First Report on Several NO-Donor Sets and Bidentate Schiff Base and Its Metal Complexes: Characterization and Antimicrobial Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
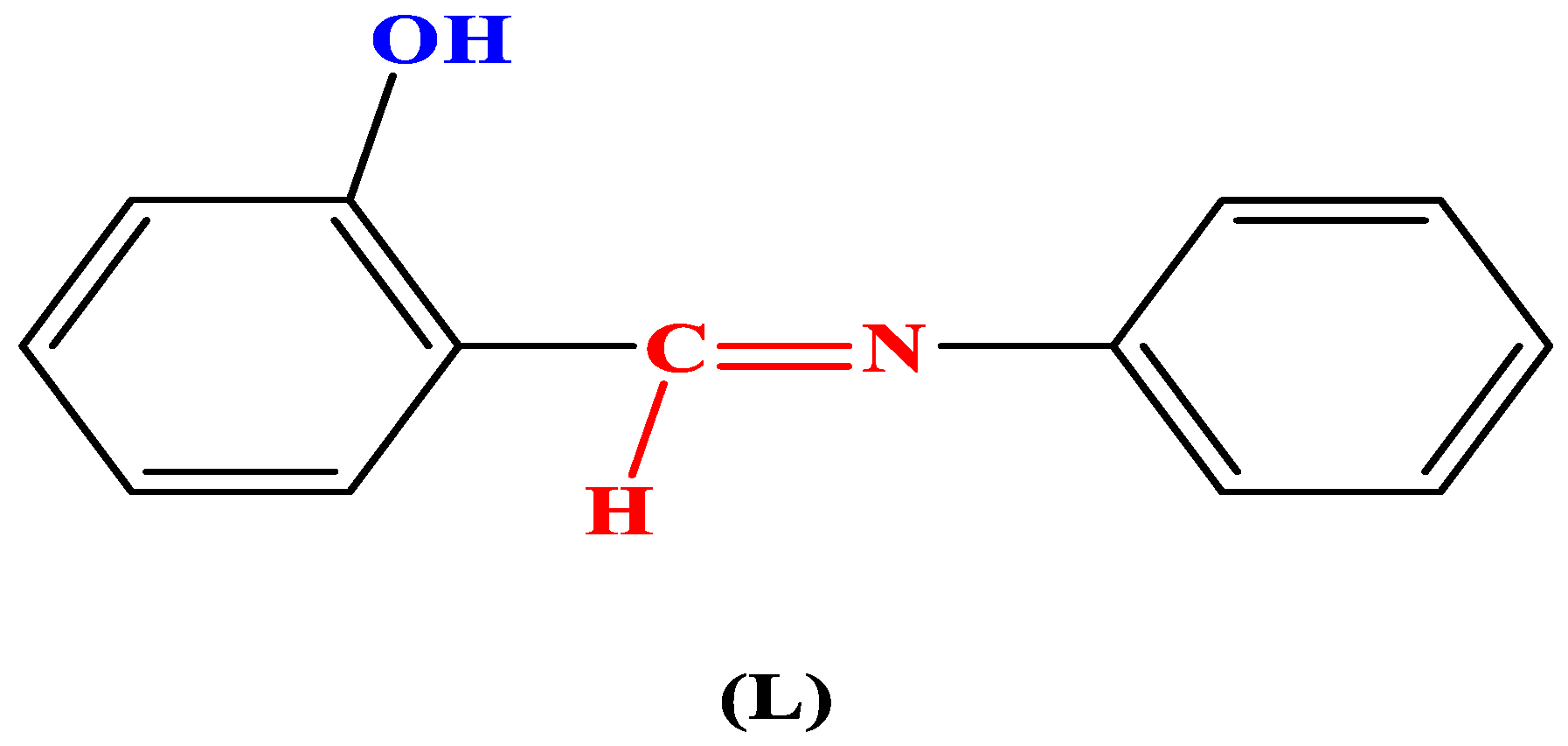
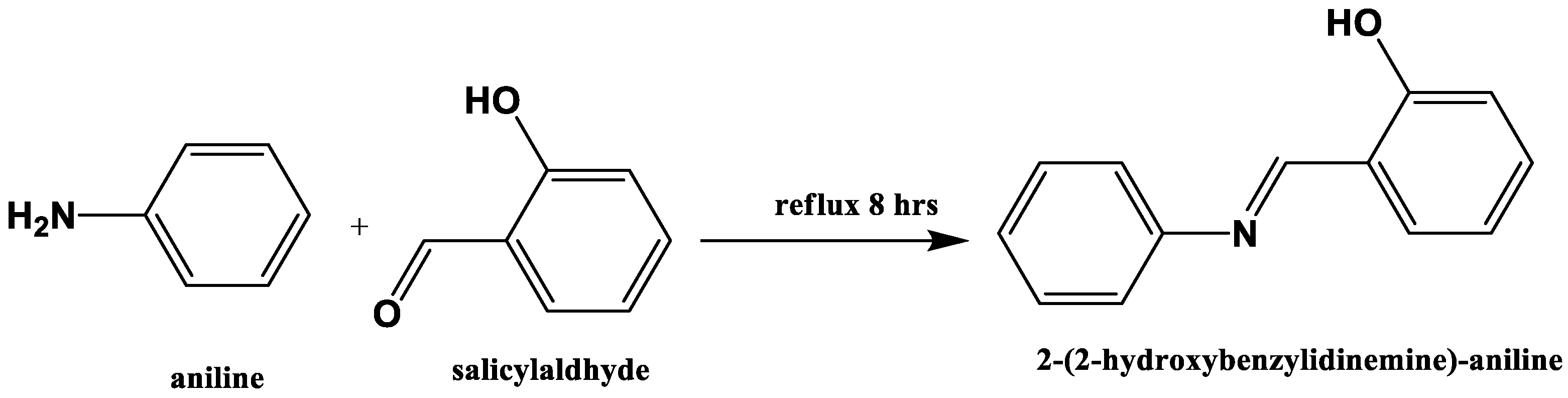
2.2. Preparation of 2-(2-Hydroxybenzylidinemine)-aniline (L)
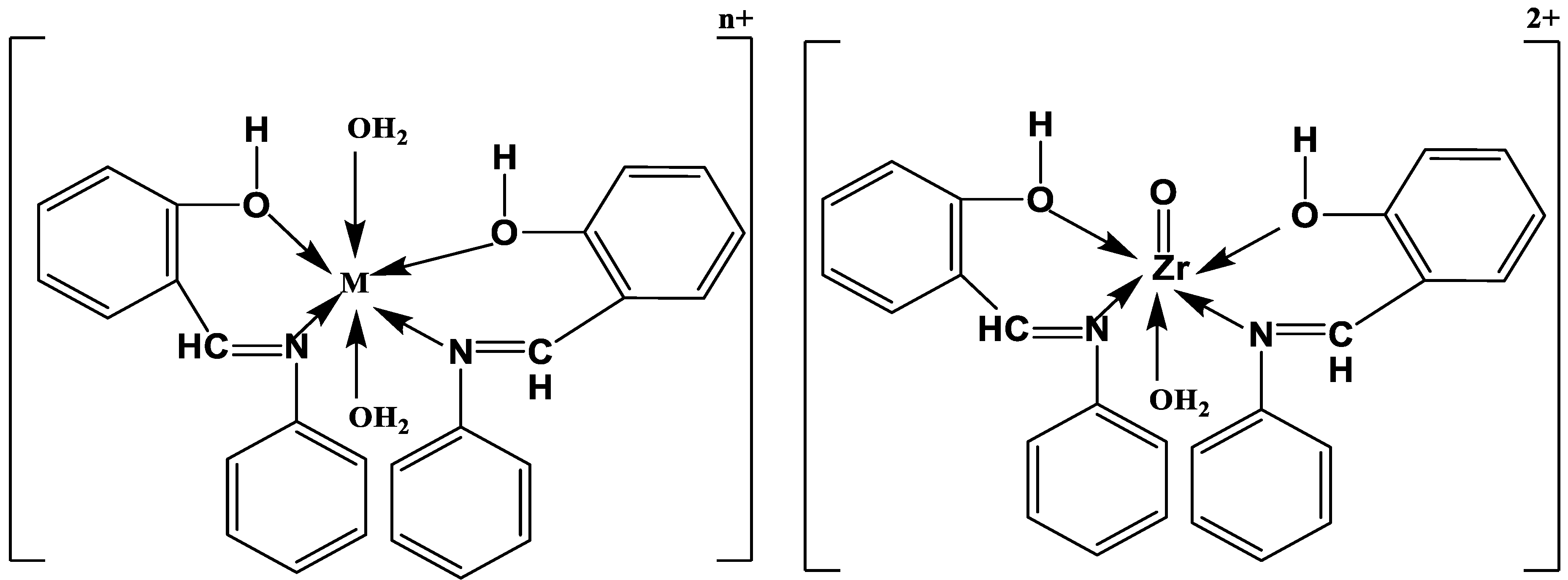
2.3. Synthesis of L Metal Complexes
2.4. Instruments
2.5. Antimicrobial Investigation
3. Results and Discussion
3.1. Elemental Composition and Molar Conductance
3.2. FT-IR Spectra and Mode of Bonding
3.3. Electronic Absorption Studies and Magnetic Moment Measurements
3.4. Nuclear Magnetic Resonance
3.5. Thermal Analysis Studies (TG and DTG)
3.6. Calculation of Thermodynamic Parameters
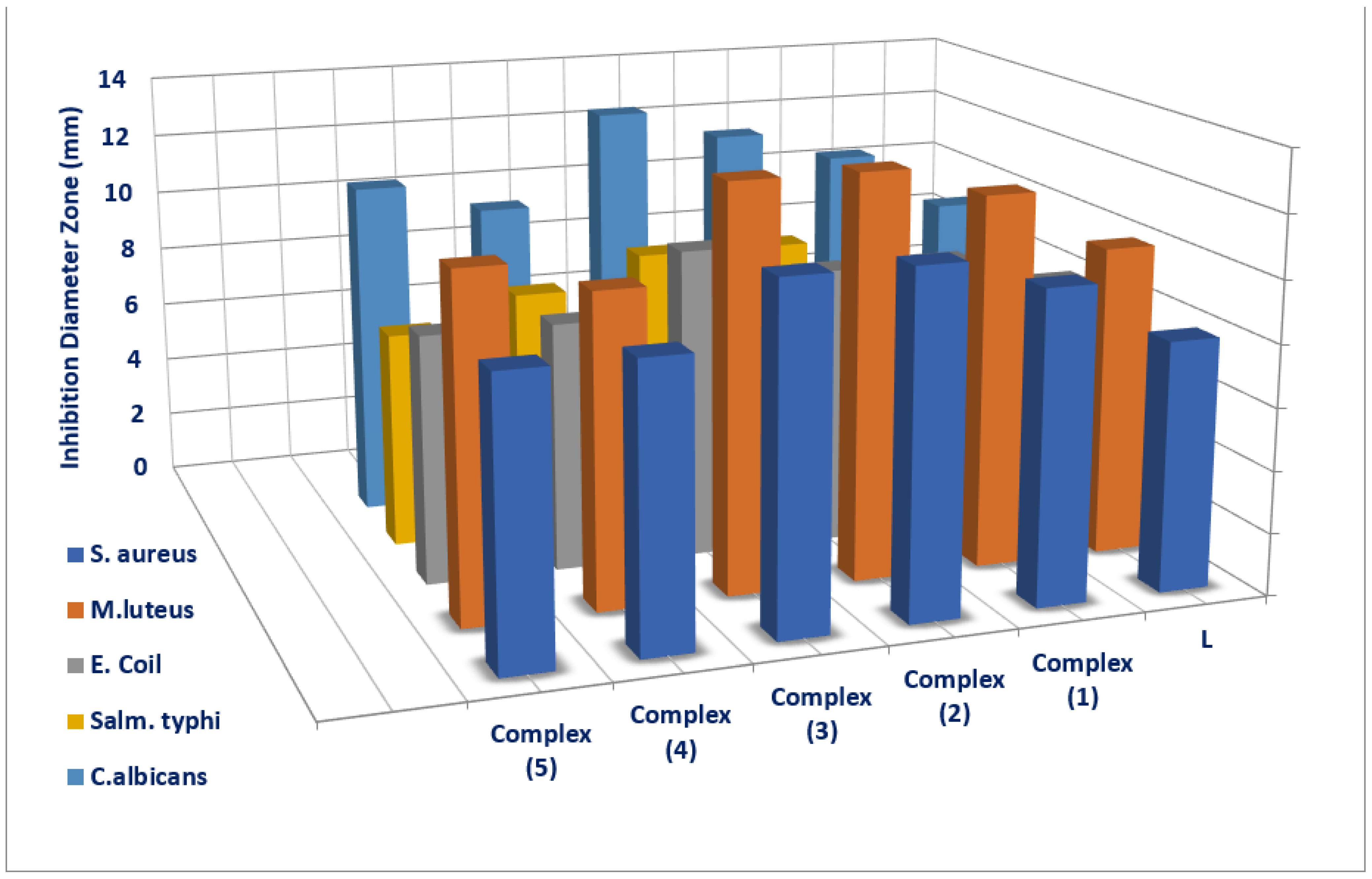
3.7. Antimicrobial Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Noor, A. Recent developments in two coordinate transition metal chemistry. Coord. Chem. Rev. 2023, 476, 214941. [Google Scholar] [CrossRef]
- Golbedaghi, R.; Tabanez, A.M.; Esmaeili, S.; Fausto, R. Biological Applications of Macrocyclic Schiff Base Ligands and Their Metal Complexes: A Survey of the Literature (2005–2019). Appl. Organomet. Chem. 2020, 34, e5884. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Omar, M.M.; Ahmed, Y.M.; Mohamed, G.G. Transition metal complexes of Schiff base ligand based on 4,6-diacetyl resorcinol. Appl. Organomet. Chem. 2020, 34, e5528. [Google Scholar] [CrossRef]
- Jali, B.R.; Baruah, J.B. Recent progress in Schiff bases in detections of fluoride ions. Dye. Pigment. 2021, 194, 109575. [Google Scholar] [CrossRef]
- Hussein, A.M.; Khowdiary, M.M. Nonionic Isatin Surfactants: Synthesis, Quantum Chemical Calculations, ADMET and Their Antimicrobial Activities. J. Surf. Deter. 2020, 23, 489–501. [Google Scholar] [CrossRef]
- Bal, S.; Bal, S.S.; Erener, A.; Halipci, H.N.; Akar, S. Synthesis, thermal stability, electronic features, and antimicrobial activity of phenolic azo dyes and their Ni(II) and Cu(II) complexes. Chem. Pap. 2014, 68, 352–361. [Google Scholar] [CrossRef]
- Osypiuk, D.; Cristóvão, B.; Bartyzel, A. New Coordination Compounds of CuII with Schiff Base Ligands—Crystal Structure, Thermal, and Spectral Investigations. Crystals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Al-Barody, S.M. Characterization and Thermal Study of Schiff-Base Monomers and Its Transition Metal Polychelates and Their Photovoltaic Performance on Dye Sensitized Solar Cells. J. Struct. Chem. 2018, 59, 53–63. [Google Scholar] [CrossRef]
- Abd El-Hamid, S.M.; Sadeek, S.A.; Mohammed, S.F.; Ahmed, F.M.; El-Gedamy, M.S. N2O2-chelate metal complexes with Schiff base ligand: Synthesis, characterisation and contribution as a promising antiviral agent against human cytomegalovirus. Appl. Organomet. Chem. 2023, 37, e6958. [Google Scholar] [CrossRef]
- Abd El-Hamid, S.M.; Sadeek, S.A.; Mohammed, S.F.; Ahmed, F.M.; El-Gedamy, M.S. Newly synthesised Schiff base metal complexes, characterisation, and contribution as enhancers of colon cancer cell apoptosis by overexpression of P53 protein. Aplp. Organomet. Chem. 2023, 37, e7129. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Attar, M.S.; Abd El-Hamid, S.M. Preparation and characterization of new tetradentate Schiff base metal complexes and biological activity evaluation. J. Mol. Struct. 2013, 1051, 30. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-khatib, R.M.; Aljohani, F.S.; Alzahrani, S.O.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Synthesis and intensive characterization for novel Zn(II), Pd(II), Cr(III) and VO(II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J. Mol. Struct. 2021, 1242, 130693. [Google Scholar] [CrossRef]
- Abd El-Hamid, S.M.; Sadeek, S.A.; Abd El-Lattif, N.S. Study of the chemical structure and their nematicidal activity of N2O2 tetradentate Schiff base metal complexes. Appl. Organo. Chem. 2019, 33, e5010. [Google Scholar] [CrossRef]
- Polarz, S.; Landsmann, S.; Klaiber, A. Hybrid Surfactant Systems with Inorganic Constituents. Angew. Chem. Int. Ed. 2014, 53, 946–954. [Google Scholar] [CrossRef]
- Hubin, T.J.; Amoyaw, P.N.A.; Roewe, K.D.; Simpson, N.C.; Maples, R.D.; Freeman, T.N.C.; Cain, A.N.; Le, J.G.; Archibald, S.J.; Khan, S.I.; et al. Synthesis and antimalarial activity of metal complexes of cross-bridged tetraazamacrocyclic ligands. Bioorg. Med. Chem. 2014, 22, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Abdallah, E.M.; Ahmed, S.A.; Rabee, M.M.; Bräse, S. Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A Review. Molecules 2023, 28, 1808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.W. Synthesis, Crystal Structures, and Antibacterial Activity of Manganese(III) Complexes with Schiff Bases. Russ. J. Coord. Chem. 2019, 45, 608–614. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Guler, E.; Sevgi, F.; Ozkalp, B. Synthesis, spectroscopic characterization and antimicrobial studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with Schiff bases derived from 5-bromo-salicylaldehyde. J. Mol. Struct. 2013, 1048, 476–481. [Google Scholar] [CrossRef]
- Keypour, H.; Shayesteh, M.; Rezaeivala, M.; Chalabian, F.; Elerman, Y.; Buyukgungor, O. Synthesis, spectral characterization, structural investigation and antimicrobial studies of mononuclear Cu(II), Ni(II), Co(II), Zn(II) and Cd(II) complexes of a new potentially hexadentate N2O4 Schiff base ligand derived from salicylaldehyde. J. Mol. Struct. 2013, 1032, 62–68. [Google Scholar] [CrossRef]
- Dhanaraj, C.J.; Johnson, J.; Joseph, J.; Joseyphus, R.S. Quinoxaline-based Schiff base transition metal complexes: Review. J. Coord. Chem. 2013, 66, 1416–1450. [Google Scholar] [CrossRef]
- EL-Shwiniy, W.A.; Ibrahim, A.G.; Sadeek, S.A.; Zordok, W.A. Synthesis, structural elucidation, molecular modeling and antimicrobial studies of 6-(2-hydroxyphenylimine)-2-thioxotetrahydropyrimidin-4 (1H)-one (L) Schiff base metal complexes. Appl. Organomet. Chem. 2021, 35, e6174. [Google Scholar] [CrossRef]
- Munshi, A.M.; Bayazeed, A.A.; Abualnaja, M.; Morad, M.; Alzahrani, S.; Alkhatib, F.; Shah, R.; Zaky, R.; El-Metwaly, N.M. Ball-milling synthesis technique for Cu(II)-Schiff base complexes with variable anions; characterization, potentiometric study and in-vitro assay confirmed by in-silico method. Inorg. Chem. Commun. 2021, 127, 108542. [Google Scholar] [CrossRef]
- Fernandes, P.; Sousa, I.; Cunha-Silva, L.; Ferreira, M.; De Castro, B.; Pereira, E.F.; Feio, M.J.; Gameiro, P. Synthesis, characterization and antibacterial studies of a copper(II) lomefloxacin ternary complex. J. Inorg. Biochem. 2014, 131, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Nagaj, J.; Starosta, R.; Jeżowska-Bojczuk, M. Acid-base characterization, coordination properties towards copper(II) ions and DNA interaction studies of ribavirin, an antiviral drug. J. Inorg. Biochem. 2015, 142, 68–74. [Google Scholar] [CrossRef]
- Chakraborty, L.; Chakraborty, N.; Choudhury, T.D.; Phani Kumar, B.V.N.; Mandal, A.B.; Rao, N.V.S. Synthesis, mesomorphic and photo-physical properties of few d- and f- block metals coordinated to polar Schiff’s bases. Liq. Crys. 2012, 39, 655–668. [Google Scholar] [CrossRef]
- Kucková, L.; Jomová, K.; Švorcová, A.; Valko, M.; Segľa, P.; Moncoľ, J.; Kožíšek, J. Synthesis, Crystal Structure, Spectroscopic Properties and Potential Biological Activities of Salicylate–Neocuproine Ternary Copper(II) Complexes. Molecules 2015, 20, 2115–2137. [Google Scholar] [CrossRef]
- Salem, A.E.; Mohammed, S.F.; Sadeek, S.A.; Zordok, W.A.; El-Attar, M.S. Synthesis, structural elucidation, molecular modeling, and antimicrobial studies of some nanoparticles mixed ligands complexes of cetirizine in presence of 2,2′-bipyridine. Appl. Organomet. Chem. 2022, 35, e6715. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Mohamed, A.A. Biochemical characterization of new gemifloxacin schiff base (GMFX-o-phdn) metal complexes and evaluation of their antimicrobial activity against some phyto- or human pathogens. Int. J. Mol. Sci. 2022, 23, 2110. [Google Scholar] [CrossRef] [PubMed]
- Beecher, D.J.; Wong, A.C. Identification of hemolysin BL-producing Bacillus cereus isolates by a discontinuous hemolytic pattern in blood agar. Appl. Environ. Microbiol. 1994, 60, 1646–1651. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 7263. [Google Scholar] [CrossRef]
- Sofo, A.; Elshafie, H.S.; Scopa, A.; Mang, S.M.; Camele, I. Impact of airborne zinc pollution on the antimicrobial activity of olive oil and the microbial metabolic profiles of Zn-contaminated soils in an Italian olive orchard. J. Trace Elem. Med. Biol. 2018, 49, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.A. CLSI Document M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Clinical and Laboratory Standard Institute: Wayne, NY, USA, 2012. [Google Scholar]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Vogel, A.I. Qualitative Inorganic Analysis, 6th ed.; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Kamal, H.M.; El-Sayed, H.A.; Sadeek, S.A.; Zordok, W.A.; El-Attar, M.S. Spectroscopic characterization, DFT modeling and antimicrobial studies of some novel nanoparticles mixed ligand complexes of NS bidentate ligand in presence of 2,2′-bipyridine. J. Mol. Liq. 2023, 376, 121404. [Google Scholar] [CrossRef]
- Emam, S.M. Spectral characterization, thermal and biological activity studies of Schiff base complexes derived from 4,4′-Methylenedianiline, ethanol amine and benzyl. J. Mol. Struct. 2017, 1134, 444. [Google Scholar] [CrossRef]
- Kavitha, N.; Lakshmi, P.V.A. Synthesis, characterization and thermogravimetric analysis of Co(II), Ni(II), Cu(II) and Zn(II) complexes supported by ONNO tetradentate Schiff base ligand derived from hydrazino benzoxazine. J. Saudi Chem. Soc. 2017, 21 (Suppl. S1), S457–S466. [Google Scholar] [CrossRef]
- Emam, S.M.; Abouel-Enein, S.A.; Abouzayed, F.I. Synthesis, spectral characterization, thermal studies and biological activity of (Z)-5-((1,5-dimethyl-3-oxo-2-phenyl-2,3- di-hydro-1H-pyrazol-4-yl)diazenyl)-6-hydroxy-2-mercaptopyrimidin-4(3H)-one and its metal complexes. Appl. Organomet. Chem. 2018, 32, e4073. [Google Scholar] [CrossRef]
- El-Morshedy, R.M.; El-Gamil, M.M.; Abou-Elzahab, M.M.; Abu El-Reash, G.M. Spectroscopic investigation, DFT, fluorescence, molecular docking and biological studies of divalent and trivalent binuclear complexes prepared from benzoyl thiosemicarbazide derivative of 2-benzylmalonohydrazide. Appl. Organomet. Chem. 2019, 33, e4871. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Nassr, A.A.; Sadeek, S.A.; Elshafie, H.S. Synthesis and Spectral, Thermal and Antimicrobial Investigation of Mixed Ligand Metal Complexes of N-Salicylidene Aniline and 1,10-phenanthroline. Compounds 2023, 3, 298–309. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Sadeek, S.A. Ligational and biological studies of Fe(III), Co(II), Ni(II), Cu(II), and Zr(IV) complexes with carbamazepine as antiepileptic drug. Appl. Organomet. Chem. 2021, 35, e6178. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Awad, H.M.; Mohamed, A.A. Biological and Spectroscopic Investigations of New Tenoxicam and 1.10-Phenthroline Metal Complexes. Molecules 2020, 25, 1027. [Google Scholar] [CrossRef]
- Sadeek, S.A.; EL-Shwiniy, W.H. Metal complexes of the third generation quinolone antibacterial drug sparfloxacin: Prepara-tion, structure, and microbial evaluation. J. Coord. Chem. 2010, 63, 3471–3482. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ahmed, F.M.; Zordok, W.A.; EL-Shwiniy, W.H.; Sadeek, S.A.; Elshafie, H.S. Novel Enrofloxacin Schiff Base Metal Complexes: Synthesis, Spectroscopic Characterization, Computational Simulation and Antimicrobial Investigation against Some Food and Phyto-Pathogens. Inorganics 2022, 10, 177. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Sadeek, S.A.; Abd El-Hamid, S.M.; Zordok, W.A.; Awad, H.M. Mixed-ligand complexes of tenoxicam drug with some transition metal ions in presence of 2,2′-bipyridine: Synthesis, spectroscopic characterization, thermal analysis, density functional theory and in vitro cytotoxic activity. J. Mol. Struct. 2019, 1197, 628–644. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Abd El-Hamid, S.M.; Mohamed, A.A.; Zordok, W.A.; El-Sayed, H.A. Spectroscopic characterization, thermogravimetry, density functional theory and biological studies of some mixed-ligand complexes of meloxicam and 2,2′-bipyridine with some transition metals. Appl. Organomet. Chem. 2019, 33, e4889. [Google Scholar] [CrossRef]
- El-Sherif, A.A.; Shehata, M.R.; Shoukry, M.M.; Barakat, M.H. Synthesis, characterization, equilibrium study and biological activity of Cu(II), Ni(II) and Co(II) complexes of polydentate Schiff base ligand. Spectrochim. Acta A 2012, 96, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Zayed, E.M.; Ismail, E.H.; Mohamed, G.G.; Khalil, M.M.H.; Kame, A.B. Synthesis, spectroscopic and structural characterization and antimicrobial studies of metal complexes of a new hexadentate Schiff base ligand. Spectrophotometric determination of Fe(III) in water samples using a recovery test. Monatsh. Chem. 2014, 145, 755–765. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Attar, M.S.; Abd El-Hamid, S.M. Complexes and Chelates of Some Bivalent and Trivalent Metals with Ciprofloxacin Schiff Base. Synth. React. Inorg. Met. Org. Chem. 2015, 45, 1412–1426. [Google Scholar] [CrossRef]
- Mohamed, G.G.; El-Gamel, N.E.A. Synthesis, investigation and spectroscopic characterization of piroxicam ternary complexes of Fe(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) with glycine and DL-phenylalanine. Spectrochim. Acta A 2004, 60, 3141. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Mohamed, A.A.; Zordok, W.A.; Awad, H.M.; Abd El-Hamid, S.M. Spectroscopic characterization, thermogravimetric, DFT and biological studies of some transition metals complexes with mixed ligands of meloxicam and 1,10 phenanthroline. Egypt. J. Chem. 2021, 64, 4197–4208. [Google Scholar] [CrossRef]
- Yağcı, N.K.; Kansız, S.; Özcandan, E. Synthesis, crystal structure, DFT studies, Hirshfeld surface analysis and drug delivery performance of bis(2-chloro-4,6-diaminopyrimidine)copper(II)-dichloride. J. Mol. Struct. 2021, 1246, 131142. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H. Metal complexes of the fourth generation quinolone antimicrobial drug gatifloxacin: Synthesis, structure and biological evaluation. J. Mol. Struct. 2010, 977, 243–253. [Google Scholar] [CrossRef]
- Ilhan, S.; Temel, H.; Yilmaz, I.; Sekerci, M. Synthesis and characterization of new macrocyclic Schiff base derived from 2,6-diaminopyridine and 1,7-bis(2-formylphenyl)-1,4,7-trioxaheptane and its Cu(II), Ni(II), Pb(II), Co(III) and La(III) complexes. Polyhedron 2007, 26, 2795–2802. [Google Scholar] [CrossRef]
- Refat, M.S. Synthesis and characterization of norfloxacin-transition metal complexes (group 11, IB): Spectroscopic, thermal, kinetic measurements and biological activity. Spectrochim. Acta A 2007, 68, 1393–1405. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Elshwiniy, W.H.; El-Attar, M.S. Synthesis, characterization and antimicrobial investigation of some moxifloxacin metal complexes. Spectrochim. Acta A 2011, 84, 99–110. [Google Scholar] [CrossRef]
- Alias, S.S.; Ismail, A.B.; Mohamad, A.A. Effect of pH on ZnO nanoparticle properties synthesized by sol–gel centrifugation. J. Alloy. Compd. 2010, 499, 231–237. [Google Scholar] [CrossRef]
- Nisansala Bandara, W.R.L.; De Silva, R.M.; Nalin de Silva, K.M.; Dahanayake, D.; Gunasekara, S.; Thanabalasingam, K. Is nano ZrO2 a better photocatalyst than nano TiO2 for degradation of plastics? RSC Adv. 2017, 7, 46155–46163. [Google Scholar] [CrossRef]
- Sagadevan, S.; Podder, J.; Das, I. Hydrothermal synthesis of zirconium oxide nanoparticles and its characterization. J. Mater. Sci. Mater. Electron. 2016, 27, 5622–5627. [Google Scholar] [CrossRef]
- Coats, A.W.; Red fern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A New Analysis of Thermogravimetric Traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Omar, M.M. Spectral, thermal and biological activity studies on ruthenium(II) complexes with some pyridyl-amines. J. Therm. Anal. Calorim. 2009, 96, 607–615. [Google Scholar] [CrossRef]
- Rahmouni, N.T.; Bensiradj, N.E.; Megatli, S.A.; Djebbar, S.; Baitich, O.B. New mixed amino acids complexes of iron(III) and zinc(II) with isonitrosoacetophenone: Synthesis, spectral characterization, DFT study and anticancer activity. Spectrochim. Acta A 2019, 213, 235–248. [Google Scholar] [CrossRef]
- Moore, J.W.; Pearson, R.G. Kinetic and Mechanism; John Wiley& Sons: New York, NY, USA, 1981. [Google Scholar]
- Lewis, D.A.; Mitjà, O. Haemophilus ducreyi: From sexually transmitted infection to skin ulcer pathogen. Curr. Opin. Infec. Dis. 2016, 29, 52–57. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M.; Shehata, M.R.; Abu-Dief, A.M. Fabrication, DFT Calculation, and Molecular Docking of Two Fe(III) Imine Chelates as Anti-COVID-19 and Pharmaceutical Drug Candidate. Int. J. Mol. Sci. 2022, 23, 3994. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Sofo, A.; Mazzone, G.; Caivano, M.; Masi, S.; Caniani, D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere 2020, 252, 126597. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.; Manzor, O.; Check, C.; Pawlak, J.; Belian, B. Antimicrobial activity of moxifloxacin, gatifloxacin, and six fluoroquinolones against Streptococcus pneumoniae. J. Antimicrob. Chemother. 2001, 47, 875. [Google Scholar] [CrossRef] [PubMed]
- Cottagnoud, P.; Acosta, F.; Cottagnoud, M.; Tauber, M.G. Gemifloxacin is efficacious against penicillin-resistant and quino-lone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 2002, 46, 1607–1609. [Google Scholar] [CrossRef]
- Sakr, S.H.; Elshafie, H.S.; Camele, I.; Sadeek, S.A. Synthesis, Spectroscopic, and Biological Studies of Mixed Ligand Com-plexes of Gemifloxacin and Glycine with Zn(II), Sn(II), and Ce(III). Molecules 2018, 23, 1182. [Google Scholar] [CrossRef] [PubMed]
- Scheld, W.M. Maintaining fluoroquinolone class efficacy: Review of influencing factors. Emerg. Infect. Dis. 2003, 9, 1–9. [Google Scholar] [CrossRef]
| Compounds MW (M.F.) | Yield% | M.P./°C | Color | (Calc.) Found (%) | Λ (S cm2 mol−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | Cl | M | |||||
| L 197 (C13H11NO) | - | 50 | Light-yellow | (79.18) 79.01 | (5.58) 5.52 | (7.10) 6.89 | - | - | 1.45 |
| (1) 631.83 (CoC26H34N2O8Cl2) | 88.34 | 215 | Gray | (49.38) 49.20 | (5.38) 5.23 | (4.43) 4.26 | (11.22) 11.13 | (9.32) 9.21 | 95.84 |
| (2) 618.44 (CuC26H32N2O7Cl2) | 80.69 | 210 | Dark-brown | (50.44) 50.28 | (5.17) 5.10 | (4.52) 4.41 | (11.46) 11.32 | (10.27) 10.18 | 92.45 |
| (3) 622.25 (YC26H26N2O4Cl3) | 90.25 | 220 | Dark-green | (50.14) 49.88 | (4.17) 4.10 | (4.49) 4.38 | (17.09) 17.00 | (14.28) 14.12 | 125.00 |
| (4) 662.12 (ZrC26H32N2O8Cl2) | 82.24 | >300 | Green | (47.12) 46.89 | (4.83) 4.97 | (4.22) 4.18 | (10.70) 10.62 | (13.77) 13.65 | 96.10 |
| (5) 737.25 (LaC26H34N2O8Cl3) | 85.75 | >300 | Pale-green | (42.31) 42.17 | (4.61) 4.51 | (3.79) 3.70 | (14.42) 14.31 | (18.84) 18.70 | 123.80 |
| L | (1) | (2) | (3) | (4) | (5) | Assignments |
|---|---|---|---|---|---|---|
| 3426 mbr | 3408 mbr | 3473 m | 3413 mbr | 3396 mbr | 3398 mbr | ν(O–H); H2O |
| 1612 vs | 1600 vs | 1603 vs | 1601 vs | 1600 vs | 1602 vs | ν(C=N) pyridine ring |
| 832 m | ν(Zr=O) | |||||
| 688 w 570 w | 687 w 575 w | 686 w 580 w | 674 vw 555 w | 686 w 565 w | ν(M–O) and ν(M–N) |
| Compounds | Peak | Assignment | Ε (M−1cm−1) × 104 | 10Dq | C`FSE | μeff (B.M) | ||
|---|---|---|---|---|---|---|---|---|
| nm | cm−1 | cm−1 | kJ/mol | |||||
| L | 270 | 37,037 | π → π* | 0.600 | ||||
| 318 | 31,446 | n → π* | 0.723 | |||||
| 340 | 29,411 | n → π* | 0.769 | |||||
| (1) | 275 | 36,363 | π → π* | 2.150 | 17,241 | 206 | 206 + 2p | 5.20 |
| 312 | 32,051 | n → π* | 1.567 | |||||
| 345 | 28,958 | n → π* | 1.425 | |||||
| 450 | 22,222 | CT | 0.825 | |||||
| 580 | 17,241 | 4T1g (F) → 4T1g (P) | 0.450 | |||||
| (2) | 287 | 41,666 | π → π* | 1.400 | 16,806 | 201 | 201 + 4p | 1.70 |
| 365 | 27,397 | n → π* | 0.900 | |||||
| 415 | 24,096 | CT | 0.443 | |||||
| 595 | 16,806 | 2B1g → 2E1g | 0.350 | |||||
| (3) | 270 | 37,037 | π → π* | 2.150 | ||||
| 325 | 30,769 | n → π* | 2.100 | |||||
| 338 | 29,585 | n → π* | 2.000 | |||||
| 440 | 22,727 | CT | 0.923 | |||||
| (4) | 266 | 37,593 | π → π* | 0.867 | ||||
| 348 | 28,735 | n → π* | 0.426 | |||||
| 465 | 21,505 | CT | 0.328 | |||||
| (5) | 270 | 37,037 | π → π* | 0.630 | ||||
| 320 | 31,250 | n → π* | 0.500 | |||||
| 337 | 29,673 | N → π* | 0.490 | |||||
| 470 | 21,276 | CT | 0.375 | |||||
| L | (1) | (2) | (3) | (4) | (5) | Assignments |
|---|---|---|---|---|---|---|
| 2.48 | 2.40 | 2.52 | 2.50 | 1.82–2.70 | 2.51 | δH, –CH aliphatic (DMSO) |
| - | 3.48 | 3.37 | 3.32 | 3.40 | 3.33 | δH, H2O |
| 6.96–7.94 | 7.23–7.88 | 7.04–7.91 | 7.00–7.91 | 7.20–7.98 | 7.00–7.90 | δH, –CH aromatic |
| 8.95 | 8.93 | 8.92 | 8.96 | 8.96 | 8.96 | δH, –H–C=N |
| 13.09 | 13.00 | 13.02 | 13.01 | 13.20 | 13.00 | δH, –OH |
| Compounds | Decay Steps | Tmax (°C) | Mass Loss (%) | Lost Species | |
|---|---|---|---|---|---|
| Calc. | Found | ||||
| L | Step one | 200 | 100.00 | 99.68 | 2C4H2 + H2O + 0.5N2 + 2.5C2H2 |
| Total loss | 100.00 | 99.68 | |||
| (1) | Step one | 57 | 11.39 | 11.38 | 4H2O |
| Step two | 129 | 5.69 | 5.68 | 2H2O | |
| Step three | 252,597 | 67.27 | 67.44 | 6C4H2 + 2HCl + H2O + 2NH3 | |
| Total loss | 84.35 | 84.50 | |||
| Residue | 15.65 | 15.50 | CoO + 2C | ||
| (2) | Step one | 75,114 | 8.73 | 8.68 | 3H2O |
| Step two | 275,402 | 74.53 | 74.63 | 11C2H2 + C2N2 + H2O + 2CO + 2HCl2 | |
| Total loss | 83.26 | 83.31 | |||
| Residue | 16.74 | 16.69 | CuO + 2C | ||
| (3) | Step one | 227 | 85.72 | 85.83 | 6C4H2 + 3HCl + 2H2O + 2NH3 + 2CO + 0.5H2 |
| Total loss | 85.72 | 85.83 | |||
| Residue | 14.28 | 14.17 | Y | ||
| (4) | Step one | 109 | 10.87 | 10.84 | 4H2O |
| Step two | 604 | 70.52 | 70.65 | 6C4H2 + 2HCl + 2H2O + C2N2 + 3H2 | |
| Total loss | 81.39 | 81.49 | |||
| Residue | 18.61 | 18.51 | ZrO2 | ||
| (5) | Step one | 95 | 9.76 | 9.73 | 4H2O |
| Step two | 150 | 45.57 | 45.53 | 6C4H2 + 2H2O | |
| Step three | 265,597 | 25.83 | 26.02 | 3HCl + 2CO + 2NH3 + 0.5H2 | |
| Total loss | 81.16 | 81.28 | |||
| Residue | 18.84 | 18.72 | La | ||
| Compounds | Decay Range (K) | Ts (K) | Method | Parameters | R a | SD b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | A (s−1) | ΔS* (kJ/mol.K) | ΔH* (kJ/mol) | ΔG* (kJ/mol) | ||||||
| L | 315–917 | 473 | CR | 27.46 | 8.1567 | −0.231 | 23.57 | 111.75 | 0.986 | 0.163 |
| HM | 43.95 | 1.43 × 101 | −0.226 | 40.02 | 147.20 | 0.970 | 0.238 | |||
| (1) | 298–393 | 330 | CR | 33.89 | 1.26 × 102 | −0.205 | 31.15 | 98.96 | 0.982 | 0.141 |
| HM | 36.36 | 3.81 ×103 | −0.177 | 33.62 | 92.10 | 0.974 | 0.168 | |||
| (2) | 463–603 | 548 | CR | 53.45 | 4.59 × 102 | −0.199 | 48.91 | 157.96 | 0.978 | 0.190 |
| HM | 63.24 | 4.48 × 103 | −0.180 | 58.68 | 157.35 | 0.966 | 0.236 | |||
| (3) | 423–610 | 500 | CR | 38.86 | 3.19 × 101 | −0.220 | 34.71 | 144.90 | 0.979 | 0.190 |
| HM | 46.26 | 2.51 × 102 | −0.203 | 42.11 | 143.73 | 0.963 | 0.250 | |||
| (4) | 355–435 | 382 | CR | 32.39 | 1.31 × 102 | −0.206 | 29.21 | 108.07 | 0.982 | 0.150 |
| HM | 45.15 | 9.240 | −0.228 | 41.94 | 129.25 | 0.972 | 0.184 | |||
| (5) | 608–463 | 538 | CR | 71.00 | 1.04 × 102 | −0.211 | 66.53 | 180.13 | 0.981 | 0.157 |
| HM | 93.51 | 7.74 × 106 | −0.117 | 89.04 | 152.48 | 0.975 | 0.181 | |||
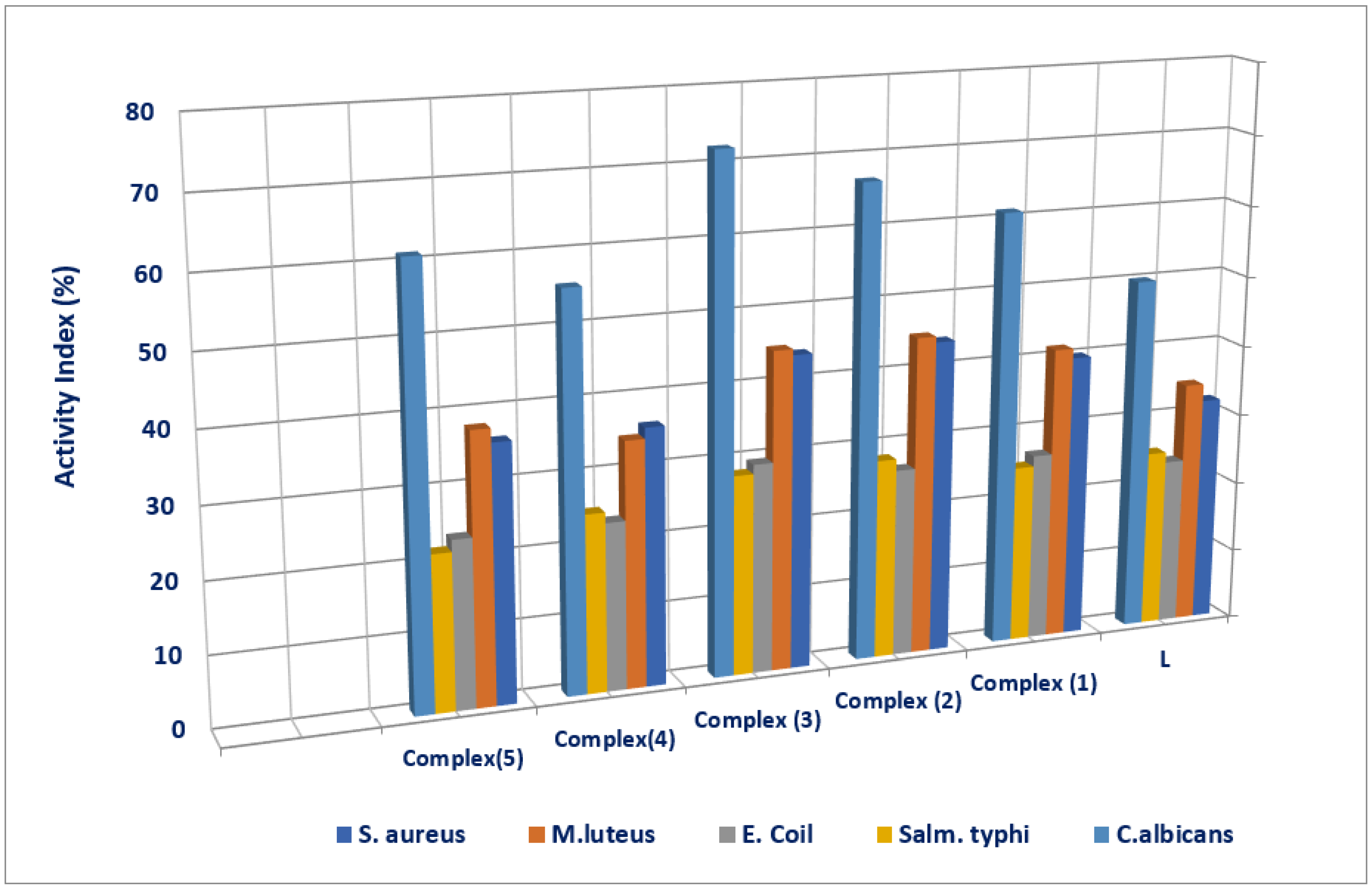
| Tested Compounds | Examined Microorganisms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G(+ve) Bacteria | G(-ve) Bacteria | Fungi | ||||||||
| S. aureus | M. luteus | E. coli | S. typhi | C. Albicans | ||||||
| D.Iz a (mm) | AI b (%) | D.Iz (mm) | AI (%) | D.Iz (mm) | AI (%) | D.Iz (mm) | AI (%) | D.Iz (mm) | AI (%) | |
| L | 8 ±0.17 | 32 | 10 ±0.08 | 34.48 | 8 ±0.12 | 23.52 | 8 ±0.14 | 25 | 9 ±0.19 | 50 |
| (1) | 10NS ±0.79 | 40 | 12NS ±0.73 | 41.37 | 9NS ±0.36 | 26.47 | 8 ±0.46 | 25 | 11NS ±0.53 | 61.11 |
| (2) | 11+1 ±0.34 | 44 | 13+1 ±0.40 | 44.82 | 9NS ±0.25 | 26.47 | 9NS ±0.28 | 28.12 | 12+1 ±0.39 | 66.66 |
| (3) | 11+1 ±0.28 | 44 | 13+1 ±0.43 | 44.82 | 10NS ±0.59 | 29.41 | 9NS ±0.32 | 28.12 | 13+1 ±0.57 | 72.22 |
| (4) | 9NS ±0.37 | 36 | 10 ±0.17 | 34.48 | 8 ±0.22 | 23.52 | 8 ±51 | 25 | 10NS ±0.29 | 55.55 |
| (5) | 9NS ±0.49 | 36 | 11NS ±0.41 | 37.93 | 8 ±0.27 | 23.52 | 7 ±0.20 | 21.87 | 11NS ±0.55 | 66.11 |
| Ciprofloxacin (control) | 25 ±0.3 | 100 | 29 ±0.2 | 100 | 34 ±1.11 | 100 | 32 ±0.98 | 100 | 0 | 0 |
| Nystatin (control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 ±0.42 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.A.; Nassr, A.A.; Sadeek, S.A.; Rashid, N.G.; Abd El-Hamid, S.M. First Report on Several NO-Donor Sets and Bidentate Schiff Base and Its Metal Complexes: Characterization and Antimicrobial Investigation. Compounds 2023, 3, 376-389. https://doi.org/10.3390/compounds3030029
Mohamed AA, Nassr AA, Sadeek SA, Rashid NG, Abd El-Hamid SM. First Report on Several NO-Donor Sets and Bidentate Schiff Base and Its Metal Complexes: Characterization and Antimicrobial Investigation. Compounds. 2023; 3(3):376-389. https://doi.org/10.3390/compounds3030029
Chicago/Turabian StyleMohamed, Amira A., Abeer A. Nassr, Sadeek A. Sadeek, Nihad G. Rashid, and Sherif M. Abd El-Hamid. 2023. "First Report on Several NO-Donor Sets and Bidentate Schiff Base and Its Metal Complexes: Characterization and Antimicrobial Investigation" Compounds 3, no. 3: 376-389. https://doi.org/10.3390/compounds3030029
APA StyleMohamed, A. A., Nassr, A. A., Sadeek, S. A., Rashid, N. G., & Abd El-Hamid, S. M. (2023). First Report on Several NO-Donor Sets and Bidentate Schiff Base and Its Metal Complexes: Characterization and Antimicrobial Investigation. Compounds, 3(3), 376-389. https://doi.org/10.3390/compounds3030029








