First Molecular Detection of the Poultry Pathogen Protozoan Histomonas meleagridis from Red Mite (Dermanyssus gallinae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
| Species | Origin | Sampling Date (DD.MM.YYYY) | Sample Type | No. of Samples | Confirmed HIS PCR Positive | Histomonas 18S rDNA NCBI Acc. No. | Dermanyssus COX1 NCBI Acc. No. |
|---|---|---|---|---|---|---|---|
| Dermanyssus gallinae | poultry farm HU/A | 05.07.2023 | mite trap | 13 | 8 | PX210515–PX210523 | PX210480–PX210485 |
| Dermanyssus gallinae | backyard flock HU/B | 17.08.2023 | mite trap | 4 | 2 | PX210524 | PX210486 |
| Gallus gallus domesticus | poultry farm HU/A | 12.04.2024 | liver, cecum | 10 | 0 * | n.d. | n.d. |
2.2. DNA Extraction
2.3. Dermanyssus-Specific PCR
2.4. Histomonas-Specific PCR and DNA Sequence Analysis
2.5. Phylogenetic Analysis
3. Results
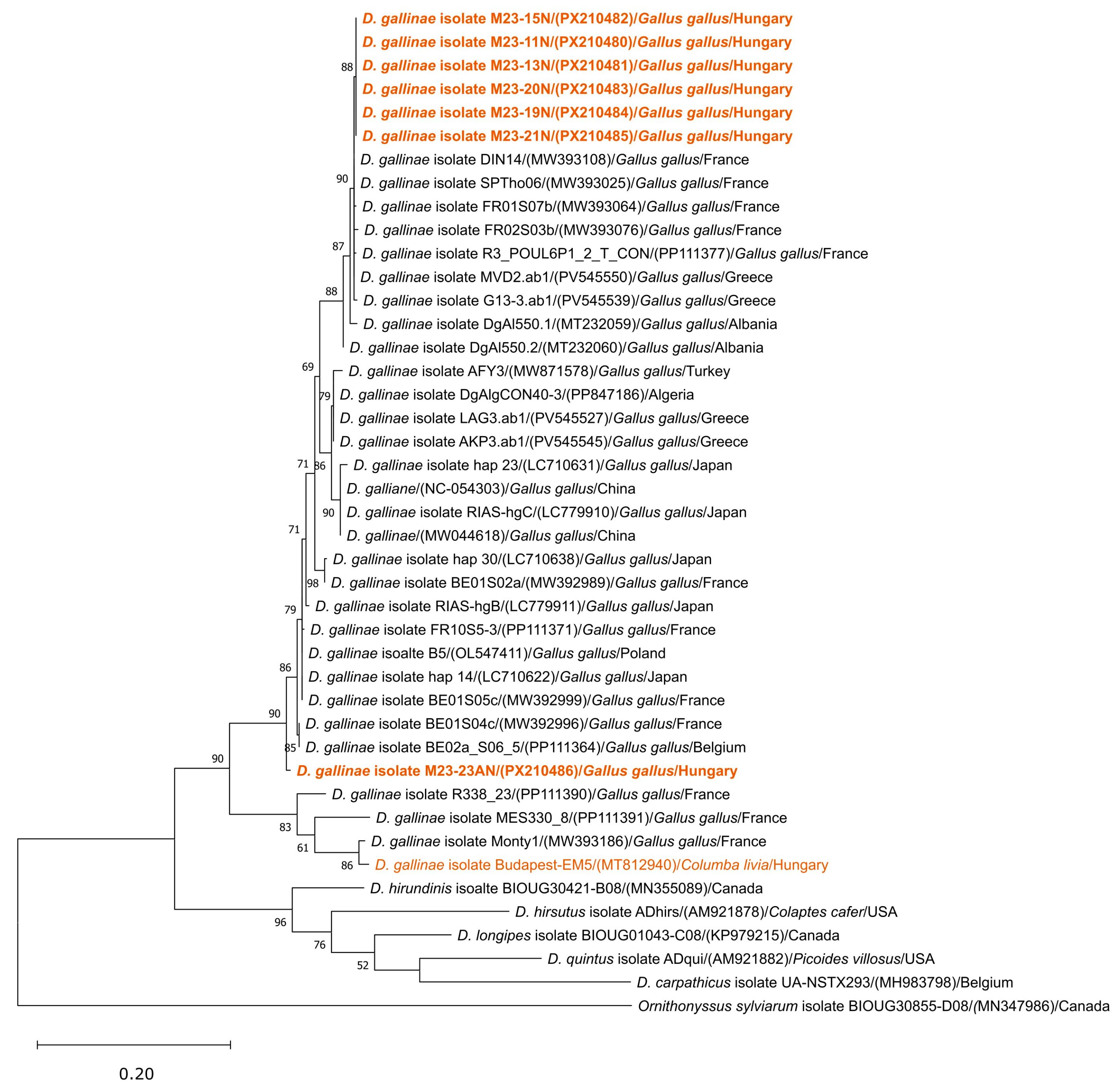
3.1. Species Identification of Mites
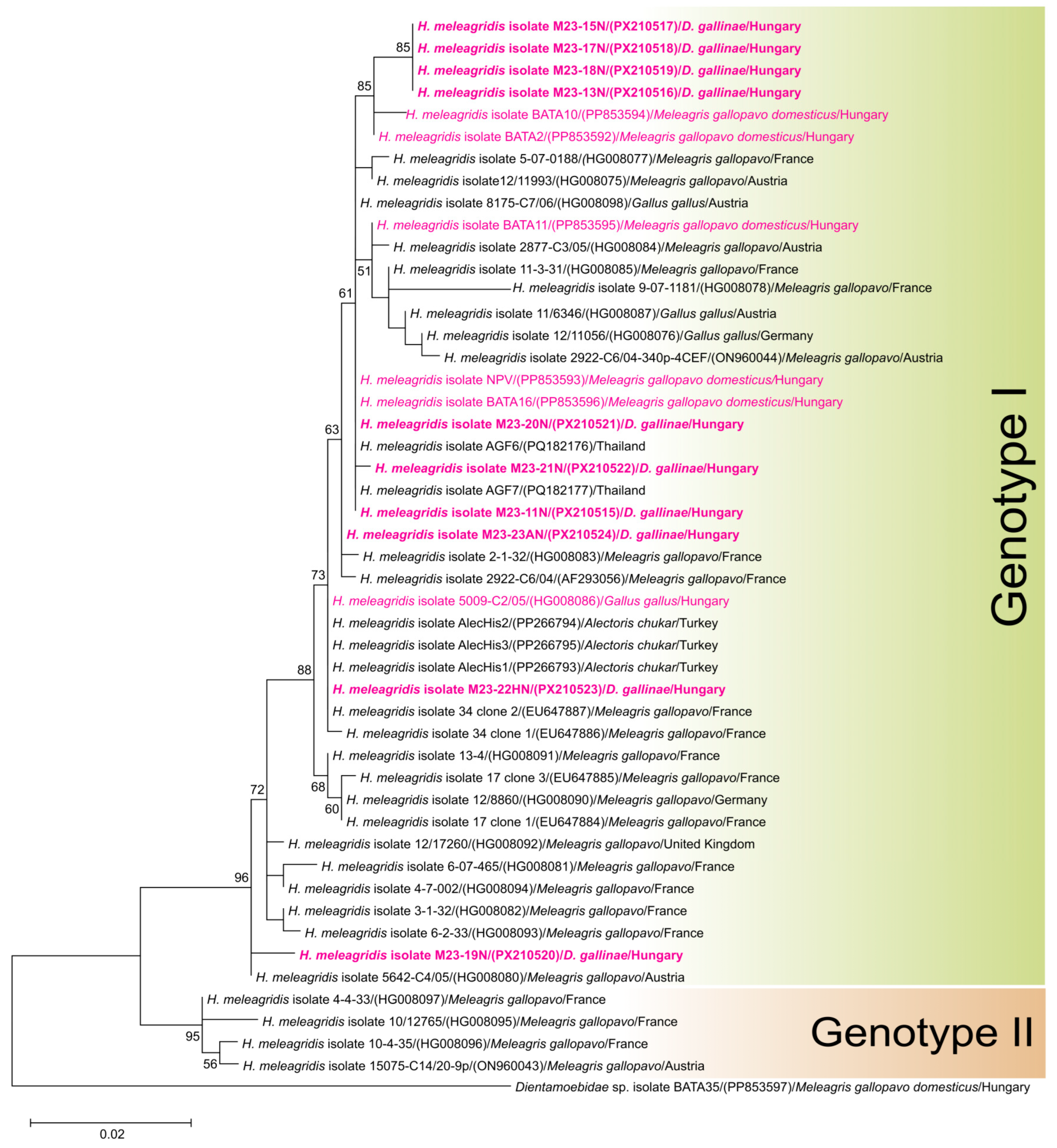
3.2. Detection of Histomonas
3.3. Histomonas meleagridis Phylogeny and Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COX1 | Cytochrome c oxidase gene subunit I |
| 18S rDNA | Small subunit ribosomal RNA gene |
| RAxML | Randomized Axelerated Maximum Likelihood |
| TBE | Transfer Bootstrap Expectation |
References
- Truong, A.T.; Yoo, M.S.; Woo, S.D.; Lee, H.; Park, Y.; Nguyen, T.T.; Youn, S.Y.; Min, S.; Lim, J.; Yoon, S.S.; et al. Evaluation of acaricidal activity in entomopathogenic fungi for poultry red mite (Dermanyssus gallinae) control. Vet. Parasitol. 2024, 331, 110292. [Google Scholar] [CrossRef]
- Schiavone, A.; Pugliese, N.; Otranto, D.; Samarelli, R.; Circella, E.; De Virgilio, C.; Camarda, A. Dermanyssus gallinae: The long journey of the poultry red mite to become a vector. Parasites Vectors 2022, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.M.; Salem, M.A.; Mohamed, H.I.; Orabi, A.; El-Bahy, M.M.; Taha, N.M. Dermanyssus gallinae as a pathogen vector: Phylogenetic analysis and associated health risks in pigeons. Vet. Parasitol. Reg. Stud. Rep. 2025, 57, 101198. [Google Scholar] [CrossRef]
- Sigognault Flochlay, A.; Thomas, E.; Sparagano, O. Poultry red mite (Dermanyssus gallinae) infestation: A broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Paras. Vectors 2017, 10, 357. [Google Scholar] [CrossRef]
- Sommer, D.; Heffels-Redmann, U.; Köhler, K.; Lierz, M.; Kaleta, E.F. Role of the poultry red mite (Dermanyssus gallinae) in the transmission of avian influenza A virus. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2016, 44, 26–33. [Google Scholar] [CrossRef]
- Ciloglu, A.; Yildirim, A.; Onder, Z.; Yetismis, G.; Duzlu, O.; Simsek, E.; Inci, A. Molecular characterization of poultry red mite, Dermanyssus gallinae lineages in Turkey and first report of Plasmodium species in the mite populations. Int. J. Acarol. 2020, 46, 241–246. [Google Scholar] [CrossRef]
- Clarke, L.L.; Beckstead, R.B.; Hayes, J.R.; Rissi, D.R. Pathologic and molecular characterization of histomoniasis in peafowl (Pavo cristatus). J. Vet. Diagn. Investig. 2017, 29, 237–241. [Google Scholar] [CrossRef]
- Friend, M.; Franson, J.C.; Ciganovich, E.A. Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds; Ciganovich, E.A., Ed.; U.S. Dept. of the Interior, U.S. Geological Survey: Washington, DC, USA, 1999; ISBN 0607880961.
- Lund, E.E.; Chute, A.M. Heterakis and Histomonas infections in young peafowl, compared to such infections in pheasants, chickens, and turkeys. J. Wildl. Dis. 1972, 8, 352–358. [Google Scholar] [CrossRef]
- Grabensteiner, E.; Hess, M. PCR for the identification and differentiation of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis spp. Vet. Parasitol. 2006, 142, 223–230. [Google Scholar] [CrossRef]
- Hatfaludi, T.; Rezaee, M.S.; Liebhart, D.; Bilic, I.; Hess, M. Experimental reproduction of histomonosis caused by Histomonas meleagridis genotype 2 in turkeys can be prevented by oral vaccination of day-old birds with a monoxenic genotype 1 vaccine candidate. Vaccine 2022, 40, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.C.; Petrone-Garcia, V.M.; Graham, B.D.; Hargis, B.M.; Tellez-Isaias, G.; Vuong, C.N. Histomonosis in Poultry: A Comprehensive Review. Front. Vet. Sci. 2022, 9, 880738. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.L. The structure and development of the protozoon Histomonas meleagridis in the male reproductive tract of its intermediate host, Heterakis gallinarum (nematoda). Parasitology 1971, 63, 439–445. [Google Scholar] [CrossRef]
- Lotfi, A.R.; Abdelwhab, E.M.; Hafez, H.M. Persistence of Histomonas meleagridis in or on materials used in poultry houses. Avian Dis. 2012, 56, 224–226. [Google Scholar] [CrossRef]
- Lund, E.E.; Wehr, E.E.; Ellis, D.J. Earthworm Transmission of Heterakis and Histomonas to turkeys and chickens. J. Parasitol. 1966, 52, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.F.; Dormitorio, T.; Oladipupo, S.O.; Bethonico Terra, M.T.; Lawrence, K.; Macklin, K.S.; Hauck, R. Heterakis gallinarum and Histomonas meleagridis DNA persists in chicken houses years after depopulation. Vet. Parasitol. 2021, 298, 109536. [Google Scholar] [CrossRef] [PubMed]
- Terra, M.T.; Macklin, K.S.; Burleson, M.; Jeon, A.; Beckmann, J.F.; Hauck, R. Mapping the poultry insectome in and around broiler breeder pullet farms identifies new potential Dipteran vectors of Histomonas meleagridis. Parasites Vectors 2023, 16, 244. [Google Scholar] [CrossRef]
- Frank, J.F. A Note on the Experimental Transmission of Enterohepatitis of Turkeys by Arthropoids. Can. J. Comp. Med. Vet. Sci. 1953, 17, 230. [Google Scholar]
- Hauck, R.; Balczulat, S.; Hafez, H.M. Detection of DNA of Histomonas meleagridis and Tetratrichomonas gallinarum in German poultry flocks between 2004 and 2008. Avian Dis. 2010, 54, 1021–1025. [Google Scholar] [CrossRef]
- Huber, K.; Gouilloud, L.; Zenner, L. A preliminary study of natural and experimental infection of the lesser mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae) with Histomonas meleagridis (Protozoa: Sarcomastigophora). Avian Pathol. 2007, 36, 279–282. [Google Scholar] [CrossRef]
- Cupo, K.L.; Beckstead, R.B. PCR detection of Heterakis gallinarum in environmental samples. Vet. Parasitol. 2019, 271, 1–6. [Google Scholar] [CrossRef]
- Son, H.-Y.; Kim, N.-S.; Ryu, S.-Y.; Shin, H.-J.; Park, M.-K.; Kim, H.-C.; Cho, J.-G.; Park, B.-K. An Outbreak of Chicken Histomoniasis in the Absence of Normal Vectors. J. Vet. Clin. 2009, 26, 591–594. [Google Scholar]
- Cortes, P.L.; Chin, R.P.; Bland, M.C.; Crespo, R.; Shivaprasad, H.L. Histomoniasis in the bursa of Fabricius of chickens. Avian Dis. 2004, 48, 711–715. [Google Scholar] [CrossRef]
- Lammers, G.A.; Bronneberg, R.G.G.; Vernooij, J.C.M.; Stegeman, J.A. Experimental validation of the AVIVET trap, a tool to quantitatively monitor the dynamics of Dermanyssus gallinae populations in laying hens. Poult. Sci. 2017, 96, 1563–1572. [Google Scholar] [CrossRef]
- Bilic, I.; Jaskulska, B.; Souillard, R.; Liebhart, D.; Hess, M. Multi-locus typing of Histomonas meleagridis isolates demonstrates the existence of two different genotypes. PLoS ONE 2014, 9, e92438. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Sugimoto, T.N.; Watanabe, K.; Egami, H.; Kageyama, D. Genetic variations and microbiome of the poultry red mite Dermanyssus gallinae. Front. Microbiol. 2022, 13, 1031535. [Google Scholar] [CrossRef]
- Bhowmick, B.; Zhao, J.; Øines, Ø.; Bi, T.; Liao, C.; Zhang, L.; Han, Q. Molecular characterization and genetic diversity of Ornithonyssus sylviarum in chickens (Gallus gallus) from Hainan Island, China. Parasites Vectors 2019, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; Hauck, R.; Lüschow, D.; McDougald, L. Comparison of the specificity and sensitivity of PCR, nested PCR, and real-time PCR for the diagnosis of histomoniasis. Avian Dis. 2005, 49, 366–370. [Google Scholar] [CrossRef]
- Huber, K.; Chauve, C.; Zenner, L. Detection of Histomonas meleagridis in turkeys cecal droppings by PCR amplification of the small subunit ribosomal DNA sequence. Vet. Parasitol. 2005, 131, 311–316. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.; Domelevo Entfellner, J.B.; Wilkinson, E.; Correia, D.; Dávila Felipe, M.; De Oliveira, T.; Gascuel, O. Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 2018, 556, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Liebhart, D.; Ganas, P.; Sulejmanovic, T.; Hess, M. Histomonosis in poultry: Previous and current strategies for prevention and therapy. Avian Pathol. 2017, 46, 1–18. [Google Scholar] [CrossRef]
- Szekeres, S.; Takács, N.; Ózsvári, L.; Tuska-Szalay, B.; Bárdos, K.; Kerek, Á.; Dobra, P.F.; Kovács, L.; Keve, G.; Molnár-Nagy, V.; et al. Molecular investigation of hindgut flagellates from turkeys and pheasants in Hungary confirms the endemicity of a new species closely related to Histomonas meleagridis. Acta Vet. Hung. 2025, 1, 199–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kóczán, P.; Kovács, L.; Örkényi, A.; Kovács, D.; Kőrösi, L.; Eszterbauer, E. First Molecular Detection of the Poultry Pathogen Protozoan Histomonas meleagridis from Red Mite (Dermanyssus gallinae). Parasitologia 2025, 5, 59. https://doi.org/10.3390/parasitologia5040059
Kóczán P, Kovács L, Örkényi A, Kovács D, Kőrösi L, Eszterbauer E. First Molecular Detection of the Poultry Pathogen Protozoan Histomonas meleagridis from Red Mite (Dermanyssus gallinae). Parasitologia. 2025; 5(4):59. https://doi.org/10.3390/parasitologia5040059
Chicago/Turabian StyleKóczán, Patrícia, László Kovács, Adrienn Örkényi, Dorottya Kovács, László Kőrösi, and Edit Eszterbauer. 2025. "First Molecular Detection of the Poultry Pathogen Protozoan Histomonas meleagridis from Red Mite (Dermanyssus gallinae)" Parasitologia 5, no. 4: 59. https://doi.org/10.3390/parasitologia5040059
APA StyleKóczán, P., Kovács, L., Örkényi, A., Kovács, D., Kőrösi, L., & Eszterbauer, E. (2025). First Molecular Detection of the Poultry Pathogen Protozoan Histomonas meleagridis from Red Mite (Dermanyssus gallinae). Parasitologia, 5(4), 59. https://doi.org/10.3390/parasitologia5040059








