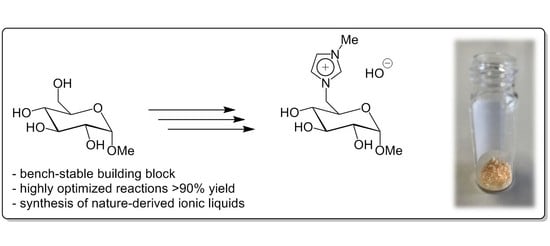
Glucosylimidazolium Hydroxide: A Bench-Stable Carbohydrate Based Building Block
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Experimental
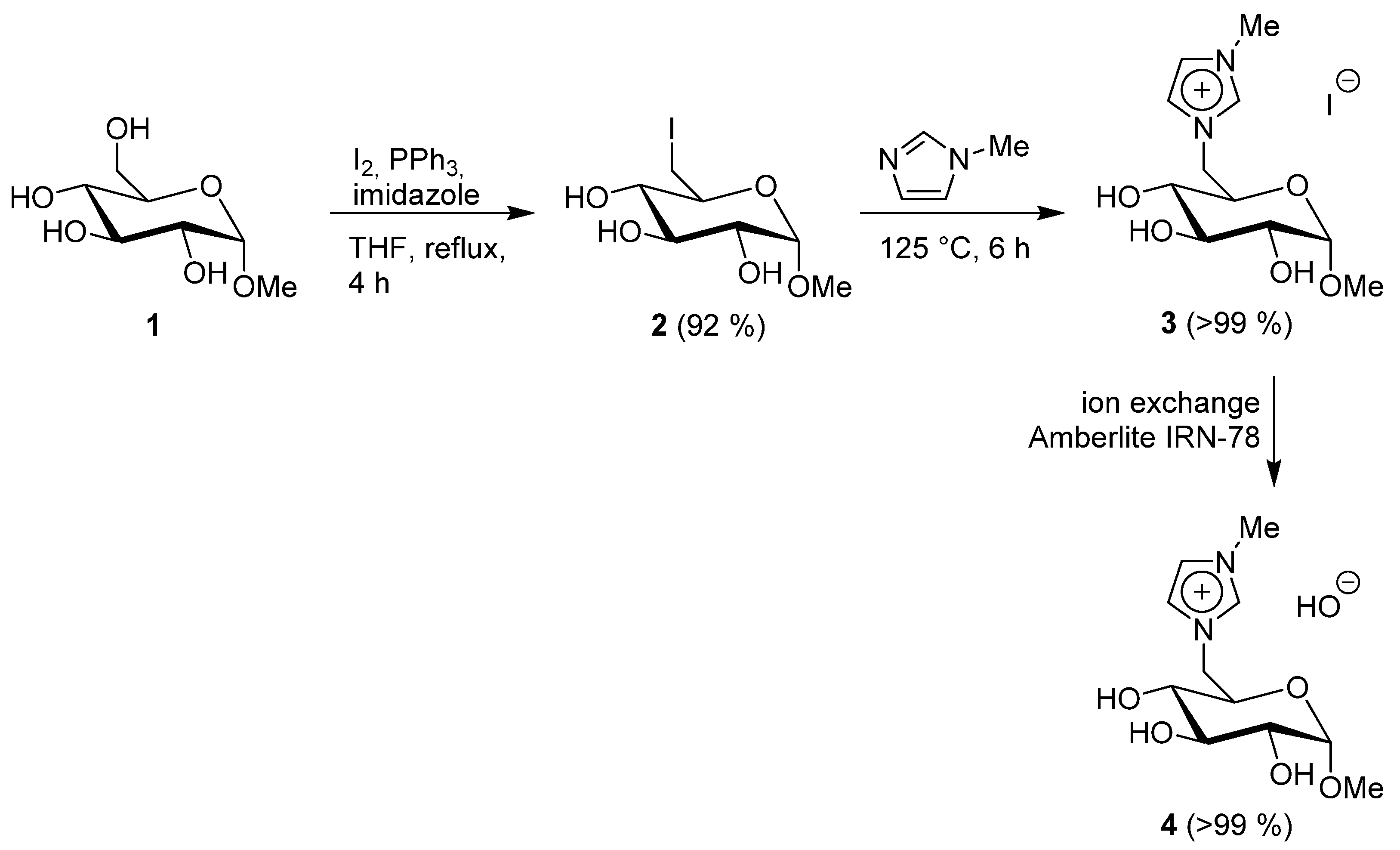
2.2.1. Methyl 6-Iodo-α-D-Glucopyranoside 2
2.2.2. 1-(Methyl 6-Deoxy-α-D-Glucopyranosid-6-yl)-3-Methylimidazolium Iodide 3
2.2.3. 1-(Methyl 6-Deoxy-α-D-Glucopyranosid-6-yl)-3-Methylimidazolium Hydroxide 4
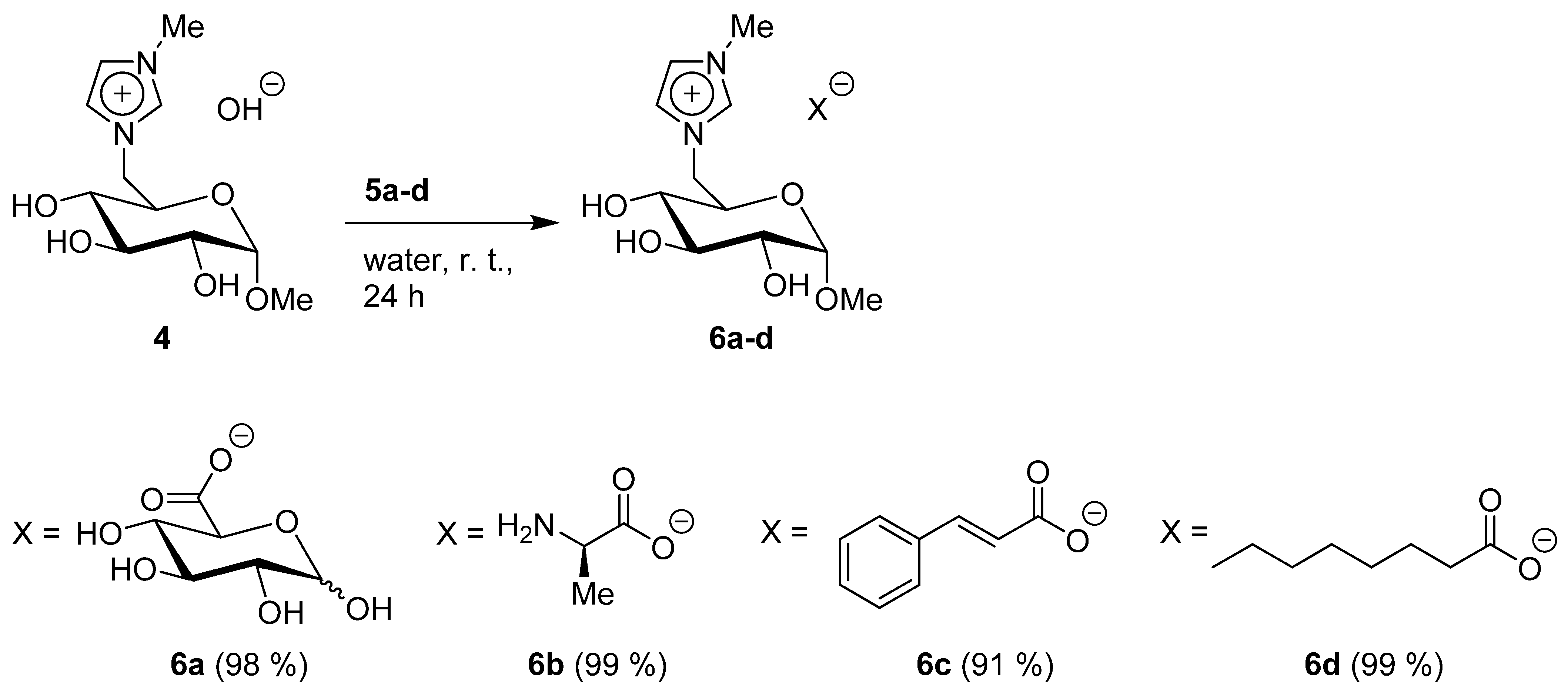
2.2.4. 1-(Methyl 6-Deoxy-α-D-Glucopyranosid-6-yl)-3-Methylimidazolium Glucuronate 6a
2.2.5. 1-(Methyl 6-Deoxy-α-D-Glucopyranosid-6-yl)-3-Methylimidazolium L-Alaninate 6b
2.2.6. 1-(Methyl 6-Deoxy-α-D-Glucopyranosid-6-yl)-3-Methylimidazolium Trans-Cinnamate 6c
2.2.7. 1-(Methyl 6-Deoxy-α-D-Glucopyranosid-6-yl)-3-Methylimidazolium Caprylate 6d
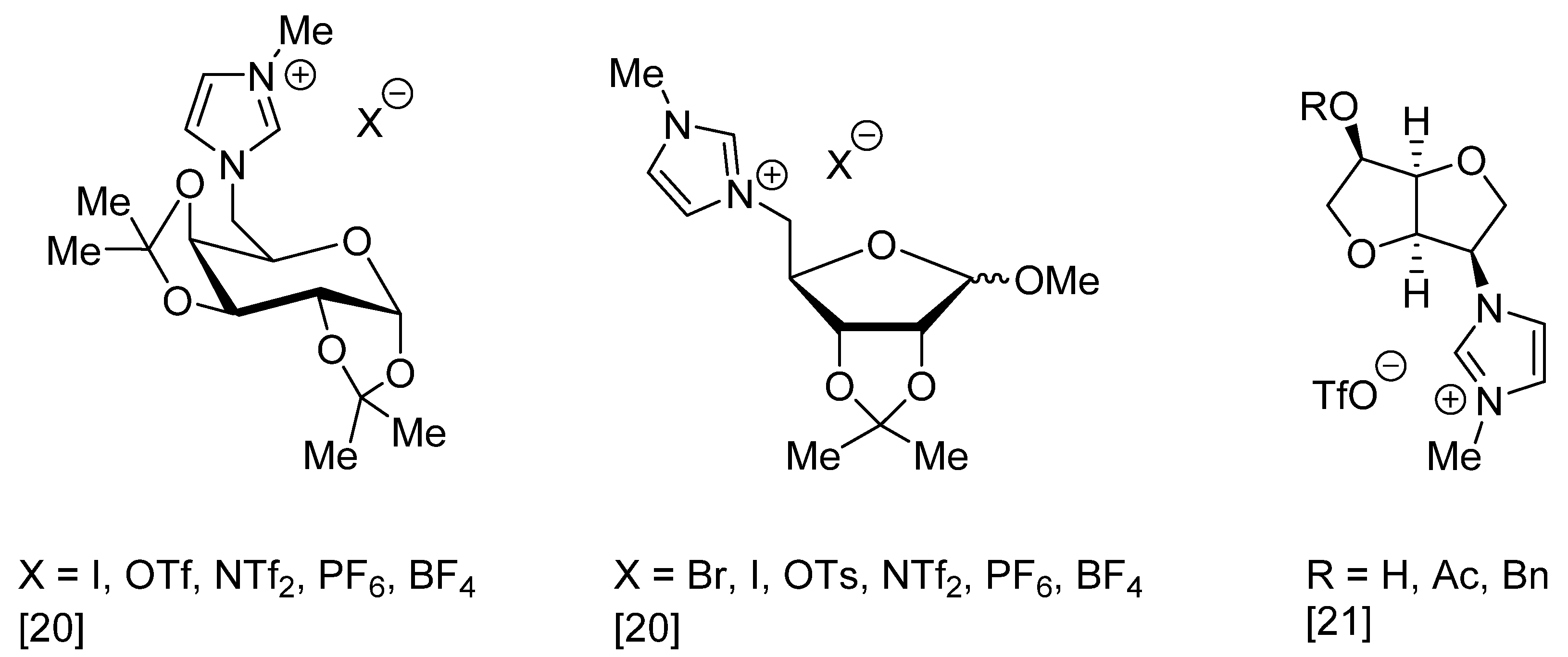
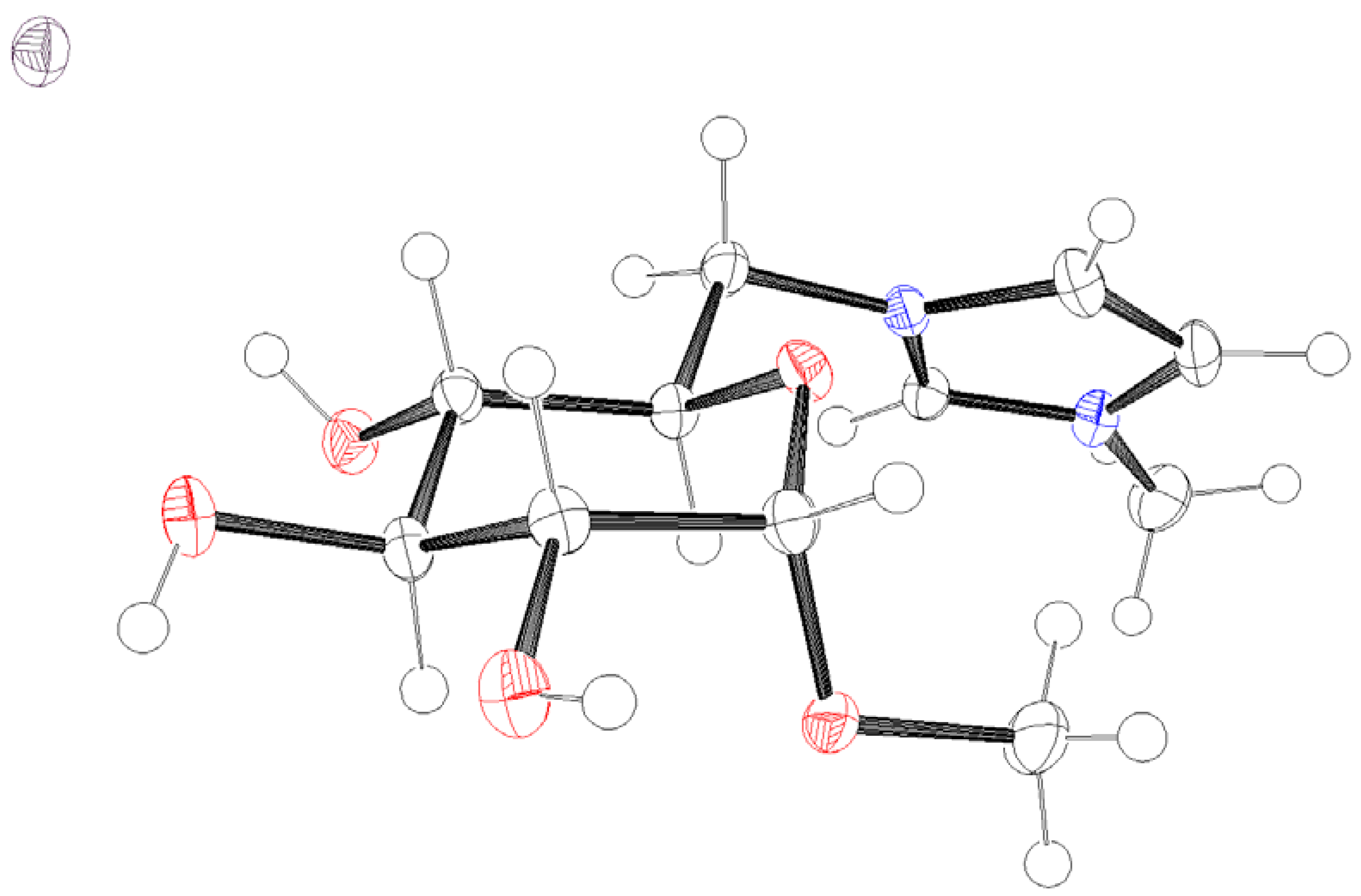
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Saito, T. Synthesis and properties of polymerized ionic liquids. Eur. Polym. J. 2017, 90, 245–272. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Jopp, S. Carbohydrate Based Ionic Liquids (CHILs): Synthesis and Applications. Eur. J. Org. Chem. 2020, 2020, 6418–6428. [Google Scholar] [CrossRef]
- Oskarsson, A.; Wright, M.C. Ionic Liquids: New Emerging Pollutants, Similarities with Perfluorinated Alkyl Substances (PFASs). Environ. Sci. Technol. 2019, 53, 10539–10541. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Reiß, M.; Brietzke, A.; Eickner, T.; Stein, F.; Villinger, A.; Vogel, C.; Kragl, U.; Jopp, S. Synthesis of novel carbohydrate based pyridinium ionic liquids and cytotoxicity of ionic liquids for mammalian cells. RSC Adv. 2020, 10, 14299–14304. [Google Scholar] [CrossRef]
- Erfurt, K.; Wandzik, I.; Walczak, K.; Matuszek, K.; Chrobok, A. Hydrogen-bond-rich ionic liquids as effective organocatalysts for Diels–Alder reactions. Green Chem. 2014, 16, 3508–3514. [Google Scholar] [CrossRef]
- Jayachandra, R.; Lakshmipathy, R.; Reddy, S.R. Hydrophobic d-galactose based ionic liquid for the sequestration of Pb2+ ions from aqueous solution. J. Mol. Liq. 2016, 219, 1172–1178. [Google Scholar] [CrossRef]
- Jayachandra, R.; Reddy, S.R.; Lakshmipathy, R. D-Galactose based hydrophobic ionic liquid: A new adsorbent for the removal of Cd2+ ions from aqueous solution. Environ. Prog. Sustain. Energy 2018, 38, S139–S145. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Jin, H.; Zha, C.; Gu, L. Direct dissolution of cellulose in NaOH/thiourea/urea aqueous solution. Carbohydr. Res. 2007, 342, 851–858. [Google Scholar] [CrossRef]
- Abe, M.; Kuroda, K.; Ohno, H. Maintenance-Free Cellulose Solvents Based on Onium Hydroxides. ACS Sustain. Chem. Eng. 2015, 3, 1771–1776. [Google Scholar] [CrossRef]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Javed, F.; Ullah, F.; Akil, H.M. Synthesis, characterization and cellulose dissolution capabilities of ammonium-based room temperature ionic liquids (RTILs). Pure Appl. Chem. 2017, 90, 1019–1034. [Google Scholar] [CrossRef] [Green Version]
- Hayouni, S.; Robert, A.; Ferlin, N.; Amri, H.; Bouquillon, S. New biobased tetrabutylphosphonium ionic liquids: Synthesis, characterization and use as a solvent or co-solvent for mild and greener Pd-catalyzed hydrogenation processes. RSC Adv. 2016, 6, 113583–113595. [Google Scholar] [CrossRef]
- Jayachandra, R.; Reddy, S.R. Synthesis of D-ribose and D-galactose derived chiral ionic liquids as recyclable chiral solvent for michael addition reaction. Trends Carbohydr. Res. 2015, 7, 60–67. [Google Scholar]
- Van Buu, O.N.; Aupoix, A.; Hong, N.D.T.; Vo-Thanh, G. Chiral ionic liquids derived from isosorbide: Synthesis, properties and applications in asymmetric synthesis. New J. Chem. 2009, 33, 2060–2072. [Google Scholar] [CrossRef]
- Madsen, R.; Skaanderup, P.R.; Poulsen, C.S.; Hyldtoft, L.; Jørgensen, M.R. Regioselective Conversion of Primary Alcohols into Iodides in Unprotected Methyl Furanosides and Pyranosides. Synthesis 2002, 2002, 1721–1727. [Google Scholar] [CrossRef]
- CCDC 2121301 Contains Supplementary Crystallographic Data for This Paper. Available online: https://www.ccdc.cam.ac.uk/structures/Search?ccdc=2121301 (accessed on 11 October 2021).
- Seiler, E.R.D.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-Fujita, M. Development of a novel cellulose solvent based on pyrrolidinium hydroxide and reliable solubility analysis. RSC Adv. 2020, 10, 11475–11480. [Google Scholar] [CrossRef] [Green Version]
- Yuen, A.K.; Masters, A.F.; Maschmeyer, T. 1,3-Disubstituted imidazolium hydroxides: Dry salts or wet carbenes? Catal. Today 2013, 200, 9–16. [Google Scholar] [CrossRef]
- Komabayashi, M.; Nokami, T.; Jopp, S. From Chitin to CHILs: First Glucosamine based Ionic Liquids. Asian J. Org. Chem. 2020, 9, 2092–2094. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Gaida, B.; Blacha-Grzechnik, A.; Matuszek, K.; Chrobok, A. Bioderived Ionic Liquids and Salts with Various Cyano Anions as Precursors for Doped Carbon Materials. Int. J. Mol. Sci. 2021, 22, 10426. [Google Scholar] [CrossRef]

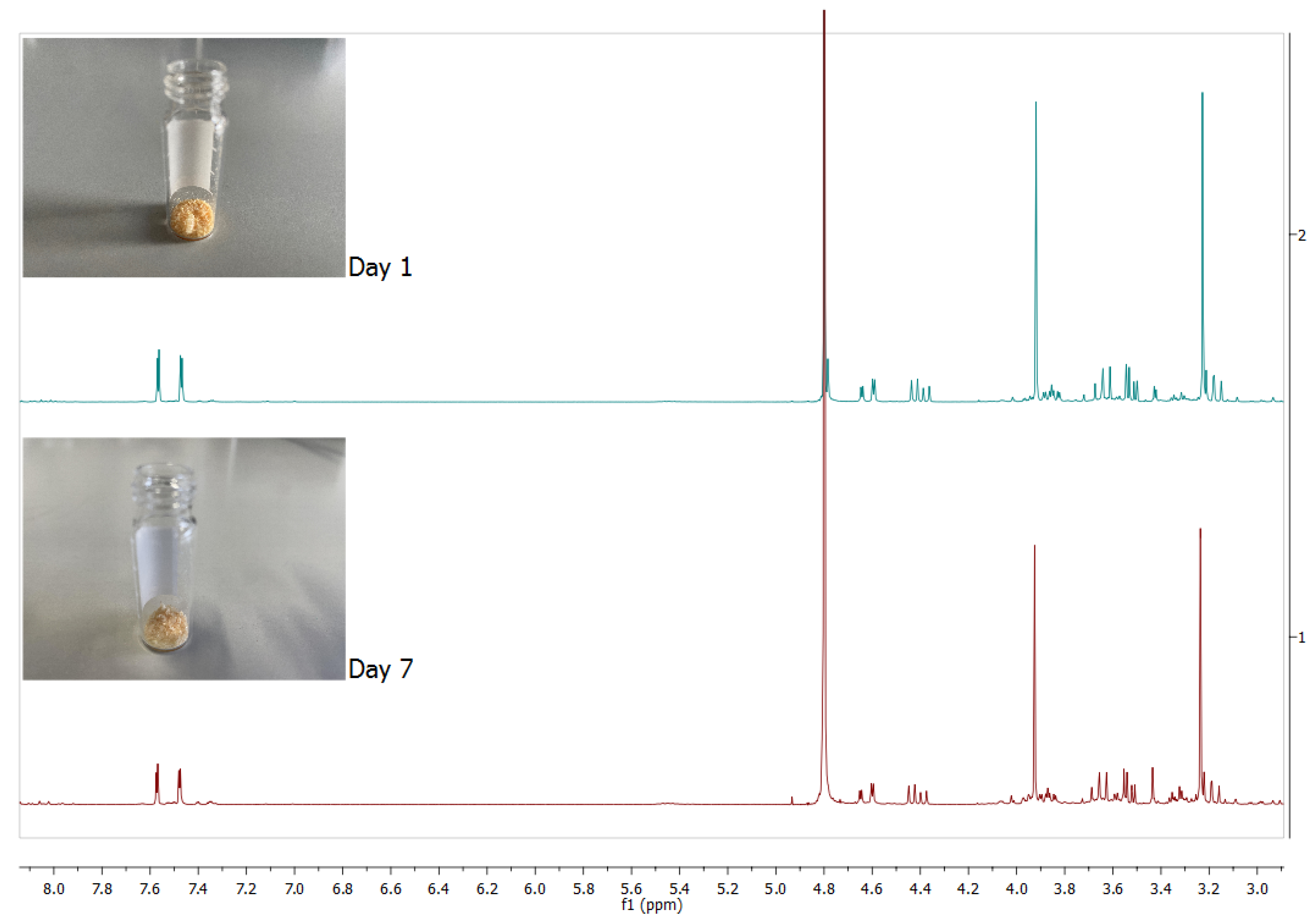
| Product | Physical State | Melting Point (°C) | Decomposition Point (°C) |
|---|---|---|---|
| 3 | yellow solid | 172–173 | 268 |
| 4 | yellow solid | / | 139–142 1 |
| 6a | off-white solid | 106–111 | 230 |
| 6b | yellow viscous liquid | / | 238 |
| 6c | off-white solid | 38–40 | 275 |
| 6d | yellow viscous liquid | / | 272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnegas, J.; Jopp, S. Glucosylimidazolium Hydroxide: A Bench-Stable Carbohydrate Based Building Block. Compounds 2021, 1, 154-163. https://doi.org/10.3390/compounds1030014
Schnegas J, Jopp S. Glucosylimidazolium Hydroxide: A Bench-Stable Carbohydrate Based Building Block. Compounds. 2021; 1(3):154-163. https://doi.org/10.3390/compounds1030014
Chicago/Turabian StyleSchnegas, Johannes, and Stefan Jopp. 2021. "Glucosylimidazolium Hydroxide: A Bench-Stable Carbohydrate Based Building Block" Compounds 1, no. 3: 154-163. https://doi.org/10.3390/compounds1030014
APA StyleSchnegas, J., & Jopp, S. (2021). Glucosylimidazolium Hydroxide: A Bench-Stable Carbohydrate Based Building Block. Compounds, 1(3), 154-163. https://doi.org/10.3390/compounds1030014