1. Introduction
Self-healing materials can repair corrosion, cracks, scratches, and other alterations independently and autonomously. This technology significantly benefits the economy, with direct consequences for social improvement in certain areas of science such as chemistry, energy, etc., by substantially increasing the life expectancy of structures and self-healing materials and significantly boosting the viability of industries as cracks and wear, in general, spontaneously disappear. The application of this technology is effective in areas that are difficult to access, whereas their repair is difficult and expensive. Therefore, in recent years, the field of material science has begun to focus on “self-healing” technology rather than “improving” technology.
Although the use of materials with self-healing properties can prevent their damage, wear and tear during their service life is inevitable. This means continuous inspection of products and structures made of such materials is necessary to assess wear evolution. So far, the interventions carried out concern either the repair of the wear or the replacement of the product, which are both time-consuming and expensive procedures. Therefore, a self-healing material capable of carrying out these procedures without external intervention would be ideal [
1,
2,
3].
For a material to qualify as a self-healing material, it must be able to eliminate cracks and other damage spontaneously after the appropriate stimulus. For this, it is required to fill the gap with “something” that will work through the self-healing process and restore the material to its original state. This filler should come from within the material itself. A small piece of the material will turn on and fill the crack. However, when these “activated pieces” approach the cracks, they should be able to “heal” the material (e.g., join the two surfaces of the damage together) and stabilize.
The phenomenon of self-healing was discovered through the use of polymers in 1969 [
4]. After ten years, Wool observed the restoration of polymers to their original state using the self-healing phenomenon [
5,
6]. White stored a polymer in a container, in which the healing substance was released autonomously to restore the cracks [
7]. Following these publications, scientists saw the potential of these new materials, and work in this area is ongoing [
2,
8]. Since the nineteenth century, the “self-healing capacity” of cement, i.e., its ability to heal itself, has been realized, and this property has taken on many different dimensions in recent years. The addition of substances offers stimulating or even mechanical functionality and is currently considered a viable method of increasing the durability of these structures to increase their service life. Many researchers have attempted to understand the mechanisms of these seals and healing cracks, which has led to several new self-healing technologies. These results have been certified both in the laboratory and in practice [
9].
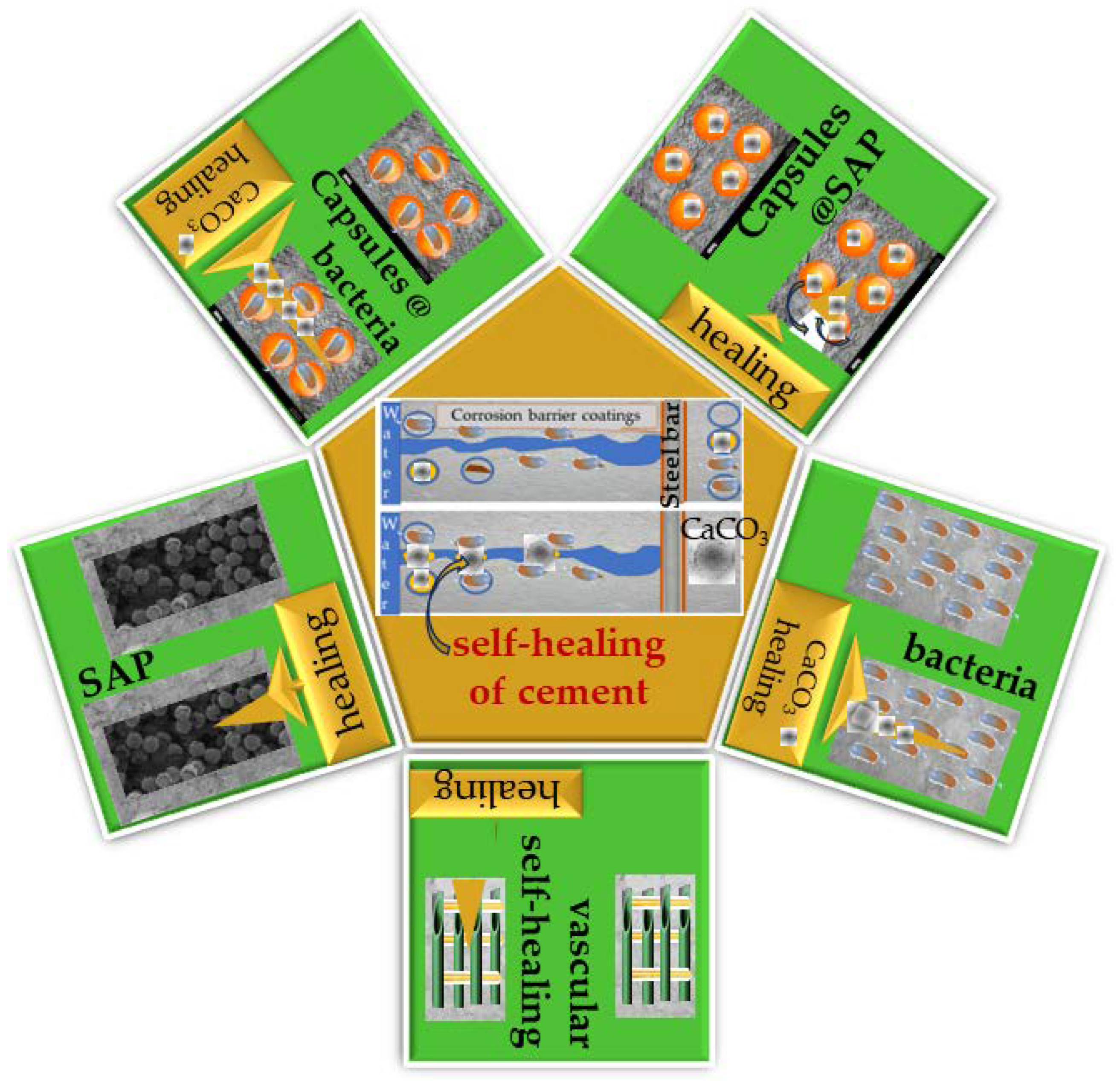
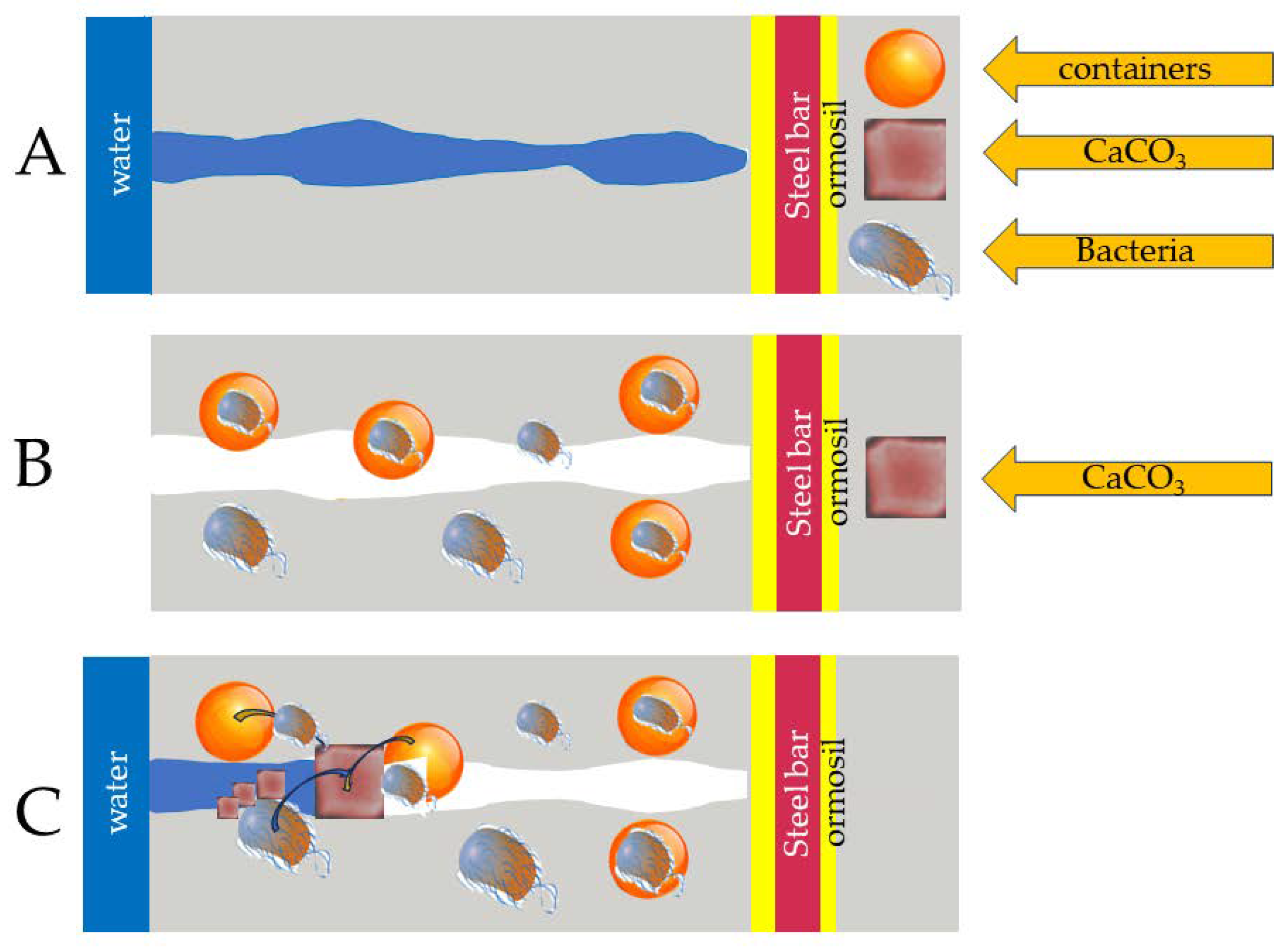
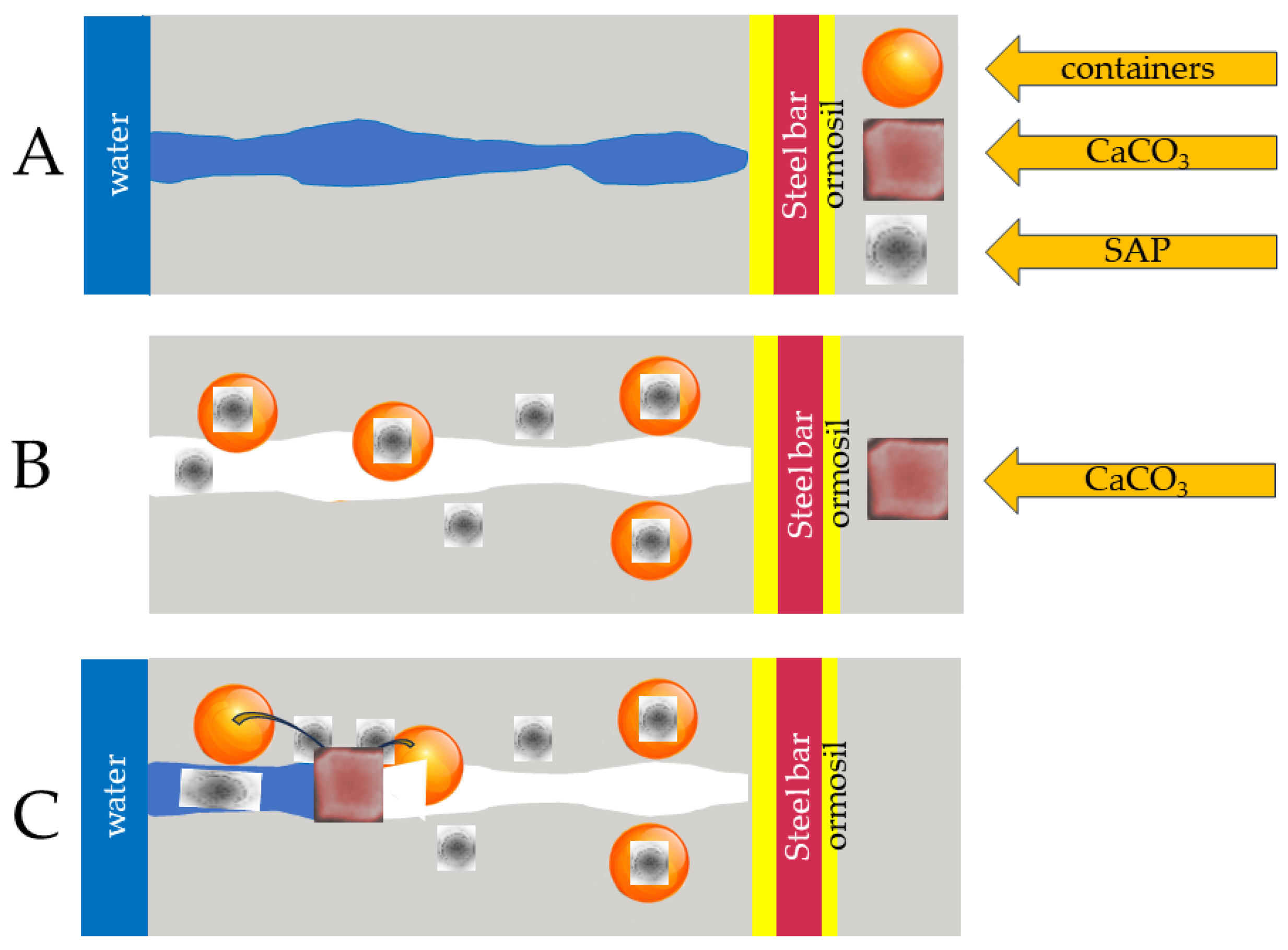
It is known that concrete can heal its cracks independently and restore its mechanical properties to a certain extent. These cracks reduce the durability of concrete, all of which affect the steel reinforcement bars, which leads to corrosion. When concrete heals itself through its autogenous healing character, cracks can heal by moisturizing the mineral or carbonizing calcium hydroxide. Additional materials can also be placed on the concrete to give it autonomous self-healing techniques, such as capsules, bacteria, vascular self-healing, and treatment with super absorbent polymers. Each autonomous self-healing mechanism is outlined in
Figure 1, which presents the various treatments used. Furthermore, this picture provides the framework of this review, including all self-healing technologies for cement instead of reviewing only the individual technologies that most of the literature uses. Such a review is helpful for a student starting their career and a practitioner who wants to decide which technology to use for a particular application. If, for example, a civil engineer wishes to inject a solution of cement with healing properties into a building, he must know the different properties of the technology to inform his choice, such as how it works, how many years it will last, whether the slurry will survive the pressure of the processing-injection method, or if the quantity of the self-healing agent will be sufficient for the desired result, etc. Of course, these are just some of the many examples that could be mentioned, but it is impossible here to discuss more examples due to a lack of space.
The first part of the process involved the incorporation of microcapsules containing the self-healing substance into polymeric material. So, when a crack encountered a capsule, it would break, and the healing substance would flow, filling it and resulting in its healing. The goal of this technology is for new materials to cost less than conventional technologies but also to increase their reliability, require less maintenance for their use, and improve their life expectancy. In other words, the cost of materials is significantly reduced [
1].
2. Cement Self-Healing
Cement is characterized as a technical stone and is a relatively cheap and durable material. In recent decades, the most used construction material’s properties have depended on its initial components, such as water and various aggregates that can vary. However, the final product has high compressive strength but not tensile strength.
Cement is produced by burning raw materials at 1500 °C, releasing large amounts of carbon dioxide worldwide. Common materials used to manufacture cement include limestone, shells, and chalk or marl combined with shale, clay, slate, blast furnace slag, silica sand, and iron ore. Cement production must be reduced or replaced with materials that demonstrate the phenomenon of self-healing. In addition, some industrial wastes, such as fly ash, blast furnace slag, and iron silicates, can partially replenish or improve their properties.
A primary cause that activates the wear mechanisms of cement is its permeability, i.e., the microstructure of the cement pores under consideration, which includes isolated or interconnected pores. Interconnected pores let water and other chemicals into the concrete matrix, leading to chemical wear and tear. For example, the penetration of sulfate ions into the concrete leads to ettringite formation [
10]. This conversion of a high-density phase into a lower-density phase creates internal stresses and the further expansion of cracks. In addition, ions entering the matrix through the networked pores destabilize the reinforcement’s passive film, ultimately achieving concrete corrosion. Similarly, the diffusion of carbon dioxide occurs, which reacts with alkaline components in a process called carbonation. This results in a decline in pH and passivation of the protective film of the reinforcement.
The above brief description makes it clear that cracks in concrete must be reduced to limit the permeability of the matrix to attacking substances. Here, the method of healing is cost-effective [
3]. The further hydration of cement particles and carbonation of dissolved calcium hydroxide guides the healing of concrete cracks. This was first observed by the French Academy of Sciences in 1836, and it was presented in a publication [
3]. At that time, there was a differentiation between “self-healing” and “self-sealing”. In one case, the original strength of the concrete is restored, while in the second case, when the cracks are filled, there is no improvement in the mechanical properties of the materials.
One observes self-healing in concrete samples exposed to freezing and thawing cycles [
11,
12,
13,
14]. In adverse climatic conditions near coastal areas, such as the Barents Sea, concrete undergoes multiple cycles of freezing and thawing. In winter, when the climate is heavy, concrete is damaged, while the properties are recovered due to self-healing during the year’s warm months [
12].
Recently, rigorous research has been carried out on the self-healing of concrete. These investigations can be classified into two categories: mixed materials (mineral mixtures, fibers, nanofillers, and curing agents); or self-healing technologies (capsules and bacteria).
2.1. Mechanisms
A substance used for self-healing cement must adhere to some characteristics. First, it must seal the crack zones to reduce the permeability of harmful substances. Additionally, the incorporation of these substances into the uterus must be continuous to ensure that the self-healing properties last for more than 50 years. Finally, this substance should be relatively inexpensive [
15].
Various physicochemical processes contribute to the self-healing of cracks in cement. These can be the formation of calcium carbonate, the blocking of water from impurities resulting from the appearance of cracking, the complete hydration of unreacted cement, and, finally, the expansion of the hydration of the cement matrix with the formation of C-S-H sealing inside the crack [
16].
From this brief discussion, one understands that the recrystallization of calcium carbonate is the primary mechanism implemented by cement hydration products dissolved in water and carbonate. Ca(OH)2 is released and diffused along the cracks. Calcium ions react with dissolved carbon dioxide and form CaCO3. In this way, they fill the gaps in the cement.
2.2. Self-Healing Methods of Cement
Recently, many studies have been conducted concerning the creation of containers added to cement that are suitable for self-healing. These materials have various forms and are charged with numerous substances, leading to cement “self-healing”. Some of them are mentioned below.
Bacteria
Self-healing of cement can be accomplished by incorporating bacteria into the concrete [
17]. The bacterium metabolizes urea and precipitates calcium carbonate into the crack environment. Microbial precipitation of calcium carbonate is evidenced by the concentration of dissolved inorganic carbonate ions, the concentration of calcium ions, and the presence of nucleation centered in the bacterium. Its walls act as centers of nucleation [
18,
19,
20,
21,
22,
23]. Using bacteria in concrete quickly reduces cracking and increases compressive strength and rigidity compared to those without bacteria. Applying microbial calcite helps reduce thawing and freezing due to the chemical process and reduces the permeability of substances, thereby reducing the freezing process. Because chemical ingress cracks are waterproof, this improves the service life of reinforced concrete [
24]. On the other hand, using bacteria in concrete doubles the cost: In the application and growth of bacteria, it requires nutrients and metabolic products to develop microorganisms. Estimating the number of bacteria that should be used in concrete facilities for optimal performance is also tricky. Furthermore, the lifetime of this technology has not been fully certified [
24]. Despite the various weaknesses of the technology, using bacteria to self-heal cement is becoming more popular in areas, such as in repairing limestone monuments and healing cracks in concrete in buildings with high strength, facilities near humid environments such as sea and rivers, and many other applications.
Bacillus sphaericus and Sporosarcinapastuerii were used in a study of the effect of bacteria on cement. After cultivation, they were planted in cement in different concentrations. The samples were cured in tap water and tested after 7 to 28 days. In cement, the increase in strength was 50% and 28% in mortar for Sphaericus and Sporosarcinapastuerii, respectively, compared to the conventional mixture. The XRD method proved the creation of calcite in cement samples produced in the presence of bacteria, which enhanced the strength and durability of cement [
25]. Concrete reinforcement was accomplished using Bacillus subtilis. The addition of bacteria improved compressive strength in the order of 12 to 30%, tensile strength of about 13 to 19%, and tensile strength bending at values from 13 to 16% at different ages [
26]. Bacillus subtilisaureolytic was used to enhance the self-healing properties of concrete. This bacterium produced calcium carbonate in an alkaline environment certified by scanning electron microscopy. The result of this method was to prove the sealing of concrete cracks [
27]. B. sphaericus, protected by silicate gel, was incorporated into a cement matrix to study calcite precipitation, the self-healing mechanism of concrete. The protection of bacteria in this way from the pH of concrete leads to calcium carbonate precipitation into the uterus. For results of this biological treatment, it was evaluated with ultrasonic pulse and optical measurements that are particularly useful without contamination of the sample [
28].
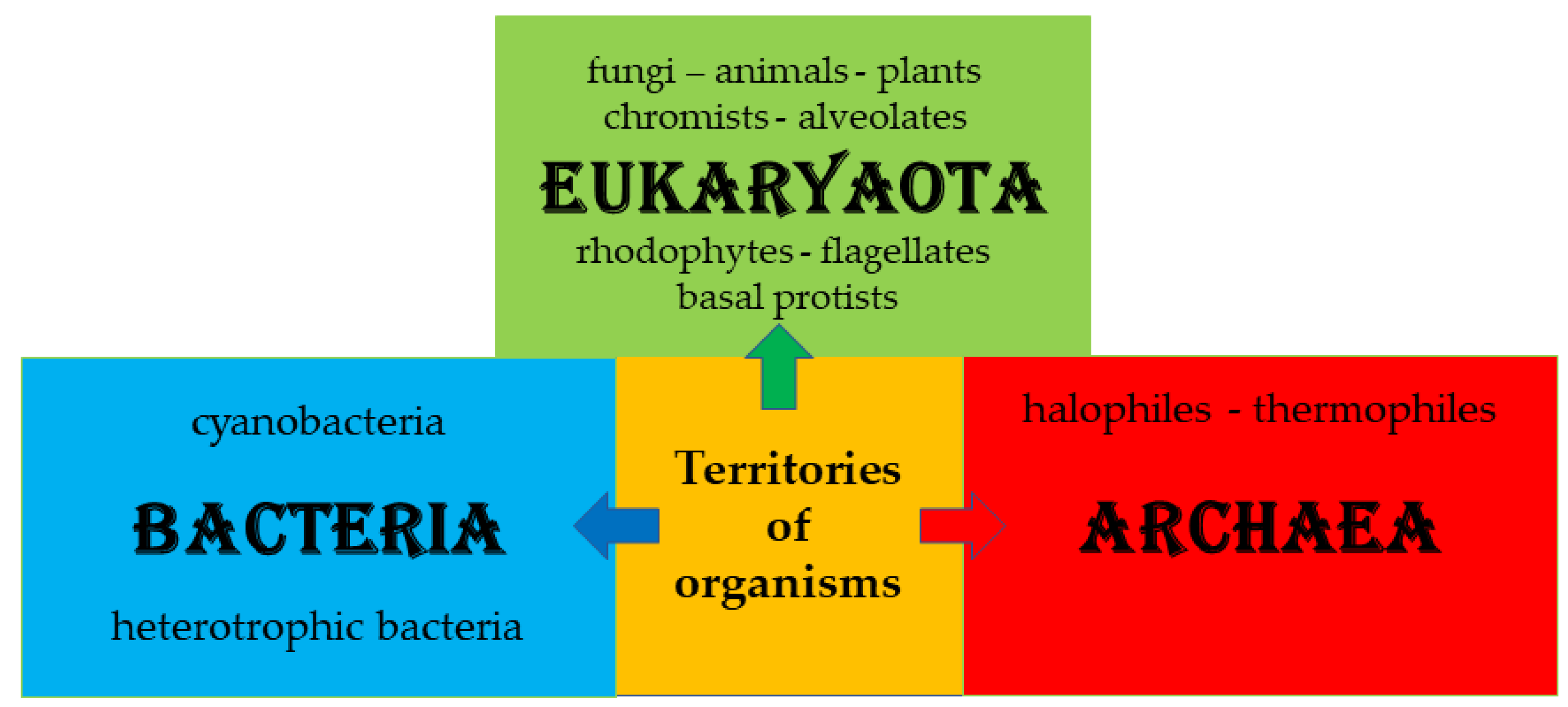
3. General about Organisms
Figure 2 shows the territories of organisms distinguished in bacteria, eukaryotes, and archaea [
29]. Each domain is divided into primary fungi, plants, and animals. The number of bacteria and archaea is not easily calculable and is mostly unknown.
There are two main categories of cells: the prokaryotic and the eukaryotic. Organisms classified as bacteria and archaea are structured based on prokaryotic cells, while other organisms belonging to protists and fungi, plants, and animals are structured based on eukaryotic cells.
Prokaryotic organisms are simple, unicellular, and wear an envelope we call a cell wall [
30]. Inside the cell wall, there is a flexible and semi-permeable cell membrane. Inside there is no organization, and only ribosomes are observed. The DNA. is diffused within the cell and not as a nucleus.
Microorganisms are a large group of microscopic organisms that live as independent single-celled organisms or groups of cells. To this complex belong those that are tiny but not cells. Unlike macroorganisms, microorganisms perform the processes of growth and reproduction independently of other cells of the same or distinct species.
Bacteria are single-celled prefabricated organisms whose diameter can reach up to 50 microns. Their morphology differs and can be, for example, spherical or the same, and so on. Bacteria are divided by Gram into Gram-positive and Gram-negative. Gram-positive bacteria acquire a violet color, while harmful bacteria acquire a pinkish-red color. This is due to the differentiation in cell wall structure between Gram-positive and Gram-negative bacteria. A relatively large set of bacteria can be used for this purpose, such as Bacillus pasteurii [
31], Bacillus sphaericus [
32], Escherichia coli [
33], Bacillus subtilus [
34], Bacillus cohnii [
35], Bacillus pseudofirmus [
36], and Bacillus halodurans [
27]. In this paper, however, we will limit our selection for the sake of clarity.
Intestinal bacteria are Gram-negative, rod-shaped, motionless, or self-propelled with cuttle flagella, optionally aerobic. Among intestinal bacteria are pathogens for humans, animals, plants, and other strains of industrial importance. Undoubtedly, Escherichia coli has more knowledge than any other type of bacterium.
Escherichia coli inhabits the intestines of humans and warm-blooded animals. Escherichia coli plays a role in nutrition, especially in synthesizing vitamin K. It contributes to oxygen consumption, making the large intestine anoxic. They have rare requirements for growth factors and can grow with a wide variety of carbon and energy, such as sugars, amino acids, organic, acute, etc. Some strains of this genus are pathogenic. Goals have been requested to be seen in infants and can cause epidemics in clinics [
37].
Staphylococcus is Gram-positive and optionally aerobic with the typical respiratory, respiratory, and metabolizing bacterium. They resist reduced water potential and tolerate drying and high salt concentrations well. This is a common parasite of humans and mammals and can cause serious infections [
37].
Bacillus is a Gram-positive, rod-shaped, aerobic, or optionally aerobic bacteria with the potential to form endospores. Due to their properties, they can live in any environment [
38]. They can be used in artificial media containing some source of carbon. The extracellular enzymes they produce can break down a lot of sugar and lipids, allowing these organisms to be used as carbon sources and electron donors. Many of them have antibiotics. This may be due to the process where the antibiotic is released when the culture enters the stagnant growth phase after starting sporulation.
3.1. Microbially Induced Carbonate Precipitation
The precipitation of calcium carbonate (CaCO3) is created by two mechanisms, one biologically controlled and the other induced. In the first case, the organism essentially possesses the precipitation process consisting of the nucleation and growth of mineral particles. In the case of the induced mechanism, the production of calcium carbonate carbon is highly dependent on environmental conditions. The nutrient composition contributes to the different precipitation mechanisms in different environments. Calcium concentration, the concentration of dissolved inorganic carbonate ions, and the availability of nucleation centers control the precipitation of calcium carbonate.
Their capability to create an alkaline environment through various physical activities plays a primary role. In fact, both autotrophic and heterotrophic paths can participate [
38]. In general, three mechanisms are associated with the bio-precipitation of carbonate ions. The first is oxidative deamination of amino acids (ODAA). The second mechanism is organic acid utilization (OAU). The latter mechanism is related to the nitrogen cycle and has to do with the hydrolysis of urea (HU: hydrolysis of urea) [
38,
39,
40].
Bacteria can influence calcium carbonate precipitation by acting as nucleation centers or enriching them with Ca2+. Due to negatively charged groups on the surface of cells (as mentioned above), positively charged ions can bind to the surface of bacteria at a neutral pH.
Such metal ions (e.g., Ca
2+) can then react with anions (e.g., CO
32−) and form insoluble salts. When there is a sufficient excess of the required cations and anions, the mineral formation begins on the cell’s surface, as the organism acts as a nucleation center. Anions can be products of the bacterium’s metabolism or exist in the external environment [
40].
3.2. CaCO3 Formation via Breakdown of Urea
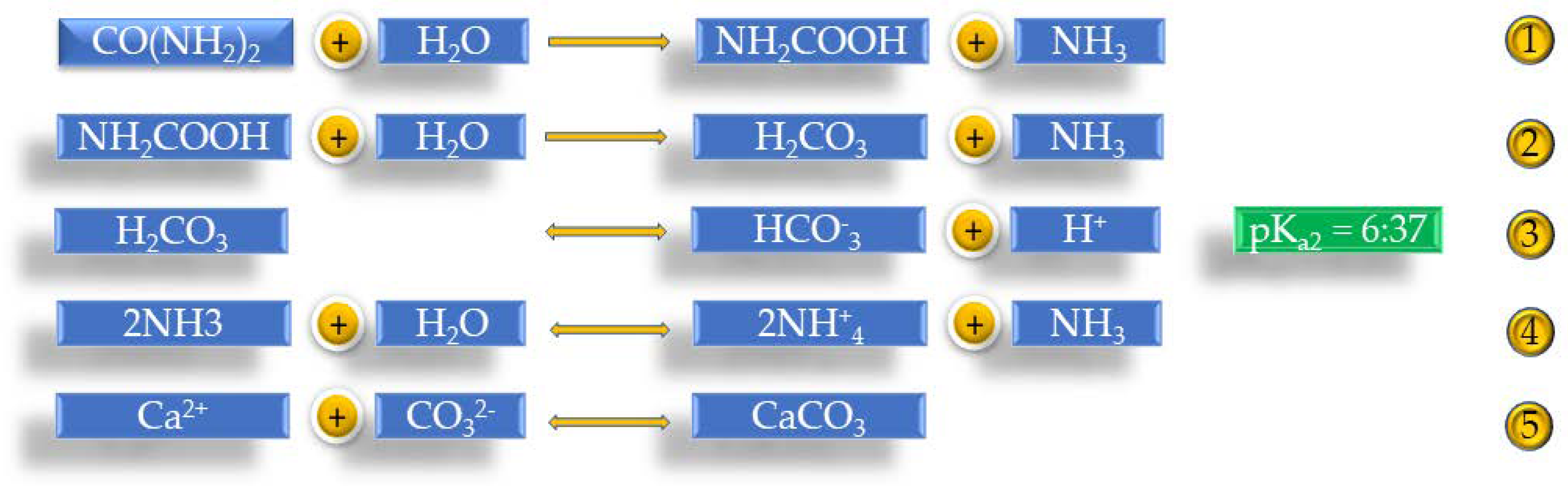
When urea is broken down locally, the pH increases and leads to the microbial deposition of carbon dioxide as calcium carbonate in a calcium-rich environment. Reaction 5 of
Figure 3 is the basis of the chemical process. The main factor for the precipitation of calcium carbonate is the degree of supersaturation, which is given by the proportion of ionic agents:
One mole of urea is hydrolyzed inside the cell, 1 mol of NH
3, and 1 mol of carbamide, which is then spontaneously hydrolyzed and given an additional mol of NH
3 and carbonic acid. These products dissociate in water, forming a mol bicarbonate ion and two ammonium and hydroxyl ions. This further increases the goal, which helps form carbonate ions. The pH increase begins in the small local environment of the bacterial wall, which then spreads to the leading solution of the bacterial suspension. This increases the concentration of carbonate ions and causes the S. In this environment, precipitation of calcium carbonate is observed around the cell where calcium is soluble [
41].
Figure 3 presents all the above reactions.
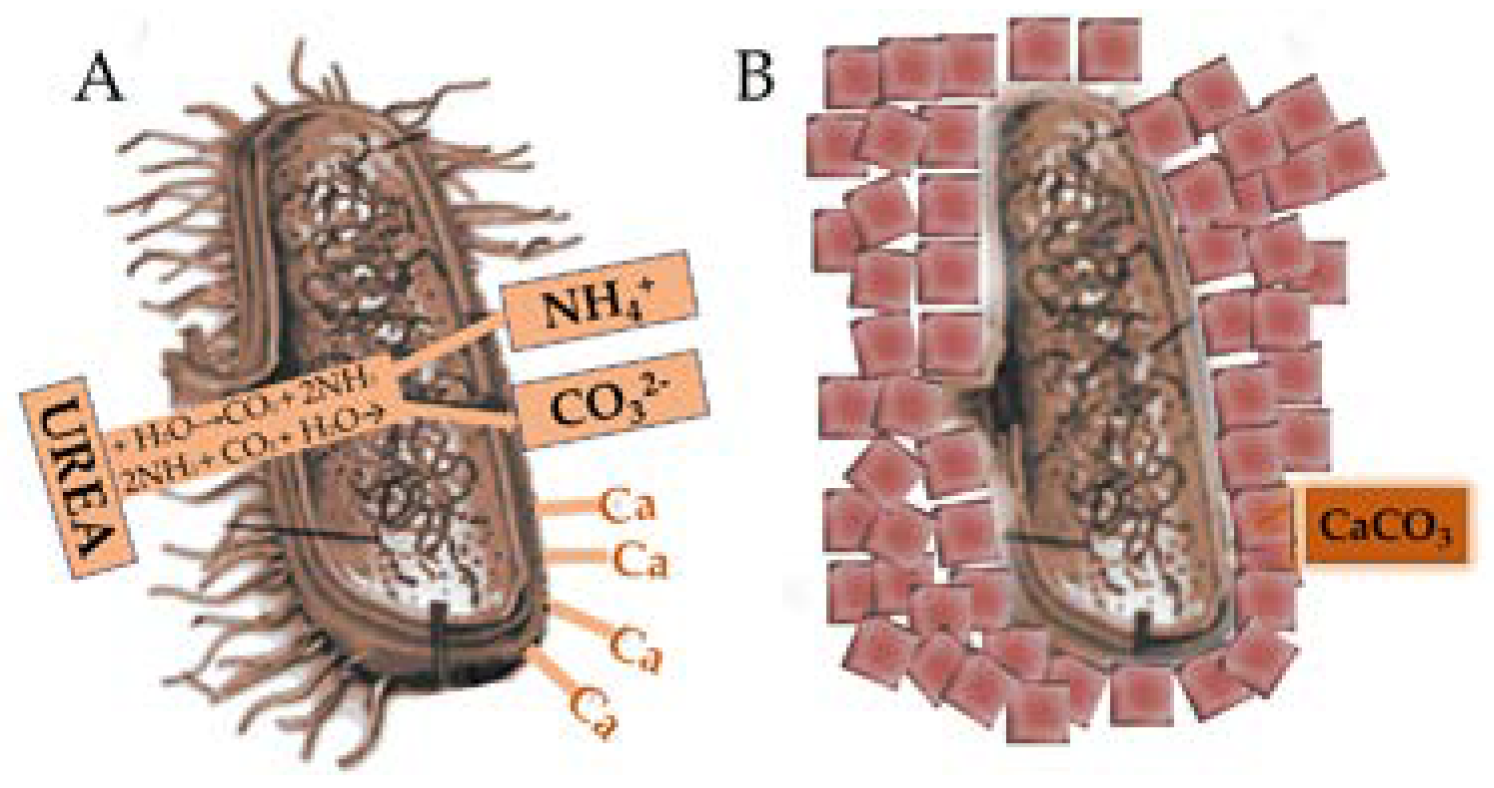
Figure 4 shows the addition of urea to a bacterial solution released into the small environment of the bacterium inorganic carbonate ions (CO
32−) and ammonia (NH
4+) (A). The bacterial wall is negatively charged and drives the adhesion of calcium onto its walls. Calcium can occur as local supersaturation, leading to the precipitation of calcium carbonate in the walls of the bacterium (A). After a while, the cell becomes trapped, limiting the transport of nutrients and, in the end, leads to death (B).
At the beginning of nucleation, calcium carbonate can precipitate into the pure form of the mineral, in amorphous stubbornness, or a combination of all 2 [
41]. Calcium carbonate exists in six different crystal structures. The amorph and two hydrates are rare due to their chemical instability. The three remaining forms are crystalline and occur in nature and living organisms. They have different thermodynamic stability and solubility. Calcite constitutes 7% of the earth’s crust in the form of marble or limestone rocks and is found at the fundament of lakes and seas due to the solubilization of lime rocks. Bacteria grow in a suitable nutrient medium that leads to calcium carbonate precipitation. For example, when the bacterium is in an environment containing urea and calcium, precipitation of calcium carbonate is observed. Depending on the source of calcium carbonate, various crystals appear at the end, sometimes rhombus and sometimes spheroidal.
4. Effect of Bacteria on Increasing the Mechanical Properties of Cement
The compressive strength of cement is satisfactory, but its tensile strength is different [
42]. As we have expressed in the previous discussion, a method of healing cracks via bacteria offers a change in concrete structures. In a recent study, two bacteria, one Bacillus subtilis, and one Bacillus halodurans were introduced at a concentration of 10
5~10
7 cells/mL in water, and the modification of concrete cracks was studied. After a few days, the concrete was subjected to compression and bending, and its mechanical properties at days 14 and 28 were measured, hardening by 7% and presenting an 18% higher compressive strength than compatible concrete, respectively. These changes indicate that rapping the cracks in the concrete, making it impermeable, has a beneficial effect on increasing mechanical properties [
42].
Some bacteria find the environment of concrete inhospitable due to its dryness and alkalinity relative to the natural environment in which they grow. However, some bacteria appear active even in highly alkaline environments due to spore formation. These are specialized cells that can withstand high mechanical stresses and chemically-intense conditions. The spores exhibit low metabolic activity and long lifespans, where it has been found in some species to produce spores that last up to 200 years. With the precipitation of dense layers of calcium carbonate atoms by bacteria, the healing of cracks is observed, which reduces the permeability of harmful molecules in them. Water penetration activates the bacteria, where they multiply and precipitate lime and calcium carbonate, which eventually seals the cracks and protects from the passage of corrosive agents [
11]. In 1995, Gollapudi applied this innovative technique of treating cracks with biological processes for the first time [
43].
Bacillus sphaericus precipitates CaCO
3 through the breakdown of urea into NH
+4 and CO
32−. This reaction leads to a local pH increase and promotes the precipitation of CaCO
3. Thus, the cracks of the cement are filled with CaCO
3, and, as a result, the passages are healed, demonstrating self-healing [
23]. Other bacteria can precipitate calcium carbonate in soil, sand, and minerals; in one study, they used Bacillus congii to precipitate calcium carbonate [
43]. Bacillus pasteurii, other groups used to precipitate calcium carbonate [
23,
44]. Dick used the Bacillus lentus and Bacillus sphaericus [
43]. This species exhibits intense ureolytic activity, continuous formation of dense CaCO
3 crystals, and a negative ζ-potential.
Many studies have been performed on the bacterial precipitation of calcium carbonate with calcium chloride (CaCl
2 2H
2O) as a source of calcium [
45]. As chlorine ions can be harmful to reinforcement, the use of calcium nitrate (Ca(NO
3)
2 4H
2O) and calcium acetate (Ca(CH
3COO)
2 H
2O) as alternative sources of calcium were explored in the study in [
45].
A cement system impregnated with Bacillus pseudofirmus B-4104 evolved as a self-healing system. After healing the cracks from self-healing, the system achieved a 112% increase in mechanical tensile strength, an 82% reduction in gas permeability, and an 87.4% increase in water absorption. SEM showed the crystalline formation of therapeutic products produced by bacteria exhibiting cubic crystallinity, which are compatible with the cement matrix [
46].
Bacillus licheniformis AK01 was investigated for calcium precipitation in concrete. This bacterium was compared with others, such as Sporosarcina pasteurii DSM-33, Pseudomonas aeruginosa MA01, and Bacillus subtilis TRPC2, for its behavior. Calcite crystal formation was observed in cement mortar and performed better than other bacteria to improve uptake by 15% and water absorption by 25% compared to others. Bacillus licheniformis AK01 is more suitable than the others used in this study and can enhance the properties of concrete, offering an environmentally friendly alternative technology [
47].
One study has investigated the biochemistry of CaCO
3 precipitation caused by non-ureolytic bacteria of the genus Bacillus. Different sources of calcium are compared for their effectiveness in bacterial mediation of precipitation. Surface treatment using this biodeposition technique is evaluated by parameters that affect the durability of cementitious materials. The results of this study disclosed that the type of calcium source has a profound impact on the biochemical process and the crystalline form, size, and morphology of bacterial-mediated CaCO
3 mineralization. An organic source of calcium, particularly calcium glutamate, is beneficial for adequate CaCO
3 precipitation. Bacterial surface treatment in the samples results in more than 50% reduction in capillary water absorption and an increase of almost 50% in carbonation resistance. One attributed this to resource-blocking effects. This new biological surface treatment shows promising prospects for increasing the strength aspects of concrete structures [
48].
Calcite formation from Bacillus subtilis in concrete was used as a laboratory model for producing calcite precipitate. They swapped three different cell concentrations and were compared in a bacteria-free sample. The study showed an increased compressive strength of concrete for cell concentrations dragged significantly, with greater compressive strength achieved at concentrations of 105 cells/mL. The study proved that bacterial calcite improves the strength of concretes [
49,
50].
Halobacillus halophilus bacteria, calcium lactate, and expanded perlite aggregate autonomously improved cracks in marine conditions. The bacteria strengthened throughout the depth of the damage due to the availability of water and oxygen in the cracks [
51].
7. Microencapsulation
Another technology that has been developed is the entrapment of substances in nanocontainers that are isolated through them and protected from unwanted interactions with the cement. These substances are released when the capsule breaks when these substances are released inside the cement and diffuse through capillary effects. This method has proven to be particularly successful for the self-healing of cement. However, it should be noted that here, too, we must be satisfied with enough so as not to alter the mechanical properties of cement.
B. sphaericus spores were first encapsulated in hydrogel incorporated in concrete, which showed that they could precipitate a large amount of calcium carbonate from oxygen consumption on crack surfaces. This technology offers adequate protection with hydrogel and spores in concrete, resulting in self-healing [
54].
It is known that bacterial activity decreases for pH by more than 12. In these cases, it is essential to protect the bacteria by incorporating silica gel or polyurethane as carriers that protect them. One study showed that silica gels containing bacteria have higher activity than polyurethane-immobilized bacteria and higher calcium carbonate production than polyurethane. However, the bacteria incorporated into polyurethane show a 60% higher strength regain and lower water permeability than silica gels. This study demonstrated that polyurethane is more suitable to house bacteria for self-healing cement [
39].
Nanocontainers were loaded with bacteria and incorporated into cement for self-healing purposes. The bacteria used were Escherichia coli and Staphylococcus aureus; both bacteria lead to the precipitation of calcium carbonate. The organism, metabolized by release from the other container and meets water, begins to metabolize, and precipitate the mineral, finally healing the crack. The microbial precipitation of calcium carbonate was based on the breakdown of (C.O. (NH
2)
2) into CO
32− and NH
3. Due to the high pK value of the NH
3/NH
4+ system (pKa = 9.2), the breakdown reaction increases the pH preceding the release of carbon ions. In an environment rich in calcium ions, calcium carbonate precipitation occurs. One publication describes in detail the production of the container. The same study shows that the containers are not toxic to human cells and the bacteria used in the study [
55].
The genus Bacillus was selected for a self-healing study that was added directly to the paste mixture and remained viable for up to 4 months. Coagulation of the concrete reduces pore size diameter during cement settings during the life of the seeds. This study showed that these bacteria survive incorporation into concrete for an extended period and act as a self-healing agent [
20].
Porous expanded clay particles were used as a reservoir of bacterial spores mixed with calcium lactate. Experiments showed crack healing in about 100 days after water immersion. This is due to the metabolic activity of bacteria that causes oxygen consumption. The work concluded that these materials might house bacteria that can be incorporated into concrete [
56].
Calcium carbonate precipitation was used to stabilize sand columns, heal granite cracks, or as a surface application to limestone. Using a bacterium to precipitate calcium carbonate proved its effectiveness for these applications. However, when a bacterium is used to heal cracks in concrete, the strong deterrent is the highly alkaline pH of the cement, which inhibits the growth of the bacterium. For this reason, it is essential to stabilize the bacterium and protect it from the high pH of concrete. Due to their mechanical strength and biochemical inertness, polyurethanes (P.U.) have been widely used as carriers to stabilize enzymes and whole cells [
44]. Bang used cylindrical-shaped polyurethane foam (diameter 10 mm and length 50 mm) with embedded bacterial cells [
45]. Polyurethane foam strips were placed in simulated cracks and used as a method of crack repair. Silica gel was applied in another study to stabilize bacteria since this method is time-dependent and used only in cases of extensive damage (Bang and colleagues created cracks 3.18 mm wide) [
45]. With its embedded bacteria, the gel can be sprayed directly into the gap with a funnel. Silica gel was previously used as a filler to heal cracks before calcium carbonate precipitation began.
In one study, bacterial spores were injected into hydrogels and incorporated in cement specimens to investigate the efficacy of self-healing. Precipitation of calcium carbonate was observed from the spores encapsulated in the hydrogel. A key question answered in this work is the upper healing size of the crack, which appeared to be up to 0.5 mm. This study observed a decrease in water permeability by 68% [
57].
With the prospect of cementitious materials being applied in marine environments and low temperatures, a crack healing study with bacteria was carried out investigating water permeability and resistance development measurements through compression testing. The material showed excellent properties in healing cracks, reducing them to a width of 0.4 mm by 95% after 56 days of immersion in seawater and a temperature of 8 °C. Clogging occurred due to the precipitation of bacteria after the swelling of containers and the precipitation of minerals based on magnesium and calcium carbonate caused by bacteria. This research comes after applying this self-healing participating type of bacteria-based composite in marine environments and at low temperatures [
58].
According to the technology developed in a publication by White, the containers break to release entrapped substances that lead to the self-healing of materials after the reaction of the two-component system. In the case of cement self-healing via containers, they must withstand intense pressures without breaking when used in industrial applications [
59]. These are planned to be incorporated into the cement, where they will stir for some time, or the cement–nano–container mass will be injected into buildings or other infrastructures with enormous forces. There is a risk of their breakage. For this purpose, double-walled vessels walled polyurethane/urea-formaldehyde (PU/UF) were prepared where healing agents were incorporated. Compared to single-walled containers, 2-wall containers exhibit better mechanical properties but are well integrated into concrete. A thorough search was made of the composition parameters of this double container, and conditions were found under which we obtain the best mechanical and thermal resistance properties. It was also observed with 5% similar self-healing vessels in concrete beams [
60].
To survive the mixing of cement with microcapsules or their incorporation into cracks, microcapsules were produced with sodium silicate solution as a core using complex emulsion water in an oil emulsion system. The final diameter ranged from 300 to 700 μm. The thickness of the capsules ranged from 5 to 20 μm. The capsules remain stable up to 190 °C and retain their structure in concrete environments. Inside, the concrete will release sodium silicate solution to achieve healing [
61].
Organic microcapsules were attached with a urea-formaldehyde (UF) shell and epoxide therapeutic agent. The microcapsules were mixed with cement and sand, and their mechanical strength to bending and pressure was tested. It turned out that enough microcapsules play an essential role in the recovery of the strength of cement. The capsules significantly affect the permeability of chlorine ions as much as the cement’s ability to heal itself [
62].
To implement nanocapsules in cement, two types were developed using silicon nanoparticles coated with an amine group and silicon capsules filled with epoxy resin. This system’s innovative character comes from using silicon shell microcapsules to improve durability and compatibility with cement and using functional nano silicon to obtain a cementitious amine matrix. The characterization of the particles suggests that they are amorphous and have the correct morphology and size to be considered as additions to cement. Moreover, the amine group shows a pozzolanic activity superior to the silica fume used as a reference. The epoxy compound is, to a high degree, stable upon reaction with lime. The system has been shown to have a pozzolanic activity superior to the silicon used as a reference. It turned out that the capsules break simultaneously with the matrix and release epoxy resin into the cement, which leads to self-healing [
63].
Steel is used to enhance the strength of concrete. Under corrosion conditions, steel corrodes, and its strength decreases with time. CeO
2 ceramic nanocontainers were developed and enriched with 5-amino-1, 3, and 4- thiadiazole-2-thiol (5-ATDT) to reduce the corrosion of the steel we use in concrete. Both Cerium and 5-ATDT act as corrosion inhibitors. The steel bars were laid in NaCl of 0.5 M solution. The anti-corrosion properties of these coatings were observed with electrochemical impedance spectroscopy (EIS) as a function of the exposure time. The EIS study showed that steel coatings exhibit the phenomenon of self-healing and protect the metal from corrosion [
64,
65].
An exciting paper describes capsules that respond to chlorine ions and cause self-healing [
66]. These capsules react to the chemical environment, and when they encounter chlorine, they break and prevent the lead of chlorine in the concrete matrix. These capsules were introduced into cementitious materials and were shown to respond to chlorine in a concrete matrix, thus reducing the harmful performance of chlorine and reducing the susceptibility of steel to corrosion. These capsules are produced from silver alginate hydrogel that synthesizes and responds to low concentrations of chloride ions (0.1 wt.%). These capsules promise a lot for steel concrete applications, such as corrosion inhibition with the phenomenon of self-healing [
66].
Figure 5 summarizes the mechanism of calcium carbonate production by bacteria embedded in concrete and protected in containers.
8. Superabsorbent Polymers (SAP)
The superabsorbent polymers in cement can control the entrained water in its cracks. SAP controls water availability and hydration reactions over its lifetime [
67]. They are hydrogels with reversible behavior that do not dissolve in water; they can retain large amounts of water depending on their composition, size, and distribution. These factors determine the ability to absorb and desorb moisture in the cement matrix. SAP has been used to stimulate autogenous self-healing behavior, sealing cracks [
68]. They enhance prolonged hydration reactions that alter cement’s microstructure and improve its durability. SAP can absorb water, seal cracks, and stimulate further precipitation hydration calcium carbonate in the way described above, leading to self-healing and sealing of cracks [
69]. The production of SAPs has been described in detail in the recent literature [
70]. There are other open issues regarding mixing them with cement, such as their ability to absorb and release water repeatedly and avoid micro pores produced due to this repetitive action (sorption-desorption) [
71,
72]. Introducing superabsorbent polymer (SAP) particles in cement samples improve their tensile strength and shrinkage. One study showed that SAP samples exhibit more than 1,5% excellent ductility compared to those without SAP. The tensile capacity and deflection with SAP increased by 92% and 77%, respectively, effectively reducing the shrinkage or drying of samples. SAP particles can reduce compressive strength by 5–15% compared to control samples [
73,
74].
Superabsorbent polymers were used to reduce damage between steel reinforcement and concrete. They used SAP with different particle sizes and chemical compositions. Smaller particle sizes positively affect compressive strength more than larger ones. Experiments showed that samples containing SAP improve healing for large crack widths (𝑤 ≥ 0.30 mm) compared to non-SAP mixtures. Strength was recovered to 100% for self-consolidating concrete mixture with w = 0.10 mm after 28 days of healing [
75].
In one study, samples containing SAP matured for ten years and were stored in different conditions to determine whether there is a possibility of self-healing for aged samples. The results showed that even after so many years, cracks could heal due to calcium carbonate crystal formations, and recovery of mechanical properties was observed after repeated 4-point bending tests. It turned out that healing is still possible even after ten years of the life of these specimens. Of course, this study must be repeated for the conclusions to be judged credible [
76].
In another study [
74] using cementitious material containing microfibers to limit the width of cracks and super absorbent polymers (SAPs), autogenous healing was studied at sample ages of 7 days, 28 days, 3 months, one year, three years, and eight years, and the mechanical properties were studied using the 4-point bending technique. SAP addition offers the healing of cracks by further hydration, leading to calcium carbonate crystallization. Of particular importance are the samples containing SAP that remain stable for up to 8 years without any degradation. This work demonstrates the increased healing capacity of cementitious materials containing SAP compared to a sample without SAP [
74]. These results are in harmony with previous work that showed that rot benefits goals even in 10 years of life [
76].
Figure 6 visualizes the mechanism of self-healing via SAP employment.
Microfiber in cement was inserted to control the cracks’ width and promote self-healing effectiveness. To these samples were added super absorbent polymers (SAP) to seal cracks in their ability to heal them. Samples exposed to 60% humidity showed the ability to heal cracks. The amount of SAP gives optimal results without reducing their mechanical properties compared to the reference sample without SAP and possesses superior sealing ability [
77].
The amount of SAP we use in a mixture must be taken seriously because it determines the samples’ workability, microstructure, and strength. The particles reduce the flow and, due to internal hardening, cause thickening of the matrix, and because micro pores are created, they affect the strength of the materials. Considering this, we must determine the amount of SAP we use in the mixture [
72].
Adding SAPs to cement improves internal hardening and reduces the risk of cracking during shrinkage. So, they will help to increase the strength of concrete structures. Various factors, such as the quantity and size of SAP particles, determine their effectiveness in the characteristic parameters we specified above. After a change in these parameters, their effect on workability, compressive strength, and hydration kinetics was assessed. This study demonstrated which SP can be chosen for a specific application [
78].
The ergonomics of the mortar were not adversely affected by the amount of water absorbed by the SAP. They showed a minimal adverse effect on the compressive strength of mortar and less impact on cement hydration. When 10–100 μm were used in the mixtures, no differences between particle sizes were observed in the mortar [
78].
The way cement mortar is made is modified by incorporating superabsorbent polymer (SAP) with two types: one dry powdered SAP and a swelled SAP were studied in terms of mortar shrinkage rate, compressive strength, and the effect on cooling–thawing resistance in cement mortar. After 28 days, it was shown that incorporating the dry SAP increased compressive strength by about 10%, reaching 50%. Repeating the same experiment with swelled SAP increases the compressive strength by about −26 virus 6%. However, when incorporated into 0.1% cement, the mortar shrinkage rate is reduced from 14.5 (wetted SAP) to 32.2% (dried SAP). It is noted that both have a positive effect on the cooling–thawing resistance of cement mortar [
79].
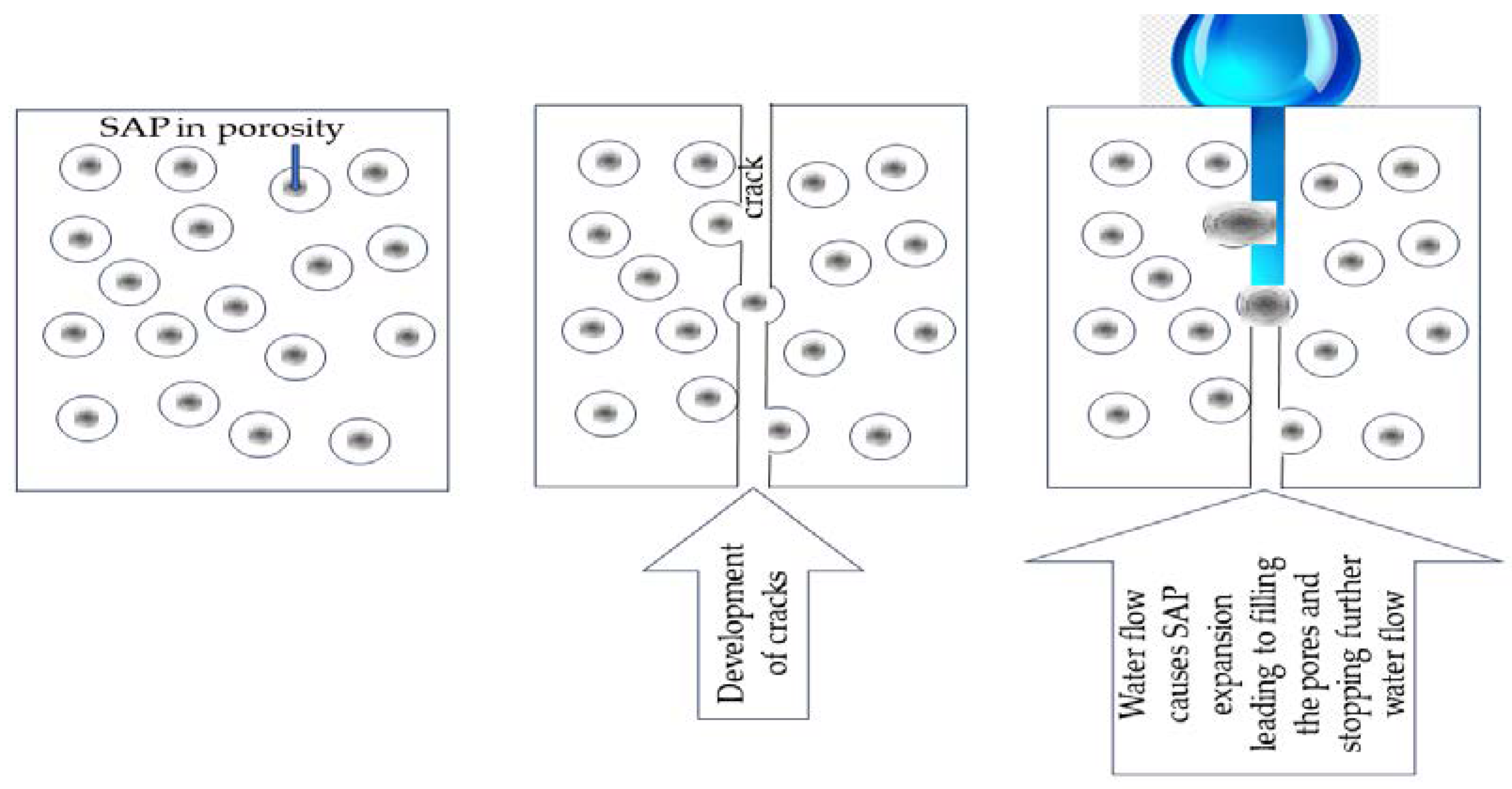
SAP was integrated into a cement sample to find the water flow mechanism through technical cracks 340 mm wide. The water flow causes swelling of the SAPs that encounter the water and stop the water flow through the cracks in the sample.
Figure 6 shows SAP sealing the crack and finally stopping water flow inside the sample. This is the mechanism used to explain the experiments presented in the literature. The experiment used about 1% vol. SAP, which was sufficient to block the water inside the samples [
80].
The use of X-rays (XRD) in cement slurry enriched with SAP showed that the amount of Ca(OH)
2 increased and the concentration of alite (C3S) decreased. SAP promotes the hydration of cement quality and accelerates the formation of C-S-H. Adding SAP to cement speeds up the hydration process and hydration product [
81]. It becomes evident from this work that products in a cementitious system affect the quantity of hydrates and the type of products produced. SAP favors the establishment of calcium hydroxide. Different shop sizes do not affect the amount of hydrates [
81].
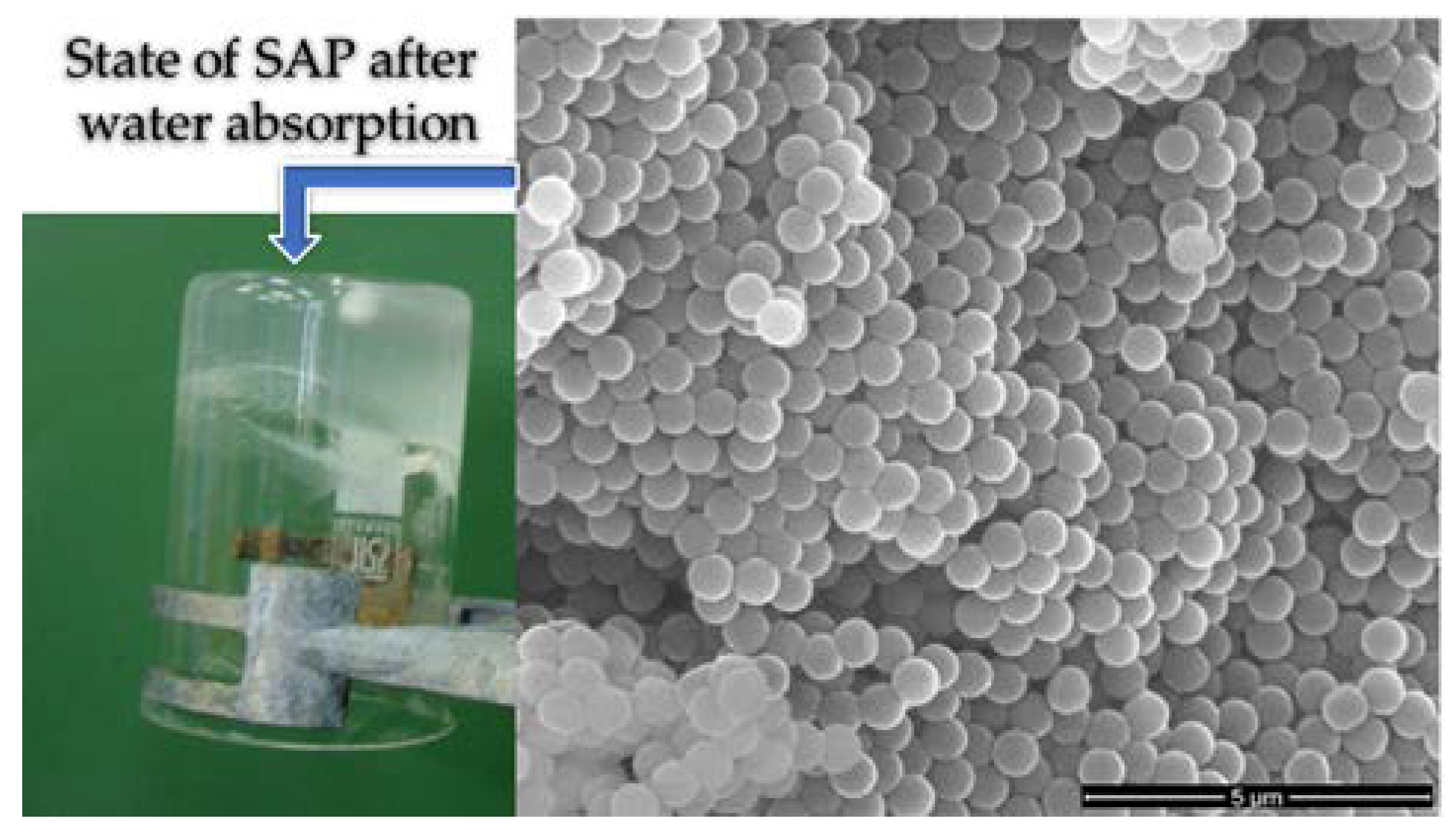
The production of SAP involves mixing Poly(methacrylic acid (PMAA) (e.g., 2 g) in deionized water (e.g., 100 mL) under ultrasonication, where sodium hydroxide can be added after homogenization. The milky solution turns transparent after stirring for 30 min at room temperature. This should be centrifuged at 10,000 rpm for 4 min, giving the product a swollen form like a hydrogel. The ethanol should then be resuspended and centrifuged at 6000 rpm for 4 min. The sediment must then be dried twice at 90 °C. This process produces spheres with a diameter of 700 μm (
Figure 7). These spheres can absorb 70 times their weight in water and return to their previous shape when dry (
Figure 7). The drying–wetting process can be repeated continuously [
70]. Similarly, Ethylene glycol dimethyl acrylate (EGDMA) can form PMAA@EDGMA spheres [
2,
82,
83,
84]. The spheres PMAA@EDGMA were prepared through the radical polymerization of MAA with EGDMA in calcium ammonium nitrate (CAN). Then, it was centrifuged at 7000 rpm for 10 min and rinsed in CAN. Details of this method can be found in the study [
84]. Coatings were made with known procedures.
The SAP spheres can be used as templates to coat them with inorganic compounds such as SiO
2, SiO
2CaO, etc. The sol–gel chemistry of these ceramic coatings has been described in numerous recent publications [
85,
86,
87,
88,
89,
90]. This way, the SAPs can be significantly constrained without altering their volume between dry–wet cycles.
After this in-depth discussion, there are significant challenges regarding composite materials in cement. The first concerns the process followed for incorporating the SAP in the cement mixture and the reduction in the workability of the mix. The second point concerns the preservation of their ability to absorb and release water often during the formation of cracks so that the healing and sealing of cracks occur continuously. The third point concerns the mixing process, which leads to the degradation of their mechanical properties due to the formation of micropores due to the absorbent/sorbent actions. Fourthly, the lack of adhesion between the phases of the matrix interface can lead to additional defects that can induce internal stresses.
9. Hybrid Organic-Inorganic Core-Shell SAPs
The previous discussion mentioned that SAPs are used in cement to stimulate self-healing or seal concrete cracks. By absorbing water, pores close due to swelling and promote the precipitation of calcium carbonate through hydration reactions, as mentioned in earlier chapters. However, this process also has negative consequences by reducing the workability of the mixture, such as the repeated absorption and release of water, which degrades the mechanical properties of concrete, and causes many other defects that occur in concrete.
An attempt was made to solve this problem based on previous work by trapping P(MAA-co-EGDMA) inside SiO
2 and CaO-SiO
2 shells and incorporating them into a cement mixture [
70,
84,
85,
86,
91,
92]. The effects were studied by various methods, which proved cement’s self-healing. The surface compositions of the shells were made to be compatible with cement; the flexural strength and compressive strength of these materials were measured at various concentrations.
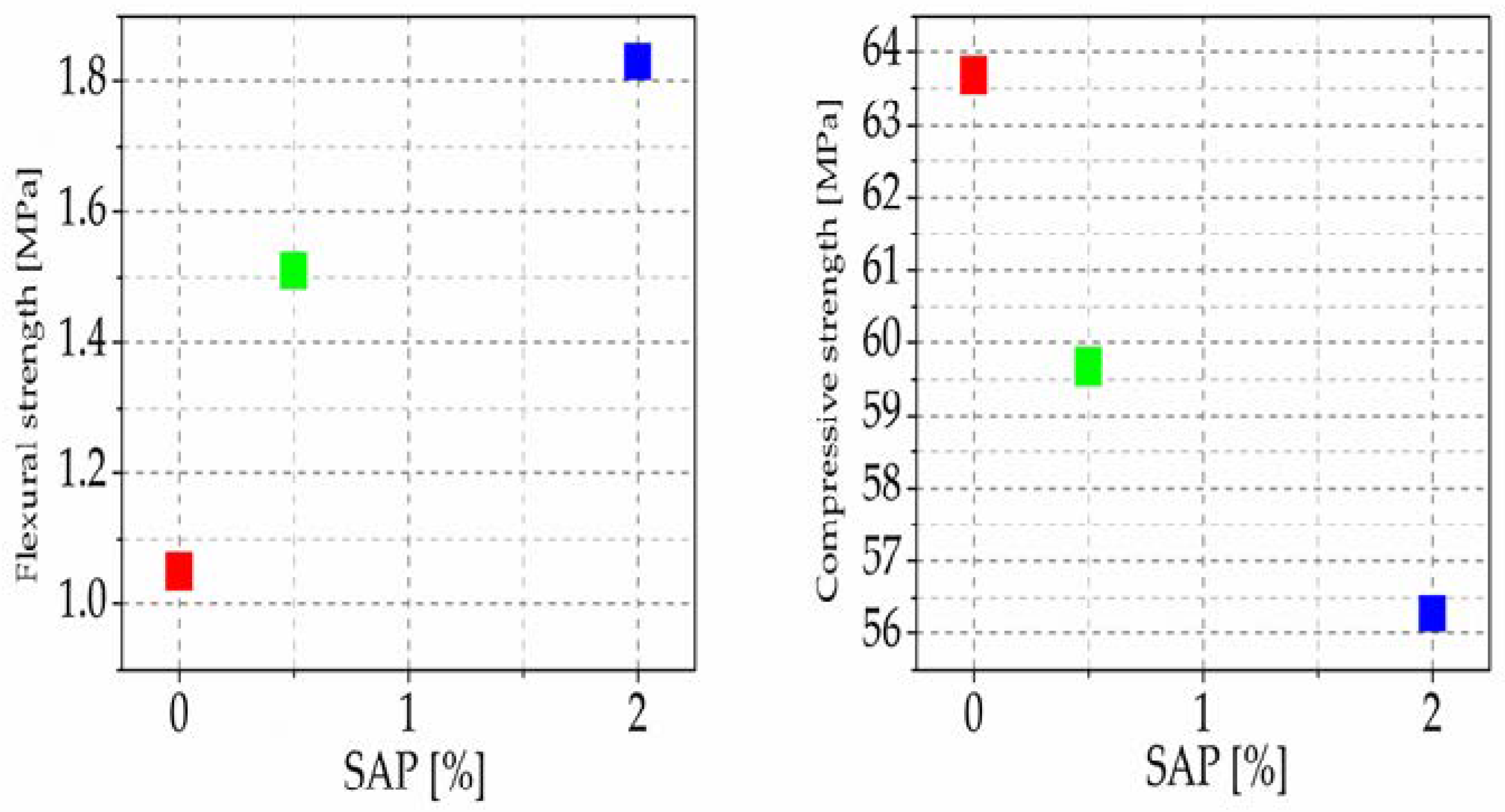
Figure 8 shows the results. The measurements proved that composite material with 2% self-healing cement after 28 days responds to problems caused by SAP stemming from repeated absorption and water release, which ultimately degrades concrete properties and causes further problems. When SAP is encapsulated in ceramic shells whose strength withstands the force pre-created during cement mixing and injecting the cement slurry into structures, it satisfactorily complies with these problems [
82,
84,
93].
Cracks in concrete can develop easily and affect the durability and safety of structures. Cement heals itself because the minerals are hydrated and close the cracks, and calcium carbonate is shattered so that water cannot flow through the cracks. This self-healing ability of cement can be further improved by adding SAP to concrete. When water enters, the SAP expands and stops the entry of harmful substances and water. SEM and TGA measurements found that the self-healing products are calcium carbonate, which can fill the cracks and heal the cement errors. The formation of self-healing products improves self-healing performance with the use of SAPs.
Figure 9 presents the phenomena that can occur in cement-containing SAP.
11. Conclusions and Recommendations
Introducing bacteria into concrete is a beneficial process that improves its properties. By producing calcium carbonate to block cracks, cracks are repaired, thus improving the properties of conventional concrete by increasing its strength and many other properties. Unlike plain concrete, this concrete benefits from mechanical properties enhanced by bacterial incorporation. To protect the bacteria from the environmental pH, they are incorporated into a container that does not destroy them. Reference is made to the SAPs, which yield self-healing, but drawbacks can be solved by integrating them into a container compatible with the concrete matrix. Many possibilities in the field need to be explored where other self-healing technologies have developed to an advanced stage, such as self-diagnosing the condition of the sample with specialized detectors [
2]. There are many prospects for improving the properties, ascertaining the potential of this technique, and applying these technologies in areas that still need to be explored. The sector requires closer cooperation with industry, which needs to understand this technology’s potential for rapid application. The new material’s necessary specifications must also be clearly defined, and the lifetime of these methods must be safely determined [
2]. Considering other properties that have evolved in similar technologies, one should consider developing and applying new artificially intelligent nanocontainers to manage bacteria and self-heal automatically for an extended period. This paper did not comment on autogenous concrete healing because it is unreliable and limited to cracks up to one hundred micrometers [
69]. Some self-healing additives, such as minerals and hydrogels, improve the intrinsic self-healing properties of cement, but this can only be achieved if the width of the cracks is limited to a few hundred microns. Another serious question is the shelf life of self-healing under field conditions, which needs to be confirmed in real applications. Additionally, transfers of knowledge produced in the laboratory must remain intact in actual conditions throughout construction. The whole community should also emphasize the development of sensors that will evaluate, throughout the life of a structure, its state of cement and self-healing. The community involved in the subject needs to determine the requirements or expectations of this technology so that incoming researchers can be directed where to look. Specifications are urgently needed, e.g., do we need ten years or more for self-healing technology to last? Furthermore, we need more companies to test abandoned technologies in literature.