Abstract
One-step reactions between squaric acid and pyrroles, such as 3-ethyl-2,4-dimethyl-pyrrole and 1,2,5-trimethylpyrrole, in water provide the corresponding pyrrol-2-yl- and pyrrol-3-yl-containing semisquaraines in high yields. These semisquaraines serve as useful precursors for the synthesis of various non-symmetric pyrrole-containing squaraine dyes.
1. Introduction
Squaraine dyes (Figure 1) could be considered privileged types of chromophores and fluorophores due to their tunable photophysical properties and diverse range of applications, which span over various areas of chemistry, biology, materials, and engineering [1,2,3,4]. Notably, non-symmetric squaraines (dyes with distinct moieties attached to the squaric acid scaffold; Figure 1) possess several advantages over symmetric squaraine dyes, including better solubility in organic solvents, as well as superior adaptability for introducing structural and functional diversity onto fluorogenic scaffolds [5]. These features and properties of non-symmetric squaraine dyes could ultimately be translated to a broader, potentially more useful, range of applications [5].
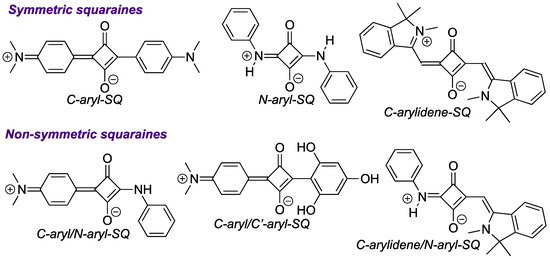
Figure 1.
General examples of symmetric and non-symmetric squaraine dyes.
Importantly, from a synthetic point of view, the accessibility of non-symmetric squaraines is typically more elaborate and problematic than for symmetric squaraines. The simultaneous condensation of two different nucleophiles with squaric acid leads to mixtures of products, which are difficult to separate. Hence, most practical routes to non-symmetric squaraine dyes rely on the use of semisquaric acids (semisquaraines) as intermediate synthons. In general, the synthesis of semisquaraines can be accomplished via two-step procedures, either starting with dialkyl-squarate or squaryl dichloride (which, in turn, are synthesized by reacting squaric acid with either an alcohol or thionyl chloride or other chlorinating agent), followed by hydrolysis (Scheme 1) [5,6]. Subsequent reaction with nucleophiles at elevated temperatures in either alcohol/toluene or iso-propanol/alkyl formate mixtures leads to non-symmetric squaraines (Scheme 1). However, general, modular, and facile approaches which could enable the synthesis of structurally and functionally diverse non-symmetric squaraines are not available. Thus, the development of facile synthetic routes to various semisquaraine scaffolds is warranted.
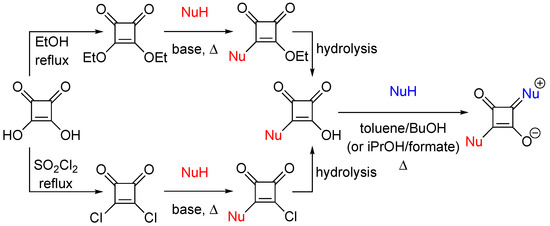
Scheme 1.
Preparations of non-symmetric squaraines, via diethylsquarate (top) or squaryl dichloride (bottom), using squaric acid and various nucleophiles (NuH) as the starting materials for each step.
Furthermore, from the sustainability and green chemistry perspectives, it would be advantageous to reduce the number of synthetic steps that are required to synthesize the semisquaraine synthons as well as to eliminate or reduce the use of hazardous chemicals and solvents. This would be especially crucial because squaraine dyes are becoming integral parts in various devices and processes; thus, the possibility of conducting larger-scale syntheses of these dyes should be taken into consideration. It should also be pointed out that only a handful of studies have addressed the synthesis of symmetric squaraines from the green and sustainable chemistry perspectives [7,8,9]. However, to the best of our knowledge, there have been no reports on green, sustainable approaches towards non-symmetric squaraines. Here, we provide a facile, efficient, and sustainable approach to some pyrrole-containing semisquaraines, which could be used as viable synthons for the synthesis of non-symmetric squaraine dyes.
2. Materials and Methods
2.1. General Information
All reagents and solvents were purchased from the commercial sources and were used as received. NMR spectra were acquired on a Bruker Ascend 400 (400 MHz) spectrophotometer using DMSO-d6 as the solvent, and the chemical shifts are reported in ppm (δ) from the residual DMSO peak (2.51 ppm) or TMS (0.00 ppm). High-resolution mass spectra (HRMS-ESI) were acquired on an Agilent 6230 instrument at the Mass Spectrometry Facility, Louisiana State University. Microwave-facilitated reactions were conducted in CEM Discover or Discover-SP microwave synthesizers using 10 mL vials. The UV–Vis absorption spectra were measured with a Cary 60 UV–Vis spectrophotometer (Agilent Technologies). The fluorescence spectra were measured with a Cary Eclipse fluorescence spectrometer (Varian Inc., Palo Alto, CA, USA).
2.2. Synthesis of Compound 1a
A round-bottomed flask was charged with a stirring bar, water (100 mL), and squaric acid (21.16 g, 185.5 mmol). Once the solution was brought to reflux, 2,4-dimethyl-3-ethyl-pyrrole (5.00 mL, 37.1 mmol) was added in one portion, and the reaction mixture was refluxed for 15 min. The reaction mixture was removed from heat, water (150 mL) was added, and the mixture brought to reflux. Next, while hot, the supernatant was decanted, and water (250 mL) was added to the residue, and the mixture was brought to reflux, followed by decantation. This sequence was repeated two more times (total volume of water used was 1000 mL for reaction and washings). After the final wash, the solid was filtered, then dried under vacuum to give 1a as a green solid (7.90 g, 97%). The water washes were combined, and charcoal (10.0 g) was added. The mixture was brought to reflux, filtered while hot, and water was removed in vacuo, to give white squaric acid (16.07 g, 95% recovery).
2.3. Synthesis of Compound 4a
A round-bottomed flask was charged with a stirring bar, water (20 mL), and squaric acid (846.5 mg, 7.42 mmol). Once the solution was brought to 80 °C, 1,2,5-trimethylpyrrole (1.00 mL, 7.39 mmol) was added in one portion, and the reaction mixture was stirred at 80 °C for 60 min. The reaction mixture was removed from heat, cooled to room temperature, and filtered. Next, the precipitate was washed with water (3 × 10 mL). Then, the precipitate was washed with diethyl ether (2 × 5 mL) and dried under vacuum to give 4a as a maroon solid (1.27 g, 84%).
2.4. General Synthetic Procedure for Synthesis of Compounds 6–18
A microwave vial was charged with a stirring bar, semisquaraine (50.0 mg, 1a or 4a), 1-BuOH (0.6 mL), and nucleophile (1.5 eq.), sealed, and subjected to MW irradiation at 90 °C for 30 min or 90 min. After the reaction was cooled to room temperature, the reaction mixture was filtered, washed with appropriate solvent, and the resulting solid was dried under vacuum, recrystallized, or subjected to column chromatography to give respective squaraine dyes as colored solids in 25–90% yield.
The Supplementary Materials contains detailed information on the synthesis, characterization, and spectral data of the reported compounds.
3. Results and Discussion
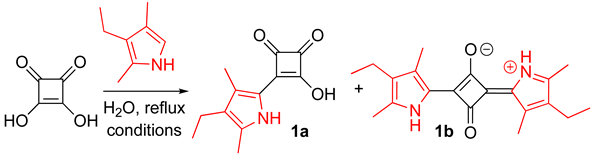
In an effort to develop modular syntheses of non-symmetric squaraines, we envisioned using squaric acid monoamines (i.e., N-alkyl/aryl-containing semisquaraines) as synthons for preparation of non-symmetric squaraines, because these semisquaraines could be prepared by simply reacting squaric acid with the amines either under thermal or microwave irradiation heating [10,11]. Regretfully, we found that the amine-containing semisquaraines were unreactive towards a number of amines (Scheme S1). However, when the amine-containing semisquaraines were reacted with kryptopyrrole (2,4-dimethyl-3-ethyl-pyrrole), either no reaction or the formation of symmetric kryptopyrrolyl-containing squaraine was noted (Scheme S1).
In view of the aforementioned results, we hypothesized that pyrrolyl-containing semisquaraines might be viable synthons for the synthesis of non-symmetric squaraines. In addition, pyrrole-containing squaraine dyes possess a variety of useful properties and have a wide range of applications [12,13,14,15,16]. Therefore, we decided to investigate the synthesis of a common pyrrole-containing synthon that would be suitable for the modular, efficient, facile, and divergent synthesis of non-symmetric squaraines. Accounts in the literature utilize a multistep procedure for obtaining pyrrole-containing semisquaraines (Scheme 2, top) [17,18,19,20,21]. In an effort to develop a more facile approach, we decided to explore the synthesis of 1a directly using squaric acid and kryptopyrrole (Scheme 2, bottom). We hypothesized that due to the poor solubility of squaric acid and good miscibility of kryptopyrrole with organic solvents, a higher local concentration of kryptopyrrole (as well as high nucleophilicity of this pyrrole) around squaric acid might favor the formation of symmetric 1,3-bis-kryptopyrrol-2-yl squaraine 1b (Scheme 2, bottom). Indeed, a reaction between equimolar amounts of squaric acid and kryptopyrrole in several organic solvents led to formation of 1b as the major product, which was consistent with accounts in the literature [16,22].
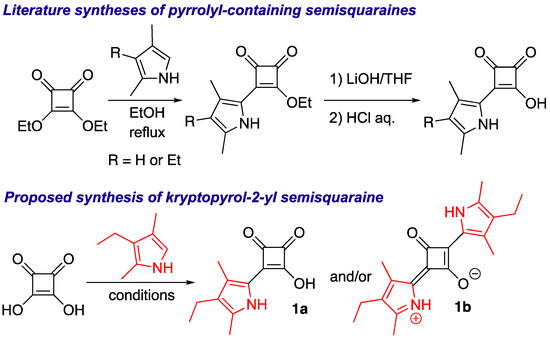
Scheme 2.
Syntheses from the literature [18,19,20] (top) and this study (bottom) of pyrrole-containing semisquaraines.
Arguably, in water, where the solubility of squaric acid would be higher than that of the pyrrole, the scenario could be reversed, which, in turn, should favor the formation of kryptopyrrole semisquaraine 1a. We found that, in water, 1a was obtained as the major product, although a substantial amount of 1b was still observed (Table 1, entry 1). Multiple attempts to separate semisquaraine 1a from squaraine 1b either via recrystallization or by washing with a range of organic solvents (including chloroform, acetone, diethyl ether, and petroleum ether) proved inefficient and non-sustainable. We also attempted to prepare 1a using the slow addition of kryptopyrrole to an equimolar amount of squaric acid, albeit with limited success, as the formation of 1a was accompanied by the formation of a number of unidentified products, which proved difficult to remove in a facile and efficient manner. However, progressively increasing the amount of squaric acid (from 2 to 5 eq.) completely suppressed the formation of 1b (entries 2–5). Subsequently, we varied the reaction time (entries 5–8); the corresponding results revealed the reaction completion to occur within 15 min. The reaction proved inefficient when the concentration of kryptopyrrole was lowered to 0.037 M (entry 9); concentrations higher than 0.37 M were not tested to retain the solubility of squaric acid. We also conducted this reaction under room temperature (entry 10), and although 1a formed as the major product, the overall results appeared to be inferior to those obtained under reflux conditions. Thus, 15 min reflux and 5 eq. of squaric acid (entry 7) appeared to be optimal conditions for the synthesis of 1a. To demonstrate the scalability of this process, the synthesis of 1a was also conducted on a multigram-scales (i.e., 1 mL (7.4 mmol), and 5 mL (37.1 mmol) scales); in both cases, 1a was obtained in high yields (entries 11 and 12), thus indicating the robust nature of this process, and potential industrial applicability.

Table 1.
Synthesis 1a: optimization studies a.
The substantial excess of squaric acid used in the synthesis of 1a deems this process unsustainable, unless squaric acid could be recovered and reused. Thus, we turned our attention to the recovery of the unreacted squaric acid. Simply removing the water in vacuo and washing the residue with acetone gave squaric acid with a 98% recovery, i.e., over 3.9 eq. of squaric acid (out of possible 4.0 eq.) were recovered. Although the color of the recovered squaric acid turned out to be pale green, which was not consistent with the authentic/commercial sample (i.e., white), the squaric acid exhibited properties and chemical reactivity (e.g., the reaction of recovered/“pale green” squaric acid with kryptopyrrole under the optimized conditions gave 1a in an approximately 95% yield) that were identical to an authentic sample (Figure S1). However, when water (i.e., combined fractions from the reaction and the washings) was treated with charcoal, followed by the removal of water in vacuo, recovered squaric acid was obtained as a white solid (95% recovery; i.e., 3.8 eq. of squaric acid were recovered out of possible 4.0 eq.), whose spectral and physical characteristics were also in line with those of the authentic sample (Figure S1).
In addition, we determined the green chemistry metrics for this process [23,24], and compared them to the literature preparation of 1a [18] (Table 2; see Supplementary Materials for considerations, calculations, and detailed results). The method developed here had an E-factor value of 0.2, atom economy of 92.4%, mass intensity of 3.1, and process mass intensity value of 46.9, which were all superior to those obtained from the literature protocol [18].

Table 2.
Green chemistry metrics for the synthesis of 1a.
To evaluate the scope of this reaction (under optimized conditions, Table 1, entry 7), we used several pyrroles to prepare the corresponding pyrrolyl-semisquaraines (Scheme 3). The choice of pyrroles was driven by commercial availability and cost, which was set to be comparable to that of kryptopyrrole (Table S1). Surprisingly, the reaction between 2,4-dimethylpyrrole and squaric acid led only to minute amounts of the corresponding semisquaraine 2a, while symmetric squaraine 2b was obtained as the major product (Scheme 3B; Figure S2). Although conducting the reaction at room temperature obtained 2a as the major product, a relatively low yield of this process made it inferior to the synthesis of 1a. Notably, the preparations of 2a in the literature rely on a two-step process (Scheme 2) [19,20,21]. Reactions between squaric acid and 2-ethylpyrrole (Scheme 3C) turned out to be inefficient, because we were unable to obtain pure semisquaraine 3a from a complex mixture of products that also included the symmetric squaraine 3b.
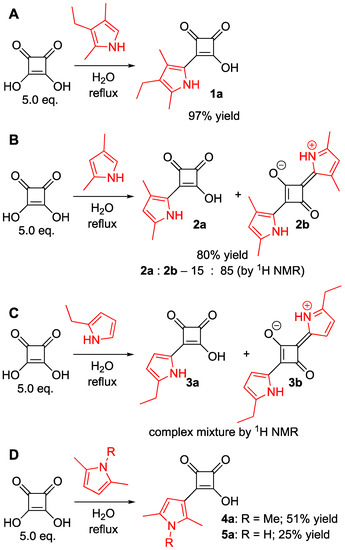
Scheme 3.
Optimized synthesis of 1a (Table 1, entry 7) (A) and the attempted syntheses of other pyrrol-2-yl- and pyrrol-3-yl semisquaraines (B–D). The 2a:2b ratio was determined by 1H NMR using the integration of NH resonances of 2a (NH) and 2b (2NHs).
However, the reaction of 1,2,5-trimethylpyrrole and 2,5-dimethylpyrrole with squaric acid led to the formation of semisquaraines 4a and 5a, respectively, albeit in low yields (Scheme 3D). These low yields were attributed to higher solubilities of these semisquaraines in water as compared with 1a. In addition, it appeared that minute amounts of squaric acid were still present in those products, and squaric acid could not be easily removed without further decreasing the yield of the desired semisquaraines.
On the other hand, because the formation of symmetric squaraines, i.e., bis-1,2,5-trimethylpyrrol-3-yl- and bis-2,4-dimethylpyrrol-3-yl-squaraines, was not observed (most likely due to lower nucleophilicity of 2,5-dimethylpyrrole and 1,2,5-trimethylpyrroles as compared with kryptopyrrole) [25], we reasoned that excess squaric acid might not be required to obtain the corresponding semisquaraines. Indeed, reacting 1,2,5-trimethylpyrrole with an equimolar amount of squaric acid enabled us to obtain 4a in an approximately 80% yield (Table S2). Similar to the synthesis of 1a (Table 1), the synthesis of 4a could also be done performed at elevated temperature or at room temperature, although it was found to be more facile under 80 °C (Table S2). Notably, 1 mL and 5 mL scale syntheses of 4a proceeded in 84% and 77% yields, respectively (Table S2, entries 9 and 10), thus highlighting the scalability and robustness of this process. However, to our regret, the aforementioned conditions appeared not to be applicable for the reaction of 2,5-dimethylyrrole with an equimolar amount of squaric acid in water to produce the corresponding semisquaraine 5a. Overall, pyrrol-2-yl- and pyrrol-3-yl-containing semisquaraines 1a and 4a could be obtained in a facile and efficient manner using water as the only solvent and with water being the only by-product.
As mentioned above (Scheme 1), non-symmetric squaraine dyes are typically prepared by reacting a semisquaraine with various nucleophiles in either alcohol/toluene or iso-propanol/alkyl formate mixtures under reflux (or at elevated temperatures) [5,6]. Here, we aimed to investigate the possibility of using 1a and 4a to generate structurally and functionally diverse non-symmetric squaraines. It is of interest to point out that although considerable research has focused on C-aryl/C’-aryl squaraines, several recent accounts have indicated that C-aryl/N-aryl-SQ (Figure 1) might exhibit unique properties, such as dual-state emission [26,27,28] and crystallization-induced emission [29], which could raise the demands for facile access to C-aryl/N-aryl-SQ dyes as well.
Thus, we screened several reactions between 1a and a few p-substituted anilines and found out that non-symmetric C-aryl/N-aryl(alkyl)-SQ dyes could be obtained in moderate to good yields using either H2O, n-BuOH or t-BuOH as a solvent (Table S3). Not surprisingly, and in line with previous reports [9,30], conducting the reaction under MW-heating proved to be more facile, and non-symmetric squaraines were obtained in good yields after 30 min of MW heating using n-BuOH as the only solvent (Table S3), which is designated as the “preferred/recommended” from the green chemistry and sustainability points of view [31]. With these optimized conditions at hand, a number of non-symmetric C-aryl/N-aryl(N-alkyl) squaraines were synthesized (Scheme 4). The reaction exhibited a great degree of functional group tolerance, as non-symmetric squaraines with electron-donating (6, 7, 10) and electron-withdrawing groups (8, 9, 11), as well as heterocyclic (12, 13), aliphatic (14), and aralkyl (15) motifs were obtained in moderate to good yields (Scheme 4). Notably, the incorporation of NH2 and CO2H groups onto the squaraine scaffolds (i.e., squaraines 9 and 15) should provide opportunities for post-functionalization, and/or conjugation to biomacromolecules or solid supports.
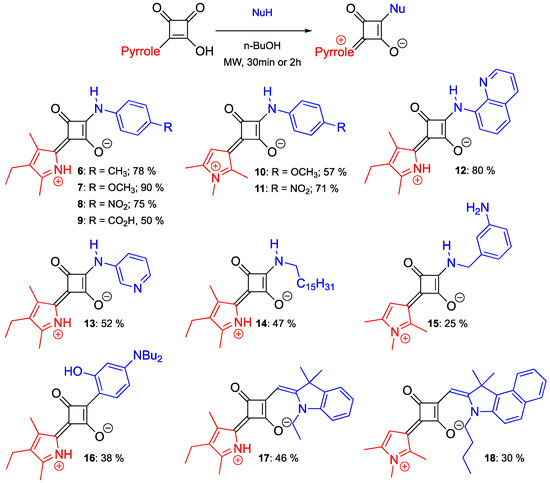
Scheme 4.
Synthesis of non-symmetric squaraines from semisquaraines 1a and 4a. Isolated yields are given.
Next, the reactivity of 1a and 4a semisquaraines with several C-nucleophiles was explored (Scheme 4). It appeared that squaraine 16 could be obtained under similar conditions as those used with amines (i.e., N-nucleophiles) to produce squaraines 6–15. The reaction with indolinium C-nucleophiles required the use of pyridine and MW reaction times of 90 min to give non-symmetric squaraines 17 and 18 in moderate yields (Scheme 4).
Some photophysical properties of non-symmetric squaraines were investigated (Table S4). In general, C-aryl/C′-aryl-squaraines appeared to exhibit absorption and emission maxima at longer wavelengths than the C-aryl/N-aryl(N-alkyl) squaraines. Complete photochemical characterization will be provided in due course.
4. Conclusions
Facile, efficient, and scalable syntheses of pyrrol-2-yl and pyrrol-3-yl semisquaraines were achieved by simply refluxing kryptopyrrole with excess squaric acid, and by refluxing 1,2,5-trimethylpyrrole with equimolar squaric acid, in water, respectively. In the case of kryptopyrrolyl-semisquaraine synthesis, the excess squaric acid could be easily recovered, with an approximately 95% efficiency) and reused without any impact on the reactivity. The synthesis of these semisquaraines had an atom economy of over 90%, with water being the only by-product. Semisquaraines were shown to undergo condensation reactions with various nucleophiles to give a range of structurally/functionally diverse non-symmetric squaraines. When amines were used as nucleophiles, efficient, facile, and chromatography-free access to C-aryl/N-aryl(N-alkyl) non-symmetric squaraines was achieved using either H2O or n-BuOH as the only solvent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/compounds3010002/s1, Experimental details, 1H and 13C NMRs of the synthesized compounds, UV–Vis and fluorescence spectra of non-symmetric squaraines, as well as green chemistry metrics and related calculations, considerations for the synthesis of 1a (PDF). References [32,33,34] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, D.D.T. and S.V.D.; methodology, D.D.T., J.M.F. and S.V.D.; formal analysis, D.D.T. and J.M.F.; investigation, D.D.T. and J.M.F.; resources, S.V.D.; data curation, S.V.D.; writing—original draft preparation, D.D.T. and S.V.D.; writing—review and editing, D.D.T., J.M.F. and S.V.D.; supervision, S.V.D.; project administration, S.V.D.; funding acquisition, S.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by NIH (1R15GM135900-01) and TCU-RCAF (Award #: 60982).
Data Availability Statement
Not applicable.
Acknowledgments
D.D.T. acknowledges TCU and the Department of Chemistry and Biochemistry for TCU-STAR graduate student scholarship. We would like to thank E. E. Simanek (TCU-CHEM) for access to the CEM Discover-SP microwave synthesizer, and Z. K. Gryczynski (TCU-PHYS) for access to the Cary 60 UV–Vis spectrophotometer (Agilent Technologies) and the Cary Eclipse fluorescence spectrometer (Varian Inc.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine dyes: Molecular design for different applications and remaining challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jo, Y.J.; Sun, X.; Qiao, W.; Ok, J.; Kim, T.-I.; Li, Z. Squaraine dyes for photovoltaic and biomedical applications. Adv. Funct. Mater. 2021, 31, 2008201. [Google Scholar] [CrossRef]
- Ta, D.D.; Dzyuba, S.V. Squaraine-based optical sensors: Designer toolbox for exploring ionic and molecular recognitions. Chemosensors 2021, 9, 302. [Google Scholar] [CrossRef]
- Qiao, W.; Li, Z. Recent progress of squaraine-based fluorescent materials and their biomedical applications. Symmetry 2022, 14, 966. [Google Scholar] [CrossRef]
- Khopkar, S.; Shankarling, G. Synthesis, photophysical properties and applications of NIR absorbing unsymmetrical squaraines: A review. Dyes Pigment. 2019, 170, 107645. [Google Scholar] [CrossRef]
- Lynch, D.E.; Hamilton, D.G. Microreview: Pyrrol-3-yl squaraines (including indol-3-yl-squaraines). J. Heterocycl. Chem. 2018, 55, 1249–1262. [Google Scholar] [CrossRef]
- Khopkar, S.; Deshpande, S.; Shankarling, G. Greener protocol for the synthesis of NIR fluorescent indolenine-based symmetrical squaraine colorants. ACS Sustain. Chem. Eng. 2018, 6, 10798–10805. [Google Scholar] [CrossRef]
- Zappimbulso, N.; Capozzi, M.A.M.; Porcheddu, A.; Farinola, G.M.; Punzi, A. Solvent-free reactions for the synthesis of indolenine-based squaraines and croconaines: Comparison of thermal heating, mechanochemical milling, and IR irradiation. ChemSusChem 2021, 14, 1363–1369. [Google Scholar] [CrossRef]
- Minkovska, S.; Burdzhiev, N.; Alexiev, A.; Deligeogiev, T. A novel fast green method for the preparation of the squaraine dye 3-oxo-4[(1,3,3-trimethyl-3Hindol-1-ium-2-yl)methylene]-2-[(1,3,3-trimethylindolin-2-ylidene)methyl]cyclobut-1-enolate, inner salt. Chem. Papers 2018, 72, 1549–1552. [Google Scholar] [CrossRef]
- Xie, J.; Comeau, A.B.; Seto, C.T. Squaric acid-based peptidic inhibitors of matrix metalloprotease-1 (MMP-1). Org. Lett. 2004, 6, 83–86. [Google Scholar] [CrossRef]
- Lopez, C.; Vega, M.; Sanna, E.; Rotger, C.; Costa, A. Efficient microwave-assisted preparation of squaric acid monoamides in water. RSC Adv. 2013, 3, 7249–7253. [Google Scholar] [CrossRef]
- Bagnis, D.; Beverina, L.; Huang, H.; Silvestri, F.; Yao, Y.; Yan, H.; Pagani, G.A.; Marks, T.J.; Facchetti, A. Marked alkyl- vs alkenyl-substitutent effect on squaraine dye solid-state structure, carrier mobility, and bulk-heterojunction solar cell efficiency. J. Am. Chem. Soc. 2010, 132, 4074–4075. [Google Scholar] [CrossRef] [PubMed]
- Anees, P.; Sreejith, S.; Ajayahgosh, A. Self-assembled near-infrared dye nanoparticles as a selective protein sensor by activation of a dormant fluorophore. J. Am. Chem. Soc. 2014, 136, 13233–13239. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, H.; Wang, Z.; Dong, G. Narrowband organic photodiodes based on green light sensitive squarylium. J. Phys. Chem. C 2017, 121, 15333–15338. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Lin, T.T.; Wu, J.; Xu, J. Triphenylethylene- and tetraphenylethylene-functionalized 1,3-bis(pyrrol-2-ul)squaraine dyes: Synthesis, aggregation-caused quenching to aggregation-induced emission, and thiol detection. ACS Omega 2018, 3, 16424–16435. [Google Scholar] [CrossRef]
- Balcerak, A.; Kwiatkowska, D.; Kabatc, J. Novel photoinitiators based on difluoroborate complexes of squaraine dyes for radical polymerization of acrylates upon visible light. Polym. Chem. 2022, 13, 220–234. [Google Scholar] [CrossRef]
- Triebs, V.A.; Jacob, K. Cyclobutenederivate der pyrrolreihe II. Liebigs Ann. Chem. 1968, 712, 123–137. [Google Scholar]
- Kiel, D.; Hartmann, H. Synthesis and characterization of a new class of unsymmetrical squaraine dyes. Dyes Pigment. 2001, 49, 161–179. [Google Scholar] [CrossRef]
- Beverina, L.; Ruffo, R.; Patriarca, G.; De Angelis, F.; Roberto, D.; Righetto, S.; Ugo, R.; Pagani, G.A. Second harmonic generation in nonsymmetrical squaraines: Tuning of the directional charge transfer character in highly delocalized dyes. J. Mater. Chem. 2009, 19, 8190–8197. [Google Scholar] [CrossRef]
- Beverina, L.; Ruffo, R.; Salamone, M.M.; Ronchi, E.; Binda, M.; Natali, D.; Sampietro, M. Panchromatic squaraine compounds for broad band light harvesting electronic devices. J. Mater. Chem. 2012, 22, 6704–6710. [Google Scholar] [CrossRef]
- Salice, P.; Arnbjerg, J.; Pedersen, B.W.; Toftegaard, R.; Beverina, L.; Pagani, G.A.; Ogilby, P.R. Photophysics of squaraine dyes: Role of charge-transfer in singlet oxygen production and removal. J. Phys. Chem. A 2010, 114, 2518–2525. [Google Scholar] [CrossRef]
- Triebs, V.A.; Jacob, K. Cyclobutenderivate der perrolreihe. Liebigs Ann. Chem. 1966, 691, 153–167. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to ‘green’ chemistry—Which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Nigst, T.A.; Westermaier, M.; Ofial, A.R.; Mayr, H. Nucleophilic reactivities of pyrroles. Eur. J. Org. Chem. 2008, 2008, 2369–2374. [Google Scholar] [CrossRef]
- Yu, F.; Yan, Q.; Liang, K.; Cong, Z.; Shao, Q.; Wang, Y.; Hong, L.; Jiang, L.; Ye, G.; Wang, H.; et al. Dual-state emission and solvatofluorochromism properties of facile squaraine dyes with cis-3,5-dimethylpiperidine. J. Lumin. 2021, 233, 117882. [Google Scholar] [CrossRef]
- Xia, G.; Shao, Q.; Liang, K.; Wang, Y.; Jiang, L.; Wang, H. A phenyl-removal strategy for accessing an efficient dual-state emitter in the red/NIR region guided by TDDFT calculations. J. Mater. Chem. C 2020, 8, 13621–13626. [Google Scholar] [CrossRef]
- Shao, Q.; Liang, K.; Ling, H.; Wang, Y.; Yan, Z.; Xia, G.; Wang, H. Tetraphenylethylene-incorporated squaraine dyes: Structural and theoretical insights into the diverse emission behaviors in solution and solid state. J. Mater. Chem. C 2020, 8, 4549–4556. [Google Scholar] [CrossRef]
- Yang, S.; Yin, P.-A.; Li, L.; Peng, Q.; Gu, X.; Gao, G.; You, J.; Tang, B.Z. Crystallization-induced reversal from dark to bright excited states for construction of solid-emission-tunable squaraines. Angew. Chem. Int. Ed. 2020, 59, 10136–10142. [Google Scholar] [CrossRef]
- Barbero, N.; Magistris, C.; Park, J.; Saccone, D.; Quagliotto, P.; Buscaino, R.; Medana, C.; Barolo, C.; Viscardi, G. Microwave-assisted synthesis of near-infrared fluorescent indole-based squaraines. Org. Lett. 2015, 17, 3306–3309. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Kazmaier, P.M.; Hamer, G.K.; Burt, R.A. Conformational isomerism in squaraines: Saturation transfer NMR studies on hydroxy squaraines. Can. J. Chem. 1990, 68, 530–536. [Google Scholar] [CrossRef]
- Gu, Y.; Fei, X.; Liu, Y.; Wang, Y.; Yang, X. Trimethine cyanine dyes with an indole nucleus: Synthesis and spectral properties studies. J. Lumin. 2013, 134, 184–190. [Google Scholar] [CrossRef]
- Law, K.Y.; Bailey, F.C. Squaraine chemistry. Synthesis of bis (4-dimethylaminophenyl) squaraine from dialkyl squarates. Mechanism and scope of the synthesis. Can. J. Chem. 1986, 64, 2267–2273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).