
| C50G50 | 40 | Semi-spherical | - | ||||
| C25G75 | 43 | Platelet | - | ||||
| C0G100 | 63 | Platelet | - | ||||
| Urea (CH4N2O) | 0.6 | 75 | Scherer | Pyramid shape (aggregated in flower-like structure) | - | [30] | |
| 1 | 55 | - | |||||
| 1.8 | 48 | - | |||||
| 5.4 | amorphous | Spongy sheet-like | - | ||||
| C. colocynthis extract | Fruit portion ** | 85 | Scherer | Nanoflakes (aggregated in flower-like structure) | 85–100 | [12] | |
| Seed portion | 27 | Hexagonal | 20–35 | ||||
| Pulp portion | 64 | Block-shaped (irregular polygons) | 30–80 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheibani, E.; Soltani Alasvand, S.; Sami, N.; Vahdati Khaki, J.; Mollazadeh Beidokhti, S. The Solution Combustion Synthesis of ZnO Nanoparticles Using Allium schoenoprasum (Chives) as a Green Fuel. Compounds 2024, 4, 503-520. https://doi.org/10.3390/compounds4030030
Sheibani E, Soltani Alasvand S, Sami N, Vahdati Khaki J, Mollazadeh Beidokhti S. The Solution Combustion Synthesis of ZnO Nanoparticles Using Allium schoenoprasum (Chives) as a Green Fuel. Compounds. 2024; 4(3):503-520. https://doi.org/10.3390/compounds4030030
Chicago/Turabian StyleSheibani, Elyas, Saman Soltani Alasvand, Neda Sami, Jalil Vahdati Khaki, and Sahar Mollazadeh Beidokhti. 2024. "The Solution Combustion Synthesis of ZnO Nanoparticles Using Allium schoenoprasum (Chives) as a Green Fuel" Compounds 4, no. 3: 503-520. https://doi.org/10.3390/compounds4030030
APA StyleSheibani, E., Soltani Alasvand, S., Sami, N., Vahdati Khaki, J., & Mollazadeh Beidokhti, S. (2024). The Solution Combustion Synthesis of ZnO Nanoparticles Using Allium schoenoprasum (Chives) as a Green Fuel. Compounds, 4(3), 503-520. https://doi.org/10.3390/compounds4030030




