Abstract
The electrodeposition of copper (Cu), silver (Ag), and their alloys has been a subject of interest since the 19th century. Primarily due to their exceptional features such as good mechanical hardness and electrical conductivity, high resistance to corrosion, and electromigration, Cu–Ag electrodeposits continue to be investigated and developed to improve their properties for different applications. This paper reviews the state of the art in the field of electroplated Cu–Ag alloys in an aqueous solution, with particular emphasis on the observed properties and variety of electrochemical processes used to produce high-quality materials. Moreover, this review paper focuses on the experimental conditions employed for Cu–Ag electrodeposition, intending to understand the basis and manipulate the processes to obtain coatings with superior characteristics and for attractive usage. Finally, the most trending applications of these coatings are discussed depending on different parameters of electrodeposition to provide prospects for potential research.
1. Introduction
A metallic alloy is a combination of two or more metallic elements resulting in either a mixture of distinct phases or a random solid solution of these elements [1]. Alloys have different properties from those of the initial metals they are composed with.
Electrodeposition, or electroplating, is an electrochemical process used to change the surface of materials. This method has a long and interesting history [2] and was first applied in 1805 by Italian inventor Luigi V. Brugnatelli. For instance, silver plating was patented in 1840 [3]. Nowadays, electrodeposition is one of the most implemented techniques utilized to fabricate different metals and alloys [4]. Using this method, the structure, shape, and thickness of the deposits are controlled on the surface of the substrates by modifying the conditions of the process. Furthermore, a large surface area of the deposited alloys can be obtained, a complex shape of the substrates can be used, and various kinetic advantages can be applied by electroplating [5].
The combination of excellent mechanical strength and high electrical conductivity makes Cu–Ag alloy materials of interest for various applications [6]. Compared to pure copper, Cu-based alloys have many advantages including higher chemical and electromigration resistance, mechanical hardness, and electrocatalytic performance [7].
2. Physico-Chemical Properties of Cu, Ag, and Their Alloys
It is widely acknowledged that the phase diagram of the Cu–Ag alloy system refers to the eutectic type with limited solubility of the components in each other (Figure 1). The maximum solubility of Cu in Ag is 14.1 at.%, and the solubility limit of Ag in Cu is 4.9 at.%. The eutectic temperature is between 778 and 779 °C [8].
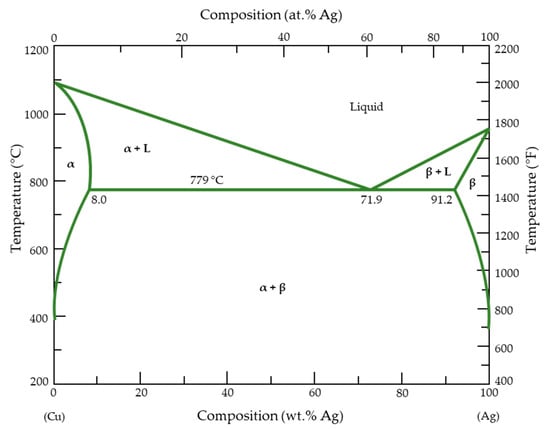
Figure 1.
Phase diagram of the Cu–Ag alloy [9].
According to previously published results [9], if the content of Ag is <6 wt.%, the Cu–Ag alloy consists of a single Cu-rich α-phase [10]. When the Ag content is >6 wt.%, an Ag-rich solid β-phase appears in the primary α-phase dendrite. At 6–15 wt.%, the Cu–Ag microstructure is usually composed of the Cu-rich solid α-phase and the eutectic phase (α-phase + β-phase) [11]. However, the microstructure of Cu–24 wt.% Ag mostly consists of the Cu-rich phase and a reticular eutectic structure [12]. By increasing Ag content up to 71.9 wt.%, the whole microstructure becomes eutectic and is composed of both α- and β-phases [13]. The phase diagram also demonstrates that these two metals are almost entirely immiscible at room temperature, as a result of their positive heat of mixing [14]. Underpotential co-deposition does not happen in the Cu–Ag electrodeposition system because of Cu–Ag unmixing, in contrast to alternative alloy systems with complete miscibility [15]. Nevertheless, under specific synthesis conditions such as mechanical alloying for different milling times [16], Cu and Ag are known to form oversaturated solid solutions. For the production of solid solutions, ball milling in an inert atmosphere for 12 h is sufficient. Additionally, Ag precipitates can nucleate and grow in the grain interiors when the Ag concentration is oversaturated in the Cu matrix. This strengthens Cu by obstructing the dislocation motion [17]. Moreover, copper and silver crystallize in a face-centered cubic structure (Figure 2) and have different lattice constants (Table 1), making the manufacture of their alloys challenging.
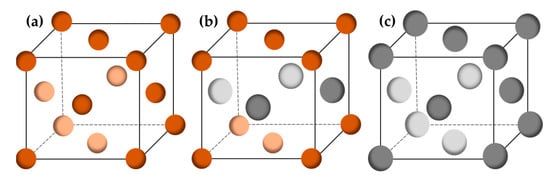
Figure 2.
Crystal structures of Cu (a), Cu0.5Ag0.5 (b), and Ag (c). Brown for Cu; grey for Ag.
Various compositions of Cu–Ag alloys have exceptional malleability, which indicates that they have presumably reached superplastic behavior. The Cu–71.9 wt.% Ag eutectic composition exhibited superplasticity in its annealed equiaxed structure. According to Cline et al. [18], complementary research is needed to replicate this effect and identify the circumstances in which other compositions and microstructures will be able to demonstrate superplasticity.

Table 1.
Lattice constants (in Å) of Ag, Cu, and Cu–Ag alloys with different atomic ratios [19].
Table 1.
Lattice constants (in Å) of Ag, Cu, and Cu–Ag alloys with different atomic ratios [19].
| Substance | a | b | c |
|---|---|---|---|
| Ag | 4.147 | 4.147 | 8.295 |
| Cu | 3.638 | 3.638 | 7.276 |
| Cu0.125Ag0.875 | 4.094 | 4.094 | 8.188 |
| Cu0.25Ag0.75 | 4.037 | 4.037 | 8.075 |
| Cu0.5Ag0.5 | 3.948 | 3.909 | 7.782 |
Cu and Ag metals have an identical electronic configuration since they are members of the same group of chemical elements in the periodic table. Jian et al. constructed Cu–Ag alloys [19] with various atomic ratios by setting several units of the Ag conventional cell and replacing one of the Ag atoms with a substitutional element—Cu. The crystal structures of Ag, Cu0.125Ag0.875, Cu0.25Ag0.75, and Cu0.5Ag0.5 were schematically shown. It was determined that Cu–Ag alloys in the investigated composition range are not thermodynamically stable and that non-equilibrium methods, for example, physical vapor deposition (PVD), should be used for their production.
The calculated lattice constants of Cu–Ag alloy unit cells are presented in Table 1. The lattice constants are 4.147 Å and 3.638 Å for Ag and Cu, respectively, which are comparable to the experimental results [20]. For the Cu–Ag alloys, their lattice constants are between that of Ag and Cu, which also gradually decrease as the Cu ratio increases. In contrast, the lattice constants for various alloy structures are nearly equivalent for the same atomic ratio.
Furthermore, due to the redox potential difference, it is difficult to manage a simultaneous reduction in Cu and Ag [21]. Another important obstacle is the instability of copper ions in an aqueous medium. Table 2 demonstrates some physical properties (intervals) of the Cu–6 wt.% Ag alloy obtained from different processes and/or metallurgical states [22], and their comparison with pure Cu and Ag at T = 20 °C.

Table 2.
Comparison of physical properties (T = 20 °C) for Cu—6 wt.% Ag alloy and pure Cu and Ag. 100% IACS (International Annealed Copper Standard) is equivalent to 58 × 106 S·m−1 at 20 °C [23].
Nowadays, Cu–Ag alloys are extensively utilized in the manufacturing of electrodes, solders for circuit boards, coins, silverware, and jewelry. These alloys, with their high Ag content, are well-recognized due to their mechanical and electrical characteristics [24]. Numerous material properties such as, for example, electrical conductivity and Brinell hardness display a plateau between 30 wt.% and 80 wt.% of Cu as was shown by Broniewski et al. in Figure 3 [25]. The mechanical properties of Cu–Ag alloys are relatively comparable over a wide range of compositions. In other words, there is no particular Cu–Ag ratio that can substantially enhance the mechanical properties of the alloy.
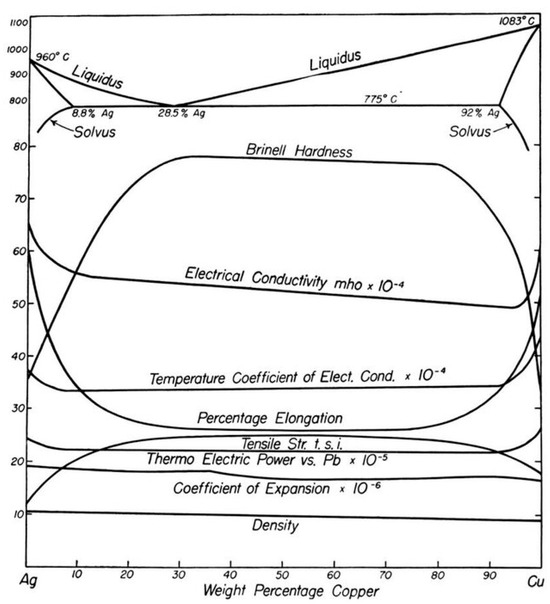
Figure 3.
Properties of annealed Cu–Ag alloys at 25 °C [25].
Considering the existence of many other beneficial properties of Ag (conductivity and antibacterial), it can also improve the Cu(II)/Cu(0) reduction kinetics. Shao et al. implemented cyclic voltammetry of different electrolytes on various metallic substrates and used an electrochemical quartz crystal microbalance to observe the first Cu reduction peak at the copper substrate [1]. It was found that the depolarization of reactive species significantly depends on the presence of silver, either in ionic form, in the electroplating bath, or as a substrate, initiating the reduction in Cu(II) at the overpotential range during Cu deposition. The authors explained that such an effect may be caused by the catalytic behavior of Ag on the substrate in the intermediate step of Cu(II)/Cu(I). So, this feature can shift the reduction rate of Cu(II)/Cu(0), from Cu(II)/Cu(I) to Cu(I)/Cu(0).
3. Different Techniques Mentioned to Date for the Production of Cu–Ag Alloys
Methods such as laser cladding [26], blasting [27], microwaves [28], ball milling [29], pulsed laser deposition [30], ion beam mixing [31], PVD [32], the wet chemical method [33], and chemical vapor deposition [34] are usually used to fabricate Cu–Ag alloys by producing a metastable solid solution that kinetically prevents the separation of the constituent metals. Also, Cu–Ag structures with various Ag contents can be obtained via in situ alloying and laser powder bed fusion additive manufacturing [35]. In this case, increased Ag content from 10 at.% to 30 at.% led to a decrease in the amount of pore defects for produced and annealed samples [36]. On the other hand, it was found that annealing may enlarge the porosity by up to 164%. Moreover, this process causes atomic lattice contractions, which lead to improved yield strength, Young’s modulus, and ultimate tensile strength (UTS).
There are many different possibilities to fabricate bulk alloys, for example, mechanical alloying [37], magnetron sputtering [38], cold spray [39], and cold drawing combined with intermediate heat treatments [40]. The last manufacturing method enabled the achievement of ultra-high strength at low reduction and large cross-section conductors. The optimized Cu–24 wt.% Ag alloy wire with a total drawing strain of η = 5.8 showed an electrical conductivity of 65% IACS and a UTS of 1.5 GPa at room temperature. The wires obtained via this technique could be suggested as prospective candidate conductors used in high-field pulse magnets [41].
A fine and dense micro-composite structure is gradually formed by the compression and elongation of two phases, a Cu-rich and an Ag-rich solid solution, during cold working operations such as forging, drawing, swaging, or rolling. The degree of deformation significantly increases the strength of this structure [40]. High strength with the maintenance of relatively high conductivity can be reached for the alloy if cold working is combined with an appropriate intermediate heat treatment [42].
Suitable quantity and process sequences of heat treatment allow the production of wires [43] composed of Cu and Ag fibers with nanometric transverse dimensions, providing the ideal possible superposition of high electrical conductivity and mechanical strength with satisfactory ductility of Cu–Ag alloys [44]. It was reported that a combination of thermal treatments and extensive cold forming achieved the required strength level [41]. The conceivable thermomechanical treatment can include homogenized annealing, prefinal cold rolling, solution annealing, and final cold rolling, as well as isochronal and isothermal annealing up to the recrystallization temperature.
Nestorovic et al. have shown that the anneal hardening effect appeared in the temperature range of 160–400 °C on the Cu–6.6 wt.% Ag alloy. Also, thermomechanical treatment influenced structural modifications and an improvement in electrical conductivity and hardness [45]. Up to 200 °C, the electrical conductivity did not change considerably; however, above 300 °C, it quickly increased. The microstructural coarsening of the heavily deformed linear band structure could be related to this improved conductivity [37].
Another research study presented the parameters of Cu–Ag alloy rods that were obtained by continuous casting [9]. The wires with a diameter of 0.2 mm exhibited a UTS of >1.25 GPa with a simultaneous electrical conductivity of 69% IACS for Cu–15 wt.% Ag, and a UTS of >1.1 GPa with 79.3% IACS for Cu–5 wt.% Ag. This study demonstrated that through reciprocal reactions of precipitation and drawing, the mutually variable solubilities of Cu in the Ag matrix and Ag in the Cu matrix, in addition to temperature changes, provide valuable opportunities for enhancing the mechanical and electrical characteristics of Cu–Ag alloys.
Zhu et al. indicated that after the continuous cold drawing, the Cu–4 wt.% Ag alloy wire with a diameter of 40 μm had a UTS of 1048 MPa, yield strength of 886 MPa, and electrical conductivity of 75.2% IACS [46]. Following the drawing deformation, the Cu–Ag alloy was strengthened by work hardening, solid solution, and grain refinement strengthening, which resulted in yield strengths of 92.8 MPa, 117.9 MPa, and 626.1 MPa, respectively.
Tian et al. discovered that a UTS of 720 MPa was reached in the Cu–8 wt.% Ag alloy after multiple equal-channel angular pressing [47]. A banded structure was observed on the cross-section, and the UTS of the Cu–8 wt.% Ag alloy rose up to ~1.1 GPa after high-pressure torsion. Chang et al. measured the yield strength and UTS of the hot extruded Cu–0.1 wt.% Ag alloy, which were 75 and 168 MPa, respectively [48]. Its elongation was approximately 38%. After cold drawing, the yield strength was increased up to 280 MPa, and the elongation was reduced to 11%.
In addition to the above-mentioned techniques, Cu–Ag films have been obtained via electron beam evaporation [49], electrodeposition in supercritical fluid [50], or by deposition from the melted stage in the vacuum [51].
4. Electroplating of Cu–Ag Alloys
Regarding the application in the interconnections of electronic devices [52], the low-temperature wet-process of Cu–Ag co-deposition is the most relevant and controllable [53]. Due to the electrochemical potential values of copper and silver systems (Figure 4), it is possible to obtain these two metals via electroreduction in aqueous solutions without hydrogen evolution. Therefore, by combining the concentrations of the copper and silver ions and complexing species, alloys of these two metals with various compositions can be obtained using a cheap and green method, i.e., electrodeposition in aqueous electrolytes. This fact explains the very few published works [54,55,56] dealing with the electrodeposition of Cu–Ag alloys in eutectic solvents, which is a more expensive method requiring more stringent conditions (no trace of water, anaerobic conditions, etc.).
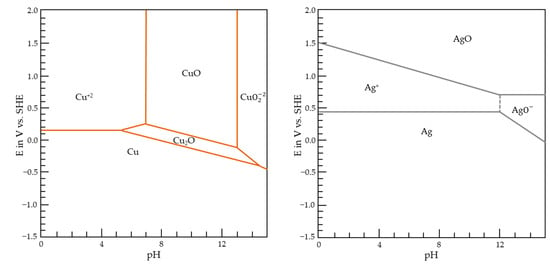
Figure 4.
Potential-pH diagrams for Cu and Ag at 25 °C. The total concentration of copper ion and silver ion at pH = 0 is 10−6 mol/L.
Alloy films are usually obtained by applying a potential or current to conductive substrates submerged in electrolytes containing multiple metal precursors. The composition of electrolytes and applied current (or potential) can manage the contents of metal components. For example, in alloy electrodeposition, it is important to correctly select the electrolyte in order to produce uniform films without any spontaneous formation of the metal precipitates.
The electroplating technique has many benefits, including the ability to produce low-cost nanocrystalline films, the possibility to deposit compact pore-free dense coatings, and the ability to control crystal particle size, microstructure, and roughness [57]. In consequence, plating on any complex shape for antimicrobial touch surface application is achievable.
Bernasconi et al. synthesized Cu–Ag alloys using the electrodeposition method [6]. Different Ag percentages (3.2–15.4 at.%) were achieved by managing the conditions of deposition. Eventually, these as-deposited alloys demonstrated high hardness. The Cu–15.4 at.% Ag alloy, for example, exhibited a hardness of 628 VHN (Vickers hardness). Bao et al. sequentially examined the UTS of Cu, Cu–2 wt.% Ag, and Cu–6 wt.% Ag vs. drawing strain [58]. It was discovered that the strength of Cu–6 wt.% Ag reached approximately 1.24 GPa, more than twice the UTS of pure Cu when the drawing strain was η = 6.
Lee et al. implemented the electrodeposition of nanocrystalline Cu–Ag foil and investigated its properties [59]. The as-deposited foil showed moderate ductility, high UTS (993 MPa), and high electrical conductivity (66.7% IACS). The strength and conductivity were further enhanced with mild annealing (1043 MPa and 68% IACS, respectively), reaching characteristics similar to deformed Cu–Ag foils of greater thicknesses. Another efficient method of strengthening the Cu–Ag alloy without a significant reduction in conductivity is micro-alloying [60].
The electroplating process can be carried out through direct current (DC), pulse current (PC), and pulse reverse current (PRC) modes [61]. All these methods can be used for producing the Cu–Ag alloys. During the DC electrodeposition process, the electrical current is applied to the system continuously in an uninterruptible manner. This conventional technique has been widely implemented for the electroplating of metals and alloys, despite the fact that, in this case, the deposition process is slow, and often it has drawbacks in film defects, such as porosity and poor adhesion [62].
In the PC mode, the current or potential quickly alternates between two different values. This leads to a sequence of pulses separated by zero current that have the same amplitude, duration, and polarity. Each pulse is composed of two phases: an ON-time (tON) when potential and/or current are exerted and an OFF-time (tOFF) when no current is applied. According to priorly attained research [63], electrodeposition via the PC method results in finer grain sizes and an improvement in adhesion to the substrate.
In the case of the PRC technique, the plating current is interrupted, and a stripping time is introduced into the plating cycle. PRC selectively dissolves the protrusions of the metal surface, ensuring uniform deposition. Also, the utilization of additives can be limited by the introduction of high-frequency PRC, which decreases the electrical conductivity and ductility of deposits. PRC avoids the disadvantages of additives, while the superimposed pulsation maintains control over the crystal structure.
Table 3 shows some examples of using electroplating in an aqueous solution with the synthesis conditions used and the following acquired properties.

Table 3.
Examples of using the electrodeposition technique (synthesis conditions used and properties obtained).
5. The Main Electrolytes Used in Electroplating of Cu–Ag Alloys
In general, copper electrodeposition in a bivalent Cu bath without complexing species occurs in two basic sequential steps, which are electrochemical reduction in the Cu(II) species to Cu(I) and then, from the Cu(I) species to Cu(0), as shown in Equations (1) and (2), respectively. The theoretical mass/charge values for these reactions are both equal to 63.6 g/mol (/1e−) [64].
where represents the Cu(I) ion, which is an intermediate species, not stable in solution but adsorbed on the surface of the electrode.
The global system (Cu2+/Cu) has a redox potential , (Cu2+/Cu) equal to +0.34 V vs. SHE—volt vs. the standard hydrogen electrode.
The actual value of the redox potential for the copper system depends on the pH of the solution, and a high value of the pH can provoke the precipitation of CuO and Cu2O, as demonstrated in the potential-pH diagram (Figure 4) and as was calculated from [65]. Copper electrodeposition can be put in competition with the electrodeposition of Cu2O and the precipitation of CuO, as seen in Equations (3) and (4), respectively:
The potential-pH diagram for Ag in an aqueous system at 25 °C is shown in Figure 4. Thus, Ag(I) electrodeposition can be described by an electrochemical reduction to Ag(0) as follows in Equation (5):
, (Ag+/Ag) = +0.80 V vs. SHE.
In particular, copper ions in aqueous baths can exist in diverse complex configurations depending on the used electrolyte.
For example, the electroplating bath prepared for Cu–Ag electrodeposition may consist of Cu(II) ions, Ag(I) ions, and ammonia (NH3) as a complexing agent. NH3 can form complexes with both Cu(II) and Ag(I) ions. The corresponding reactions for the copper complexes are shown in Equations (6) and (7) [7]:
Cu(NH3)42+ + e− ⇄ Cu(NH3)2+ + 2NH3,
Cu(NH3)2+ + e− ⇄ Cu0 + 2NH3.
The values of the standard redox potentials of these two systems are 0.00 V vs. SHE and −0.11 V vs. SHE, respectively.
A one-electron reduction in the silver complex occurs as in Equation (8):
Ag(NH3)2+ + e− ⇄ Ag0 + 2NH3,
This has a standard potential equal to 0.373 V vs. SHE.
The main positive point of the complexation of the ions by ammonia lies in the mass transport control of the kinetic velocities of the reduction reactions that allow us to obtain the desired composition of deposits without affecting the potential but only due to the concentrations of the ions in the electrolyte.
The delivery of a highly effective electrolyte composition is the most challenging direction in the development of Cu–Ag alloys’ electrodeposition [66]. Generally, Cu electrodeposition is performed in an acidic sulfate bath, which frequently contains halogen ions in order to obtain better characteristics of the films [67]. However, Ag cannot be deposited in the electrolyte containing halogen ions since precipitation from their spontaneous reaction with Ag(I) ions will form too easily. The electrolytes for Cu–Ag electroplating require suitable complexing agents (additives) to produce smooth and uniform Cu–Ag coatings. It was noted that the electrodeposition of Cu–Ag alloys from the H2SO4-based bath comprised of CuSO4 and AgNO3 without any complexing agents led to an increase in the surface roughness and demixing of Cu and Ag into separate phases. The addition of thiourea (TU), a complexing agent for both Cu(II) and Ag(I) ions [68], allows the deposition of a homogeneous solid solution via a reduction in [Cu4[SC(NH2)2]9]4+ and [Ag[SC(NH2)2]4]+, respectively. Furthermore, cyanide [69] and citrate ions [70], as well as organic acids, give complexes with Cu(II) [71] and Ag(I) species [72]. Bernasconi et al. obtained a Cu–Ag alloy utilizing two different complexing agents [73]. It was realized from a solution containing pyrophosphate and iodide ions, which react with Cu(II) and Ag(I) ions to form the species Cu(P2O7)2− and AgI43− or AgI32−, respectively [6].
Cyanide-based electrolytes are favorable in Cu–Ag super-filling, which is possible with the assistance of potassium selenocyanate (KSeCN) and TU [74]. However, researchers nowadays are looking for cyanide-free baths due to their extreme toxicity and the limitations of practicing ionic cyanide in the industry [70]. Less toxic pyrophosphate- and ammonia-based electrolytes can potentially replace the cyanide bath [53]. Previous studies focused on ammonia solution [75], hydrazine sulfate [76], methanesulfonic acid (MSA) [77], and protic ionic liquid baths [4]. Although, the results are not encouraging. For example, the parameters for dendritic or nodular cluster growth are still unknown.
Figure 5 presents the main aqueous electrolytes employed for the electroplating of Cu–Ag alloys with their principal advantages (in green) and disadvantages (in red).
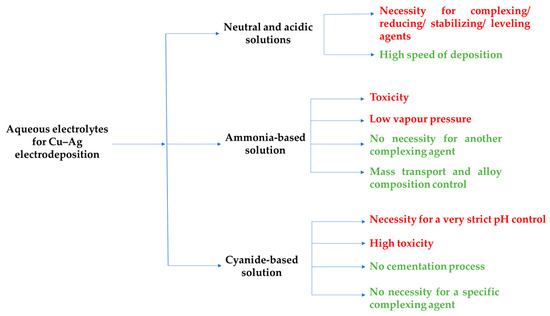
Figure 5.
The summary of aqueous solutions used for electrodeposition of Cu–Ag alloys. Advantages are in green; disadvantages are in red.
Traditionally, electrodeposition is conducted in aqueous solutions containing additives to alleviate the deposition of metals with high negative reduction potentials, which is impeded by low current efficiencies and hydrogen embrittlement of the substrate [78]. The need to deposit refractory metals was the primary driving force behind the development of non-aqueous electrolytes, and molten salts—so-called ionic liquids—have recently been employed for this purpose [79].
Many fundamental issues remain to be resolved, so this ionic liquid-based technology has not found broad industrial application. Firstly, the double-layer structure requires comprehensive analysis and the influence of electrolytes, temperature, composition, and brighteners must be further quantified [80]. The next step is to determine how complexing agents affect the redox characteristics of metals since this is essential for the creation of innovative methods in alloy plating [81]. To enhance mass transport properties, conductivity, and nucleation characteristics, diluents will likely be investigated [82]. The current systems used in aqueous solutions will likely be implemented first, and to produce materials with optimal interfacial properties, it will be necessary to rely on the results obtained from the aforementioned research [83].
For the electrodeposition of nanostructures, deep eutectic solvents (DESs) have been proposed as an alternative sustainable solution [84]. These substances are presently acknowledged as a variety of ionic liquids [56]. DESs are a mixture of Lewis and Brønsted acids and bases that considerably lower the freezing point compared to their constituent parts [85]. Due to their generally high conductivities, DESs can be beneficial in electrochemical processes [86]. Numerous metals were effectively solo electrodeposited in DESs [87] and also several bimetallic compounds [88]. Nevertheless, the electroplating of noble bi- or multimetallic nanostructures in these fluids is still a subject in its formative stages [89]. In addition, the use of a DES as a solvent slows the growth process [90]. Generating a current comparable to that in an aqueous medium requires a higher concentration and temperature in the DES [91].
Ternary Sn–Ag–Cu alloy coatings were electrodeposited by Huang et al. by employing DES-based electrolytes at various deposition potentials [54]. According to the experimental results, when the deposition potential rose, the crystal grain and the coating became finer and denser, respectively. Moreover, higher cathodic deposition potentials promoted the presence of the Cu6Sn5 phase, whereas lower potential values indicated the Ag3Sn phase. The Sn–7.6 wt.% Ag–1.5 wt.% Cu alloy had a melting point of ~215 °C and was suggested as an improved carrier printed circuit board.
Reyna-González et al. performed an electroplating of the Ag–22.5 wt.% Cu alloy on a glassy carbon electrode from a pyridinium-based ionic liquid The co-deposit was obtained at high overpotentials from the extracted phase Ag+–Cu2+–[3-BuPyr][NTf2]–H2O [55].
There is a possibility to acquire ionic liquids that are non-flammable, green, and financially affordable to produce materials that cannot be reached in standard H2O-based electrochemical baths. Despite previous research, the improvement of electrolytes for high-end Cu–Ag electrodeposition remains essential. Table 4 shows some examples of using the above-mentioned types of electroplating baths with the synthesis conditions used and the achievement of properties following prospective future applications.

Table 4.
Examples of using different types of electroplating baths.
6. Various Applications of Electrodeposited Cu–Ag Alloys
Different structures can be formed by modifying the morphology of electrodeposited Cu–Ag alloys. This enables numerous applications from surface-enhanced Raman spectroscopy (EC-SERS) [94] to electrocatalysis [95]. Rajashekhar et al. have reported that a two-step reduction process was used to develop the Cu–Ag nanocrystalline coating via chronoamperometric co-deposition, optimizing precursor composition and deposition time/current [96]. The rate of nucleation and growth of the nanocrystalline alloy cluster were analyzed using scanning electron microscopy. The controlled 15 mA electro co-deposition led to a better overlay of Cu–Ag nanocrystallites with an average particle size in the range of 200–500 nm compared to the 5 mA for Si wafer of the 2.25 × 10−4 m2 mediated alloy. This Cu–Ag coating was performed as a SERS probe. The facile nanocrystallite cluster formation resulting from electro co-deposition could be utilized for other bimetallic components advantageous in biomarker, agro-livestock sensing, and trace chemical investigations.
The composition and surface structure of Cu–Ag catalysts can be regulated by adjusting the quantity of precursors in the electroplating bath. Włodarczyk et al. synthesized the Cu–Ag catalyst applied to the cathode using the electrodeposition method [97]. A Cu foam served as a substrate for the catalyst (Cu–Ag alloy). In their work, the bio-electricity production from yeast wastewater in membrane-less microbial fuel cells with a Cu–Ag cathode was described. According to the measurements, the most favorable catalytic parameters—a power of 6.38 mW and a cell voltage of 1.09 V—have been reached with a Cu–5 wt.% Ag alloy that was oxidized over 6 h and after 3 cycles of anodic charge. Also, compared to pure Cu, which has a film-like structure with a prominence-terminated surface, and pure Ag, which has a multi-pod structure, Cu–Ag catalysts were found to have hierarchical dendritic structures [95].
In addition, the morphology of Cu–Ag deposits is readily modulable from smooth films that were achieved at low electrodeposition rates to nanoparticles or dendrites [98], allowing their implementation in the electrochemical reduction in CO2. The nanoparticles of Cu–Ag alloy have demonstrated great optical, electronic, and catalytic characteristics [99]. Hoang et al. obtained nanoporous Cu–Ag alloys via electrochemical deposition with 3,5-diamino-1,2,4-triazole (DAT) as an inhibitor [100]. The addition of DAT to the electrolyte leads to a nanowire morphology of the Cu–Ag alloy and results in catalysts with a large surface area that are relevant for selective and active CO2 electroreduction to multicarbon hydrocarbons and oxygenates. In comparison to Cu–Ag poly and monometallic copper nanowires, the Cu–6at.%Ag alloy film exhibited the highest reduction current density (nearly −300 mA/cm2 at a cathode potential of −0.7 V vs. RHE—volt vs. the reference hydrogen electrode). Even though the concentration of generated hydrogen has not considerably changed, the Faradaic efficiency (FE) for C2 products was enhanced, reaching ~60% of C2H4 and 25% of C2H5OH [101]. Carbon monoxide is one of the most beneficial outputs of CO2 reduction, and the electroreduction of CO2 to CO was painstakingly researched [102]. Chen et al. electrodeposited Cu–Ag alloy films by using the same DAT inhibitor [103]. The results show that CO production begins at approximately −0.2 V vs. RHE and increases at more negative potentials to achieve a maximum FE value of ~60% at a cathode potential of −0.4 V vs. RHE. However, the FE improves the production of both C2H4 and C2H5OH at increasing negative potentials, while the FE for CO production decreases. This trend confirms the hypothesis that the adsorbed CO serves as an important intermediate in the synthesis of C2 products [104].
Sulfate-, nitrate-, and cyanide-based electrolytes containing Cu(II) and Ag(I) ions are widely used for the mentioned applications of Cu–Ag alloys including great prospects for electronic interconnections [105]. Since one of the major concerns in metal interconnection is electromigration failure, the Cu–Ag interconnects are good candidates to solve this problem without severe deterioration of the electrical performance [106]. Recently, the Cu–Ag alloy has been studied as a potential alternative for interconnections in microelectronic circuits and can possibly be implemented in high-field magnets [107]. Advanced research can be effectively conducted at extremely high magnetic fields (80–100 T) with the help of non-destructive pulsed high-field magnets. The Cu–Ag micro-composites, among other materials, are considered to be promising for usage in the above-mentioned magnets [34].
Regarding the application in ultra-large-scale-integration interconnects, Cu–Ag alloys can increase reliability [108]. This may be the result of the interconnect architecture developed and tailoring copper microstructure in terms of the low-grain boundary and interface diffusion [109], low electrical resistivity, and high mechanical strength [110]. For complete utilization of their alloying effects [111], while keeping a moderately low electrical resistance, an auspicious material modification is necessary. However, alloying additions usually have detrimental effects on one or more of the important characteristics such as thermal stability, electrical resistivity, texture, grain size, and surface roughness. Therefore, the Cu–Ag alloy must offer enhanced mechanical strength and reliability, reduced electrical resistivity, and increased resistance against electromigration effects in order to be accepted as interconnections in future microelectronic devices. Fortunately, it was discovered that in comparison to other copper alloys, alloying Cu with Ag would lead to the lowest increase in resistivity [106].
Further uses of Cu–Ag alloys include the electrodeposition of their particles to ensure improved performance for Zn-air batteries. Also, Cu–Ag films can be used as antibacterial coatings for stainless steel [112].
In addition to the above-mentioned applications, researchers have reported that Cu–Ag alloys have exhibited success in fields such as bactericides [113], decorative artifacts [114], electrocatalysis (H2O2 reduction [115] and ammonia oxidation [116]), electrical contacts (flexible electronics [117] and conductive inks [118]), and electrochemical sensors [119], usually in the shape of nanoparticles or core-shell nanowires.
Also, the metal alloys containing Cu (and Ag) were found to have high antibacterial activity against several types of bacteria: E. coli; S. aureus; MRSA; L. monocytogenes; M. tuberculosis strain R432 and R267; C. albicans; K. pneumoniae; P. aeruginosa; A. baumannii; Salmonella; Enteritidis; S. Typhimurium DT193 S9, DT120 S19 and DT66 S20; S. typhimurium; Streptococcus sp. BY1; Enterococcus sp. BY2; and B. cereus BY3. Metallic materials with antibacterial properties can effectively inhibit bacterial adhesion, growth, and proliferation by using element alloying, suitable metal forming, and heat treatment [27].
Table 5, Table 6 and Table 7 include examples of implementing Cu–Ag alloys as interconnections, catalysts, and SERS investigations with the synthesis conditions used and the properties obtained.

Table 5.
The use of Cu–Ag alloys as interconnections.

Table 6.
The use of Cu–Ag alloys as catalysts.

Table 7.
The use of Cu–Ag alloys for SERS investigations.
7. Conclusions
This review article summarizes different aspects of Cu–Ag alloys’ electrodeposition from aqueous solutions. The process, effective parameters, and related properties were described. Although much research in terms of synthesis, as well as applications, has already been conducted and reported in the literature, there are still many avenues that deserve exploration to gain a better understanding of Cu–Ag systems. Despite the previously carried out studies, it is necessary to further develop electrolytic baths for high-quality Cu–Ag electrodeposition. Moreover, the effects of aging treatment on the mechanical and electrical properties of these alloys remain unclear. Future research is also needed to investigate how Ag contributes to strengthening and creating nano-lamellar structures with a coherent interface that may be a practical method for obtaining Cu–Ag alloys with high strengths and stability. The main applications for these superior alloys are wires and microwires, conductors for DC and pulsed high magnetic fields or fusion magnets, metal sheets, cables for diagnostics, power supplies, and transformers, and electrodes used in resistance welding. To broaden the utilization of Cu–Ag alloys, more studies will be required to determine how organic additives affect Cu–Ag electrodeposition and surface morphology control. Furthermore, the production of materials with excellent resistance against electromigration effects in interconnected metallization is still one of the most promising directions.
Indeed, controlling the electrodeposition processes aims at adjusting the competing forces involved to achieve the desired mass transport. For instance, the formation of quasi-equilibrium structures becomes accessible via the close control of the driving force and is beneficial for the growth of ordered phases or intrinsic compounds. Furthermore, the degree of metastability of the structure during formation can be continuously tuned. This enables the corresponding modification of properties via dopant density, impurity concentration, and defects in products, as well as through alloy composition and atomic configuration in metals.
Therefore, the use of ultrasound during the electroplating process is suggested to improve the composition and physical properties of materials, in particular, corrosion resistance, magnetization, wear, and microhardness. Ultrasound technology showed a high potential in promoting the growth of smooth and uniform surfaces with homogeneously distributed particle content. Also, the imposition of ultrasound induces a decrease in the size of porous structures due to hydrogen absorption in the deposit. Regarding the stability of adhesion between the film and substrate along with the orientation of crystals, ultrasonic waves play an important role.
Concentration polarization and hydrogen evolution throughout electroplating readily affect the deposition efficiency and surface compactness. To improve this efficiency and avoid the cracking of fragile coatings caused by introducing ultrasonic energy, other physical treatments are proposed [127]. For example, the use of moving electrodes allows us to enhance mass transport and deal with slight reactant and ionic concentrations. However, such a method has limited effects and requires intricate cell construction.
The application of an external magnetic field develops the functionalization of the surfaces during the electrodeposition process and is advantageous for obtaining dense alloy films and refining grain size. Moreover, by changing the surface roughness and accelerating the rate of ion mass transfer, the Lorentz force produced through an interaction of the electric and magnetic fields will lead to magnetohydrodynamic phenomena on the cathodic surface, affecting the current distribution and increasing film thickness and hardness as well as the deposition efficiency.
Enhancing the characteristics of Cu–Ag alloys obtained via the electrodeposition technique may be an appropriate way to achieve materials with specific properties and large surface area, as was discussed in this review article.
Author Contributions
Conceptualization, A.-L.D. and J.-P.C.; formal analysis, S.E., F.S.L. and A.-L.D.; investigation, S.E., F.S.L., A.-L.D. and F.D.; writing—original draft preparation, S.E., F.S.L., A.-L.D. and J.-P.C.; writing—review and editing, A.-L.D.; visualization, S.E. and J.-P.C.; supervision, A.-L.D., F.S.L. and J.-P.C. funding acquisition, A.-L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by URCA under the SURFMED grant.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shao, W.; Sun, Y.; Xu, Y.; Zangari, G. Depolarization of Cu Electrodeposition in the Presence of Ag: A Cyclic-Voltammetry Study. Electrochim. Acta 2022, 405, 139796. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe Alloys, Composites, and Nano Coatings—A Review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Elkington, G.; Elkington, H. Improvements in Coating, Covering, or Plating Certain Metals. British Patent 8447, 25 March 1840. [Google Scholar]
- Costa, J.G.d.R.d.; Costa, J.M.; Almeida Neto, A.F.d. Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes. Metals 2022, 12, 2095. [Google Scholar] [CrossRef]
- Tench, D.M.; White, J.T. A New Periodic Displacement Method Applied to Electrodeposition of Cu-Ag Alloys. J. Electrochem. Soc. 1992, 139, 443–446. [Google Scholar] [CrossRef]
- Bernasconi, R.; Hart, J.L.; Lang, A.C.; Magagnin, L.; Nobili, L.; Taheri, M.L. Structural Properties of Electrodeposited Cu-Ag Alloys. Electrochim. Acta 2017, 251, 475–481. [Google Scholar] [CrossRef]
- Jeon, Y.; Choe, S.; Kim, H.C.; Kim, M.J.; Kim, J.J. Electrodeposition of Cu-Ag Films in Ammonia-Based Electrolyte. J. Alloys Compd. 2019, 775, 639–646. [Google Scholar] [CrossRef]
- Hamana, D.; Hachouf, M.; Boumaza, L.; Biskri, Z.E.A. Precipitation Kinetics and Mechanism in Cu-7 Wt% Ag Alloy. Mater. Sci. Appl. 2011, 2, 899–910. [Google Scholar] [CrossRef]
- Kawecki, A.; Knych, T.; Sieja-Smaga, E.; Mamala, A.; Kwasniewski, P.; Kiesiewicz, G.; Smyrak, B.; Pacewicz, A. Fabrication, Properties and Microstructures of High Strength and High Conductivity Copper-Silver Wires. Arch. Metall. Mater. 2012, 57, 1261–1270. [Google Scholar] [CrossRef]
- Benghalem, A.; Morris, D.G. Microstructure and Strength of Wire-Drawn Cu-Ag Filamentary Composites. Acta Mater. 1997, 45, 397–406. [Google Scholar] [CrossRef]
- Yang, H.Y.; Ma, Z.C.; Lei, C.H.; Meng, L.; Fang, Y.T.; Liu, J.B.; Wang, H.T. High Strength and High Conductivity Cu Alloys: A Review. Sci. China Technol. Sci. 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Freudenberger, J.; Lyubimova, J.; Gaganov, A.; Witte, H.; Hickman, A.L.; Jones, H.; Nganbe, M. Non-Destructive Pulsed Field CuAg-Solenoids. Mater. Sci. Eng. A 2010, 527, 2004–2013. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Zhang, Z.F. Stability of Interfaces in a Multilayered Ag-Cu Composite during Cold Rolling. Scr. Mater. 2013, 68, 542–545. [Google Scholar] [CrossRef]
- Barmak, K.; Lucadamo, G.A.; Cabral, C.; Lavoie, C.; Harper, J.M.E. Dissociation of Dilute Immiscible Copper Alloy Thin Films. J. Appl. Phys. 2000, 87, 2204–2214. [Google Scholar] [CrossRef]
- Shao, W.; Sun, Y.; Zangari, G. Electrodeposition of Cu-Ag Alloy Films at n-Si(001) and Polycrystalline Ru Substrates. Coatings 2021, 11, 1563. [Google Scholar] [CrossRef]
- Spassov, T.; Lyubenova, L.; Liu, Y.; Bliznakov, S.; Spassova, M.; Dimitrov, N. Mechanochemical Synthesis, Thermal Stability and Selective Electrochemical Dissolution of Cu-Ag Solid Solutions. J. Alloys Compd. 2009, 478, 232–236. [Google Scholar] [CrossRef]
- Li, A.; Szlufarska, I. Morphology and Mechanical Properties of Nanocrystalline Cu/Ag Alloy. J. Mater. Sci. 2017, 52, 4555–4567. [Google Scholar] [CrossRef]
- Cline, H.E.; Lee, D. Strengthening of Lamellar vs. Equiaxed Ag-Cu Eutectic. Acta Metall. 1970, 18, 315–323. [Google Scholar] [CrossRef]
- Jian, C.C.; Zhang, J.; Ma, X. Cu-Ag Alloy for Engineering Properties and Applications Based on the LSPR of Metal Nanoparticles. RSC Adv. 2020, 10, 13277–13285. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Saunders College: Philadelphia, PA, USA, 1976; ISBN 0-03-049346-3. [Google Scholar]
- Antipas, A.; Dolphin, D.; Gouterman, M.; Johnson, E. Redox Potentials, Charge Transfer, and Emission of Copper, Silver, and Gold Complexes. J. Am. Chem. Soc. 1978, 100, 7705–7709. [Google Scholar] [CrossRef]
- European Copper Institute Basic Properties of Cu94Ag6 Alloy. Available online: http://conductivity-app.org/alloy-sheet/9 (accessed on 27 June 2024).
- Copper Wire Tables, Technical report. In Circular of the Bureau of Standards No. 31, 3rd ed.; United States Department of Commerce: Washington, WA, USA, 1 October 1914.
- Taylor, S.L. An Investigation of the Mechanical and Physical Properties of Copper-Silver Alloys and the Use of These Alloys in Pre-Columbian America. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2017. [Google Scholar]
- Broniewski, W.; Koslacz, S. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences; Académie des Sciences: Paris, France, 1932; p. 973. ISBN 07-052070-4. [Google Scholar]
- Hans, M.; Támara, J.C.; Mathews, S.; Bax, B.; Hegetschweiler, A.; Kautenburger, R.; Solioz, M.; Mücklich, F. Laser Cladding of Stainless Steel with a Copper-Silver Alloy to Generate Surfaces of High Antimicrobial Activity. Appl. Surf. Sci. 2014, 320, 195–199. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial Metals and Alloys for Potential Biomedical Implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Alford, T.L. Microwave Assisted Low Temperature Encapsulation of Ag Films by Cu Reactions Using Ag-Cu Alloy Structures. Mater. Lett. 2012, 89, 163–165. [Google Scholar] [CrossRef]
- Ren, F.; Bellon, P.; Averback, R.S. Nanoscale Self-Organization Reaction in Cu–Ag Alloys Subjected to Dry Sliding and Its Impact on Wear Resistance. Tribol. Int. 2016, 100, 420–429. [Google Scholar] [CrossRef]
- Rao, S.V.; Podagatlapalli, G.K.; Hamad, S. Ultrafast Laser Ablation in Liquids for Nanomaterials and Applications. J. Nanosci. Nanotechnol. 2014, 14, 1364–1388. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, B.Y.; Lau, S.S.; Mayer, J.W. Continuous Series of Metastable Ag-Cu Solid Solutions Formed by Ion-Beam Mixing. Appl. Phys. Lett. 1980, 36, 823–826. [Google Scholar] [CrossRef]
- Higgins, D.; Wette, M.; Gibbons, B.M.; Siahrostami, S.; Hahn, C.; Escudero-Escribano, M.; García-Melchor, M.; Ulissi, Z.; Davis, R.C.; Mehta, A.; et al. Copper Silver Thin Films with Metastable Miscibility for Oxygen Reduction Electrocatalysis in Alkaline Electrolytes. ACS Appl. Energy Mater. 2018, 1, 1990–1999. [Google Scholar] [CrossRef]
- Tan, K.S.; Cheong, K.Y. Advances of Ag, Cu, and Ag-Cu Alloy Nanoparticles Synthesized via Chemical Reduction Route. J. Nanoparticle Res. 2013, 15, 1–29. [Google Scholar] [CrossRef]
- Szymańska, I.B.; Piszczek, P.; Bała, W.; Bartkiewicz, K.; Szłyk, E. Ag/Cu Layers Grown on Si(111) Substrates by Thermal Inducted Chemical Vapor Deposition. Surf. Coat. Technol. 2007, 201, 9015–9020. [Google Scholar] [CrossRef]
- Chen, Q.; Jing, Y.; Yin, J.; Li, Z.; Xiong, W.; Gong, P.; Zhang, L.; Li, S.; Pan, R.; Zhao, X.; et al. High Reflectivity and Thermal Conductivity Ag–Cu Multi-Material Structures Fabricated via Laser Powder Bed Fusion: Formation Mechanisms, Interfacial Characteristics, and Molten Pool Behavior. Micromachines 2023, 14, 362. [Google Scholar] [CrossRef]
- Robinson, J.; Arjunan, A.; Stanford, M.; Lyall, I.; Williams, C. Effect of Silver Addition in Copper-Silver Alloys Fabricated by Laser Powder Bed Fusion in Situ Alloying. J. Alloys Compd. 2021, 857, 157561. [Google Scholar] [CrossRef]
- Lim, M.S.; Song, J.S.; Hong, S.I. Microstructural and Mechanical Stability of Cu-6 wt.% Ag Alloy. J. Mater. Sci. 2000, 35, 4557–4561. [Google Scholar] [CrossRef]
- Gohil, S.; Banerjee, R.; Bose, S.; Ayyub, P. Influence of Synthesis Conditions on the Nanostructure of Immiscible Copper-Silver Alloy Thin Films. Scr. Mater. 2008, 58, 842–845. [Google Scholar] [CrossRef]
- Coddet, P.; Verdy, C.; Coddet, C.; Debray, F. Effect of Cold Work, Second Phase Precipitation and Heat Treatments on the Mechanical Properties of Copper-Silver Alloys Manufactured by Cold Spray. Mater. Sci. Eng. A 2015, 637, 40–47. [Google Scholar] [CrossRef]
- Sakai, Y.; Schneider-Muntau, H.-J. Ultra-High Strength, High Conductivity Cu-Ag Alloy Wires. Acta Mater. 1997, 45, 1017–1023. [Google Scholar] [CrossRef]
- Freudenberger, J.; Klauß, H.J.; Heinze, K.; Gaganov, A.; Schaper, M.; Schultz, L. Fatigue of Highly Strengthened Cu-Ag Alloys. Int. J. Fatigue 2008, 30, 437–443. [Google Scholar] [CrossRef]
- Sakai, Y.; Inoue, K.; Maeda, H. New High-Strength, High-Conductivity Cu-Ag Alloy Sheets. Acta Metall. Mater. 1995, 43, 1517–1522. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, H.; Hu, J.; Yang, X.; Li, M.; Chen, Y.; Ma, C.; Guo, N. Microstructure and Defects of Rectangular Cu-Ag Wires Fabricated by the Continuous Extrusion Forming Process. Can. Metall. Q. 2023, 63, 46–57. [Google Scholar] [CrossRef]
- Liu, J.B.; Zhang, L.; Meng, L. Relationships between Mechanical Strength and Electrical Conductivity for Cu-Ag Filamentary Microcomposites. Appl. Phys. A Mater. Sci. Process 2007, 86, 529–532. [Google Scholar] [CrossRef]
- Nestorović, S.; Marković, I.; Marković, D. Influence of Thermomechanical Treatment on the Hardening Mechanisms and Structural Changes of a Cast Cu-6.6 Wt.%Ag Alloy. Mater. Des. 2010, 31, 1644–1649. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, Z.; An, J.; Jiang, H.; Jiang, Y.; Li, Z. Microstructure and Properties of Cu-Ag Alloy Prepared by Continuously Directional Solidification. J. Alloys Compd. 2021, 883, 160769. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Zhang, Z.F. Bulk Eutectic Cu-Ag Alloys with Abundant Twin Boundaries. Scr. Mater. 2012, 66, 65–68. [Google Scholar] [CrossRef]
- Chang, L.L.; Wen, S.; Li, S.L.; Zhu, X.D.; Shang, X.J. Strain Softening during Tension in Cold Drawn Cu-Ag Alloys. Mater. Charact. 2015, 108, 145–151. [Google Scholar] [CrossRef]
- Zhao, B.; Kim, H.; Shimogaki, Y. Effects of Ag Addition on the Resistivity, Texture and Surface Morphology of Cu Metallization. Jpn. J. Appl. Phys. Part 2 Lett. 2005, 44, L1278. [Google Scholar] [CrossRef]
- Zhao, B.; Momose, T.; Shimogaki, Y. Deposition of Cu-Ag Alloy Film by Supercritical Fluid Deposition. Jpn. J. Appl. Phys. Part 2 Lett. 2006, 45, L1296–L1299. [Google Scholar] [CrossRef]
- Lin, J.; Meng, L. Effect of Aging Treatment on Microstructure and Mechanical Properties of Cu-Ag Alloys. J. Alloys Compd. 2008, 454, 150–155. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, C.; Cai, D.; Huang, Y.; Adi, K.; Hong, Y.; Chen, Y.; Zhou, G.; Armini, S.; De Gendt, S.; et al. Hydroquinone Oriented Growth Control to Achieve High-Quality Copper Coating at High Rate for Electronics Interconnection. J. Taiwan. Inst. Chem. Eng. 2020, 112, 130–136. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, H.J.; Yong, S.H.; Kwon, O.J.; Kim, S.-K.; Kim, J.J. Facile Formation of Cu-Ag Film by Electrodeposition for the Oxidation-Resistive Metal Interconnect. J. Electrochem. Soc. 2012, 159, D253–D259. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Xiang, Q.; Qin, S.; Wang, P.; Mitsuzaki, N.; Chen, Z. Effect of Deposition Potential on Electrodeposition of Sn-Ag-Cu Ternary Alloy Solderable Coating in Deep Eutectic Solvent. J. Electroanal. Chem. 2023, 943, 117613. [Google Scholar] [CrossRef]
- Reyna-González, J.M.; Reyes-López, J.C.; Aguilar-Martínez, M. Silver and Silver-Copper Electrodeposition from a Pyridinium-Based Ionic Liquid. Electrochim. Acta 2013, 94, 344–352. [Google Scholar] [CrossRef]
- Alhaji, A.; Alkarekshi, W.; Hammadi, F.; Tunsi, B. Electrodeposition of Cu-Ag Alloys Using Ionic Liquid (Ethaline) as Deep Eutectic Solvents. Libya J. Appl. Sci. Technol. 2018, 5, 7–16. [Google Scholar]
- Li, K.; Chen, W. Recent Progress in High-Entropy Alloys for Catalysts: Synthesis, Applications, and Prospects. Mater. Today Energy 2021, 20, 100638. [Google Scholar] [CrossRef]
- Bao, G.; Xu, Y.; Huang, L.; Lu, X.; Zhang, L.; Fang, Y.; Meng, L.; Liu, J. Strengthening Effect of Ag Precipitates in Cu–Ag Alloys: A Quantitative Approach. Mater. Res. Lett. 2016, 4, 37–42. [Google Scholar] [CrossRef]
- Lee, K.H.; Kong, W.; Han, M.; Park, D.J.; Ahn, J.H.; Han, S.Z.; Park, Y.B.; Lee, K.H.; Choe, S. Properties of Nanocrystalline CuAg Foil Prepared via Electrodeposition. J. Alloys Compd. 2021, 881, 160522. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, L. Microstructure and Properties of Cu–Ag, Cu–Ag–Cr and Cu–Ag–Cr–RE Alloys. Mater. Sci. Technol. 2003, 19, 75–79. [Google Scholar] [CrossRef]
- Okonkwo, B.O.; Jeong, C.; Jang, C. Advances on Cr and Ni Electrodeposition for Industrial Applications—A Review. Coatings 2022, 12, 1555. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Wendrock, H.; Acker, J.; Gemming, T.; Wetzig, K. Thermo-Mechanical Behavior and Microstructural Evolution of Electrochemically Deposited Low-Alloyed Cu(Ag) Thin Films. Microelectron. Eng. 2004, 76, 205–211. [Google Scholar] [CrossRef]
- Chandrasekar, M.S.; Pushpavanam, M. Pulse and Pulse Reverse Plating-Conceptual, Advantages and Applications. Electrochim. Acta 2008, 53, 3313–3322. [Google Scholar] [CrossRef]
- Celante, V.G.; Freitas, M.B.J.G. Electrodeposition of Copper from Spent Li-Ion Batteries by Electrochemical Quartz Crystal Microbalance and Impedance Spectroscopy Techniques. J. Appl. Electrochem. 2010, 40, 233–239. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas D’équilibres Électrochimiques; Gauthier-Villars & Cie: Paris, France, 1963; pp. 384–398. ISBN 0915567989. [Google Scholar]
- Jie, S.; Ting-Yun, M.; Hui-Xuan, Q.; Qi-Song, L. Preparation of Copper–Silver Alloy with Different Morphologies by a Electrodeposition Method in 1-Butyl-3-Methylimidazolium Chloride Ionic Liquid. Bull. Mater. Sci. 2019, 42, 227. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Zhai, Q.; Kong, D. Mechanical Properties of Cu/Ag Multilayered Composites. Mater. Sci. Eng. 1998, 255, 20–32. [Google Scholar] [CrossRef]
- Satpathy, B.; Kayal, N.; Jena, S.; Das, S.; Das, K. Structural and Mechanical Characterization of Nano-Structured Cu–Ag Bimetallic Coatings Developed from a Novel Thiourea-Based Electroplating Bath. Mater. Charact. 2022, 189, 112011. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M.J.; Lim, T.; Park, K.J.; Kim, J.J.; Kwon, O.J. Electrodeposition of Cu-Ag Film in Cyanide-Based Electrolyte. ECS Trans. 2011, 33, 5–10. [Google Scholar] [CrossRef]
- El Sayed, M.A.; Ibrahim, M.A.M.; Elazab, T.N.; Gassoumi, M. Electrochemical Synthesis of Nanocrystalline CuAg Coatings on Stainless Steel from Cyanide-Free Electrolyte. Processes 2022, 10, 2134. [Google Scholar] [CrossRef]
- Michailova, E.; Vitanova, I.; Stoychev, D.; Milchev, A. Initial Stages of Copper Electrodeposition in the Presence of Organic Additives. Electrochim. Acta 1993, 38, 2455–2458. [Google Scholar] [CrossRef]
- Liang, D.; Shao, W.; Zangari, G. Selection of Phase Formation in Electroplated Ag-Cu Alloys. J. Electrochem. Soc. 2016, 163, D40–D48. [Google Scholar] [CrossRef]
- Bernasconi, R.L.; Nobili, L. Magagnin Electrodeposition of Supersaturated CuAg Alloys in Pyrophosphate-Iodide Electrolytes. ECS Trans. 2014, 58, 53–60. [Google Scholar] [CrossRef]
- Akben, H.K.; Timur, S.I. A Comparative Study of Silver Electrodeposition from Pyrophosphate-Cyanide and High Concentration Cyanide Electrolytes in the Presence of Brighteners. Turk. J. Chem. 2020, 44, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.T.; Neufeld, P.; Young, K. The Electrodeposition of Copper-Silver and Copper-Nickel Alloy Powders from Aqueous Ammoniacal Solutions. J. Appl. Electrochem. 1984, 14, 605–613. [Google Scholar] [CrossRef]
- Abdul Salam, A.; Singaravelan, R.; Vasanthi, P.; Bangarusudarsan Alwar, S. Electrochemical Fabrication of Ag–Cu Nano Alloy and Its Characterization: An Investigation. J. Nanostruct. Chem. 2015, 5, 383–392. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Lin, C.-C.; Hu, C.-C. Electrodeposition and Microstructure Characterization of Bimetallic Copper-Silver Films from the Methanesulfonic Acid Baths. J. Electrochem. Soc. 2018, 165, D550–D556. [Google Scholar] [CrossRef]
- Plaza-Mayoral, E.; Sebastián-Pascual, P.; Dalby, K.N.; Jensen, K.D.; Chorkendorff, I.; Falsig, H.; Escudero-Escribano, M. Preparation of High Surface Area Cu-Au Bimetallic Nanostructured Materials by Co-Electrodeposition in a Deep Eutectic Solvent. Electrochim. Acta 2021, 398, 139309. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of Ionic Liquids to the Electrodeposition of Metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Electroplating Using Ionic Liquids. Annu. Rev. Mater. Res. 2013, 43, 335–358. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P. Model for the Conductivity of Ionic Liquids Based on an Infinite Dilution of Holes. ChemPhysChem 2005, 6, 2502–2505. [Google Scholar] [CrossRef]
- Al-Murshedi, A.Y.M.; Hartley, J.M.; Abbott, A.P.; Ryder, K.S. Effect of Water on the Electrodeposition of Copper on Nickel in Deep Eutectic Solvents. Trans. Inst. Metal. Finish. 2019, 97, 321–329. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P. Deep Eutectic Solvents and Their Application in Electrochemistry. Curr. Opin. Green. Sustain. Chem. 2022, 36, 100649. [Google Scholar] [CrossRef]
- Liu, A.; Shi, Z.; Reddy, R.G. Mechanism Study of Cu-Zn Alloys Electrodeposition in Deep Eutectic Solvents. Ionics 2020, 26, 3161–3172. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep Eutectic Solvents for the Production and Application of New Materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Abbott, A.P.; Alhaji, A.I.; Ryder, K.S.; Horne, M.; Rodopoulos, T. Electrodeposition of Copper-Tin Alloys Using Deep Eutectic Solvents. Trans. Inst. Metal. Finish. 2016, 94, 104–113. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, P.; Vallés, E.; Gómez, E. First Stages of Silver Electrodeposition in a Deep Eutectic Solvent. Comparative Behavior in Aqueous Medium. Electrochim. Acta 2013, 112, 149–158. [Google Scholar] [CrossRef]
- Sebastián, P.; Vallés, E.; Gómez, E. Copper Electrodeposition in a Deep Eutectic Solvent. First Stages Analysis Considering Cu(I) Stabilization in Chloride Media. Electrochim. Acta 2014, 123, 285–295. [Google Scholar] [CrossRef]
- Kong, W.; Park, T.; Kim, K.-T.; Kim, Y.-D.; Lee, K.H.; Park, I.; Choe, S. High Strength CuAg Foil with a Nano-Lamellar Structure Prepared via Pulse Electrodeposition in an Environmental-Friendly Methanesulfonic Acid-Based Bath. J. Alloys Compd. 2023, 934, 167893. [Google Scholar] [CrossRef]
- Shao, W.; Sun, Y.; Giurlani, W.; Innocenti, M.; Zangari, G. Estimating Electrodeposition Properties and Processes: Cu-Ag Alloy at n-Si(001) and Ru Substrates from Acidic Sulfate Bath. Electrochim. Acta 2022, 403, 139695. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, H.; Barrow, C.J.; Yang, W. Electrochemical Synthesis of Fractal Bimetallic Cu/Ag Nanodendrites for Efficient Surface Enhanced Raman Spectroscopy. Chem. Commun. 2016, 52, 10968–10971. [Google Scholar] [CrossRef]
- Choi, J.; Kim, M.J.; Ahn, S.H.; Choi, I.; Jang, J.H.; Ham, Y.S.; Kim, J.J.; Kim, S.K. Electrochemical CO2 Reduction to CO on Dendritic Ag-Cu Electrocatalysts Prepared by Electrodeposition. Chem. Eng. J. 2016, 299, 37–44. [Google Scholar] [CrossRef]
- Rajashekhar, B.; Pandey, G.; Sekar, R.; Veerapandian, M. Electro Co-Deposition of Copper-Silver Nanocrystallite Alloy Cluster: A Way for Tunable SERS Substrate Development. Mater. Lett. X 2022, 15, 100157. [Google Scholar] [CrossRef]
- Włodarczyk, B.; Włodarczyk, P.P. Electricity Production from Yeast Wastewater in Membrane-Less Microbial Fuel Cell with Cu-Ag Cathode. Energies 2023, 16, 2734. [Google Scholar] [CrossRef]
- Kim, K.O.; Kim, S. Surface Morphology Control of Cu-Ag Alloy Thin Film on W Diffusion Barrier by Seedless Electrodeposition. J. Nanosci. Nanotechnol. 2016, 16, 11701–11706. [Google Scholar] [CrossRef]
- Singh, M.; Sinha, I.; Mandal, R.K. Synthesis of Nanostructured Ag-Cu Alloy Ultra-Fine Particles. Mater. Lett. 2009, 63, 2243–2245. [Google Scholar] [CrossRef]
- Ye, W.; Guo, X.; Ma, T. A Review on Electrochemical Synthesized Copper-Based Catalysts for Electrochemical Reduction of CO2 to C2+ Products. Chem. Eng. J. 2021, 414, 128825. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Verma, S.; Ma, S.; Fister, T.T.; Timoshenko, J.; Frenkel, A.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous Copper-Silver Alloys by Additive-Controlled Electrodeposition for the Selective Electroreduction of CO2 to Ethylene and Ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.; Masel, R.I.; Kenis, P.J.A. The Effect of Electrolyte Composition on the Electroreduction of CO2 to CO on Ag Based Gas Diffusion Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Chen, X.; Henckel, D.A.; Nwabara, U.O.; Li, Y.; Frenkel, A.I.; Fister, T.T.; Kenis, P.J.A.; Gewirth, A.A. Controlling Speciation during CO2 Reduction on Cu-Alloy Electrodes. ACS Catal. 2020, 10, 672–682. [Google Scholar] [CrossRef]
- Ma, S.; Sadakiyo, M.; Luo, R.; Heima, M.; Yamauchi, M.; Kenis, P.J.A. One-Step Electrosynthesis of Ethylene and Ethanol from CO2 in an Alkaline Electrolyzer. J. Power Sources 2016, 301, 219–228. [Google Scholar] [CrossRef]
- Volov, I.; Swanson, E.; O’Brien, B.; Novak, S.W.; van den Boom, R.; Dunn, K.; West, A.C. Pulse-Plating of Copper-Silver Alloys for Interconnect Applications. J. Electrochem. Soc. 2012, 159, D677–D683. [Google Scholar] [CrossRef]
- Strehle, S.; Bartha, J.W.; Wetzig, K. Electrical Properties of Electroplated Cu(Ag) Thin Films. Thin Solid. Film. 2009, 517, 3320–3325. [Google Scholar] [CrossRef]
- Wei, M.Z.; Xu, L.J.; Shi, J.; Pan, G.J.; Cao, Z.H.; Meng, X.K. Achieving High Strength and High Electrical Conductivity in Ag/Cu Multilayers. Appl. Phys. Lett. 2015, 106, 011604. [Google Scholar] [CrossRef]
- Hsieh, J.; Hung, S. The Effect of Cu: Ag Atomic Ratio on the Properties of Sputtered Cu-Ag Alloy Thin Films. Materials 2016, 9, 914. [Google Scholar] [CrossRef]
- Lee, K.P.; Tran, D.P.; Chen, F.C.; Hsu, W.Y.; Lin, Y.Q.; Liu, H.C.; Chen, C. Mechanical Strengthening of Nanotwinned Cu Films with Ag Solid Solution. Mater. Lett. 2022, 313, 131775. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Wetzig, K.; Bartha, J.W. Microstructure of Electroplated Cu(Ag) Alloy Thin Films. Thin Solid. Film. 2011, 519, 3522–3529. [Google Scholar] [CrossRef]
- Spolenak, R.; Kraft, O.; Arzt, E. Effects of Alloying Elements on Electromigration. Microelectron. Reliab. 1998, 38, 1015–1020. [Google Scholar] [CrossRef]
- Ciacotich, N.; Din, R.U.; Sloth, J.J.; Møller, P.; Gram, L. An Electroplated Copper–Silver Alloy as Antibacterial Coating on Stainless Steel. Surf. Coat. Technol. 2018, 345, 96–104. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and Anti-Bacterial Activity of Cu, Ag and Cu-Ag Alloy Nanoparticles: A Green Approach. Mater. Res. Bull. 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Giurlani, W.; Zangari, G.; Gambinossi, F.; Passaponti, M.; Salvietti, E.; Di Benedetto, F.; Caporali, S.; Innocenti, M. Electroplating for Decorative Applications: Recent Trends in Research and Development. Coatings 2018, 8, 260. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, F. Preparation of Ag-Cu Bimetallic Dendritic Nanostructures and Their Hydrogen Peroxide Electroreduction Property. J. Appl. Electrochem. 2013, 43, 667–677. [Google Scholar] [CrossRef]
- Gang, L.; Anderson, B.G.; van Grondelle, J.; van Santen, R.A.; van Gennip, W.J.H.; Niemantsverdriet, J.W.; Kooyman, P.J.; Knoester, A.; Brongersma, H.H. Alumina-Supported Cu-Ag Catalysts for Ammonia Oxidation to Nitrogen at Low Temperature. J. Catal. 2002, 206, 60–70. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Lin, Y.; Yang, Z.; Liu, L. Cu-Ag Core-Shell Nanowires for Electronic Skin with a Petal Molded Microstructure. J. Mater. Chem. C Mater. 2015, 3, 9594–9602. [Google Scholar] [CrossRef]
- Lee, C.; Kim, N.R.; Koo, J.; Lee, Y.J.; Lee, H.M. Cu-Ag Core-Shell Nanoparticles with Enhanced Oxidation Stability for Printed Electronics. Nanotechnology 2015, 26, 455601. [Google Scholar] [CrossRef] [PubMed]
- Easow, J.S.; Selvaraju, T. Unzipped Catalytic Activity of Copper in Realizing Bimetallic Ag@Cu Nanowires as a Better Amperometric H2O2 Sensor. Electrochim. Acta 2013, 112, 648–654. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, K.J.; Lim, T.; Kwon, O.J.; Kim, J.J. Fabrication of Cu-Ag Interconnection Using Electrodeposition: The Mechanism of Superfilling and the Properties of Cu-Ag Film. J. Electrochem. Soc. 2013, 160, D3126–D3133. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Bartha, J.W.; Wetzig, K. Electroplating of Cu(Ag) Thin Films for Interconnect Applications. Microelectron. Eng. 2010, 87, 180–186. [Google Scholar] [CrossRef]
- Kim, K.O.; Kim, S. Nano-Nucleation Characteristic of Cu-Ag Alloy Directly Electrodeposited on w Diffusion Barrier for Microelectronic Device Interconnect. J. Nanosci. Nanotechnol. 2016, 16, 5173–5178. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, F.; Lei, Y.; Wu, X. A Silver-Copper Alloy as an Oxygen Reduction Electrocatalyst for an Advanced Zinc-Air Battery. ChemCatChem 2015, 7, 2377–2383. [Google Scholar] [CrossRef]
- Dutta, A.; Montiel, I.Z.; Erni, R.; Kiran, K.; Rahaman, M.; Drnec, J.; Broekmann, P. Activation of Bimetallic AgCu Foam Electrocatalysts for Ethanol Formation from CO2 by Selective Cu Oxidation/Reduction. Nano Energy 2020, 68, 104331. [Google Scholar] [CrossRef]
- Kottakkat, T.; Klingan, K.; Jiang, S.; Jovanov, Z.P.; Davies, V.H.; El-Nagar, G.A.M.; Dau, H.; Roth, C. Electrodeposited AgCu Foam Catalysts for Enhanced Reduction of CO2 to CO. ACS Appl. Mater. Interfaces 2019, 11, 14734–14744. [Google Scholar] [CrossRef]
- Clarke, O.J.R.; St. Marie, G.J.H.; Brosseau, C.L. Evaluation of an Electrodeposited Bimetallic Cu/Ag Nanostructured Screen Printed Electrode for Electrochemical Surface-Enhanced Raman Spectroscopy (EC-SERS) Investigations. J. Electrochem. Soc. 2017, 164, B3091–B3095. [Google Scholar] [CrossRef]
- Scott, K. Process Intensification: An Electrochemical Perspective. Renew. Sustain. Energy Rev. 2018, 81, 1406–1426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).