Reduction of the Plasma Uric Acid Level in Potassium Oxoate-Induced Hyperuricemic Rats by Heat-Concentrated Prunus mume Fruit Extract Containing Three Chlorogenic Acid Isomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Mei Extract Production and Crude Extraction
2.3. HPLC Analysis
2.4. Mass Spectrometric Analysis
2.5. Xanthine Oxidase Inhibitory Assay
2.6. Animals
2.7. Hyperuricemia Model and Drug Administration
2.8. Blood and Urine Collection and Determination of Uric Acid Content
2.9. Statistical Analysis
3. Results
3.1. Chlorogenic Acid Isomers in Fresh Prunus mume Juice and Mei Extract
3.2. In Vitro XO Assay
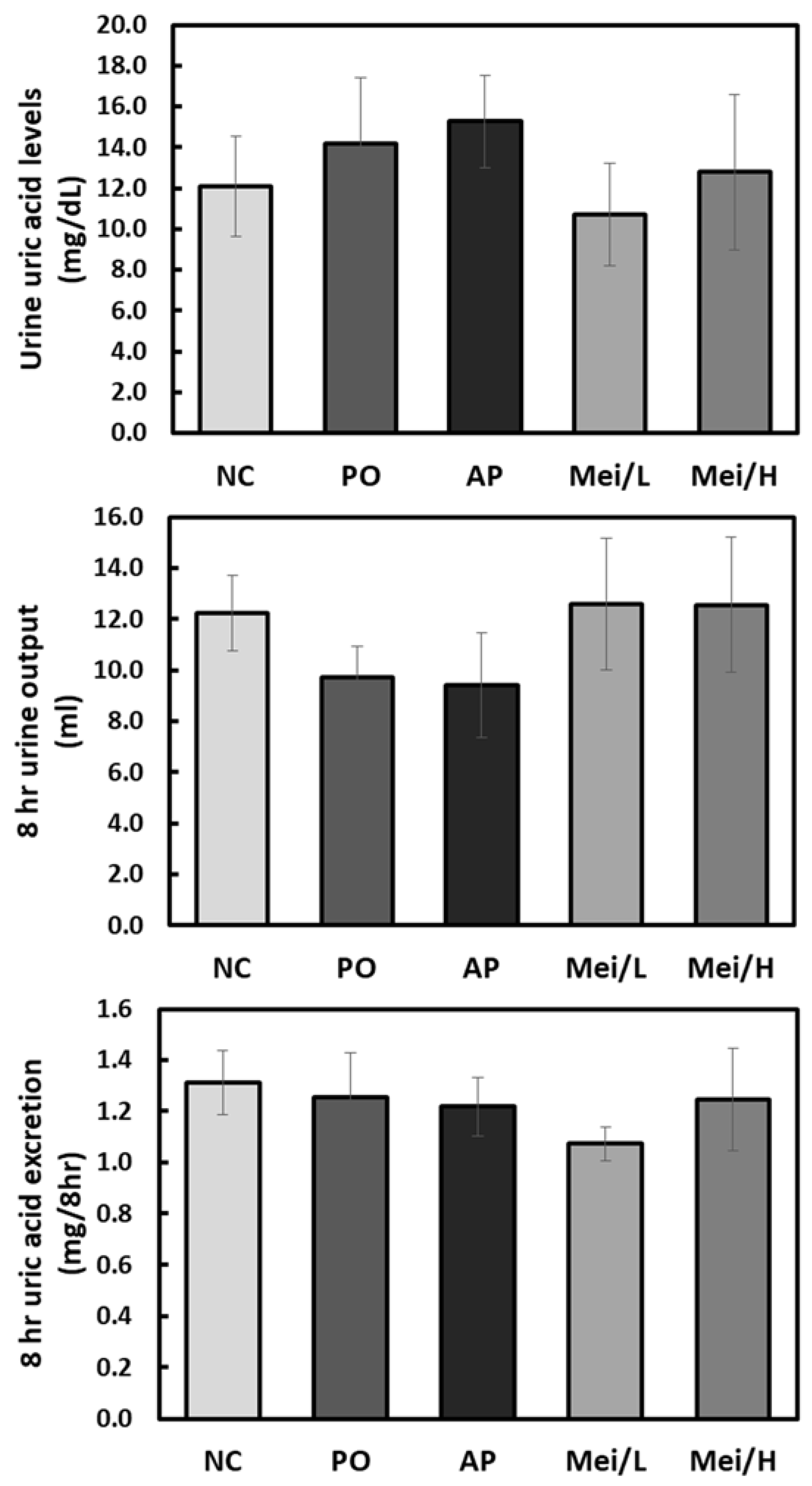
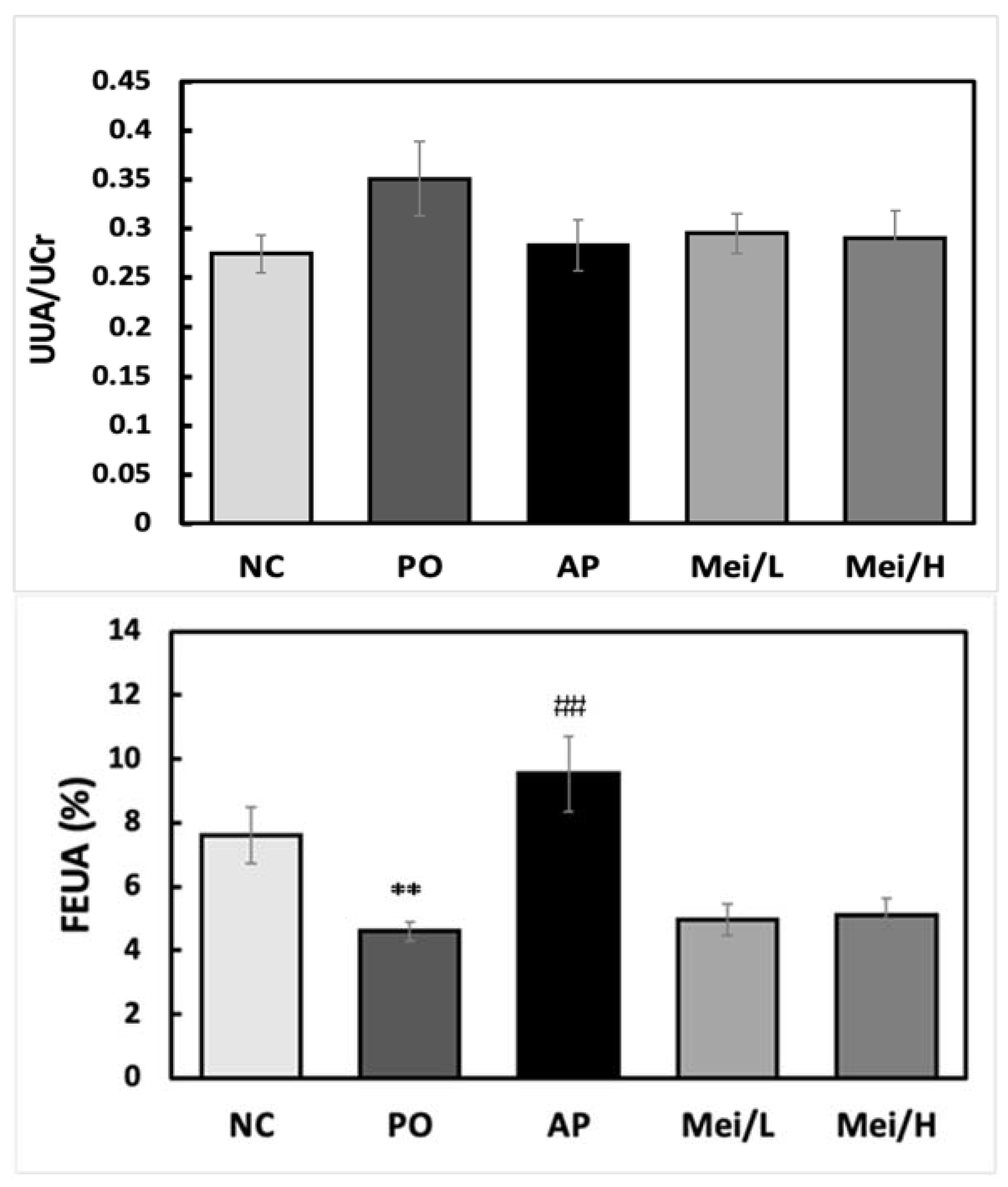
3.3. Effects of Mei Extract on Serum and Urinary Uric Acid Levels in Hyperuricemic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Torralba, K.D.; De Jesus, E.; Rachabattula, S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: Current and future directions. Int. J. Rheum. Dis. 2012, 15, 499–506. [Google Scholar]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Awale, S.; Tezuka, Y.; Ueda, J.Y.; Tran, Q.L.; Kadota, S. Xanthine oxidase inhibitors from the flowers of Chrysanthemum sinense. Planta Med. 2006, 72, 46–51. [Google Scholar]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar]
- Gong, X.P.; Tang, Y.; Song, Y.Y.; Du, G.; Li, J. Comprehensive review of phytochemical constituents, pharmacological properties, and clinical applications of Prunus mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer properties of Prunus mume extracts (Chinese plum, Japanese apricot). J. Ethnopharmacol. 2020, 246, 112215. [Google Scholar]
- Yi, L.T.; Li, J.; Su, D.X.; Dong, J.F.; Li, C.F. Hypouricemic effect of the methanol extract from Prunus mume fruit in mice. Pharm. Biol. 2012, 50, 1423–1427. [Google Scholar]
- Lo, Y.H.; Chen, Y.J.; Chang, C.I.; Lin, Y.W.; Chen, C.Y.; Lee, M.R.; Lee, V.S.; Tzen, J.T.C. Teaghrelins, unique acylated flavonoid tetraglycosides in Chin-shin oolong tea, are putative oral agonists of the ghrelin receptor. J. Agric. Food Chem. 2014, 62, 5085–5091. [Google Scholar] [CrossRef]
- Hsieh, S.K.; Lo, Y.H.; Wu, C.C.; Chung, T.Y.; Tzen, J.T.C. Identification of biosynthetic intermediates of teaghrelins and teaghrelin-like compounds in oolong teas, and their molecular docking to the ghrelin receptor. J. Food Drug Anal. 2015, 23, 660–670. [Google Scholar]
- Lin, P.Y.; Jhuo, C.F.; Lin, N.H.; Chen, W.Y.; Tzen, J.T.C. Assessing anti-psoriatic effects of bitter Pu’er tea and its three major compounds, strictinin, theacrine and epigallocatechin gallate in imiquimod-treated mice. Compounds 2022, 2, 293–306. [Google Scholar]
- Chau, Y.T.; Chen, H.Y.; Lin, P.H.; Hsia, S.M. Preventive effects of fucoidan and fucoxanthin on hyperuricemic rats induced by potassium oxonate. Mar. Drugs 2019, 17, 343. [Google Scholar]
- Hongyan, L.; Suling, W.; Weina, Z.; Yajie, Z.; Jie, R. Antihyperuricemic effect of liquiritigenin in potassium oxonate-induced hyperuricemic rats. Biomed. Pharmacother. 2016, 84, 1930–1936. [Google Scholar]
- Wang, Y.; Wen, J.; Zheng, W.; Zhao, L.; Fu, X.; Wang, Z.; Xiong, Z.; Li, F.; Xiao, W. Simultaneous determination of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid and geniposide in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study after administration of Reduning injection. Biomed. Chromatogr. 2015, 29, 68–74. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef]
- Murugaiyah, V.; Chan, K.L. Mechanisms of antihyperuricemic effect of Phyllanthus Niruri and its lignan constituents. J. Ethnopharmacol. 2009, 124, 233–239. [Google Scholar]
- Marković, S.; Tošović, J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chem. 2016, 210, 585–592. [Google Scholar] [CrossRef]
- Ferraz-Filha, Z.S.; Ferrari, F.C.; Araújo, M.C.P.M.; Bernardes, A.C.F.P.F.; Saúde-Guimarães, D.A. Effects of the aqueous extract from Tabebuia roseoalba and phenolic acids on hyperuricemia and inflammation. Evid. Based Complement. Alternat. Med. 2017, 2017, 2712108. [Google Scholar]
- Jaiswal, R.; Matei, M.F.; Golon, A.; Witt, M.; Kuhnert, N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food Funct. 2012, 3, 976–984. [Google Scholar] [CrossRef]
- Meng, Z.Q.; Tang, Z.H.; Yan, Y.X.; Guo, C.R.; Cao, L.; Ding, G.; Huang, W.Z.; Wang, Z.Z.; Wang, K.D.G.; Xiao, W.; et al. Study on the anti-gout activity of chlorogenic acid: Improvement on hyperuricemia and Gouty inflammation. Am. J. Chin. Med. 2014, 42, 1471–1483. [Google Scholar] [CrossRef]
- Honda, S.; Miura, Y.; Masuda, A.; Masuda, T. Identification of crypto- and neochlorogenic lactones as potent xanthine oxidase inhibitors in roasted coffee beans. Biosci. Biotechnol. Biochem. 2014, 78, 2110–2116. [Google Scholar] [CrossRef]
- Saito, J.; Matsuzawa, Y.; Ito, H.; Omura, M.; Ito, Y.; Yoshimura, K.; Yajima, Y.; Kino, T.; Nishikawa, T. The alkalizer citrate reduces serum uric acid levels and improves renal function in hyperuricemic patients treated with the xanthine oxidase inhibitor allopurinol. Endocr. Res. 2010, 35, 145–154. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, L.; Lin, D.; Ma, Z.; Deng, X. Lemon fruits lower the blood uric acid levels in humans and mice. Sci. Hortic. 2017, 220, 4–10. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Wu, J.L.; Li, J.X.; Ma, Z.C. Effect of lemon water soluble extract on hyperuricemia in a mouse model. Food Funct. 2019, 10, 6000–6008. [Google Scholar] [CrossRef]
- Chen, Q. Development of local mei specialties. J. Taichung District Agric. Res. Ext. Station Council Agric. 2010, 99, 197–203. [Google Scholar]
- Yan, H.; Ma, Y.; Liu, M.; Zhou, L. The dual actions of Paederia scandens extract as a hypouricemic agent: Xanthine oxidase inhibitory activity and uricosuric effect. Planta Med. 2008, 74, 1345–1350. [Google Scholar]
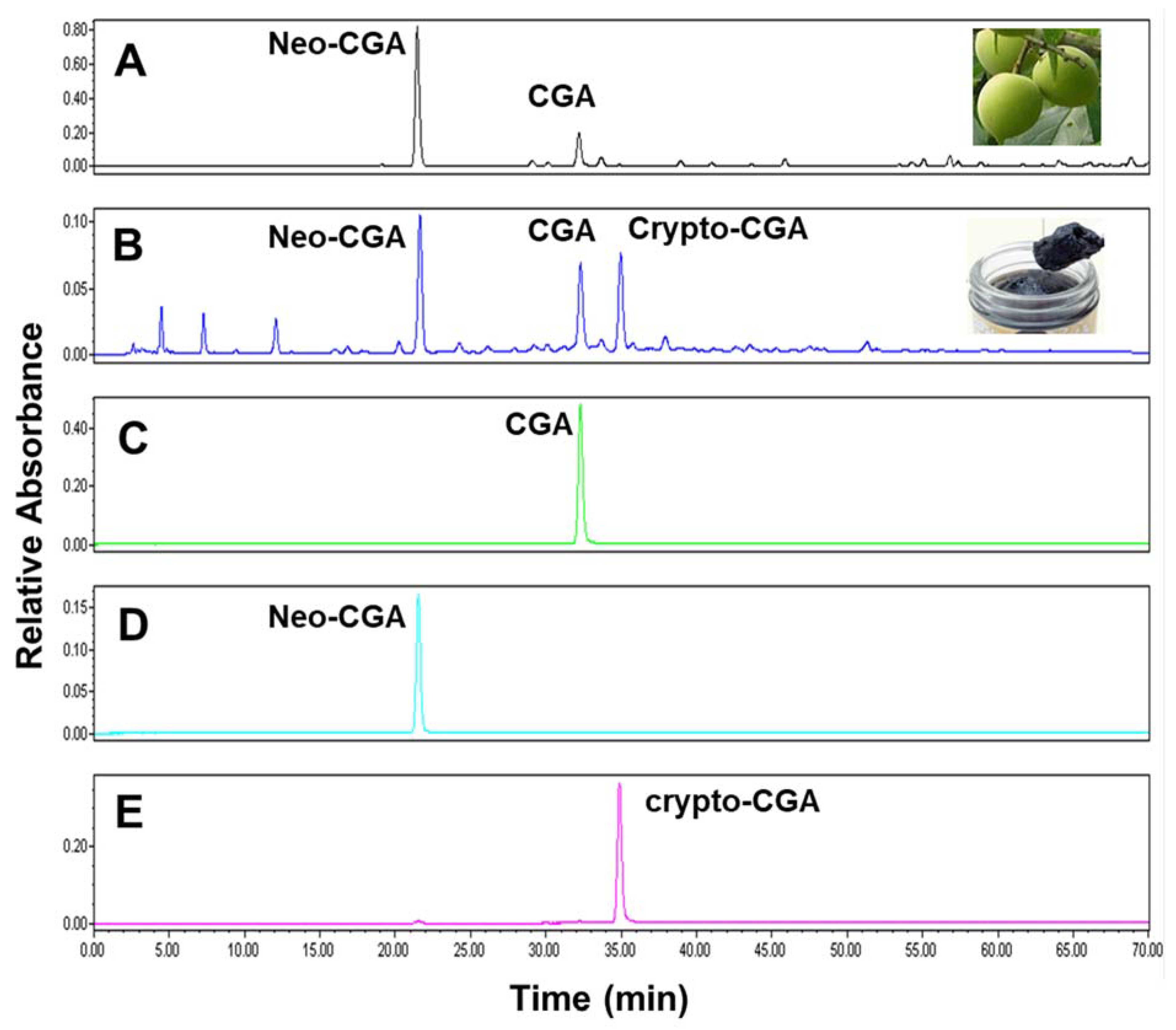
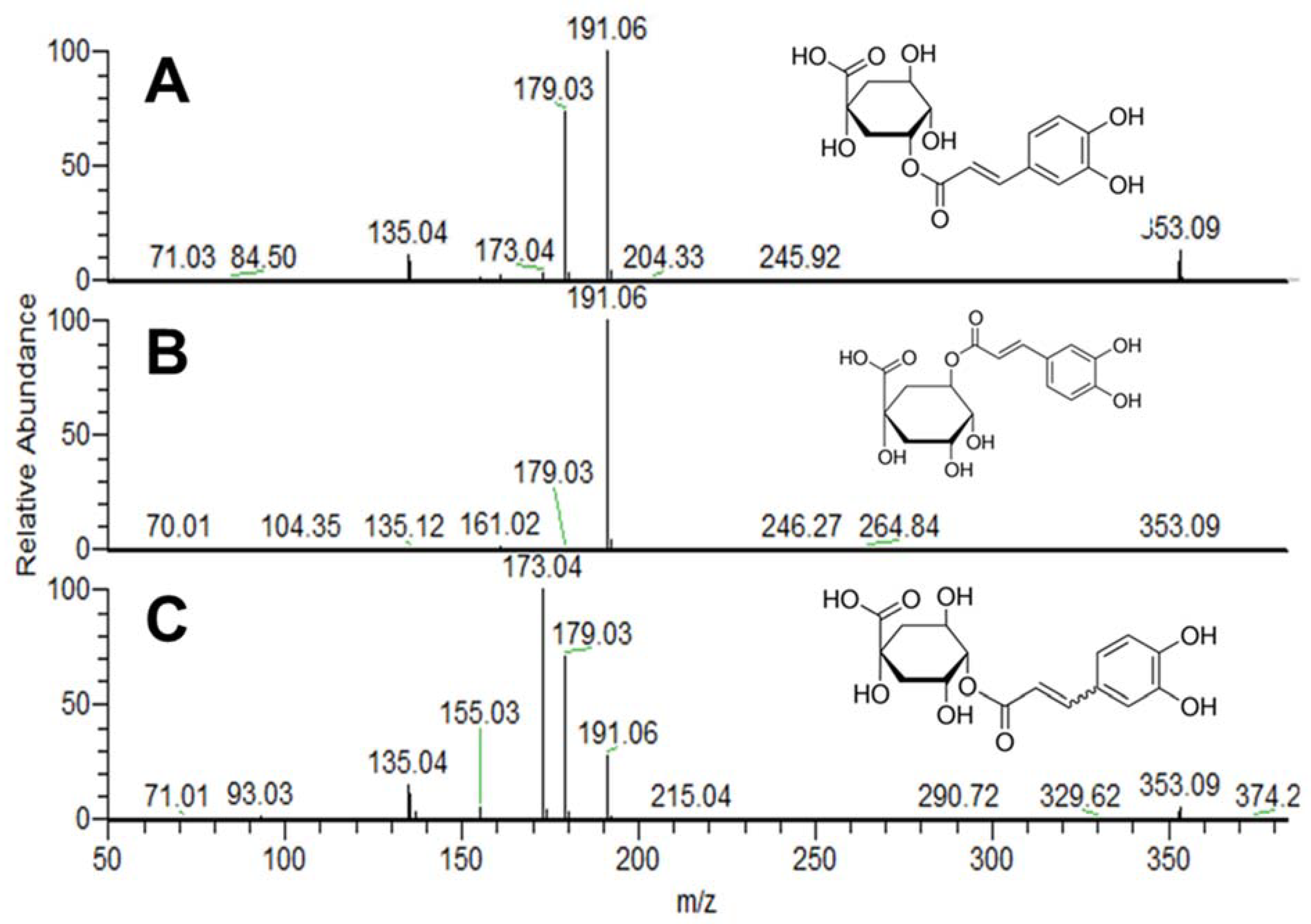
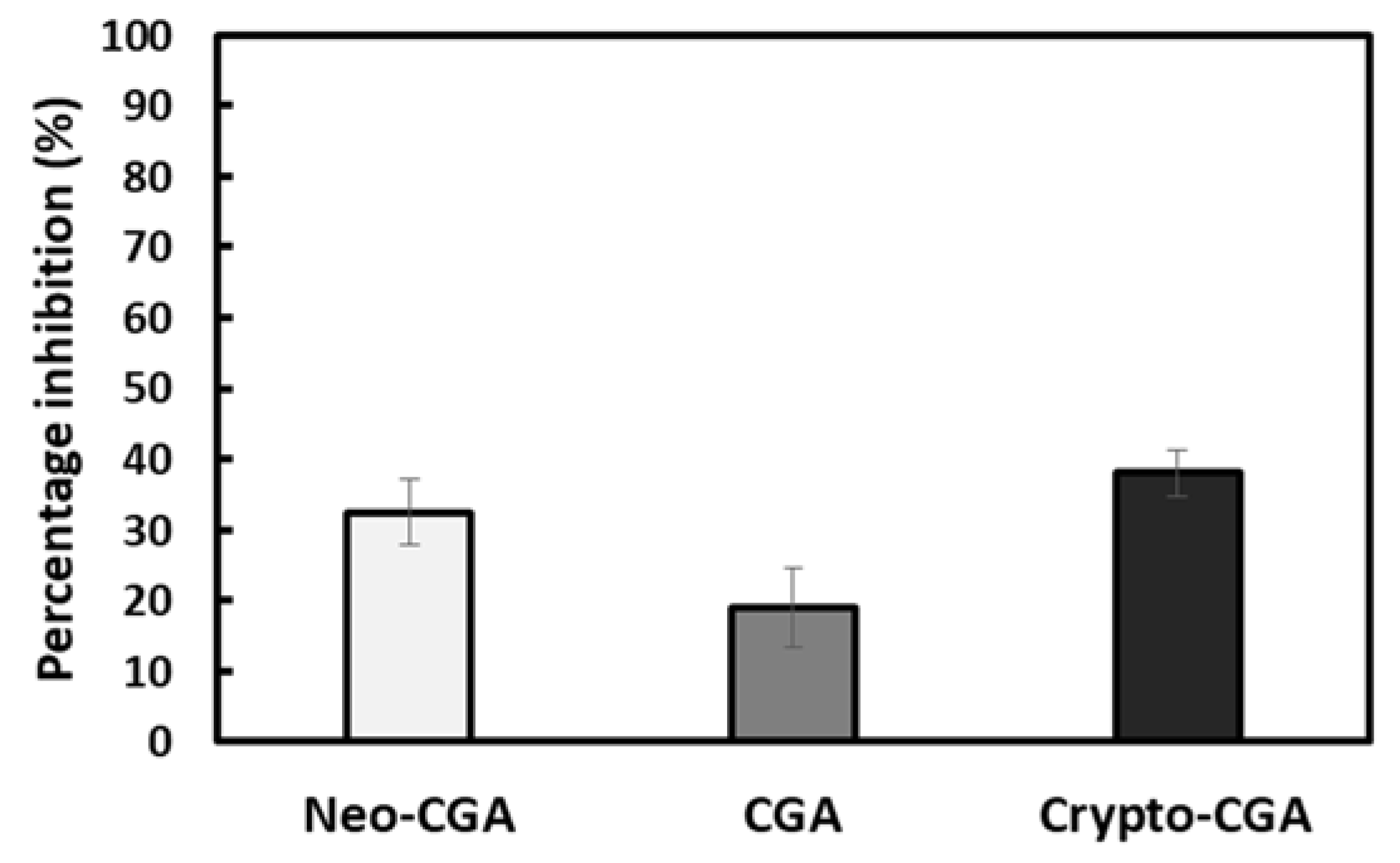
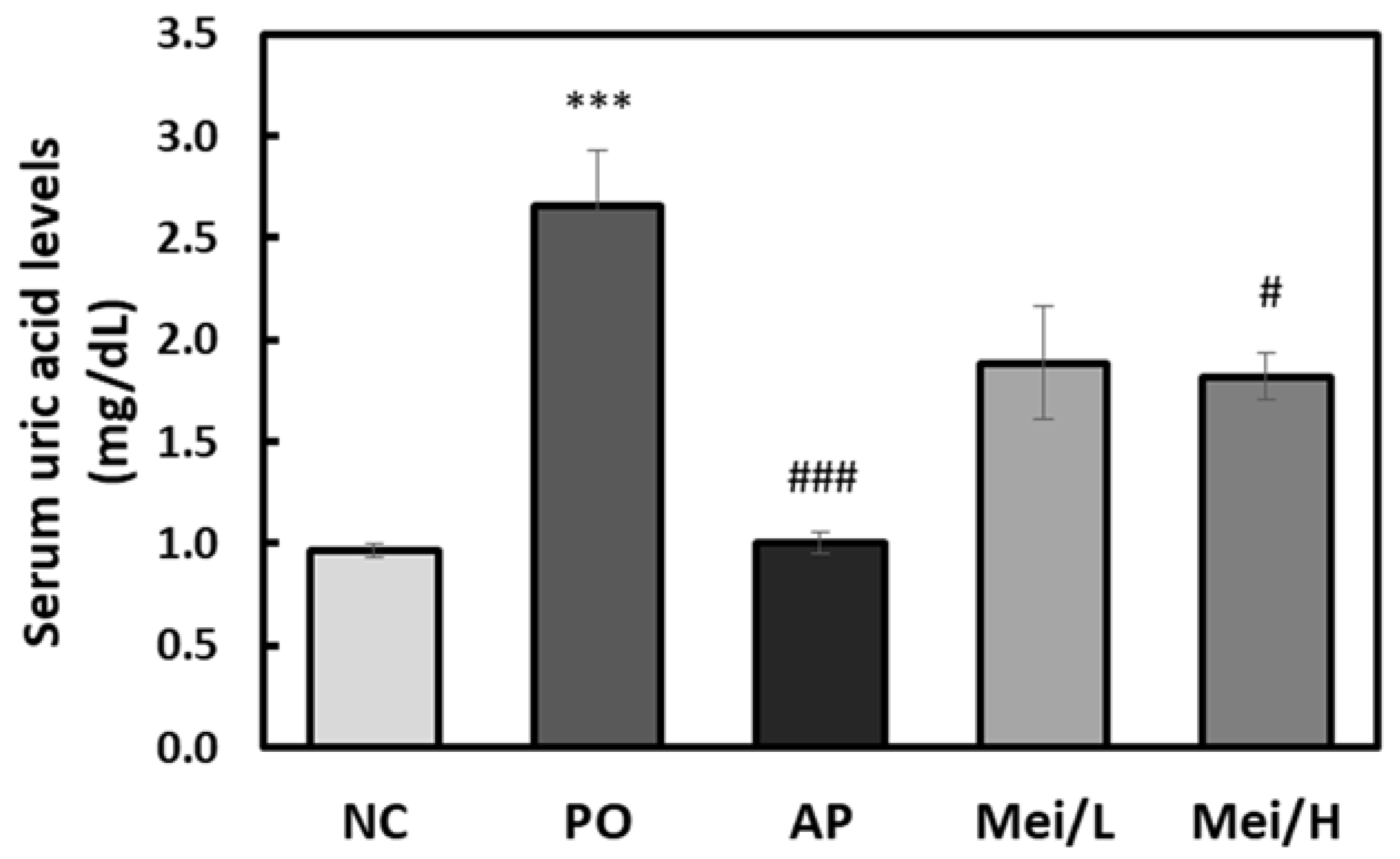
| Neo-CGA | CGA | Crypto-CGA | Total | |
|---|---|---|---|---|
| Fresh juice of cored fruit | 0.46 ± 0.01 | 0.16 ± 0.01 | ND | 0.62 ± 0.02 |
| Heat-concentrated mei extract | 0.90 ± 0.03 | 0.62 ± 0.01 | 0.70 ± 0.03 | 2.22 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Kuo, P.-C.; Chen, W.-Y.; Tzen, J.T.C. Reduction of the Plasma Uric Acid Level in Potassium Oxoate-Induced Hyperuricemic Rats by Heat-Concentrated Prunus mume Fruit Extract Containing Three Chlorogenic Acid Isomers. Compounds 2023, 3, 169-179. https://doi.org/10.3390/compounds3010014
Wu Y-C, Kuo P-C, Chen W-Y, Tzen JTC. Reduction of the Plasma Uric Acid Level in Potassium Oxoate-Induced Hyperuricemic Rats by Heat-Concentrated Prunus mume Fruit Extract Containing Three Chlorogenic Acid Isomers. Compounds. 2023; 3(1):169-179. https://doi.org/10.3390/compounds3010014
Chicago/Turabian StyleWu, Yi-Ching, Ping-Chung Kuo, Wen-Ying Chen, and Jason T. C. Tzen. 2023. "Reduction of the Plasma Uric Acid Level in Potassium Oxoate-Induced Hyperuricemic Rats by Heat-Concentrated Prunus mume Fruit Extract Containing Three Chlorogenic Acid Isomers" Compounds 3, no. 1: 169-179. https://doi.org/10.3390/compounds3010014
APA StyleWu, Y.-C., Kuo, P.-C., Chen, W.-Y., & Tzen, J. T. C. (2023). Reduction of the Plasma Uric Acid Level in Potassium Oxoate-Induced Hyperuricemic Rats by Heat-Concentrated Prunus mume Fruit Extract Containing Three Chlorogenic Acid Isomers. Compounds, 3(1), 169-179. https://doi.org/10.3390/compounds3010014







