Abstract
This study presents a comprehensive analysis of the synthesis and photophysical properties of thiazolidine-functionalized chiral ionic liquids (CILs) derived from L-cysteine. The synthesis involves a four-step route, encompassing N-protection, coupling reactions with bromoalcohols, and ionic liquid formation. The optical properties of the compounds were evaluated using UV–Vis absorption and fluorescence emission spectroscopies, revealing distinct behavior for different heterocycles and counter-ions. Notably, the investigation reveals that thiazolidine-based CILs exhibit unconventional intrinsic luminescence characteristics. Building upon these photophysical properties, an interaction study was conducted between copper (II) and the CILs. The findings exhibit a robust linear relationship between the optical response and the concentration of the metal ion. Through the calculation of the Stern–Volmer quenching constant, it was determined that the 1:1 binding model is applicable. This research underscores the potential of UV–Vis absorption spectroscopy as a highly sensitive method for detecting metal ions. By elucidating the synthesis, photophysical behavior, and metal ion interaction of thiazolidine-based CILs, this study contributes valuable insights into the field of functionalized ionic liquids and their potential applications in various areas.
1. Introduction
The thiazolidine moiety has emerged as a significant component in the field of biology [1], finding extensive exploration in medicinal chemistry as a fundamental building block for synthesizing various pharmaceutical compounds, including antibiotic drugs such as penicillin [2], protease inhibitors, and antitumor agents [3]. It has also been employed in the synthesis of pesticides and flavoring agents [4]. On the other hand, ionic liquids (ILs) have found extensive application in material science and engineering, capitalizing on their distinctive physical properties, such as their very low vapor pressure under ambient conditions, a wide range of solubility for solids and gases, and low viscosity [5,6]. The notable relevance of both thiazolidine and ILs has made them intriguing subjects for investigation, aiming to discover compounds with new properties and applications. Recent studies have primarily focused on the synthesis of novel IL architectures, aiming to modulate specific properties, such as their photophysical characteristics, acidity, or thermal stability. These efforts have targeted potential applications in the fields of chemistry, biology, and engineering [4,7]. Similarly, the optical properties of thiazolidine rings have received attention due to their electronic characteristics [8,9,10].
Therefore, constructing and investigating ionic liquids containing the thiazolidine heterocycle have the potential to uncover compounds that combine the advantageous properties of both classes. Our specific interest lies in chiral ILs and bio-ionic liquids among the various structural classifications of ILs, which include chiral ILs, bio-ILs, polymeric ILs, protic ILs, basic ILs, and metallic ILs [11]. Thiazolidine-functionalized ionic liquids can be designed from cyclic derivatives of amino acids, providing a chiral center within the IL's structure. These derivatives offer promising catalytic activity in asymmetric reactions, chiral discrimination, and the sensing of biocompounds [12,13,14,15]. Additionally, the use of bio-precursors improves environmental considerations concerning toxicity and biodegradability, expanding their potential applications [16,17,18]. The amino acid derivative can be incorporated into either the cation or the anion component of the IL. The synthesis of ILs incorporating the amino acid in the anion component can be achieved through acid–base reactions. This approach enables the utilization of a wide range of amino acids, resulting in various chiral ILs [19,20]. On the other hand, imparting chirality to the cation requires more intricate synthetic routes. Recently, chiral ionic liquids derived from L-cysteine derivatives have demonstrated high efficiency as catalysts in the enantioselective addition of diethylzinc to aldehydes [21]. Other derivatives derived from L-phenylalanine and L-tyrosine have been reported, aiming to develop agents with antimicrobial activity [22], and sensors for bovine serum albumin (BSA) were prepared from L-serine [23].
Within this context, the present work focuses on the synthesis of chiral ionic liquids and their application for optical metal sensing. All chiral components in the ILs were derived from L-cysteine, which is readily accessible, cost-effective, and abundantly available. The chosen heterocycles bearing the positive charge (imidazolium and pyridinium) and selected anions (Br- and N(Tf)2−) have been extensively studied in the literature and confer desirable properties to ionic liquids [24]. Furthermore, previous investigations conducted by our research group have revealed the presence of non-traditional intrinsic luminescence (NTIL) in similar structures. Therefore, this study of experimental and theoretical photophysical characteristics contributes to a better understanding of the phenomenon exhibited by these derivatives.
2. Experimental Section
2.1. Materials and Methods
The commercially available reagents bis(trifluoromethanesulfonyl)imide lithium salt (LiNTf2), L-cysteine, formaldehyde, tert-butyloxycarbonyl (Boc), 6-bromohexanol, 4-dimethylaminopyridine (DMAP), N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI), 1,2-dimethylimidazole, 1-methylimidazole, pyridine, and inorganic salts were purchased from commercial suppliers and were used as received. The solvents ethyl acetate, hexane, acetonitrile, toluene, methanol, and dichloromethane were either used as received or were purified using standard procedures.
The hydrogen nuclear magnetic resonance (1H NMR) and carbon-13 nuclear magnetic resonance (13C NMR) spectra were obtained using a Varian 400 MHz spectrometer, and the measurements were conducted in deuterated chloroform (CDCl3) solutions. Chemical shifts (δ) are reported in parts per million relative to the peak of tetramethylsilane (δ = 0.00 ppm), which served as the internal standard for 1H NMR, or relative to the solvent peak of CDCl3 (δ = 77.23 ppm) for 13C NMR. The data are presented as follows: chemical shift (δ), multiplicity, coupling constant (J) in Hertz, and integrated intensity. The original spectra are presented as supplementary material (Figures S1–S16). Optical rotations were determined using a Jasco P-2000 polarimeter. High-resolution mass spectra (HMRS) were recorded using a Micromass Q-Tof spectrometer with electrospray ionization (ESI). Column chromatography was performed on silica gel (230–400 mesh). For the photophysical characterization, spectroscopic grade solvents were employed. UV–Vis absorption spectra were acquired using a Shimadzu UV-2450 spectrophotometer at a concentration of 10−5 M, and steady-state fluorescence spectra were measured on a Shimadzu spectrofluorometer, model RF-5301PC. The maximum absorption wavelength (WL) was used as the excitation WL for fluorescence emission measurements. All measurements were conducted at room temperature (25 °C).
2.2. Synthesis of Thiazolidine Precursor (4)
A dry Schlenk tube under argon was charged with compound 3 (0.47 g, 2.0 mmol), dry dichloromethane (CH2Cl2) (10 mL), EDCI (0.40 g; 2.0 mmol), and DMAP (catalytic). The mixture was stirred at room temperature for 30 min. Then, 6-bromohexan-1-ol (0.36 g; 2.0 mmol) was added, and the reaction mixture was stirred at room temperature for 24 h. After, an additional amount of CH2Cl2 was added. The organic layer was washed three times with saturated sodium hydrogen carbonate (NaHCO3) solution, dried with magnesium sulfate (MgSO4), filtered, and the solvent evaporated. The product was purified via column chromatography using ethyl acetate/hexane (10:90) as eluent to provide a yellow oil.
(4R)-(6-bromohexyl) 3-(tert-butyl) thiazolidine-3,4-dicarboxylate (4)
Yield: 46%. = −70 (c 1, CH2Cl2). 1H NMR (400 MHz, CDCl3, mixture of conformers) δ: [4.91–4.84 (m) and 4.71–4.65 (m), 1H], [4.65–4.61 (m), 4.60–4.54 (m), 4.50–4.44 (m) and 4.44–4.37 (m), 2H], 4.22–4.09 (m, 2H), 3.41 (t, J = 6.4 Hz, 2H), 3.36–3.23 (m, 1H), 3.23–3.13 (m, 1H), 1.92–1.81 (m, 2H), 1.72–1.61 (m, 2H), 1.51–1.34 (m, 13H). 13C NMR (100 MHz, CDCl3) δ: 170.8, 170.3, 153.2, 153.1, 81.0, 65.4, 61.6, 48.9, 48.1, 34.7, 33.6, 33.3, 32.5, 32.4, 28.4, 28.3, 27.7, 26.4, 25.0. HRMS: calcd. for [C15H26BrNO4S]+ 395.0766; found 395.0746.
2.3. General Procedure for Chiral Ionic Liquids
Method A: In a mono-tubulated, round-bottomed flask equipped with a reflux condenser, compound 5, acetonitrile, and the respective N-heterocycle were added. The reaction mixture was stirred at 65 °C for 66–72 h. Then, the solvent was removed, and the material was solubilized in a small amount of water. The solution was washed with dichloromethane, and the water was evaporated under a vacuum.
Method B: In a mono-tubulated, round-bottomed flask equipped with a reflux condenser, compound 5 and pyridine were added. The reaction mixture was stirred at 65 °C for 24 h. Then, toluene was added to the mixture, leading to the segregation of the ionic liquids in an oil phase. The supernatant was removed with a pipette. Methanol was then added to the flask for solubilization of the ionic product, and toluene was added again. The process was repeated three times.
(R)-1-(6-((3-(tert-butoxycarbonyl)thiazolidine-4-carbonyl)oxy)hexyl)-2,3-dimethylimidazolium bromide (5a)
Prepared according to method A using compound 4 (0.10 g, 0.25 mmol), acetonitrile (1.5 mL), and 1,2-dimethylimidazole (0.03 g; 0.30 mmol). The reaction mixture was heated for 72 h. A yellow oil was obtained. Yield: 81%. = −12 (c 1, CH2Cl2). 1H NMR (400 MHz, CDCl3, mixture of conformers) δ: 7.64–7.60 (m, 1H), 7.51–7.45 (m, 1H), [4.85–4.77 (m) and 4.70–4.62 (m), 4.62–4.49 (m) and 4.47–4.34 (m), 2H], 4.27–4.16 (m, 2H), 4.16–4.07 (m, 2H), 3.96 (s, 3H), 3.36–3.20 (m, 1H), 3.20–3.08 (m, 1H), 2.76 (s, 3H), 2.46 (ls), 1.87–1.73 (m, 2H), 1.69–1.57 (m, 2H), 1.51–1.30 (m, 13H). 13C NMR (100 MHz, CDCl3, mixture of conformers) δ: 170.9, 170.6, 153.3, 153.2, 143.8, 123.1, 123.0; 121.2, 121.2, 81.2, 65.3, 65.2, 61.6, 48.9, 48.2, 36.2, 36.1, 34.7, 33.4, 29.7, 29.7, 28.3, 25.9, 25.8, 25.4, 25.2, 10.9, 10.9. HRMS: calcd. for [C20H34N3O4S]+ 412.2265; found 412.2203.
(R)-1-(6-((3-(tert-butoxycarbonyl)thiazolidine-4-carbonyl)oxy)hexyl)-3-methylimidazolium bromide (5b)
Prepared according to method A using compound 4 (0.10 g, 0.25 mmol), acetonitrile (1.5 mL), and 1-methylimidazole (0.02 mL; 0.30 mmol). The reaction mixture was heated for 66 h. A yellow oil was obtained. Yield: 75%. = −48 (c 1, CH2Cl2). 1H NMR (400 MHz, CDCl3, mixture of conformers) δ: 10.24–10.07 (m, 1H), 7.56 (s, 1H), 7.50 (s, 1H), [4.92–4.81 (m) and 4.72–4.66 (m), 1H], [4.66–4.60 (m), 5.60–4.54 (m) and 4.50–4.39 (m), 2H], 4.36 (t, J = 7.2 Hz, 2H), 4.22–4.13 (m, 4H), 3.37–3.24 (m, 1H), 3.23–3.14 (m, 1H), 2.48 (sl, 1H), 1.94 (q, J = 7.2 Hz, 2H), 1.71–1.60 (m, 2H), 1.52–1.32 (m, 13H). 13C NMR (100 MHz, CDCl3, mixture of conformers) δ: 170.9, 170.6, 153.3, 153.2, 137.3, 123.6, 123.6, 122.1, 122.1, 81.2, 65.5, 65.2, 61.6, 49.9, 48.9, 36.8, 36.8, 34.7, 32.5, 30.1, 30.1, 28.3, 25.7, 25.6, 25.3, 25.0. HRMS: calcd. for [C19H32N3O4S]+ 398.2108; found 398.2134.
(R)-1-(6-((3-(tert-butoxycarbonyl)thiazolidine-4-carbonyl)oxy)hexyl)pyridinium bromide (5c)
Prepared according to method B using compound 4 (0.30 g, 0.75 mmol) and pyridine (2 mL). The reaction mixture was heated for 24 h. A yellow oil was obtained. Yield: 83%. = −5 (c 1, CH2Cl2). 1H NMR (400 MHz, CDCl3, mixture of conformers) δ: 9.60–9.51 (m, 2H), 8.58 (t, J = 8.0 Hz, 2H), 8.19 (t, J = 7.2 Hz, 2H), 5.07–4.94 (m, 2H), [4.87–4.79 (m) and 4.73–4.65 (m), 1H], [4.65–4.52 (m) and 4.49–4.37 (m), 2H], 4.13 (t, J = 6.4 Hz, 2H), 3.40–3.26 (m, 1H), 3.24–3.13 (m, 1H), 2.61 (sl, 1H), 2.15–2.01 (m, 2H), 1.72–1.58 (2H), 1.51–1.32 (m, 13H). 13C NMR (100 MHz, CDCl3, mixture of conformers) δ: 170.8, 170.5, 153.3, 153.2, 145.3, 145.2; 128.5, 81.2, 65.3, 65.2, 61.6, 61.6, 48.9, 48.2, 34.7, 33.3, 31.8, 31.7, 28.3, 25.3, 25.0. HRMS: calcd. for [C20H31N2O4S]+ 395.1999; found 395.2036.
2.4. General Procedure for Anion Exchange
The respective bromide was solubilized in distilled water and LiNTf2 was added. The reaction mixture was kept under stirring at room temperature for 12h. At the end of the reaction time, the precipitation of the product as an oil was observed. Then, six extractions were carried out with dichloromethane. The organic layers were combined, dried over MgSO4, and the solvent was evaporated. The product was dried under a vacuum.
(R)-1-(6-((3-(tert-butoxycarbonyl)thiazolidine-4-carbonyl)oxy)hexyl)-2,3-dimethylimidazolium bis((trifluoromethyl)sulfonyl)amide (6a)
Reagents and amounts used: compound 5a, (0.05 g, 0.10 mmol), LiNTf2 (0.06 g, 0.20 mmol), distilled water (0.5 mL). A yellow oil was obtained. Yield: 97%. = −7 (c 1, CH2Cl2). 1H NMR (400 MHz, CDCl3, mixture of conformers) δ: 7.22–7.20 (m, 2H), [4.87–4.81 (m) and 4.72–4.66 (m), 1H], [4.66–4.54 (m) and 4.48–4.39 (m), 1H], 4.18–4.10 (m, 2H), 4.18–4.10 (m, 2H), 3.80 (s, 3H), 3.39–3.24 (m, 1H), 3.22–3.13 (m, 1H), 2.61 (s, 3H), 1.80 (q, J = 6.8 Hz, 2H), 1.66 (t, J = 6.8 Hz, 2H), 1.51–1.32 (m, 13H). 13C NMR (100 MHz, CDCl3, mixture of conformers) δ: 170.8, 170.6, 153.3, 153.2, 143.7, 122.6, 122.5, 120.9, 120.8, 119.7 (q, J = 310.7 Hz, CF3), 81.2, 65.1, 61.6, 61.6, 48.6, 48.2, 35.3, 35.3, 34.6, 33.3, 29.3, 28.2, 25.8, 25.6, 25.2, 25.0, 9.6, 9.5. HRMS: calcd. for [C20H34N3O4S]+ 412.2265; found 412.2278. HRMS: calcd. for [C2F6NO4S2]− 279.9178; found 279.9188.
(R)-1-(6-((3-(tert-butoxycarbonyl)thiazolidine-4-carbonyl)oxy)hexyl)-3-methylimidazolium bis((trifluoromethyl)sulfonyl)amide (6b)
Reagents and amounts used: compound 5b, (0.65 g, 0.31 mmol), LiNTf2 (0.18 g, 0.62 mmol), distilled water (1 mL). A yellow oil was obtained. Yield: 46%. = −7 (c 1 CH2Cl2). 1H NMR (400 MHz, CDCl3, mixture of conformers) δ: 8.74 (s, 1H), 7.40–7.36 (m, 1H), 7.36–7.33 (m, 1H), [4.91–4.79 (m) and 4.72–4.65 (m), 1H], [4.65–4.54 (m) and 4.49–4.38 (m), 2H], 4.25–4.06 (m, 4H), 3.94 (s, 3H), 3.39–3.24 (m, 1H), 3.23–3.13 (m, 1H), 1.93–1.82 (m, 2H), 1.72–1.61 (m, 2H), 1.56–1.29 (m, 13H). 13C RMN (100 MHz, CDCl3, mixture of conformers) δ: 170.9, 170.7, 153.4, 153.3, 136.0, 123.8, 122.4, 119.8 (q, J = 319.5 Hz, CF3), 81.2, 65.5, 65.3, 61.6, 49.9, 48.9, 48.2, 36.3, 34.7, 33.3, 29.8, 29.8, 28.3, 28.2, 25.6, 25.4, 25.1, 24.9. HRMS: calcd. for [C19H32N3O4S]+ 398.2108; found 398.2160. HRMS: calcd. for [C2F6NO4S2]− 279.9178; found 279.9107.
(R)-1-(6-((3-(tert-butoxycarbonyl)thiazolidine-4-carbonyl)oxy)hexyl)pyridinium bis((trifluoromethyl)sulfonyl)amide (6c)
Reagents and amounts used: compound 5c, (0.06 g, 0.12 mmol), LiNTf2 (0.07 g, 0.24 mmol), distilled water (1 mL). A yellow oil was obtained. Yield: 60%. = −14 (c 1, CH2Cl2). 1H NMR (400 MHz, CDCl3, mixture of conformers) δ: 8.90–8.78 (m, 2H), 8.47 (t, J = 8.0 Hz, 1H), 8.04 (t, J = 6.4 Hz, 2H), [8.34–8.74 (m) and 4.70–4.63 (m), 1H], 4.62–4.50 (m, 3H), 4.45–4.34 (m, 1H), 4.18–4.05 (m, 2H), 3.39–3.21 (m, 1H), 3.19–3.08 (m, 1H), 2.07–1.94 (m, 2H), 1.68–1.55 (m, 2H), 1.51–1.29 (m, 13H). 13C NMR (100 MHz, CDCl3, mixture of conformers) δ: 170.8, 170.6, 153.4, 153.3, 145.5, 144.4, 128.7, 119.8 (q, J = 319.3 Hz, CF3), 81.2, 65.2, 65.1, 62.3, 61.6, 48.9, 48.2, 34.6, 33.3, 31.4, 31.3, 28.3, 28.1, 25.5, 25.3, 25.1, 24.9. HRMS: calcd. for [C20H31N2O4S]+ 395.1999; found 395.2060. HRMS: calcd. for [C2F6NO4S2]− 279.9178; found 279.9125.
2.5. Metal Sensing Investigation
The candidate selection for the optical sensing investigation was determined by considering the findings from the previous photophysical study and evaluating the optical properties of each compound. The metal sensing experiments were conducted in acetonitrile, and no significant alterations were detected in the optical properties concerning the impact of the heterocycles and counter-ions. Hence, compound 5a was chosen as the primary candidate to validate the potential of chiral ionic liquids as sensors. In this way, a stock solution of 5a [ca. 1.0 × 10−4 M] in acetonitrile was prepared. Stock metal ion solutions of nitrate salts [ca. 1.0 × 10−3 M] of silver, lithium, zinc, cadmium, copper (II), cobalt (II), and iron (III) were prepared in deionized water. An initial exploratory study was performed by adding 2.0 mL (20 equivalents) of metal ion solution to 1.0 mL of the ionic liquid solution. Titration experiments were performed by adding 10 to 100 μL (0.1 to 1 equivalent) of the metal ion solution to 1.0 mL of the ionic liquid solution. The final volume was adjusted to 3.0 mL by adding acetonitrile. The final solutions were mixed for 5 min before recording the absorption and emission spectra. This study was conducted in triplicate, and the average of three readings was taken into consideration.
The limits of detection (LD) and quantification (LQ) were calculated based on I/I0 vs. [Cu2+] and F/F0 vs. [Cu2+] plots from the data in absorption and emission spectra of the titration experiment according to Equations (1) and (2) [25]:
where σ is the standard deviation of the curve linear coefficient, and S is the angular coefficient.
2.6. Theoretical Calculations
The main techniques employed in this study were density functional theory (DFT) and ab initio calculations, which were primarily conducted using version 5.0.3 of the ORCA quantum chemistry package [26,27,28]. Initial molecular geometries for molecules 6a to 6c were obtained through conformational sampling performed by the semiempirical CREST software (version 2.12) [29]. Subsequently, the most stable conformers underwent further optimization to determine their ground (S0) and first excited (S1) states using the ωB97X-D3 [30]/Def2-TZVP [31] level of theory, employing tight convergence criteria and the DefGrid3 integration grid. The resulting ground-state relaxed geometries exhibited no imaginary vibrational modes, indicating the achievement of a true energy minimum. To study electronic transitions, we primarily utilized the domain-based local pair natural orbitals–similarity transformed equation-of-motion coupled-cluster singles and doubles (DLPNO-STEOM-CCSD) method [32]. For the first 5 electronic transitions, we employed the Def2-TZVPP diffuse triple-ζ basis set. This method offers an efficient and highly accurate approach for calculating excited state properties. To enhance interpretability and computational efficiency, our analysis of electronic transitions incorporated natural transition orbitals (NTOs) [33]. The visualization of charge transfer via hole–particle formalism was achieved through the electronic density difference (EDD) method. Furthermore, we investigated the orbital properties within the DLPNO-STEOM-CCSD method by applying natural bond orbitals (NBOs) [34]. To confirm the obtained results, we performed a validation using the MOPAC 2022 software [35]. The validation involved employing a complete active space (CAS) procedure with the ZINDO Hamiltonian, considering 500 CI states, and utilizing an active space comprising 30 electrons and 15 orbitals. Time-dependent density functional theory (TD-DFT) absorption spectra using CPCM implicit solvation calculated using the same ωB97X-D3/Def2-TZVP level of theory for the first 120 electronic transitions with the Tamm–Dancoff approximation [36] active served as a comparison to the DLPNO-STEOM-CCSD method. The nature of the intermolecular interactions with bromine was examined using the second order symmetry-adapted perturbation theory (SAPT2) [37] method available on the Psi4 software version 1.8 [38] and the aug-cc-pVDZ basis sets [39]. This method is known for its ability to provide insight into the physical nature of intermolecular interactions.
3. Results and Discussion
3.1. Synthesis
The synthesis of thiazolidine-functionalized chiral ionic liquids was carried out using a four-step route, as illustrated in Scheme 1. Initially, L-cysteine (1) underwent a reaction with formaldehyde to yield compound 2 (80% yield), which was then subjected to N-protection using a tert-butyloxycarbonyl (Boc) group (90% yield) [40,41], resulting in the formation of cyclic derivative 3. Subsequently, a coupling reaction between compound 3 and the bromoalcohols facilitated the insertion of a linker into the cyclic amino acid, enabling nucleophilic substitution and attachment of various N-heterocycles. For the esterification step, compound 3 was reacted with 6-bromohexanol using 4-dimethylaminopyridine (DMAP) as a catalyst and N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) as a coupling agent, resulting in the formation of compound 4 with a yield of 46%. The preparation of chiral ionic liquids involved reacting compound 4 with 1,2-dimethylimidazole, 1-methylimidazole, or pyridines in acetonitrile at 65 °C. This reaction led to the formation of compounds 5a–5c with respective yields of 81%, 75%, and 83%. Finally, the anion exchange of the chiral ionic liquids 5a–5c from bromide (Br-) to bis(trifluoromethanesulfonyl)imide (N(Tf)2−) was achieved by treating them with LiN(Tf)2 in water, resulting in the formation of compounds 6a (97%), 6b (46%), and 6c (60%).
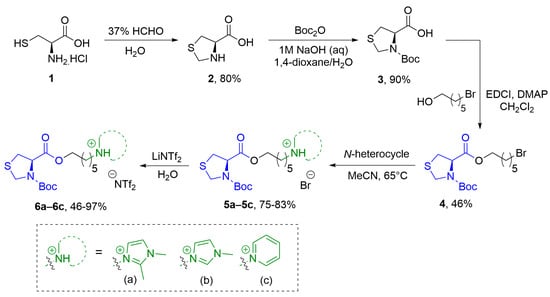
Scheme 1.
Synthetic route for thiazolidine containing chiral ionic liquids 6a–6c.
3.2. Photophysics
With the emergence of the concept of non-traditional intrinsic luminescence, there has been increasing interest in understanding the optical properties of various chemical systems that exhibit fluorescence even in the absence of traditional luminophores [42,43,44]. These features are reported for simple molecules with heteroatoms, macromolecules, supramolecular assemblies, as well as nonaromatic amino acids [45,46,47]. Additionally, it has been reported that electron-rich compositions and/or functional groups containing heteroatoms, such as nitriles [48], maleimides [49,50], aliphatic tertiary amines [51,52,53], double-bonding containing compounds [54,55], and various heteroatoms (N, O, P, and S) [56,57,58], can contribute to the photophysical behavior of these systems. Consequently, several studies have begun to explore the photophysical behavior of these unconventional compounds, focusing on specific structural characteristics. The NTIL phenomenon is primarily associated with the presence of electron-rich functional groups and the capacity for molecular organization in aggregates or clusters. Remarkably, all these criteria are observed in the thiazolidine-based ionic liquids synthesized in our study. In this way, the optical properties of compounds 5a–5c and 6a–6c were investigated through a photophysical study using UV–Vis absorption and steady-state fluorescence spectroscopies. This analysis aimed to evaluate the impact of different positively charged heterocycles, counter-ions, and structural modifications in comparison to the previously studied CIL derivatives [23]. The photophysical characterization was conducted using diluted solutions (10−5 M) in ethanol and acetonitrile as the solvents. Detailed information regarding the electronic ground and excited states can be found in Table 1. Figure 1 presents the UV–Vis absorption spectra of the thiazolidine-containing ionic liquids 5a–5c and 6a–6c in both ethanol and acetonitrile.

Table 1.
Photophysical data of ethanol and acetonitrile [ca. 10−5 M] of the bromide 5a–5c and triflate 6a–6c chiral ionic liquids where ε is the molar extinction coefficient, λabs is the absorption band, and λem is the emission maxima, respectively.
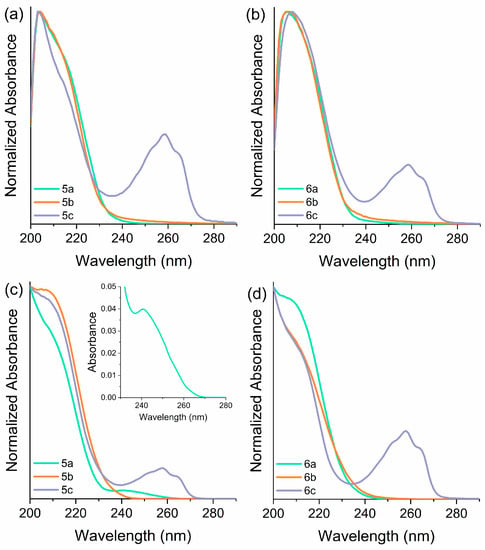
Figure 1.
UV–Vis absorption spectra in (a,b) ethanol and (c,d) acetonitrile solutions [ca. 10−5 M] of thiazolidine-based CILs 5a–5c (left) and 6a–6c (right).
These compounds exhibit transparency to visible light, as previously observed in other ILs containing imidazolium and pyridinium moieties [59,60]. Interestingly, these ILs displayed very low molar extinction coefficients in this spectral region. The optical properties of the imidazolium-based ILs exhibited distinct behavior depending on the anion and solvent parameters. In particular, compounds 6a–6b, which contain N(Tf)2 as the anion, demonstrated a similar absorption profile with a single band centered at 211 nm in both solvents (see supporting information). In contrast, compound 5a, when solubilized in acetonitrile, displayed an additional well-defined absorption band with a slightly lower intensity centered at 241 nm in addition to the band around 210 nm. The appearance of this redshifted band can be attributed to the formation of a charge transfer complex, indicating a strong interaction between the positive and negative components of the compound. Similarly, compound 5b, when solubilized in ethanol, exhibited a distinct absorption band with a redshifted shoulder, suggesting an additional, low-intensity band also associated with charge transfer. This observed profile may also be indicative of the presence of various assembled species with differing energy levels. The pyridinium-based ILs, namely compounds 5c and 6c, exhibited similar behavior regardless of the solvent or anion used. As depicted in Figure 1, a well-defined absorption band centered at 258 nm was observed, corresponding to the S1(ππ*)←S0 electronic transition of the pyridinium cation. Additionally, both compounds 5c and 6c displayed an additional absorption peak around 210 nm in both ethanol and acetonitrile. This phenomenon has been previously observed in other pyridinium-based ILs and is attributed to the charge transfer to the solvent [61].
Figure 2 illustrates the emission spectra of compounds 5a–5c and 6a–6c in ethanol and acetonitrile. In general, these compounds do not exhibit fluorescence emission when excited at the wavelengths observed in the UV–Vis absorption spectra except for compounds 5c and 6c. For these 2 compounds, excitation at 258 nm resulted in emission in the UV to violet region. It is worth noting that non-traditional emission, as described in the literature, is typically associated with high-energy transitions corresponding to excitation wavelengths ranging from 225 to 400 nm. Therefore, various excitation wavelengths within this energy range were tested to investigate their optical response, as already reported in the literature [53]. In this sense, steady-state emission spectra were acquired using 220 nm, 250 nm, 280 nm, 310 nm, and 340 nm as excitation wavelengths (Figures S17 and S18). The investigation unveiled that all compounds exhibited greater intensities when excited at higher energies, with the highest emission intensity recorded at an excitation wavelength of 280 nm. These results are consistent with what was observed in the excitation spectra of the studied compounds, which exhibit maximum values around 280 nm (Figures S19 and S20). Furthermore, the location of the emission maxima (λem = 290–390 nm) exhibits changes depending on the excitation wavelength (λex = 250–340 nm). As the excitation wavelength is shifted towards longer wavelengths, corresponding to the tail portion of the absorption band, the fluorescence maximum gradually shifts towards longer wavelengths, accompanied by a progressive decrease in overall intensity. Interestingly, the effect of the positively charged heterocycles, counter-ions, and solvent does not seem to influence the excited state properties significantly. The compounds predominantly emit in the UV region with a peak around 330 nm. However, compound 5c in ethanol displays a distinct behavior, exhibiting a dual emission in the UV to blue region with maxima at 353 nm and 453 nm. Considering the photophysical behavior exhibited by these ionic liquids with their structural characteristics, it is evident that these compounds belong to the category of molecules that display non-traditional luminescence. The noteworthy characteristics include the ability to tune the emission of the system based on the excitation wavelength, as well as the fluorescence response within an excitation region where the compounds exhibit minimal absorption. However, it should be noted that fluorescence emission is suppressed at concentrations exceeding 10−3 M (see supporting information). Based on these observations, it would not be surprising that the studied compounds formed a non-emissive complex since the NTILs exhibited numerous distinct properties that set them apart from traditional fluorescent materials, such as concentration-increasing emission, excitation-dependent fluorescence (EDF) or even excitation-independent fluorescence (EIF) [62].
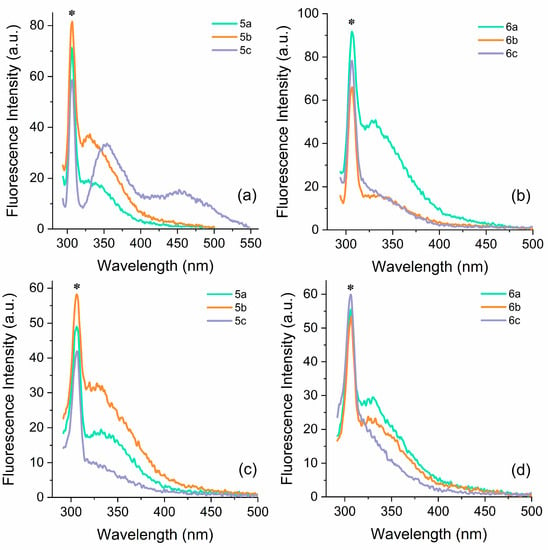
Figure 2.
Steady-state fluorescence emission spectra in (a,b) ethanol and (c,d) acetonitrile solutions [ca. 10−5 M] of thiazolidine-based CILs 5a–5c (left) and 6a–6c (right). The asterisk indicates the Raman signal.
3.3. Theoretical Calculations
3.3.1. Molecular Geometries and Electronic Properties
Given the flexibility of the initial molecules used for the conformational sampling, as depicted in Figure 3, the resulting conformational space is extensive. For instance, molecule 6a yields 1968 conformers, 641 of which are considered non-degenerate, and 41 configurations lie below the thermodynamic threshold of 2.5 kcal. The conformer with the lowest energy for 6a is deemed the most statistically significant, accounting for 94.1% of the Boltzmann population distribution. Molecule 6b is even more complex, with 3218 potential conformers, 1465 of which are non-degenerate, and 147 fall below the 2.5 kcal threshold. The conformer with the lowest energy for 6b also carries a substantial Boltzmann weight, constituting 59% of the distribution. Finally, molecule 6c exhibits 2673 unique conformers, 1587 of which are non-degenerate, and 100 fall below the 2.5 kcal threshold. The conformer with the lowest energy for 6c holds a 47.8% weight in the Boltzmann distribution. Due to the greater statistical significance of the lowest energy conformer and to manage the high computational costs, only the lowest conformer was used for subsequent computational calculations for each molecule.
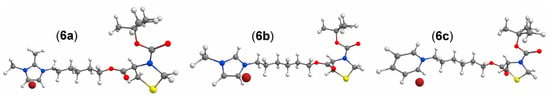
Figure 3.
Initial molecules used for conformational sampling.
After DFT re-optimization of the lowest conformer to the ground and first excited states, it becomes clear that, due to their flexibility, molecules 6a–6c tend to curl upon themselves, reaching greater stability due to the formation of intramolecular interactions. The bromine anion is electrostatically bound to the positively charged imidazolium or pyridinium moieties, and the second-order symmetry adapted perturbation theory (SAPT2) confirms that electrostatics is the main intermolecular interaction active on the molecule–bromine system (see Table S1 for the full energy decomposition). For the three molecules, 6a–6c, the highest occupied molecular orbital (HOMO) is the lone electron pair strongly localized over the bromine anion, and the lowest unoccupied molecular orbital (LUMO) is an easily identifiable π* orbital over the charged imidazolium or pyridinium moieties (Figure 4). The HOMO-LUMO gaps for the ground-states are quite similar and relatively large for 6a and 6b, which indicates that the molecules are more kinetically stable. On the other hand, molecule 6c appears with a HOMO-LUMO gap that is almost 2 eV smaller than its counterparts. When optimized to the first excited state, all molecules experienced a HOMO-LUMO gap reduction of about 2 eV, and despite being considerably large molecules, the geometric displacements upon excitation remained small, with RMSDs in the 0.548–0.866 Å range, as depicted in Figure 4. The S0 calculated dipole moments for molecules 6a–6c are, respectively, 9.743, 9.269, and 9.660 Debye and suffered a significant change upon optimization to the first excited state, reducing to 3.972, 3.729, and 4.276 Debye, as can be seen in Table S2.
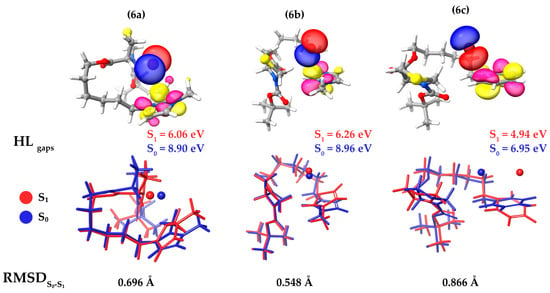
Figure 4.
Highest occupied molecular orbitals color-coded in red and blue phases and lowest unoccupied molecular orbitals color-coded in pink and yellow phases with ρ = 0.05 e/Å3 (top). Ground-state geometries are in blue, and the first excited state geometries, are in red (bottom).
3.3.2. Electronic Excitations
In comparison with experimental UV–Vis spectra, the computational absorption spectra offer interesting insights and confirmations. As noted in the experimental measurements, the solvents—ethanol and acetonitrile—exhibit similar effects on the absorption profile. A similar trend is observed for the computational spectra with implicit CPCM solvation in stark contrast to the absorptions calculated in a vacuum where solvation effects are deactivated (Figure S21). This suggests that the absorption profile is not solely dictated by molecular properties but is significantly influenced by the solvation environment. All employed methods used for absorption simulations showed good accuracy compared to the experimental results. More advanced procedures, such as STEOM and large MOPAC CASSCF calculations, revealed, as seen in Figure S22, the existence of absorption bands at much larger wavelengths in addition to the experimentally plotted UV–Vis 200–260 nm range. The absence of such lower energy bands in the experimental results is likely due to the large emission-active range observed for the compounds.
In compounds 6a–6c, the excited states comprise rather complex canonical electronic transitions. For molecule 6a, STEOM predicts an excited state with an oscillator strength (fosc) of 0.002 appearing at ~210 nm, remarkably close to the experimental 207 nm in ethanol. This state consists of 15 simultaneous electronic transitions. The complex nature of this electronic state is more conveniently interpreted in the natural transition orbital (NTO) framework as a 99% HOMO→LUMO and 1% HOMO-1→LUMO+1 transition. As shown in Figure 5, this transition corresponds to an intermolecular charge transfer from the bromine anion to the imidazolium moiety. Similarly, molecule 6b exhibits a transition with fosc equal to 0.006 at 211 nm, which is described in the NTO framework as 97.5% HOMO→LUMO. This transition also corresponds to an intermolecular charge transfer from the bromine anion to the imidazolium moiety (Figure 5). Molecule 6c features a transition with fosc of 0.094 at ~214 nm (experimental 208 nm) and another transition with a fosc of 0.036 at ~277 nm (experimental 258 nm). The 214 nm excited state is described by NTO as 87% HOMO→LUMO and 11% HOMO-1→LUMO+1, with several minor contributions involving transitions from HOMO-2 to HOMO-6 and LUMO+2 through LUMO+6. Additionally, the 277 nm transition appears as 99% HOMO→LUMO. The EDDs for 6c suggest that the electronic transitions occurring at 277 nm, similar to those for molecules 6a and 6b, account for an intermolecular bromine–pyridinium charge transfer. Meanwhile, the transitions at 214 nm are largely localized over the pyridinium moiety (Figure 5).
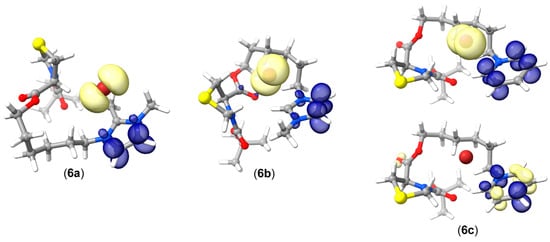
Figure 5.
Electronic density differences (EDD) for the relevant excited states on molecules 6a–6c. Light yellow depicts electronic density lost (hole) and dark blue electronic density gained (particle). Ρ = 0.005 e/Å3.
3.4. Optical Sensing
Based on the chemical structure of the studied CILs, as well as their optical properties, it was decided to explore their ability for metal sensing. In this context, their UV–Vis absorption and fluorescence emission properties were examined in response to a range of metal ions, including Ag+, Li+, Zn2+, Cd2+, Cu2+, Co2+, and Fe3+. Initially, the experiments were conducted at room temperature by adding 20 equivalents of the metal ions as aqueous solutions of their nitrate salts. Since the photophysical behavior of compounds 5a–5c and 6a–6c exhibited similar characteristics irrespective of the anions or positively charged heterocycles present, our focus for further investigation was solely on compound 5a, considering its potential application as a metal sensor. The presence of Zn2+, Co2+, and Cu2+ ions resulted in changes to the photophysical properties of compound 5a, while no significant alterations were observed with other metal ions. The interaction with zinc, cobalt, and copper ions induced a redshift in the absorption band at 210 nm, and an additional absorption band at 312 nm was observed only in the presence of Cu2+. Furthermore, a similar trend was observed in all three cases where the fluorescence emission was suppressed. These preliminary investigations demonstrated the response of ionic liquid 5a towards Zn2+, Co2+, and Cu2+ ions. Based on the structural characteristics of the studied CILs, the acid–base complex formation in ion sensing primarily depends on the hardness of the species involved, the ion size, and the hindrance at the interaction site. [63,64] Our observations reveal that the studied CIL exhibits a higher affinity for species with intermediate hardness parameter values [65]. While the binding constant indicates the formation of the CIL-Cu2+ complex, the specific mechanism by which the metal ion interacts with the ligand remains unknown. In this context, we hypothesize that both nitrogen and sulfur atoms of the thiazolidine ring are involved in the complexation process. If the interaction was solely mediated by the sulfide or tertiary amine moiety, we would expect selectivity towards soft acids (lower hardness parameter values) or hard acids (higher hardness parameter values), respectively. Thus, the participation of both nitrogen and sulfur atoms suggests a combined effect in the complexation. Furthermore, the variance in optical response among Co2+, Cu2+, and Zn2+ ions (with d7, d9, and d10 configurations, respectively) can be attributed to the magnitude of charge transfer, leading to either a gradual or complete fluorescence deactivation [66,67].
To gain insights into quantitative detection parameters, titrations were performed using a mixture of varying amounts of the aqueous solution of each respective ion (10−3 M) with a solution of 5a in acetonitrile (10−4 M). The evaluation was conducted using both UV–Vis absorption and fluorescence emission spectra. Figure 6 presents the results of the Cu2+ titration, while the spectra for Zn2+ and Co2+ can be found in the supporting information (Figures S23 and S24, respectively).
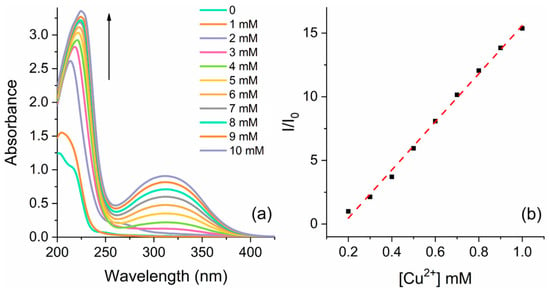
Figure 6.
(a) UV–Vis absorption spectra of compound 5a [ca. 10−4 M] at different amounts of Cu2+ in a CH3CN/H2O solution and (b) the respective linear response with increasing concentration of Cu2+@310 nm.
In each of the three cases, the intensity of the absorption band at 210 nm exhibited a gradual increase accompanied by a redshift as the ion concentration increased from 0.1 to 1.0 equiv. Figure 3 shows the arising of an additional absorption band at 310 nm upon the addition of Cu2+, which was not observed for the other ions. Conversely, the fluorescence emission exhibited an inverse trend with quenching observed as the ion concentration increased (Figure S25). It is worth noting that the behavior observed for the interaction of 5a with Cu2+ differs from that of Zn2+ and Co2+. For Zn2+ and Co2+, the fluorescence emission significantly decreased with the addition of initial amounts of the ions and then remained stable (Figures S23 and S24), whereas a linear response was observed for Cu2+ (Figure S25). Both the UV absorption and emission spectra demonstrated a good linear relationship between the optical response and the concentration of Cu2+. By employing linear regression to model the relationship between I/I0 and [Cu2+] for the absorption spectra, the LOD and LOQ were calculated. The absorption spectra data yielded the equation y = 18910x − 3.31 (R2 = 0.995 and σ = 0.29), resulting in an LOD of 50 μM or 3.2 ppm and an LOQ of 153 μM or 9.7 ppm. Similarly, the analysis conducted on the fluorescence spectra yielded the equation y = −857.2x + 1.02 (R2 = 0.915 and σ = 0.0627). The corresponding values for the LOD and LOQ were determined as 241 μM (15.3 ppm) and 731 μM (46.5 ppm), respectively. These results indicate that higher sensitivity is achieved when detection is performed using UV–Vis absorption spectroscopy. In light of the obtained results concerning the LOD and LOQ, the ionic liquids' biological activity and physical properties, such as water solubility and high stability, have demonstrated significant potential. Moreover, recent investigations into their photophysical properties have unveiled new avenues for the advancement of sensors in biological environments. The literature reports that conventional organic sensors face a low bioavailability disadvantage related to the low sensor biopermeability and poor water solubility [68,69,70], making their application as sensors in biological media unfeasible. To deepen our comprehension of the interplay between metal ions and the chiral ionic liquid, we conducted titration experiments with a metal ion and calculated the Stern–Volmer quenching constant (KSV). This calculation allows us to assess the extent of quenching and gain additional insights into the interaction dynamics between the metal ions and the ionic liquid [71]. The KSV was determined from the slope of the linear Stern–Volmer plot, which was found to be 2615 M−1 (Table S3). The linear fitting observed with Cu2+ suggests a static quenching mechanism, indicating that the binding between the metal ion and the ionic liquid leads to the formation of a nonfluorescent complex in the ground state. The emergence of a new absorption band in the UV–Vis spectrum provides evidence for the formation of the CIL-Cu2+ complex. The obtained KSV aligns with values reported in previous studies on static metal ion interactions with ionic liquids [72,73]. Furthermore, the fluorescence titration data were subjected to analysis using the BindFit v0.5 open-access program available at supramolecular.org [74,75,76]. Two binding models were considered based on the ratio of the ionic liquid to metal ion (1:1 and 2:1). The covariance of the fit and residues indicated that the 1:1 model provided a better fit, yielding a calculated binding constant of 3565 M−1.
4. Conclusions
In this study, we have presented a synthesis and photophysical investigation of thiazolidine-functionalized chiral ionic liquids derived from L-cysteine. A four-step synthetic route was employed, involving N-protection, coupling reactions with bromoalcohols, and formation of an ionic liquid. The optical properties of the chiral ionic liquids were examined using UV–Vis and fluorescence spectroscopies. It was observed that the compounds exhibited transparency to visible light with low molar extinction coefficients in this range. The UV–Vis spectra exhibited distinct characteristics based on the heterocycles and counter-ions used, and the presence of charge transfer complexes was detected in some cases. The emission spectra of the compounds were observed in the UV to violet region. These findings suggest that the thiazolidine-based chiral ionic liquids possess non-traditional intrinsic luminescence properties, which can be attributed to the presence of electron-rich functional groups. Furthermore, the interaction between copper ions and the chiral ionic liquids was investigated using UV–Vis absorption and fluorescence spectroscopies. The results demonstrated a linear correlation between the optical response and the concentration of the copper ion. The limits of detection and quantification were calculated, revealing that UV–Vis absorption spectroscopy provided lower values compared to fluorescence. Additionally, the Stern–Volmer quenching constant was determined, and the obtained value aligned with the static binding mechanism reported in previous studies on metal ion–ionic liquid interactions. Overall, these experimental findings shed light on the nature of the interaction between copper (II) ions and chiral ionic liquids, highlighting their potential as optical sensors for metal ions. The computational calculations revealed that the studied molecules, due to their flexibility, span a large conformational space despite the lowest conformer having most of the Boltzmann population weight. Ab initio and DFT calculations revealed that the electronic transitions are remarkably complex but always involve a clear charge transfer from the bromine anion to the positively charged molecular moiety. Additionally, the comparison between the vacuum and CPCM-active absorption spectra showed that solvation effects have a huge impact on their electronic excitations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/compounds3030032/s1, additional experimental procedures; Figures S1–S16: original nmr spectra from the spectroscopic characterization; Figures S17–S20: additional emission and excitation spectra from the CILs; Figures S21–S22: theoretical absorption spectra; Figures S23–S25: additional photophysical data from the titrations; Table S1: SAPT2 energy decomposition for a two-body system molecule-bromine; Table S2: Dipole moments comparison for the fully optimized S0 and S1 geometries; Table S3: Binding models for investigation of the interaction between ionic liquid and copper ion.
Author Contributions
Conceptualization, F.S.R., F.L.C. and P.H.S.; methodology, C.H.G., F.L.C., M.F.B. and H.C.S.J.; validation, F.S.R. and P.H.S.; formal analysis, C.H.G., H.C.S.J., M.F.B. and F.L.C.; resources, F.S.R. and P.H.S.; writing—original draft preparation, C.H.G., H.C.S.J. and F.L.C.; writing—review and editing, F.S.R. and P.H.S.; supervision, F.S.R. and P.H.S.; project administration, P.H.S.; funding acquisition, P.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPERGS (PRONEX and RITEs-RS), CNPq (404503/2021-7, 308487/2021-4, 305954/2019-9 and 163912/2020-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and INCT-CNM.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Theoretical calculations were performed using the Lobo Carneiro supercomputer from Núcleo Avançado de Computação de Alto Desemprenho (NACAD) under the Project ID a20006 and the Sagarana Cluster from CEPAD—Centro de Processamento de Alto Desempenho ICB/UFMG. The authors would also like to thank the National Laboratory for Scientific Computing (LNCC/MCTI, Brazil) for providing HPC resources for the SDumont supercomputer that have contributed to the research results reported in this paper. URL: http://sdumont.lncc.br.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zumbrägel, N.; Merten, C.; Huber, S.M.; Gröger, H. Enantioselective reduction of sulfur-containing cyclic imines through biocatalysis. Nat. Commun. 2018, 9, 1949. [Google Scholar] [CrossRef]
- Elander, R.P. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Nakatani, S.; Hidaka, K.; Ami, E.; Nakahara, K.; Sato, A.; Nguyen, J.T.; Hamada, Y.; Hori, Y.; Ohnishi, N.; Nagai, A.; et al. Combination of non-natural D-amino acid derivatives and allophenylnorstatine-dimethylthioproline scaffold in HIV protease inhibitors have high efficacy in mutant HIV. J. Med. Chem. 2008, 51, 2992–3004. [Google Scholar] [CrossRef]
- Sahiba, N.; Sethiya, A.; Soni, J.; Agarwal, D.K.; Agarwal, S. Saturated five membered thiazolidines and their derivatives: From synthesis to biological applications. Top. Curr. Chem. 2020, 378, 34. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Balakrishnan, C.; Theetharappan, M.; Natarajan, S.; Thalamuthu, S.; Neelakantan, M.A. Fluorescence response of a thiazolidine carboxylic acid derivative for the selective and nanomolar detection of Zn(II) ions: Quantum chemical calculations and application in real samples. RSC Adv. 2015, 5, 105453. [Google Scholar] [CrossRef]
- Bilgiçli, H.G.; Bilgiçli, A.T.; Günsel, A.; Tüzün, B.; Ergön, D.; Yarasir, M.N.; Zengin, M. Turn-on fluorescent probe for Zn2+ ions based on thiazolidine derivative. Appl. Organometal. Chem. 2020, 34, e5624. [Google Scholar]
- Aydin, D.; Karakilic, E.; Karakurt, S.; Baran, A. Thiazolidine based fluorescent chemosensors for aluminum ions and their applications in biological imaging. Spectrochim. Acta A 2020, 238, 118431. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Avtar, S.; Kumar, C.H. Chiral ionic liquids: Design, synthesis and applications in asymmetric organo-catalysis. Curr. Org. Synth. 2017, 14, 488–510. [Google Scholar]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Suzuki, Y.; Wakatsuki, J.; Tsubaki, M.; Sato, M. Imidazolium-based chiral ionic liquids: Synthesis and application. Tetrahedron 2013, 69, 9690–9700. [Google Scholar] [CrossRef]
- Rodríguez-Cárdenas, E.; Cardoso-Martínez, J.; Nieto-Camacho, A.; Frontana-Uribe, B.A. Physical-chemical properties of chiral ionic liquids derived from the phenylethylamine enantiomers. J. Mol. Liq. 2017, 236, 435–444. [Google Scholar] [CrossRef]
- Klejdysz, T.; Łęgosz, B.; Czuryszkiewicz, D.; Czerniak, K.; Pernak, J. Biobased ionic liquids with abietate anion. ACS Sustain. Chem. Eng. 2016, 4, 6543–6550. [Google Scholar] [CrossRef]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155–1157. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Lévêque, J.M.; Estager, J.; Draye, M.; Bonrath, W. A speedy one-pot synthesis of second-generation ionic liquids under ultrasound and/or microwave irradiation. Aust. J. Chem. 2007, 60, 946–950. [Google Scholar] [CrossRef]
- Rahman, M.B.A.; Jumbri, K.; Basri, M.; Abdulmalek, E.; Sirat, K.; Salleh, A.B. Synthesis and physico-chemical properties of new tetraethylammonium-based amino acid chiral ionic liquids. Molecules 2010, 15, 2388. [Google Scholar] [CrossRef]
- Bach, M.F.; Griebeler, C.H.; Jacoby, C.G.; Schneider, P.H. Design of a chiral ionic liquid system for the enantioselective addition of diethylzinc to aldehydes. Eur. J. Org. Chem. 2017, 2017, 6997–7004. [Google Scholar] [CrossRef]
- Jordan, A.; Haiß, A.; Spulak, M.; Karpichev, Y.; Kümmerer, K.; Gathergood, N. Synthesis of a series of amino acid derived ionic liquids and tertiary amines: Green chemistry metrics including microbial toxicity and preliminary biodegradation data analysis. Green Chem. 2016, 18, 4374–4392. [Google Scholar] [CrossRef]
- Borba, L.C.; Griebeler, C.H.; Bach, M.F.; Barboza, C.A.; Nogara, P.A.; da Rocha, G.B.T.; Amaral, S.S.; Rodembusch, F.S.; Schneider, P.H. Non-traditional intrinsic luminescence of amphiphilic-based ionic liquids from oxazolidines: Interaction studies in phosphatidylcholine-composed liposomes and BSA optical sensing in solution. J. Mol. Liq. 2020, 313, 113525. [Google Scholar] [CrossRef]
- Vidal, M.; Schmitzer, A.R. Thermophysical properties of imidazolium-functionalized binols and their application in asymmetric catalysis. Organometallics 2014, 33, 3328–3340. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated Exploration of the Low-Energy Chemical Space with Fast Quantum Chemical Methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Lin, Y.S.; Li, G.D.; Mao, S.P.; Chai, J.D. Long-Range Corrected Hybrid Density Functionals with Improved Dispersion Corrections. J. Chem. Theory Comput. 2013, 9, 263–272. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Lechner, M.H.; Neese, F.; Izsák, R. An excited state coupled-cluster study on indigo dyes. Mol. Phys. 2021, 119, 21–22. [Google Scholar] [CrossRef]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural Bond Orbital Analysis Program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC2016, Computational Chemistry, Colorado Springs, CO, USA. Available online: http://OpenMOPAC.net (accessed on 29 June 2023).
- Hirata, S.; Head-Gordon, M. Time-Dependent Density Functional Theory within the Tamm–Dancoff Approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Parker, T.M.; Burns, L.A.; Parrish, R.M.; Ryno, A.G.; Sherrill, C.D. Levels of Symmetry Adapted Perturbation Theory (SAPT). I. Efficiency and performance for interaction energies. J. Chem. Phys. 2014, 140, 094106. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; Di Remigio, R.; Alenaizan, A.; et al. Psi4 1.4: Open-source software for high-throughput quantum chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Vishnumaya, M.R.; Singh, V.K. Highly efficient small organic molecules for enantioselective direct aldol reaction in organic and aqueous media. J. Org. Chem. 2009, 74, 4289–4297. [Google Scholar] [CrossRef]
- Vishnumaya, M.R.; Ginotra, S.K.; Singh, V.K. Highly enantioselective direct aldol reaction catalyzed by organic molecules. Org. Lett. 2006, 8, 4097–4099. [Google Scholar]
- Zhu, S.; Song, Y.; Shao, J.; Zhao, X.; Yang, B. Non-conjugated polymer dots with crosslink-enhanced emission in the absence of fluorophore units. Angew. Chem. Int. Ed. 2015, 54, 14626–14637. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yang, T.; Zhao, Z.; Zhu, T.; Zhang, Q.; Hou, W.; Yuan, W.Z. Nonconventional luminophores: Characteristics, advancements and perspectives. Chem. Soc. Rev. 2021, 50, 12616–12655. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Jia, H.; Xie, W.; Wu, H.; Li, J.; Wang, H. Nontraditional organic/polymeric luminogens with red-shifted fluorescence emissions. Macromol. Chem. Phys. 2022, 223, 2100425. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Klajnert-Maculewicz, B.; Johnson, K.A.M.; Brinkman, H.F.; Janaszewsk, A.; Hedstrand, D.M. Non-traditional intrinsic luminescence: Inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Progr. Polym. Sci. 2019, 90, 35–117. [Google Scholar] [CrossRef]
- Chen, X.; Luo, W.; Ma, H.; Peng, Q.; Yuan, W.Z.; Zhang, Y. Prevalent intrinsic emission from nonaromatic amino acids and poly(amino acids). Sci. China Chem. 2018, 61, 351–359. [Google Scholar] [CrossRef]
- Yi, M.; Qi, P.; Fan, Q.; Hao, J. Ionic liquid crystals based on amino acids and gemini surfactants: Tunable phase structure, circularly polarized luminescence and emission color. J. Mater. Chem. C 2022, 10, 1645–1652. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, B.; Zhu, C.; Xu, S.; Gong, Y.; Yuan, W.Z.; Zhang, Y. Clustering-triggered emission of nonconjugated polyacrylonitrile. Small 2016, 12, 6586–6592. [Google Scholar] [CrossRef]
- Ji, X.; Tian, W.; Jin, K.; Diao, H.; Huang, X.; Song, G.; Zhang, J. Anionic polymerization of nonaromatic maleimide to achieve full-color nonconventional luminescence. Nat. Commun. 2022, 13, 3717. [Google Scholar] [CrossRef]
- Lee, W.I.; Bae, Y.; Bard, A.J. Strong blue photoluminescence and ECL from OH-terminated PAMAM dendrimers in the absence of gold nanoparticles. J. Am. Chem. Soc. 2004, 126, 8358–8359. [Google Scholar] [CrossRef]
- Mingkang, S.; Francesca, L.; Rui, Y.; Sajjad, D.S.; Tomasz, K.; Krzysztof, M. Assemblies of polyacrylonitrile-derived photoactive polymers as blue and green light photo-cocatalysts for Cu-catalyzed ATRP in water and organic solvents. Front. Chem. 2021, 9, 734076. [Google Scholar]
- Sun, M.; Hong, C.Y.; Pan, C.Y. A unique aliphatic tertiary amine chromophore: Fluorescence, polymer structure, and application in cell imaging. J. Am. Chem. Soc. 2012, 134, 20581–20584. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Zhang, Y. Nonconventional macromolecular luminogens with aggregation-induced emission characteristics. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 560–574. [Google Scholar] [CrossRef]
- Zhao, E.; Lam, J.W.Y.; Meng, L.; Hong, Y.; Deng, H.; Bai, G.; Huang, X.; Hao, J.; Tang, B.Z. Poly[(maleic anhydride)-alt-(vinyl acetate)]: A pure oxygenic nonconjugated macro-molecule with strong light emission and solvatochromic effect. Macromolecules 2015, 48, 64–71. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B.Z. Catalyst-free, atom-economic, multicomponent polymerizations of aromatic diynes, elemental sulfur, and aliphatic diamines toward luminescent polythioamides. Macromolecules 2015, 48, 7747–7754. [Google Scholar] [CrossRef]
- Miao, X.; Liu, T.; Zhang, C.; Geng, X.; Meng, Y.; Li, X. Fluorescent aliphatic hyperbranched polyether: Chromophore-free and without any N and P atoms. Phys. Chem. Chem. Phys. 2016, 18, 4295–4299. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.G.; Li, N.; Ling, Y.; Hua, K.B.; Geng, S.; Li, N.B.; Luo, H.Q. pH-mediated fluorescent polymer particles and gel from hyperbranched polyethylenimine and the mechanism of intrinsic fluorescence. Langmuir 2016, 32, 1881–1889. [Google Scholar] [CrossRef]
- Larson, C.L.; Tucker, S.A. Intrinsic fluorescence of carboxylated-terminated polyamido amine dendrimers. Appl. Spectrosc. Rev. 2001, 55, 679–683. [Google Scholar] [CrossRef]
- Katoh, R. Absorption spectra of imidazolium ionic liquids. Chem. Lett. 2007, 36, 1256–1257. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, L.; Wang, Y.; Xu, X.; Peng, X. Interactions between pyrene and pyridinium ionic liquids studied by ultraviolet–visible spectroscopy. J. Mol. Liq. 2016, 213, 289–293. [Google Scholar] [CrossRef]
- Ogura, T.; Akai, N.; Shibuya, K.; Kawai, A. Charge-transfer electronic absorption spectra of 1-ethylpyridinium cation and halogen anion pairs in dichloromethane and as neat ionic liquids. J. Phys. Chem. B 2013, 117, 8547–8554. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, R.; Cao, L.; Wang, H.; Zhang, L.; Lan, S.; Peng, F.; Liu, C.; Jia, D.; Wang, D. Three distinct concentration-dependent chromophores of non-traditional intrinsic luminescence: The mechanism and special properties. J. Lumin. 2021, 239, 118401. [Google Scholar] [CrossRef]
- Ahmad, M.G.; Chanda, K. Ionic liquid coordinated metal-catalyzed organic transformations: A comprehensive review. Coord. Chem. Rev. 2022, 472, 214769. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Metal complexation in ionic liquids. Annu. Rep. Prog. Chem. Sect. A Inorg. Chem. 2008, 104, 21–45. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Juliá, F. Ligand-to-metal charge transfer (LMCT) photochemistry at 3d-metal complexes: An emerging tool for sustainable organic synthesis. ChemCatChem 2022, 14, e202200916. [Google Scholar] [CrossRef]
- Fedorova, O.A.; Shepel, N.E.; Tokarev, S.D.; Lukovskaya, E.V.; Sotnikova, Y.A.; Moiseeva, A.A.; D’Aléo, A.; Fages, F.; Maureld, F.; Fedorov, Y.V. Intramolecular electron transfer in Cu(II) complexes with aryl-imidazo-1,10-phenanthroline derivatives: Experimental and quantum chemical calculation studies. New J. Chem. 2019, 43, 2817–2827. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Z.; Wang, C.; Zhu, J.; Wang, S.; Liu, Y.; Wei, P.; Yi, T. A lysosome-targeted near-infrared fluorescent probe for cell imaging of Cu2+. Dyes Pigm. 2022, 204, 110472. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, X.; Yang, H.; Zhang, T.; Han, A.; Zang, L. A Cu2+-selective probe based on phenanthro-imidazole derivative. Sensors 2017, 17, 35. [Google Scholar] [CrossRef]
- Duarte, L.G.T.A.; Coelho, F.L.; Germino, J.C.; da Costa, G.G.; Berbigier, J.F.; Rodembusch, F.S.; Atvars, T.D.Z. A selective proton transfer optical sensor for copper II based on chelation enhancement quenching effect (CHEQ). Dyes Pigm. 2020, 181, 108566. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gohil, H.; Suresh, E.; Paital, A.R. Copper(II)-specific fluorogenic task-specific ionic liquids as selective fluorescence probes and recyclable extractants. Chem. Eur. J. 2015, 21, 13943–13948. [Google Scholar] [CrossRef]
- Gohil, H.; Yadav, S.; Rajpurohit, D.; Bhojani, G.; Chatterjee, S.; Paital, A.R. Sensing vs. extraction: Functionalized ionic liquid as a single platform for dual applications with biological implications. ACS Sustain. Chem. Eng. 2021, 9, 13096–13105. [Google Scholar] [CrossRef]
- Available online: http://supramolecular.org (accessed on 1 December 2022).
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).