Abstract
The increasing global incidence of infections caused by free-living amoebae (FLA) and the lack of effective, safe, and approved treatments highlight the urgent need for novel amoebicidal compounds with pharmacological potential. Despite a growing body of literature on the anti-FLA properties of various compounds, comprehensive reviews summarizing this progress remain scarce. This study aimed to identify the most promising compounds tested in vitro and/or in vivo for anti-FLA activity. A systematic review was conducted, analyzing 108 studies published between 1986 and 2024, selected from an initial pool of 23,653 database results. A total of 537 compounds were evaluated for their in vitro anti-FLA activity. Compounds exhibiting ≥50% reduction in amoeba viability relative to untreated controls were classified as promising if they showed low toxicity in mammalian cell models, particularly when active at concentrations ≤ 10 µM, consistent with predicted favorable pharmacokinetic and pharmacodynamic profiles. The most promising compounds for drug and disinfectant development include ten trophocidal agents against B. mandrillaris, thirty-two trophocidal and four cysticidal agents against N. fowleri, and sixty-two trophocidal and nineteen cysticidal agents against Acanthamoeba spp. Compounds active at low concentrations (≤10 µM or <0.014 mg/mL) prioritized for in vivo drug development studies include: against Balamuthia mandrillaris, trophocidal 515, 531, 533; against Naegleria fowleri, trophocidal 421, 416, 518, 46, 254, 522, 111–120 and cysticidal 16; and against Acanthamoeba spp., trophocidal 498, 499, 500, 535, 107, 347, 348, and 340. Future studies should evaluate their efficacy, safety, pharmacokinetics, and pharmacodynamics toward developing effective drugs, antiseptics, and disinfectants.
1. Introduction
Free-living amoebae (FLA), particularly Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris, Vermamoeba vermiformis, and Sapinia pedata, are known to cause severe opportunistic infections in humans and other animals. Among these infections is amoebic keratitis, an ocular infection primarily caused by Acanthamoeba spp. and, more rarely, by V. vermiformis [1,2]. Brain infections linked to these amoebae include primary amoebic meningoencephalitis (PAM), caused by N. fowleri [3], and granulomatous amoebic encephalitis (GAE), caused by Acanthamoeba spp., B. mandrillaris, and S. pedata [4]. PAM is a rapidly progressive infection with an extremely high mortality rate of 95–98% [3]. GAE, although typically characterized by a more insidious onset and slower progression, is likewise highly lethal, with mortality estimates of approximately 90% for cases caused by B. mandrillaris and 94% for those due to Acanthamoeba spp. [4]. FLA can also lead to disseminated infections, especially in immunocompromised or transplant patients, affecting various tissues such as the skin, lungs, liver, bones, kidneys, lymph nodes, breast, heart, spleen, testicles, thyroid, and urethra [5,6,7,8,9].
The ubiquity of FLA in natural and anthropogenic environments [10,11,12,13,14], including extreme and hospital environments [15,16], makes human exposure to these microorganisms almost inevitable. FLA’s marked resistance to various physical and chemical biocidal agents [17,18,19] makes its environmental control and treatment of related infections challenging. Beyond their own pathogenicity, FLA are clinically important for their role in protecting, proliferating, and promoting antimicrobial pseudo-resistance of various viral, bacterial, and fungal pathogens [16,17,18,19,20,21,22].
Despite recent approvals, such as polihexanide 0.08% for Acanthamoeba keratitis (in 2024) and corifungin for PAM (in 2025), effective therapies for FLA infections remain generally unavailable. Current treatment relies on combinations of antifungals (amphotericin B, fluconazole, flucytosine, pentamidine) and antibacterials (azithromycin, rifampin, sulfadiazine), yet evidence of their efficacy is limited and these regimens are often poorly tolerated [3,4,23]. This underscores the value and pressing need for identifying molecules with antiamoebic activity that could potentially be developed into specific treatments for FLA infections. Although considerable research has been conducted over the years to discover new compounds with anti-FLA activity [24,25,26,27,28,29,30], to our knowledge, no literature has yet summarized these findings to highlight the most promising compounds for prioritization in future in vitro or in vivo studies. This study aimed to answer the question, “Which are the most promising compounds tested in vitro and in vivo against FLA?” through a systematic review. This review identifies the most promising compounds based on their amoebicidal potency and toxicological profiles in non-target mammalian cells, and offers recommendations for future research aimed at developing drugs, antiseptics, and/or disinfectants.
2. Materials and Methods
2.1. Objectives of the Review
This review was conducted to identify the most promising new chemical compounds (those not yet established as drugs) with potential pharmacological applications that have been tested in in vitro or in vivo studies against FLA. It aims to provide insights relevant to the field of antimicrobial drug development, particularly for the treatment of infections caused by FLA.
Additionally, this review seeks to identify compounds that, beyond their potential as therapeutic drugs, may also be suitable for clinical use as antiseptic agents or environmental disinfectants.
Furthermore, the review aims to highlight molecules that, although not yet fully optimized for clinical or environmental applications, may serve as valuable scaffolds or starting points for future chemical engineering efforts aimed at enhancing their biological activity, selectivity, or physicochemical properties, thus improving their suitability for therapeutic, prophylactic, or sanitation purposes.
The specific questions guiding this review are as follows:
- -
- Which novel chemical compounds (those not yet established as drugs) have been tested in vitro and/or in vivo against FLA?
- -
- Which tested compounds demonstrate promising performance in terms of amoebicidal activity, effective concentration, and mammalian toxicological profile?
2.2. Data Collection
The planning, conduct, and reporting of this review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines (Table S1. PRISMA 2020 checklist) [31]. The review protocol was registered in the Protocols.io under registration ID number 227843, (https://dx.doi.org/10.17504/protocols.io.4r3l213y3g1y/v1). Primary studies were identified through a comprehensive search strategy applied across PubMed, EMBASE, ProQuest, and Web of Science, using the following search strategy “Acanthamoeba* OR Balamuthia OR Naegleria* OR Vermamoeba”. Two authors validated the performance of the search strategy, and one author conducted the formal search for articles in the databases on 11 June 2024.
Inclusion Criteria: Studies were selected based on the following criteria: (1) full texts were accessible online, including those provided by authors upon request; (2) written in English, Portuguese, Spanish, or another language translatable with online tools; and (3) reporting in vitro or in vivo evaluation of a novel compound against FLA, specifically Acanthamoeba, Balamuthia, Naegleria, or Vermamoeba (Figure 1).
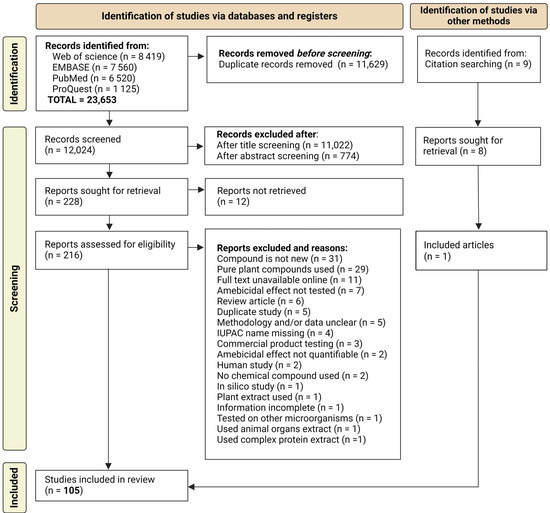
Figure 1.
Summary of the search and selection process for scientific articles included in this review.
Exclusion Criteria: Studies were excluded if they were (1) non-primary research, (2) had unclear or unreliable methodology or data, (3) tested a complex mixture of chemical compounds, (4) did not report quantifiable amoebicidal effects, (5) tested approved drugs or commercial disinfectants, or (6) if they presented only the chemical structures of the compounds without corresponding names, or if the names (whether common or IUPAC—International Union of Pure and Applied Chemistry) were unavailable or inaccessible. Studies involving pure compounds extracted from plants were also not included, as these have already been addressed in a specific review [32].
The references of the selected articles were evaluated in search of additional relevant studies that may not have been identified through the primary database search.
2.3. Screening Stages
The screening of the identified records was carried out in three main stages:
(1) Removal of duplicates—Duplicated titles were removed using semi-automated tools available in Mendeley Reference Manager. (2) Title screening—The titles were read and those clearly unrelated to the scope of the study were excluded. Titles that did not allow a clear judgment about their relevance were retained for further evaluation. (3) Abstract screening—Abstracts were reviewed to identify and exclude studies whose content, based on the abstract, was evidently outside the scope of the review.
All screening stages were conducted independently by at least three authors. Discrepancies were resolved through consensus meetings. Studies selected by consensus were then included in the next steps of full-text retrieval and data extraction.
The quality of the included studies was evaluated based on methodological robustness, clarity, and reproducibility of procedures, as conventional risk-of-bias tools designed for clinical trials were not applicable to the laboratory-based nature of these studies. Studies with low quality, limited reliability, or insufficient methodological reproducibility were not selected for inclusion.
2.4. Data Extraction Quantification of Compound Efficacy
Data extraction was initially carried out by one author, followed by verification for accuracy by two or more independent authors.
The extracted data from the articles included: reference, country (of all study authors), compound name, FLA (genus or species), amoebae form (cyst or trophozoite), density of FLA used in the tests, effective concentration (exhibiting the most relevant effect), exposure time, FLA mortality, concentration causing 50% FLA mortality (IC50), highest concentration tested for toxicity in mammalian cells (CT-toxicity), cytotoxicity in mammalian cells, and the cell line used in the toxicity test.
For each compound identified in the included publications, biocidal efficacy data were extracted as reported by the original study authors. Whenever available, values for the MIC, IC50, or the concentration corresponding to the maximum observed effect (Emax) were collected.
When multiple measurements were available, the value indicating the greatest efficacy against amoebae (according to the source article) was selected. These measurements were used as comparative indicators of the compounds’ amoebicidal activity. No extrapolation or artificial standardization was applied to homogenize the data across studies, due to methodological heterogeneity among them. The compounds were then grouped according to the highest and lowest reported effects, aiming to provide a useful comparative reference for future studies investigating these molecules in greater depth.
2.5. Data Analysis Procedure
Prior to analysis, compounds were ordered alphabetically and assigned numeric codes starting from 0 using the label encoder function in Scikit-learn. The full list of compounds and their corresponding codes is provided in Table S2 (Names and compounds codes).
Compounds were subsequently ranked according to their Effective Concentration and Mortality values. Ranking was based on the observed value ranges (minimum and maximum) and the relative distances between each compound’s Effective Concentration or Mortality. Rankings started at 1, with compounds exhibiting lower Effective Concentration values and higher Mortality values receiving the top ranks (i.e., a rank of 1 or the closest possible value).
In other words, compounds demonstrating the highest amoebicidal activity (e.g., 100% mortality) or lowest concentrations (e.g., sub-micromolar levels) were assigned ranks equal to or nearest to 1. An average ranking value was calculated for each compound based on its Effective Concentration and Mortality rankings. These mean rank values (rankAvg) were visualized using scatterplots. For interactive visualization, refer to Figure S1. (Ranking of all compounds by code), Figure S2. (Mortality of all compounds by code), and Figure S3. (Effective Concentration of all compounds by code).
Mean values and standard deviations (with 95% confidence intervals) were calculated for the variables: effective concentration, exposure time, FLA mortality, IC50, CT-toxicity, and cytotoxicity. These calculations focused on compounds that exhibited mortality rates ≥50%. Mean values were further grouped and analyzed by compound, species, and amoebae form.
The primary data and analyses are summarized in Table S3 (Data and analysis). All analyses were conducted using Python 3 and GraphPad Prism 8.02 software.
Compounds mentioned more than once in the same or different graphs (Figure S1.1–S1.28) for the same amoeba genus mean that they were tested in more than one species.
3. Results
A total of 108 studies were selected from an initial pool of 23,653 document titles retrieved from scientific databases (Figure 1). These studies involved authors from 35 countries across five continents. The distribution of studies by country, as well as the number of studies published per year, are shown in Figure 2 [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140] (Table S3).
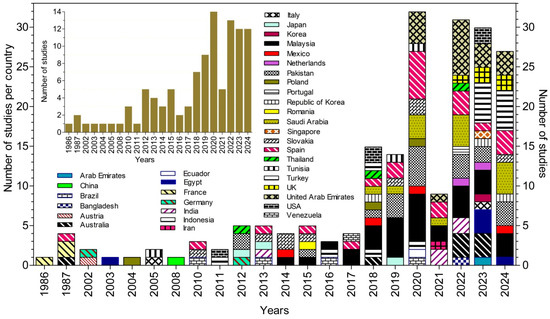
Figure 2.
Annual distribution of studies by participating countries (based on the nationalities and/or affiliations of all contributing authors).
3.1. Ranking of Tested Compounds Based on Their Amoebicidal Potency
A total of 537 different chemical compounds were evaluated for their anti-FLA activity in vitro (Figure 3). These compounds exhibited amoebicidal activity (0–100%) across a wide concentration range, from 0.015 to 5600 µM (S1). Among the tested compounds, 375 exhibited a mean rank position <200, indicating a significant amoebicidal effect (≥50%). These compounds include those coded as 0, 2, 13–18, 20, 21, 25, 27, 28, 30, 31, 32, 34, 36, 37, 39, 40–55, 57, 58, 60, 61, 64–79, 81–83, 88–103, 107, 111, 113–120, 123–125, 127–130, 132–134, 137, 138, 140, 143, 148, 150, 152, 155–157, 159, 164, 167, 170–189, 192, 200, 205, 206, 208–211, 214, 218, 219–229, 237–244, 246–250, 252–258, 260–266, 268–269, 272–282, 284, 286–295, 297, 299, 302, 304–312, 315–334, 337–344, 346–349, 361, 363–372, 374–381, 383–387, 389, 391–399, 403–404, 408, 411, 412, 414, 416–419, 421–428, 431, 432, 434–466, 468–504, 513, and 514. The compounds with the lowest average ranking values are those that present a relatively greater amoebicidal effect at relatively lower concentrations (Figure 3, refer to Figures S1 and S2 for the interactive version).
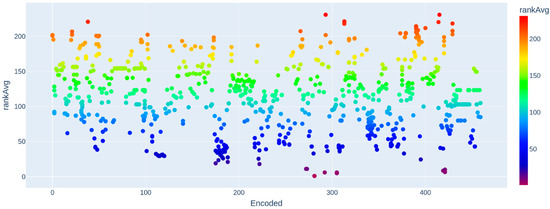
Figure 3.
Ranking of compounds according to effective concentration and amoebae mortality.
3.2. In Vitro Amoebicidal Activity of Compounds
The amoebicidal activity of all tested compounds is presented in the Supplementary Materials (Figures S2 and S3 and Table S3), while the names of the compounds corresponding to the codes reported throughout the manuscript are provided in Table S2.
Detailed performance data for compounds showing ≥50% amoebicidal activity in vitro are provided in Supplementary Materials S1. Compounds with anti-FLA activity (≥50%) at concentrations ≤10 µM or <0.2 mg/mL are highlighted in the subsequent sections (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9). The structural chemical formulas of the most promising compounds are presented in Table S4.
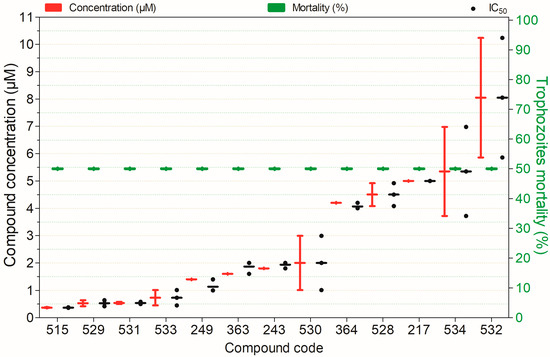
Figure 4.
Amebicidal activity of the tested compounds against Balamuthia mandrillaris trophozoites after 74 h of exposure. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
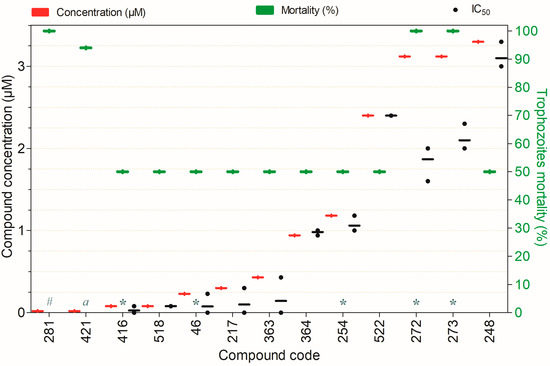
Figure 5.
Amebicidal activity of the compounds against Naegleria fowleri trophozoites, tested at concentrations ranging from 0.02 to 3.3 µM after 72 h of exposure, except for the following: (a)—24 h, (#)—36 h (*)—48 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
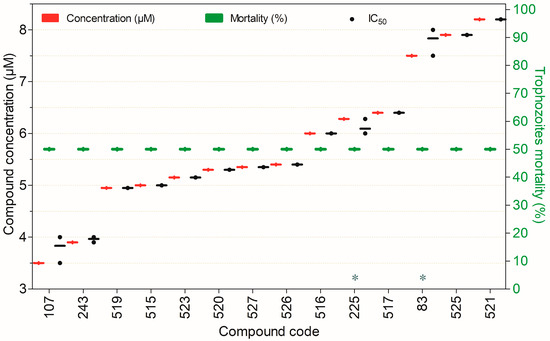
Figure 6.
Trophocidal activity of compounds against Naegleria fowleri at 3.5–8.2 µM after 72 h of exposure, except for (*) tested after 24 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
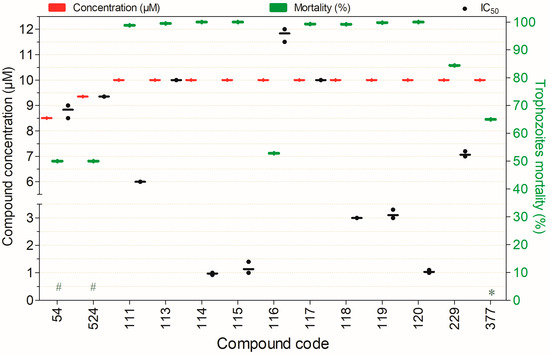
Figure 7.
Compounds exhibiting trophocidal activity against Naegleria fowleri at concentrations of 8.5–10 µM after 48 h of exposure, except for (*) compounds tested after 24 h and (#) after 72 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
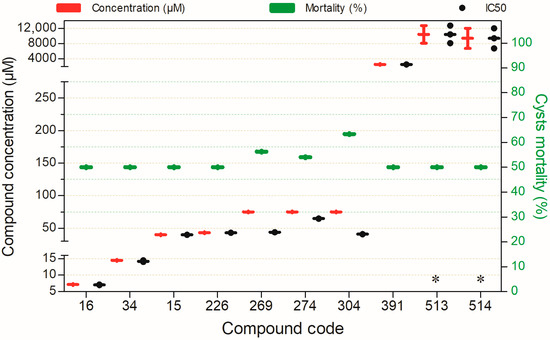
Figure 8.
Compounds exhibiting ≥50% cysticidal activity against Naegleria fowleri after 24 h of exposure, except for (*)—72 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
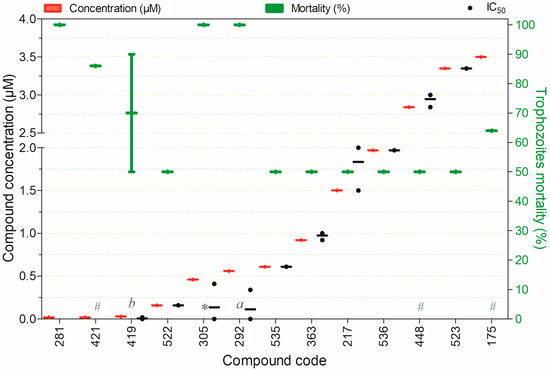
Figure 9.
Compounds exhibiting ≥50% trophocidal activity against Acanthamoeba spp. at concentrations ranging from 0.02 to 3.5 µM after 72 h of exposure, except for (a)—6 h, (*)—12 h, (#)—24 h, and (b)—48 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
3.2.1. Amebicidal Activity Against Balamuthia mandrillaris
Twenty-seven compounds showed ≥50% amoebicidal activity against B. mandrillaris trophozoites (Figure S1.1). Of these, 13 were active at concentrations <10 µM (Figure 4). Except for compound 534, all displayed notable activity; compounds 217, 243, 363, 364, 515, 528, 529, 530, 531, 532, and 533 also showed low cytotoxicity to non-target mammalian cells (Figure 4).
3.2.2. Compounds Exhibiting Trophocidal Activity Against Naegleria fowleri
Among the compounds tested against N. fowleri, 108 demonstrated the ability to inactivate ≥50% of trophozoites at concentrations ranging from 0.02 to 5489.38 µM (S1, Figures S1.2–S1.8). The compounds exhibiting trophocidal activity at concentrations below 10 µM are listed below.
Anti-N. fowleri Activity from 0.02 to 10 µM
A total of 27 compounds demonstrated the capacity to inactivate ≥50% of N. fowleri trophozoites at low concentrations (0.02–10 µM), as presented in Figure 5, Figure 6 and Figure 7. These compounds exhibited pronounced amoebicidal activity, achieving 50–100% trophozoite inactivation while generally displaying low cytotoxicity toward mammalian cells.
Figure 5 depicts the compounds capable of inactivating ≥50% of N. fowleri trophozoites at concentrations ranging from 0.02 to 3.3 µM. With the exception of compounds 272, 273, 248, and 281 (whose cytotoxicity has not yet been assessed) all demonstrated potent anti-Naegleria activity combined with minimal toxicity to non-target mammalian cells. It is noteworthy that compounds 46, 217, 254, 281, 364, 416, 421, and 518 exhibited amoebicidal activity at sub-micromolar concentrations. Among them, compounds 281 and 421 were particularly prominent, achieving 100% and 94% trophozoite inactivation within 36 and 24 h, respectively, at a concentration of only 0.02 µM (Figure 5).
The compounds that exhibited ≥50% trophocidal activity against N. fowleri at concentrations ranging from 3.5 to 8.2 µM are presented in Figure 6. Notably, for all compounds shown, potency is expressed as the concentration required to inhibit (kill) 50% of the parasites (IC50). All demonstrated pronounced anti-N. fowleri activity, and (excepting the compound 225, which also displayed cytotoxicity) they exhibited remarkably low toxicity in mammalian cell models.
Thirteen compounds exhibited substantial trophocidal activity against N. fowleri (≥50%) at concentrations of 8.5–10.0 µM. Among those depicted in Figure 7, several displayed strong anti-N. fowleri effects. Notably, compounds 54, 111, 113–119, and 229 stood out for their potent amoebicidal activity combined with low toxicity toward mammalian cell models (Figure 7).
Compounds Exhibiting ≥50% Cysticidal Activity Against N. fowleri
Eight compounds exhibited considerable cysticidal activity (≥50%) against N. fowleri at concentrations ranging from 7 to 75 µM, except for compound 391, 513, and 514 which showed activity at 2551, 10,440, and 9410 µM, respectively (S1, Figure S1.8). Notably, compounds 15, 34, 25, 226, 513, and 514 stood out for their favorable toxicological profiles in mammalian cells.
As shown in Figure 8, no compound exhibited substantial cysticidal activity (≥50%) against N. fowleri at concentrations ≤10 µM. Only compound 34 displayed the lowest cysticidal IC50 (14.46 µM) while maintaining an acceptable toxicity profile in mammalian cell models.
3.2.3. Compounds Exhibiting Trophocidal Activity Against Acanthamoeba spp.
A total of 246 compounds exhibited significant acanthamoebicidal activity (≥50%) against Acanthamoeba spp. trophozoites across a wide concentration span, ranging from 0.02 to 3536 µM (S1).
The compounds identified as most promising, based on their acanthamoebicidal potency and low toxicity are presented. The following paragraphs focus on those exhibiting ≥50% anti-Acanthamoeba activity at concentrations ranging from 0.02 to 10 µM (Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13).
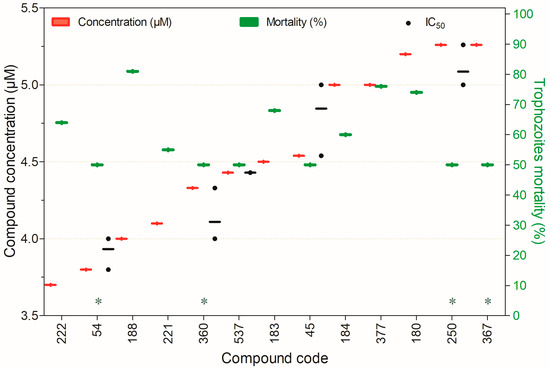
Figure 10.
Compounds displaying ≥50% trophocidal activity against Acanthamoeba spp. at 3.7–5.26 µM after 24 h of exposure, except for (*) compounds assessed at 72 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
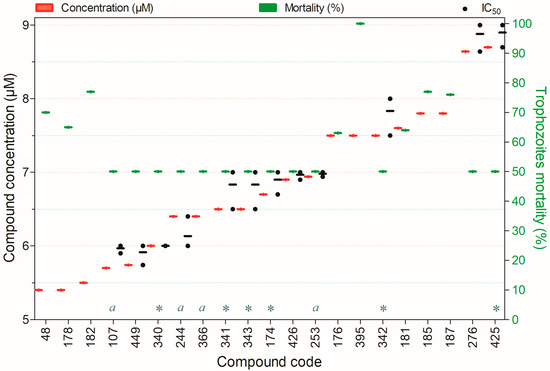
Figure 11.
Compounds exhibiting ≥50% trophocidal activity against Acanthamoeba spp. at concentrations ranging from 5. to 8.7 µM after 24 h of exposure, except for (a)—72 h and (*)—96 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
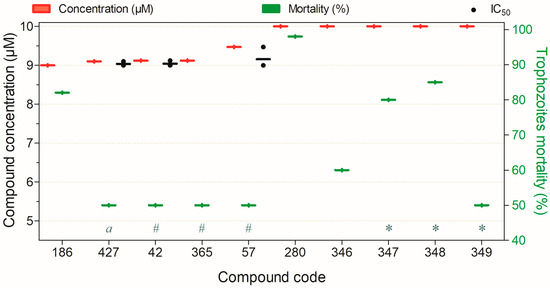
Figure 12.
Compounds showing ≥50% trophocidal activity against Acanthamoeba spp. at 9–10 µM after 24 h, except (*) 2 h, (#) 72 h, and (a) 96 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
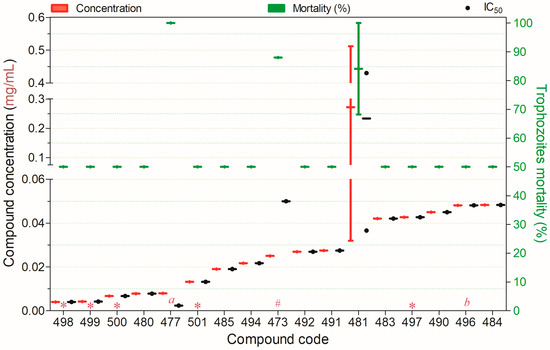
Figure 13.
Compounds displaying ≥50% trophocidal activity against Acanthamoeba spp. at concentrations ranging from 0.004 to 0.05 mg/mL after 24 h of exposure, except for (a)—4 h, (#)—72, (*)—72 h, (b)—168 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
Compounds Exhibiting Trophocidal Activity Against Acanthamoeba spp. at Concentrations ≤10 μM
Figure 9 presents compounds exhibiting ≥50% trophocidal activity against Acanthamoeba spp. at concentrations of 0.02–3.5 µM. Notably, compound 421 demonstrated rapid activity (24 h) with low toxicity to mammalian cell models, while compounds 217, 363, 522, 535, and 536 also showed minimal toxicity. Compound 305 displayed rapid trophocidal action (12 h) but lacks a characterized toxicity profile in non-target mammalian cells. Similarly, compound 281 achieved 100% trophozoite inactivation at 0.02 µM after 48 h of exposure (Figure 9).
Figure 10 shows compounds achieving ≥50% trophocidal activity against Acanthamoeba spp. at 3.7–5.26 µM. Compound 377 was notable for its rapid action (24 h) and low toxicity, while compounds 54 and 537 also displayed minimal cytotoxicity. For the remaining compounds, although their trophocidal activity was significant, their toxicity profiles in non-target mammalian cells remain unreported.
Figure 11 illustrates twenty-one compounds that inactivated ≥50% of Acanthamoeba spp. trophozoites at concentrations ranging from 5.4 to 8.7 µM. Among these, compounds 107, 174, 340, 341, 342, 343, 425, and 426 exhibited pronounced acanthamoebicidal activity while maintaining low cytotoxicity in mammalian cell models. The remaining compounds also demonstrated substantial trophocidal effects; however, their toxicological profiles in mammalian cells remain uncharacterized, except for compound 395, which showed marked toxicity to mammalian cells (Figure 11).
Ten compounds showed ≥50% trophocidal activity against Acanthamoeba spp. at 9–10 µM (Figure 12). Compounds 348 and 427 combined high activity with low mammalian cytotoxicity; notably, 348 induced ≥50% trophozoite death within 2 h. Compounds 346, 347, and 349 also displayed favorable toxicological profiles, while 186 and 280 still require cytotoxicity assessment (Figure 12).
Compounds with Anti-Acanthamoeba spp. Activity at Concentrations of 0.004–0.05 mg/mL
Seventeen compounds exhibited ≥50% trophocidal activity against Acanthamoeba spp. at 0.004–0.05 mg/mL (Figure 13). Compounds 477, 480, and 497–501 showed low cytotoxicity toward mammalian cells (HeLa or HaCaT). Among these, compound 477 achieved complete trophozoite inactivation within 4 h, demonstrating exceptional potency. Compounds 492 and 494 also exhibited strong trophocidal activity with favorable cytotoxicity profiles.
Figure 14 presents fourteen compounds that inactivated ≥50% of Acanthamoeba spp. trophozoites at concentrations ranging from 0.06 to 0.51 mg/mL. Among these, only compound 489 exhibited low toxicity toward mammalian cells. Similarly, compounds 479 and 486 showed a favorable balance between trophozoite inactivation and cytotoxicity in non-target mammalian cells (Figure 14).
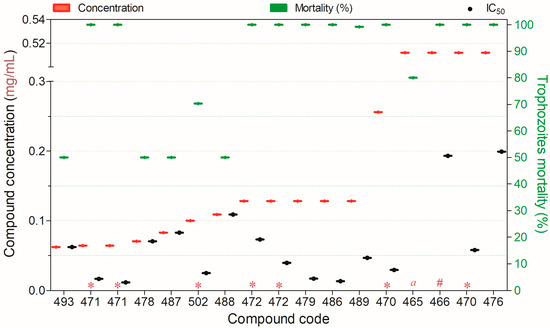
Figure 14.
Compounds displaying ≥50% trophocidal activity against Acanthamoeba spp. at concentrations ranging from 0.06 to 0.512 mg/mL after 24 h of exposure, except for (*)—48 h, (#)—72 h, (a)—144 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
3.2.4. Compounds That Displayed Cysticidal Activity Against Acanthamoeba spp.
Among all the different compounds evaluated for their cysticidal activity, 33 inactivated ≥50% of Acanthamoeba spp. cysts at concentrations ranging from 3.5 to 2243.10 µM, these results are presented in detail in Supplementary Materials S1. The paragraphs below present compounds that exerted proven cysticidal activity (≥50%) at concentrations ≤10 µM and 0.05 to 0.5 mg/mL (Figure 15).
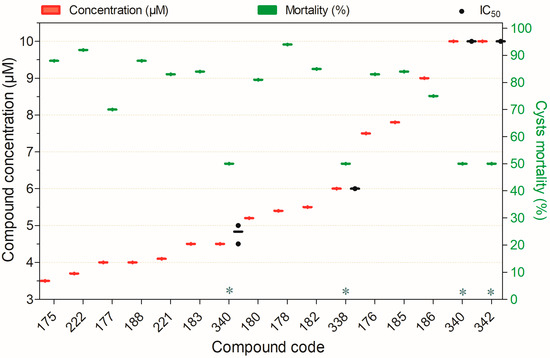
Figure 15.
Compounds displaying ≥50% cysticidal activity against Acanthamoeba spp. at concentrations ranging from 3.5 to 10 µM after 24 h of exposure, except for (*)—96 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
Fifteen compounds inactivated ≥50% of Acanthamoeba spp. cysts at concentrations ranging from 3.5 to 10 µM (Figure 15). Among these, compounds 338, 340, and 342 demonstrated low toxicity to non-target mammalian cells. While the remaining compounds showed high cysticidal potency, their cytotoxic profiles in mammalian cells have yet to be characterized (Figure 15).
Seventeen compounds inactivated ≥50% of Acanthamoeba spp. cysts at concentrations ranging from 0.05 to 0.5 mg/mL (Figure 16). Among these, all compounds evaluated for their selectivity potential (479, 480, 486, 489, and 492) were cytotoxic to non-target mammalian cells. The remaining compounds demonstrated high cysticidal potency, but their toxicity in mammalian cells has yet to be determined.
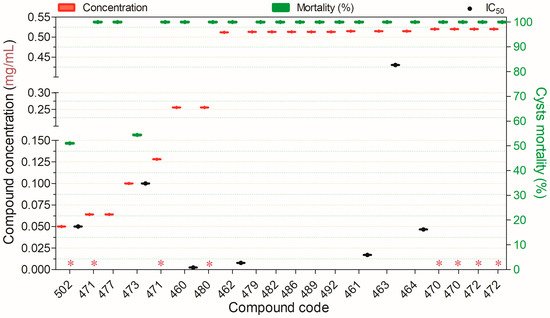
Figure 16.
Compounds displaying ≥50% cysticidal activity against Acanthamoeba spp. at concentrations ranging from 0.05 to 0.5 mg/mL after 24 h of exposure, except for (*)—48 h. Compounds are presented on the horizontal axis (x) in descending order of rank, with values expressed as means; bars (Concentration and Mortality) and point spacing (IC50) represent the standard deviation.
3.2.5. Photochemical Inactivation of Free-Living Amoebae
All photobioactive chemical compounds that had their anti-FLA activity evaluated targeted Acanthamoeba spp. Twelve different compounds (474, 475, 495, 503, 504–512) were tested against trophozoites, and three (495, 504, and 505) against cysts (Table 1). Among these, the trophocidal (474, 475, 505, 507, 510–512) and cysticidal (504) compounds appear promising; however, their cytotoxicological profile in non-target cells needs to be established. It is important to highlight that although compound 495 shows strong trophocidal and cysticidal potency, it is extremely cytotoxic in non-target cells.

Table 1.
Photochemical inactivation efficacy of various compounds against Acanthamoeba spp.
4. Discussion
The treatment of infections caused by FLA remains a significant challenge, as no approved drugs currently offer both effectiveness and safety. The existing treatment options rely on nonspecific drugs, often leading to unfavorable outcomes and frequent adverse effects. Amidst a rising incidence of FLA infections [3,23,141,142] and projections of an epidemiological worsening partly driven by climate change [143], the search for amoebicidal compounds for drug development is increasingly critical. This study reviews new compounds tested in vitro for their anti-FLA activity, aiming to identify the most promising candidates based on amoebicidal potency and potential selectivity.
Our findings (Table 2) indicate that compounds 53, 83, 218, 223, 277, 358, 515, 529, 531, and 533 exhibit the most potent trophocidal activity against B. mandrillaris, while also demonstrating notable selectivity. B. mandrillaris is a FLA with well-characterized virulence mechanisms toward animal cells, including the capacity to phagocytose neural cells [144]. It has been implicated in numerous cases of encephalitis in immunocompetent individuals [145,146] as well as in skin infections [147].

Table 2.
Most promising compounds with anti-Balamuthia mandrillaris effects.
Collectively, these results highlight ten compounds as promising leads for the future development of therapeutic agents and disinfectants targeting B. mandrillaris. Notably, compounds 515, 531, 529, and 533 emerge as the most promising candidates for drug development, exhibiting potent biocidal activity at sub-10 µM concentrations, consistent with the range predicted to confer favorable ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) profiles in biological systems.
Compounds 515 (1,1-dioxide 1-thioflavone, MMV1006203), 531 (MMV1582495), 529 (MMV1580844), and 533 (MMV1634399) belong to the thioflavone class, in which a sulfur atom substitutes the oxygen in the canonical flavone scaffold [148]. Included in the MMV Pandemic Response Box, these compounds exhibit activity against Nematocida parisii [149], multiple developmental stages of Plasmodium [150], and B. mandrillaris. Although their precise molecular mechanisms remain to be elucidated, current evidence suggests pleiotropic effects potentially mediated through redox pathways or oxidative enzymes, such as NADH oxidase [149]. To date, no validated biochemical target has been identified in amoebae.
The promising activity of these compounds represents a significant advance in the search for targeted treatments against B. mandrillaris. However, further studies are required to confirm their in vitro efficacy and, crucially, to assess their safety, pharmacokinetics, mechanisms of action, and in vivo effectiveness, both as monotherapies and in combination with other candidate compounds or approved drugs. Of particular interest is the exceptionally high selectivity of compound 277 (cellulase). Its potential for combinatorial use with other compounds, as well as strategies to enhance its bioavailability given its high active concentration, warrants further investigation.
Regarding N. fowleri, our findings (Table 3) identify compounds 13, 15, 16, 18, 34, 47, 53, 79, 83, 96, 103, 111, 113–120, 206, 214, 218, 229, 306, 325, 358, 393, 416, 421, 513, 514, and 518 as the most promising trophocidal candidates for prioritization in future studies aimed at developing therapeutic agents and disinfectants, owing to their trophocidal activity and potentially high selectivity. For cysticidal activity, compounds 15, 34, and 226 are particularly notable. It is important to note that compounds 15, 16, and 34 exhibit both anti-N. fowleri trophocidal and cysticidal activity. Compounds 53, 83, and 354 also demonstrate significant anti-B. mandrillaris activity.

Table 3.
Most promising compounds with anti-Naegleria fowleri activity.
These compounds, particularly those exhibiting anti-Naegleria activity at concentrations ≤10 µM and 0.02 mg/mL, represent high-priority candidates for future research, encompassing confirmatory in vitro studies and in vivo evaluation. It is crucial to note that infections caused by N. fowleri are increasing worldwide. Although they are relatively rare compared to other FLA infections, they are by far the most lethal [151,152]. The rarity, high lethality, and rapid health deterioration in patients affected by PAM make clinical trials unfeasible. Consequently, research using various animal models of PAM is both urgent and essential. Future studies should focus on evaluating the isolated and combined effects of these promising compounds, including their safety and pharmacokinetics.
Among the most promising compounds for anti-Naegleria fowleri activity and selectivity, trophocides 421, 416, 518, 46, 254, 522, 111, 113–120, 229, and cysticide 16 should be prioritized for future studies, exhibiting substantial activity at concentrations ≤10 µM, compatible with favorable pharmacokinetic and pharmacodynamic profiles.
Compound 421 (Tyrocidine-derived peptide) is a synthetic antimicrobial based on the amphipathic cyclic decapeptide Tyrocidine A from Bacillus brevis. Its amphipathic nature allows insertion into microbial membranes, forming ionic pores that disrupt integrity and induce cell death. Tyrocidine-derived peptides retain this mechanism and are active against various pathogens [153].
Compound 416 (staurosporine) an indole alkaloid from Streptomyces spp., is a potent inhibitor of serine/threonine kinases, including PKC, CDKs, and MAPKs [154]. The presence and critical roles of these kinases in FLA, including N. fowleri [155,156], likely underlie its observed trophocidal activity against N. fowleri.
Compound 518 (MMV1578884 or REP3123) is a promising antimalarial candidate identified by Medicines for Malaria Venture (MMV), functioning as an inhibitor of methionyl-tRNA synthetase (MetRS) from Plasmodium falciparum [157]. The presence of MetRS in free-living amoebae, such as A. polyphaga [158], may partly account for the anti-N. fowleri activity and mechanism of action of compound 518.
Compound 46 (K252a), an indole alkaloid from Nocardiopsis, is a kinase inhibitor that disrupts proliferation, differentiation, and cell death [159,160]. Its mechanism likely affects similar processes in amoebae, accounting for its observed naeglericidal activity.
Compound 254 (7-oxostaurosporine), an indole alkaloid derived from staurosporine, originates from Streptomyces platensis subsp. malvinus RK-1409. It is a potent PKC inhibitor that disrupts the cell cycle and induces apoptosis in various human tumor cells [161,162]. Its trophocidal effect against A. castellanii trophozoites has been attributed to increased membrane permeability and mitochondrial damage [163], mechanisms that may likewise underlie its activity against N. fowleri.
Compound 522 ((2R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(tetrazol-1-yl)-1-[5-[4-(2,2,2-trifluoroethoxy)phenyl]pyridin-2-yl]propan-2-ol) belongs to the azole class and is identified as oteseconazole, a selective inhibitor of the enzyme lanosterol 14α-demethylase (CYP51A1), which is essential for sterol biosynthesis in free-living amoebae (FLA), including Acanthamoeba spp. and Naegleria spp. This enzyme catalyzes the removal of the methyl group at the 14α position of lanosterol, a critical step in the synthesis of ergosterol, a key structural component of the cell membranes of protozoa such as Acanthamoeba and fungi [164]. The inhibition of CYP51A1 by compound 522 may therefore account for its amoebicidal effect against both Acanthamoeba and N. fowleri.
Compounds 111–120 (various 3-((4-(tert-butyl)benzyl)amino)-substituted picolinic acids) are benzylamine picolinate derivatives exhibiting potent trophocidal activity against N. fowleri, likely through interference with essential energy and signaling pathways, as well as mitochondrial or redox enzymes. These compounds were predicted to have good blood–brain barrier permeability by parallel artificial membrane permeability assay (PAMPA) [107]. Further studies are needed to clarify the precise anti-FLA mechanisms of these compounds.
Compound 16 ((E)-3-cyano-N-(4-methoxyphenyl)acrylamide) is a cyanoacrylamide belonging to the subclass of α,β-unsaturated amides. This chemical scaffold functions as a Michael acceptor, in which the electrophilic β-carbon is highly susceptible to nucleophilic attack, particularly by thiol groups of cysteine residues. Such interactions enable covalent, and in some cases reversible, inhibition of enzymes containing catalytic cysteines, including transforming growth factor-β–activated kinase 1 (TAK1) [165,166]. The behavior of molecules within this class may, therefore, partially account for the anti-N. fowleri activity observed for compound 16; nonetheless, further studies are required to elucidate its precise mechanism of action in FLA.
The top-ranked compounds active against Acanthamoeba spp. trophozoites and cysts are summarized in Table 4. Compounds with notable trophocidal activity include 60, 69, 107, 123, 125, 127–130, 132–134, 137, 138, 141, 143, 148, 150, 152, 155–157, 159, 164, 167, 200, 208, 209, 211, 237–241, 256–258, 275, 279, 284, 286, 294, 299, 302, 306, 318, 347, 348, 374, 377, 385, 392, 394, 396–398, 498–501, 522, and 535. Those with cysticidal activity include 275, 338–344, and 374–384.

Table 4.
Most promising compounds with anti-Acanthamoeba spp. Activity.
These candidates warrant prioritization for confirmatory studies as potential therapeutics or disinfectants. Compounds active at ≤10 µM are particularly promising for drug development, including assessment of administration, safety, and pharmacokinetics/pharmacodynamics in animal models of FLA infection.
Notably, compounds 275 and 374 exhibit exceptional potency, showing both trophocidal and cysticidal activity against Acanthamoeba spp. with no detectable cytotoxicity. However, because their activity occurs at concentrations above 10 µM, further studies are needed to address predictable bioavailability challenges.
Acanthamoeba infections are among the most prevalent FLA infections. These primarily include Acanthamoeba keratitis (AK), which predominantly occurs in contact lens wearers (86%) and individuals with ocular trauma (27%). Granulomatous amoebic encephalitis, another form of acanthamoebiasis, carries a mortality rate exceeding 90% [167,168]. Additionally, disseminated infections affecting various organs have been documented in the literature [1,2,3,4,5].
The absence of approved drugs that are both effective and safe for the treatment of Acanthamoeba infections underscores the urgency and importance of developing targeted therapeutics. Among the promising candidates, particular attention should be given to trophocidal compounds 498, 499, 500, 501, 522, 535, 377, 107, 347, and 348, as well as the cysticidal compounds 340 and 338.
Compounds 498, 499, 500, and 501 belong to the class of amino acid-based rhamnolipids, derived from arginine (RLMIX_Arg) or lysine (RLMIX_Lys). Owing to the cationic nature of these amino acids, these molecules can strongly interact with negatively charged components of the Acanthamoeba membrane, including lipoproteins, thereby disrupting their structural and functional integrity at the concentrations reported [169]. This membrane-targeting mechanism likely accounts for their potent amebicidal activity [62]. Assessing the potential synergistic effects of these compounds in combination with drugs acting on non-membrane targets, such as nitroxoline, could represent a promising strategy for enhancing therapeutic efficacy.
Compound 535 ([4-(benzylamino)-5-chloro-2,6-difluorobenzene-1,3-dicarbonitrile]) is included in the Pandemic Response Box and corresponds to OSU-03012 (also known as AR-12), a compound classically recognized as an inhibitor of 3-phosphoinositide-dependent kinase-1 (PDK1) [170]. OSU-03012/AR-12 has also been reported as a potent inhibitor of fungal and parasitic acetyl-CoA synthetase, while exhibiting only weak inhibition of the human isoform ACCS2 [171]. Additional mechanisms of action, including inhibition of dihydroorotate dehydrogenase (DHODH) and equilibrative nucleoside transporter 1 (ENT1) (both involved in nucleotide metabolism) have also been proposed [172].
Although there is currently no experimental evidence confirming the presence of PDK1, DHODH, or ENT1 in FLA, there is also no basis to associate the amoebicidal activity of compound 535 with the inhibition of these proteins. Conversely, the documented presence of acetyl-CoA synthetase in Naegleria gruberi [173], suggests that inhibition of this key enzyme could explain the amoebicidal activity of compound 535, further indicating its potential activity against N. fowleri.
The amoebicidal activity of compound 377 (oleic acid-AgNPs) likely results from the combined biocidal effects of oleic acid and silver nanoparticles (AgNPs). Oleic acid has been reported to exert a strong inhibitory effect on glucosyltransferase [174] and to disrupt the integrity of the plasma membrane, consistent with its well-documented bactericidal properties [175]. Silver nanoparticles, in turn, have been extensively characterized for their potent antimicrobial activity, exhibiting fungicidal [176], bactericidal [177], and antiviral effects [178]. In human colorectal carcinoma cells (HCT-116), AgNPs have been shown to induce cell death through deterioration of the cytoskeletal structure and membrane nanostructure [179]. In parasitic protozoa, AgNPs have been associated with a wide range of deleterious effects, including plasma membrane disruption, DNA damage mediated by reactive oxygen species (ROS), and inhibition of ATP synthesis due to the release of silver ions.
Although direct evidence of oleic acid-mediated inhibition of amoebic glucosyltransferase is lacking, the documented presence of this enzyme in Entamoeba histolytica [180] and A. castellanii [181], combined with evidence suggesting galactose as a molecular target in FLA [182], strongly supports the hypothesis that the acanthamoebicidal effect of compound 377 is associated with the inhibition of glucosyltransferase, synergistically enhanced by the multifaceted cytotoxic effects of AgNPs on eukaryotic cells.
Compound 107 (3-((4-(2-Cyclopropyl-6-morpholinopyrazolo[1,5-b]-pyridazin-3-yl)pyrimidin-2-yl)amino)benzonitrile) is an aminopyrimidine derivative containing a pyrazolo[1,5-b]pyridazine core. Molecules of this class have been identified as inhibitors of cyclin-dependent kinases (CDKs), particularly CDK2 and CDK4, and exhibit antiproliferative activity against Trypanosoma brucei [183]. Considering the presence of these essential regulatory proteins in FLA, including Acanthamoeba [184], it is plausible that the acanthamoebicidal activity of compound 107 arises from its inhibitory effect on CDKs.
Compound 347 is a hybrid formulation consisting of two distinct components: methyltrioctylammonium chloride and ethylene glycol. Ethylene glycol, commonly used as an antifreeze agent, is known to be toxic but exhibits lower cytotoxicity than diethylene glycol and propylene glycol in human oral squamous cell carcinoma cells [185]. Methyltrioctylammonium chloride, on the other hand, is a quaternary ammonium compound (QAC) widely employed as an environmental disinfectant. QACs possess broad-spectrum antimicrobial activity primarily associated with their ability to disrupt cell membranes through mechanisms such as pore formation, lysis, and protein denaturation [186]. Therefore, the acanthamoebicidal activity of compound 347 likely results from the combined action of both constituents, with a predominant contribution from methyltrioctylammonium chloride, whose anti-FLA activity has been previously reported [187].
Compound 348 is structurally analogous to 347, differing only by the substitution of ethylene glycol with fructose. Given that fructose functions primarily as a metabolic substrate and lacks reported amoebicidal properties, it is reasonable to infer that methyltrioctylammonium chloride is the principal active component responsible for the amoebicidal activity observed in compound 348.
Compounds 338 (methyl 4-chloro-1H-indole-3-carboxylate) and 340 (methyl 6-chloro-1H-indole-3-carboxylate) are methyl indole-3-carboxylate derivatives featuring a chlorine atom at positions 4 and 6 of the indole ring, respectively. Scaffolds of this class have been recognized as promising in drug design, exhibiting antimicrobial, antiparasitic, and antitumor activities [188]. Although both compounds remain underexplored, compound 340 has been reported to induce programmed cell death in Acanthamoeba spp. via mitochondrial dysfunction [131].
The data presented in Table 1 indicates that, among the thirteen compounds tested for their photoactive biocidal effects, eight showed promising acanthamoebicidal activity, comprising seven trophocidal and one cysticidal compound. Nonetheless, further studies are required to evaluate their selectivity and determine safe conditions for potential therapeutic applications.
Photoactivated chromophore compounds used in corneal crosslinking therapy have emerged as a promising adjuvant treatment for AK [189]. However, additional research is essential to fully realize and enhance their clinical potential.
Compound 511 (0.5 mM + 10.8 J/cm2, visible light) demonstrated significant in vitro trophocidal activity and exhibited a synergistic acanthamoebicidal effect when combined with amphotericin B (1 ppm) and polyhexamethylene biguanide (100 µg/mL). However, no synergistic effect was observed when combined with voriconazole (400–1000 µg/mL) [100]. Similarly, compound 505 showed promising results in in vivo studies with rabbits, achieving complete recovery from AK in 58% of animals. However, the authors highlighted the need for further research to optimize conditions for maximum therapeutic efficacy [144]. Finally, compound 507 is one of the most extensively studied photoactive compounds, with evidence supporting its potential as an adjunctive therapy to reduce the need for therapeutic penetrating keratoplasty in AK cases [190,191,192,193]. Nonetheless, additional studies, including clinical trials are required.
Overall, the compounds highlighted throughout the article represent a significant achievement in the search for effective drugs to treat FLA infections. Developing formulations based on these compounds that incorporate nanomaterials could be a promising strategy to enhance biocidal potency and/or reduce cytotoxic effects on non-target cells, as previously demonstrated [45,76,100,129].
It has been suggested that the shape of nanomaterials influences their amoebicidal activity. For example, silver nanoparticles exhibited trophocidal and cysticidal effects, reaching inactivation rates ranging from 40% to 100%. Among these, silver nanowires (AgNWs) exhibited the highest amoebicidal efficacy, outperforming silver nanorings (AgNRs), which in turn were significantly more effective than silver nanospheres (AgNSs) [71]. Therefore, it is desirable that the shape of nanoparticles be considered in studies seeking to develop nanobased formulations of the promising compounds discussed.
Among the 537 compounds tested, 123 were generally selected as suitable candidates for the development of drugs and disinfectants based on their efficacy and low cytotoxicity. The most active against Balamuthia mandrillaris were ten trophocidal compounds (53, 83, 218, 223, 277, 358, 515, 529, 531, and 533). For N. fowleri, thirty trophocidal (13, 15, 18, 34, 47, 53, 79, 83, 96, 103, 111, 113–120, 206, 214, 218, 229, 306, 325, 358, 393, 416, 421, and 518) and four cysticidal (15, 16, 34, and 226) compounds were identified. Against Acanthamoeba spp., sixty-two trophocidal (60, 69, 107, 123, 125, 127–130, 132–134, 137, 138, 141, 143, 148, 150, 152, 155–157, 159, 164, 167, 200, 208, 209, 211, 237–241, 256–258, 275, 279, 284, 286, 294, 299, 302, 306, 318, 347, 348, 374, 377, 385, 392, 394, 396–398, 498–501, 522, and 535) and nineteen cysticidal compounds (275, 338–344, 374, and 384) were highlighted.
Limitations
The findings of this study were obtained under certain limiting factors that may have influenced the strength and direction of the results: (1) The anti-FLA potential of many compounds was based on findings from a single study (and/or tests performed on a single FLA strain) available in the literature. (2) Compounds that showed <50% anti-FLA activity in vitro were classified as unpromising; however, this does not necessarily preclude their potential to demonstrate significant anti-FLA activity and selectivity in in vivo studies. (3) Compounds with uncharacterized cytotoxicological profiles in non-target cells could not be included in the list of top-ranked compounds, despite their high anti-FLA potency.
We acknowledge that Emax, MIC, and IC50 values may not fully represent a compound’s intrinsic potency, as they can be influenced by physicochemical properties like solubility and permeability. Moreover, the highest tested concentrations may be limited by toxicity or compound stability.
5. Conclusions
This study aimed to identify the most promising chemical compounds with anti-FLA activity reported in the indexed literature, recommending them for future in vitro confirmation and primarily in vivo studies to evaluate their efficacy, mechanisms of action, safety, pharmacokinetics, and pharmacodynamics (Table A1). Future studies focused on drug development should prioritize the following compounds:
Against B. mandrillaris: trophocidal compounds 515 (1,1-dioxide 1-thioflavone, MMV1006203), 531 (MMV1582495), 529 (MMV1580844), and 533 (MMV1634399).
Against N. fowleri: trophocidal compounds 421 (tyrocidine-derived peptide), 416 (staurosporine), 518 (MMV1578884 or REP3123), 46 (K252a), 254 (7-oxostaurosporine), 522 ((2R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(tetrazol-1-yl)-1-[5-[4-(2,2,2-trifluoroethoxy)phenyl]pyridin-2-yl]propan-2-ol), and compounds 111–120 (a series of 3-((4-(tert-butyl)benzyl)amino)-substituted picolinic acids). The cysticidal compound 16 ((E)-3-cyano-N-(4-methoxyphenyl)acrylamide) also deserves special attention.
Against Acanthamoeba spp.: trophocidal compounds 498 (mono-rhamnolipids–arginine), 499 (mono-rhamnolipids–arginine + di-rhamnolipids–arginine), 500 (mono-rhamnolipids–lysine), 535 ([4-(benzylamino)-5-chloro-2,6-difluorobenzene-1,3-dicarbonitrile]), 377 (oleic acid–AgNPs), 107 (3-((4-(2-cyclopropyl-6-morpholinopyrazolo [1,5-b]-pyridazin-3-yl)pyrimidin-2-yl)amino)benzonitrile), 347 (methyltrioctylammonium chloride + ethylene glycol, 1:1), and 348 (methyltrioctylammonium chloride + fructose, 1:1.25), as well as cysticidal compounds 4-chloro-1H-indole-3-carboxylate and 340 (methyl 6-chloro-1H-indole-3-carboxylate).
To the best of our knowledge, this is the first comprehensive review to systematically compile and analyze in vitro studies aimed at identifying bioactive compounds with anti-FLA activity, thereby contributing to the acceleration of efforts toward the development of safe and effective therapeutic agents and disinfectants against FLA infections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/parasitologia5040056/s1, Figure S1. Rank All Compounds By Code; Figure S2. Mortality of All Compounds By Code; Figure S3. Concentration of All Compounds By Code; Table S1. PRISMA 2020 checklist; Table S2. Names and compound codes; Table S3. Data and analysis; Table S4. Most promising compounds; S1. Detailed results of compounds that exhibit amebicidal activity equal to or greater than 50%.
Author Contributions
Conceptualization, B.J.M.C.; methodology, B.J.M.C. and M.B.R.; validation, B.J.M.C., T.C.B.d.S., L.B.C., L.F.G.K., P.E.R.B. and C.J.G.; formal analysis, B.J.M.C. and A.C.B.C.; investigation, B.J.M.C., T.C.B.d.S., L.B.C., L.F.G.K., P.E.R.B. and C.J.G.; software, B.J.M.C. and A.C.B.C.; resources, R.A.Z. and J.R.G.; data curation, B.J.M.C., T.C.B.d.S., L.B.C., L.F.G.K., P.E.R.B. and C.J.G.; writing—original draft preparation, B.J.M.C.; writing—review and editing, B.J.M.C., M.B.R. and R.A.Z.; visualization, B.J.M.C. and A.C.B.C.; supervision, B.J.M.C.; project administration, B.J.M.C.; funding acquisition, J.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), grant number 88887.154231/2025-00. The APC was funded by (Human Resources Training Project to Support the Development of Clinical Research in Brazil) grant number 88887.800.657/2022-00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3T3 | Murine fibroblasts |
| A549 | Lung carcinoma cells |
| ADMET | Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| C6 | Rat glial cells |
| CHO-K1 | Chinese Hamster Ovary cells |
| CT-toxicity | Highest concentration tested for toxicity in mammalian cells |
| ESRC | Epithelial and Stromal Rabbit Cells |
| FLA | Free-Living Amoebae |
| HaCaT | Human keratinocytes. |
| HBEC-5i | Human brain microvascular endothelial cell |
| HCEC | Human Corneal Epithelial Cells |
| HeLa | Human cervical adenocarcinoma |
| IC50 | Concentration causing half of mortality |
| J774A.1 | Murine macrophages |
| L6 | Rat skeletal muscle cell line |
| L929 | Fibroblastos de camundongos |
| MCF-7 | Human breast adenocarcinoma |
| MRC-5 | Epithelial lung cells. |
| NB1RGB | Human neonate dermal fibroblast |
| pHCEC | Primary corneal epithelial cell |
| RBCs | Horse Red Blood Cell |
| SH-SY5Y | Human neuroblastoma |
| SRB | Sheep Red Blood cells |
Appendix A

Table A1.
Codes and Names of the Most Promising Compounds with Anti-FLA Activity.
Table A1.
Codes and Names of the Most Promising Compounds with Anti-FLA Activity.
| Code | Name |
|---|---|
| 13 | (E)-2-(6,6-Dimethyl-1-phenyl-1,5,6,7-tetrahydro-4H-indazol-4-4ylidene)hydrazine-1-carbothioamide |
| 15 | (E)-3-Cyano-N-(4-methoxybenzyl)acrylamide |
| 16 | (E)-3-Cyano-N-(4-methoxyphenyl)acrylamide |
| 18 | (E)-3-phenylprop-2-enoic acid |
| 34 | 1-Benzyl-5-Imino-1H-Pyrrol-2(5H)-One |
| 46 | 2,3,9S,10R,11,12R-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, methyl ester |
| 47 | 2,3-dimethylacridin-9(10H)-one |
| 53 | 2,6-dichlorobenzonitrile |
| 60 | 2-(3-Cyano-6-methyl-4,6-dip-tolyl-5,6-dihydropyridin-2(1H)-ylidene)malono-nitrile |
| 69 | 2-(N-methylazepanio-1-yl)ethyl pentadecyloxycarbonylphosphonate |
| 79 | 2-Bromo-4-(di (1H-indol-3-yl)methyl)-6-methoxyphenol |
| 83 | 2-N-tert-butyl-6-chloro-4-N-ethyl-1,3,5-triazine-2,4-diamine |
| 96 | 2-ethylacridin-9(10H)-one |
| 103 | 3,7-Dichloro-8-quinolinecarboxylic acid |
| 107 | 3-((4-(2-Cyclopropyl-6-morpholinopyrazolo[1,5-b]- pyridazin-3-yl)pyrimidin-2-yl)amino)benzonitrile |
| 111 | 3-((4-(tert-butyl)benzyl)amino)-5-chloropicolinic acid |
| 113 | 3-((4-(tert-butyl)benzyl)amino)-5-fluoropicolinic acid |
| 114 | 3-((4-(tert-butyl)benzyl)amino)-5-methoxypicolinic acid |
| 115 | 3-((4-(tert-butyl)benzyl)amino)-5-methylpicolinic acid |
| 116 | 3-((4-(tert-butyl)benzyl)amino)-5-morpholinopicolinic acid |
| 117 | 3-((4-(tert-butyl)benzyl)amino)-5-phenylpicolinic acid |
| 118 | 3-((4-(tert-butyl)benzyl)amino)picolinic acid |
| 119 | 3-((4-(tert-butyl)phenethyl)amino)-5-chloropicolinic acid |
| 120 | 3-((4-(tert-butyl)phenethyl)amino)-5-methoxypicolinic acid |
| 123 | 3-(2,3-Dichlorophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 125 | 3-(2,4-Difluorophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 127 | 3-(2,4-Dimethylphenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 128 | 3-(2,5-Dimethoxyphenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 129 | 3-(2,5-Dimethoxyphenyl)-8-methylquinazolin-4(3H)-one |
| 130 | 3-(2,6-Diethylphenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 132 | 3-(2-Bromophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 133 | 3-(2-Chlorophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 134 | 3-(2-Iodophenyl)-8-methylquinazolin-4(3H)-one |
| 137 | 3-(3,4-Dichlorophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 138 | 3-(3,5-Dimethylphenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 141 | 3-(3-Bromophenyl)-8-methylquinazolin-4(3H)-one |
| 143 | 3-(3-Fluorophenyl)-8-methylquinazolin-4(3H)-one |
| 148 | 3-(4-Bromophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 150 | 3-(4-Butylphenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 152 | 3-(4-Chlorophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 155 | 3-(4-Fluorophenyl)-6,7-dimethoxyquinazolin-4(3H)-one |
| 156 | 3-(4-Fluorophenyl)-8-methylquinazolin-4(3H)-one |
| 157 | 3-(4-Iodophenyl)-8-methylquinazolin-4(3H)-one |
| 159 | 3-(4-Methoxyphenyl)-8-methylquinazolin-4(3H)-one |
| 164 | 3-([1,1′-Biphenyl]-4-yl)-2H-benzo[b] [1,4]thiazine |
| 167 | 3-Phenyl-2-thioxo-2,3-dihydroquinazolin-4(1H)-one |
| 200 | 4-(Cycloheptanecarboxamido)phenyl benzenesulfonate |
| 206 | 4-(Cyclohexanecarboxamido)phenyl dimethylsulfamate |
| 208 | 4-(Cyclohexanecarboxamido)phenyl methanesulfonate |
| 209 | 4-(Cyclohexanecarboxamido)phenyl methylsulfamate |
| 211 | 4-(Cyclohexanecarboxamido)phenyl sulfamate |
| 214 | 4-(Cyclopentanecarboxamido)phenyl 4-methylbenzenesulfonate |
| 218 | 4-(biphenyl-4-yl)-2-(2-(1-(pyridin-4-yl)ethylidene)hydrazinyl)thiazole |
| 223 | 5-(3-Chlorobenzyl)-1H-tetrazole |
| 226 | 5-Imino-1-Phenyl-1H-Pyrrol-2(5H)-One |
| 229 | 5-bromo-3-((4-(tert-butyl)benzyl)amino)picolinic acid |
| 237 | 6,7-Dimethoxy-3-(4-(methylthio)phenyl)quinazolin-4(3H)-one |
| 238 | 6,7-Dimethoxy-3-(4-methoxyphenyl)quinazolin-4(3H)-one |
| 239 | 6,7-Dimethoxy-3-(m-tolyl)quinazolin-4(3H)-one |
| 240 | 6,7-Dimethoxy-3-(o-tolyl)quinazolin-4(3H)-one |
| 241 | 6,7-Dimethoxy-3-(p-tolyl)quinazolin-4(3H)-one |
| 254 | 7-oxostaurosporine |
| 256 | 8-Methyl-3-(3-(Methylthio)Phenyl)QUINAZOLIN-4(3H)-One |
| 257 | 8-Methyl-3-(o-tolyl)quinazolin-4(3H)-one |
| 258 | 8-Methyl-3-(p-tolyl)quinazolin-4(3H)-one |
| 275 | Cationic Steroid Antibiotic—13 |
| 277 | Cellulase: (2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6S)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol |
| 279 | Chitosan |
| 284 | Co3(PO4)2•8H2O |
| 286 | Cobalt phosphate octahydrate (Co3(PO4)2⋅8H2O) |
| 294 | Dodecyltriphenylphosphonium Bromide |
| 299 | Ethyl(5-decyl-2-amino-1,3-thiazol-4-yl)acetate |
| 302 | Ethyl(5-octyl-2-amino-1,3-thiazol-4-yl)acetate |
| 306 | Gallic acid-ZnO |
| 318 | Isobenzofuran-1(3H)-one QOET-3 |
| 325 | Isobenzofuran-1(3H)-one QOET-1 |
| 338 | Methyl 4-chloro-1H-indole-3-carboxylate |
| 339 | Methyl 5-methyl-1H-indole-3-carboxylate |
| 340 | Methyl 6-chloro-1H-indole-3-carboxylate |
| 341 | Methyl 6-methyl-1H-indole-3-carboxylate |
| 342 | Methyl 7-chloro-4-fluoro-1H-indole-3-carboxylate |
| 343 | Methyl 7-fluoro-1H-indole-3-carboxylate |
| 344 | Methyl Indol-3-carboxylate |
| 347 | Methyltrioctylammonium chloride: Ethylene Glycol (1:1) |
| 348 | Methyltrioctylammonium chloride: Fructose (1:1.25) |
| 358 | N-(2,3-dihydro-2,6-dimethyl-1H-inden-1-yl)-6-(1-fluoroethyl)-1,3,5-triazine-2,4-diamine |
| 374 | Nano-chitosan |
| 377 | Oleic acid-AgNPs |
| 384 | PANI/MoS2 (1:5) |
| 385 | Patuletin |
| 392 | Polyaniline—hexagonal boron nitride (1:5) |
| 394 | Polyhomoarginine (10 homoarginines) |
| 396 | Polypyrrole |
| 397 | Polypyrrole-Co3O4 |
| 398 | Polypyrrole-Co3O4-AgNPs |
| 416 | Staurosporine |
| 421 | Tyrocidine-derived peptide |
| 498 | Mono-rhamnolipids-Arginine |
| 499 | Mono-rhamnolipids-Arginine + Di-rhamnolipids-Arginine |
| 500 | Mono-rhamnolipids-Lysine |
| 501 | Mono-rhamnolipids-Lysine + Di-rhamnolipids-Lysine |
| 515 | 1,1-dioxide 1-thioflavone |
| 518 | MMV1578884 |
| 522 | (2R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(tetrazol-1-yl)-1-[5-[4-(2,2,2-trifluoroethoxy)phenyl]pyridin-2-yl]propan-2-ol |
| 529 | MMV1580844 |
| 531 | MMV1582495 |
| 533 | MMV1634399 |
| 535 | MMV1582496 (4-(Benzylamino)-5-chloro-2,6-difluoro-benzene-1,3-dicarbonitrile) |
References
- Raghavan, A.; Rammohan, R. Acanthamoeba keratitis—A review. Indian J. Ophthalmol. 2024, 72, 473–482. [Google Scholar] [CrossRef]
- Aykur, M.; Selver, O.B.; Dagci, H.; Palamar, M. Vermamoeba vermiformis as the etiological agent in a patient with suspected non-Acanthamoeba keratitis. Parasitol. Res. 2024, 123, 323. [Google Scholar] [CrossRef]
- Hall, A.D.; Kumar, J.E.; Golba, C.E.; Luckett, K.M.; Bryant, W.K. Primary amebic meningoencephalitis: A review of Naegleria fowleri and analysis of successfully treated cases. Parasitol. Res. 2024, 123, 84. [Google Scholar] [CrossRef]
- Haston, J.C.; Cope, J.R. Amebic encephalitis and meningoencephalitis: An update on epidemiology, diagnostic methods, and treatment. Curr. Opin. Infect. Dis. 2023, 36, 186–191. [Google Scholar] [CrossRef]
- Schafer, K.R.; Shah, N.; Almira-Suarez, M.I.; Reese, J.M.; Hoke, G.M.; Mandell, J.W.; Roy, S.L.; Visvesvara, G. Disseminated Balamuthia mandrillaris Infection. J. Clin. Microbiol. 2015, 53, 3072–3076. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.; Li, B.; Jian, Z.; Qi, X.; Sun, D.; Gao, J.; Lu, X.; Yang, Y.; Lin, K.; et al. Balamuthia mandrillaris infection in China: A retrospective report of 28 cases. Emerg. Microbes Infect. 2020, 9, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Łanocha-Arendarczyk, N.; Kosik-Bogacka, D. Immunopathogenicity of Acanthamoeba spp. in the Brain and Lungs. Int. J. Mol. Sci. 2021, 22, 1261. [Google Scholar] [CrossRef] [PubMed]
- Stetkevich, S.A.; Le, S.T.; Ford, A.R.; Brassard, A.; Kiuru, M.; Fung, M.A.; Tartar, D.M. Isolated cutaneous acanthamoebiasis under prophylactic anticryptococcal treatment in an immunocompromised patient. JAAD Case Rep. 2022, 28, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Haston, J.C.; O’Laughlin, K.; Matteson, K.; Roy, S.; Qvarnstrom, Y.; Ali, I.K.M.; Cope, J.R. The Epidemiology and Clinical Features of Non-Keratitis Acanthamoeba Infections in the United States, 1956–2020. Open Forum Infect. Dis. 2023, 10, ofac682. [Google Scholar] [CrossRef]
- Aksozek, A.; McClellan, K.; Howard, K.; Niederkorn, J.Y.; Alizadeh, H. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J. Parasitol. 2002, 88, 621–623. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; Dos Santos, D.L.; Anvari, D.; Rott, M.B. Prevalence of free-living amoebae in swimming pools and recreational waters, a systematic review and meta-analysis. Parasitol. Res. 2022, 121, 3033–3050. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; da Silva, T.C.B.; Dos Santos, D.L.; Benitez, G.B.; Chaúque, L.G.H.; Benetti, A.D.; Zanette, R.A.; Rott, M.B. Global prevalence of free-living amoebae in solid matrices—A systematic review with meta-analysis. Acta Trop. 2023, 247, 107006. [Google Scholar] [CrossRef]
- da Silva, T.C.B.; Chaúque, B.J.M.; Benitez, G.B.; Rott, M.B. Global prevalence of potentially pathogenic free-living amoebae in sewage and sewage-related environments—Systematic review with meta-analysis. Parasitol. Res. 2024, 123, 148. [Google Scholar] [CrossRef]
- Glotova, A.A.; Loiko, S.V.; Istigechev, G.I.; Kulemzina, A.A.; Abakumov, E.V.; Raiko, M.V.; Lapidus, A.L.; Smirnov, A.V. Diversity and abundance of naked lobose amoebae (Amoebozoa: Tubulinea, Discosea, Variosea) in highly productive soil of Chernevaya taiga (Western Siberia, Russia). Protistology 2024, 18, 22–30. [Google Scholar] [CrossRef]
- Pérez-Pérez, P.; Reyes-Batlle, M.; Rodríguez-Expósito, R.L.; Perdomo-González, A.; Sifaoui, I.; Díaz-Peña, F.J.; Morchón, R.; Maciver, S.K.; Piñero, J.E.; Lorenzo-Morales, J. First Report of Acanthamoeba Genotype T4 from the Newly Formed Tajogaite Volcano Tephra (La Palma, Canary Islands). Pathogens 2024, 13, 626. [Google Scholar] [CrossRef]
- Mahdavi, F.; Fatemi, M.; Mohammad Rahimi, H.; Niyyati, M.; Yadegar, A.; Mirjalali, H. Identification of Candida albicans and non-MRSA Staphylococcus aureus in free-living amoebae isolated from the hospital wards; an alarm for distribution of nosocomial infections via FLA. Int. J. Environ. Health Res. 2024, 34, 3749–3759. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; Rott, M.B. Photolysis of Sodium Chloride and Sodium Hypochlorite by Ultraviolet Light Inactivates the Trophozoites and Cysts of Acanthamoeba castellanii in the Water Matrix. J. Water Health 2021, 19, 190–202. [Google Scholar] [CrossRef]
- Ramírez-Flores, E.; Bonilla-Lemus, P.; Carrasco-Yépez, M.M.; Ramírez-Flores, M.A.; Barrón-Graciano, K.A.; Rojas-Hernández, S.; Reyes-Batlle, M.; Lorenzo-Morales, J. Saline-Tolerant Pathogenic Acanthamoeba spp. Isolated from a Geothermal Power Plant. Pathogens 2023, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.L.; Chaúque, B.J.M.; Matiazo, F.F.; de Miranda Ribeiro, L.; Rott, M.B. Agar dehydration: A simple method for long-term storage of Acanthamoeba spp. collection at room temperature. Parasitol. Res. 2024, 123, 153. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Zhou, Z.; Wang, S.; Wei, Z.; Ravanbakhsh, M.; Shen, Q.; Xiong, W.; Kowalchuk, G.A.; Jousset, A. Protist predation promotes antimicrobial resistance spread through antagonistic microbiome interactions. ISME J. 2024, 18, wrae169. [Google Scholar] [CrossRef]
- Ali, M.; Rice, C.A.; Byrne, A.W.; Paré, P.E.; Beauvais, W. Modelling dynamics between free-living amoebae and bacteria. Environ. Microbiol. 2024, 26, e16623. [Google Scholar] [CrossRef]
- Choi, A.; Seong, J.W.; Kim, J.H.; Lee, J.Y.; Cho, H.J.; Kang, S.A.; Park, M.K.; Jeong, M.J.; Choi, S.Y.; Jeong, Y.J.; et al. Presence and diversity of free-living amoebae and their potential application as water quality indicators. Parasites Hosts Dis. 2024, 62, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Spottiswoode, N.; Haston, J.C.; Hanners, N.W.; Gruenberg, K.; Kim, A.; DeRisi, J.L.; Wilson, M.R. Challenges and advances in the medical treatment of granulomatous amebic encephalitis. Ther. Adv. Infect. Dis. 2024, 11, 20499361241228340. [Google Scholar] [CrossRef]
- Lemke, A.; Kiderlen, A.F.; Petri, B.; Kayser, O. Delivery of amphotericin B nanosuspensions to the brain and determination of activity against Balamuthia mandrillaris amebas. Nanomedicine 2010, 6, 597–603. [Google Scholar] [CrossRef]
- Aqeel, Y.; Iqbal, J.; Siddiqui, R.; Gilani, A.H.; Khan, N.A. Anti-Acanthamoebic properties of resveratrol and demethoxycurcumin. Exp. Parasitol. 2012, 132, 519–523. [Google Scholar] [CrossRef]
- Tiewcharoen, S.; Phurttikul, W.; Rabablert, J.; Auewarakul, P.; Roytrakul, S.; Chetanachan, P.; Atithep, T.; Junnu, V. Effect of synthetic antimicrobial peptides on Naegleria fowleri trophozoites. Southeast Asian J. Trop. Med. Public Health 2014, 45, 537–546. [Google Scholar]
- Rabablert, J.; Tiewcharoen, S.; Auewarakul, P.; Atithep, T.; Lumlerdkij, N.; Vejaratpimol, R.; Junnu, V. Anti-amebic activity of diosgenin on Naegleria fowleri trophozoites. Southeast Asian J. Trop. Med. Public Health 2015, 46, 827–834. [Google Scholar]
- Wehelie, Y.I.; Khan, N.A.; Fatima, I.; Anwar, A.; Kanwal, K.; Khan, K.M.; Siddiqui, R.; Tong, Y.K.; Anwar, A. Novel Tetrazoles against Acanthamoeba castellanii Belonging to the T4 Genotype. Chemotherapy 2022, 67, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; El-Gamal, M.I.; Saeed, B.Q.; Oh, C.H.; Abdel-Maksoud, M.S.; Khan, N.A.; Alharbi, A.M.; Alfahemi, H.; Siddiqui, R. Antiamoebic Activity of Imidazothiazole Derivatives against Opportunistic Pathogen Acanthamoeba castellanii. Antibiotics 2022, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; El-Gamal, M.I.; Boghossian, A.; Saeed, B.Q.; Oh, C.H.; Abdel-Maksoud, M.S.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. Imidazothiazole Derivatives Exhibited Potent Effects against Brain-Eating Amoebae. Antibiotics 2022, 11, 1515. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; da Silva, T.C.B.; Rott, E.B.; Rott, F.B.; Leite, A.P.M.C.; Benitez, G.B.; Neuana, N.F.; Goldim, J.R.; Rott, M.B.; Zanette, R.A. Effectiveness of phytoproducts against pathogenic free-living amoebae—A scoping and critical review paving the way toward plant-based pharmaceuticals. Fitoterapia 2025, 182, 106404. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasir, S.; Mungroo, M.R.; Shahabuddin, S.; Siddiqui, R.; Khan, N.A.; Anwar, A. Polyaniline-Conjugated Boron Nitride Nanoparticles Exhibiting Potent Effects against Pathogenic Brain-Eating Amoebae. ACS Chem. Neurosci. 2021, 12, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasir, S.; Mungroo, M.R.; Shahabuddin, S.; Siddiqui, R.; Khan, N.A.; Ahmad, I.; Anwar, A. Polyaniline (PANI)-conjugated tungsten disulphide (WS2) nanoparticles as potential therapeutics against brain-eating amoebae. Appl. Microbiol. Biotechnol. 2022, 106, 3279–3291. [Google Scholar] [CrossRef]
- Abdelnasir, S.; Mungroo, M.R.; Chew, J.; Siddiqui, R.; Khan, N.A.; Ahmad, I.; Shahabuddin, S.; Anwar, A. Applications of Polyaniline-Based Molybdenum Disulfide Nanoparticles against Brain-Eating Amoebae. ACS Omega 2023, 8, 8237–8247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Ho, K.Y.; Simon, S.E.; Saad, S.M.; Ong, S.K.; Anwar, A.; Tan, K.O.; Sridewi, N.; Khan, K.M.; Khan, N.A.; et al. Potential anti-acanthamoebic effects through inhibition of CYP51 by novel quinazolinones. Acta Trop. 2022, 231, 106440. [Google Scholar] [CrossRef]
- Ahmed, U.; Manzoor, M.; Qureshi, S.; Mazhar, M.; Fatima, A.; Aurangzeb, S.; Hamid, M.; Khan, K.M.; Khan, N.A.; Rashid, Y.; et al. Anti-amoebic effects of synthetic acridine-9(10H)-one against brain-eating amoebae. Acta Trop. 2023, 239, 106824. [Google Scholar] [CrossRef]
- Ahmed, U.; Ong, S.K.; Khan, K.M.; Siddiqui, R.; Khan, N.A.; Shaikh, M.F.; Alawfi, B.S.; Anwar, A. Effect of embelin on inhibition of cell growth and induction of apoptosis in A. castellanii. Arch. Microbiol. 2023, 205, 360. [Google Scholar] [CrossRef]
- Ahmed, U.; Ong, S.K.; Tan, K.O.; Khan, K.M.; Khan, N.A.; Siddiqui, R.; Alawfi, B.S.; Anwar, A. Alpha-Mangostin and its nano-conjugates induced programmed cell death in Acanthamoeba castellanii belonging to the T4 genotype. Int. Microbiol. 2024, 27, 1063–1081. [Google Scholar] [CrossRef]
- Akbar, N.; Kaman, W.E.; Sarink, M.; Nazmi, K.; Bikker, F.J.; Khan, N.A.; Siddiqui, R. Novel Antiamoebic Tyrocidine-Derived Peptide against Brain-Eating Amoebae. ACS Omega 2022, 7, 28797–28805. [Google Scholar] [CrossRef]
- Akbar, N.; Siddiqui, R.; El-Gamal, M.I.; Zaraei, S.O.; Alawfi, B.S.; Khan, N.A. The anti-amoebic potential of carboxamide derivatives containing sulfonyl or sulfamoyl moieties against brain-eating Naegleria fowleri. Parasitol. Res. 2023, 122, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Siddiqui, R.; El-Gamal, M.I.; Zaraei, S.O.; Saeed, B.Q.; Alawfi, B.S.; Khan, N.A. Potential anti-amoebic activity of sulfonate- and sulfamate-containing carboxamide derivatives against pathogenic Acanthamoeba castellanii belonging to the genotype T4. Parasitol. Int. 2024, 98, 102814. [Google Scholar] [CrossRef]
- Alishba; Ahmed, U.; Taha, M.; Khan, N.A.; Salar, U.; Khan, K.M.; Anwar, A.; Siddiqui, R. Potential anti-amoebic effects of synthetic 1,4-benzothiazine derivatives against Acanthamoeba castellanii. Heliyon 2024, 10, e23258. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Gold Nanoparticle-Conjugated Cinnamic Acid Exhibits Antiacanthamoebic and Antibacterial Properties. Antimicrob. Agents Chemother. 2018, 62, e00630-18. [Google Scholar] [CrossRef]
- Anwar, A.; Abdalla, S.A.O.; Aslam, Z.; Shah, M.R.; Siddiqui, R.; Khan, N.A. Oleic acid-conjugated silver nanoparticles as efficient antiamoebic agent against Acanthamoeba castellanii. Parasitol. Res. 2019, 118, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Chi Fung, L.; Anwar, A.; Jagadish, P.; Numan, A.; Khalid, M.; Shahabuddin, S.; Siddiqui, R.; Khan, N.A. Effects of Shape and Size of Cobalt Phosphate Nanoparticles against Acanthamoeba castellanii. Pathogens 2019, 8, 260. [Google Scholar] [CrossRef]
- Anwar, A.; Numan, A.; Siddiqui, R.; Khalid, M.; Khan, N.A. Cobalt nanoparticles as novel nanotherapeutics against A. castellanii. Parasites Vectors 2019, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Shahbaz, M.S.; Saad, S.M.; Kanwal; Khan, K.M.; Siddiqui, R.; Khan, N.A. Novel antiacanthamoebic compounds belonging to quinazolinones. Eur. J. Med. Chem. 2019, 182, 111575. [Google Scholar] [CrossRef]
- Anwar, A.; Mungroo, M.R.; Khan, S.; Fatima, I.; Rafique, R.; Kanwal; Khan, K.M.; Siddiqui, R.; Khan, N.A. Novel Azoles as Antiparasitic Remedies against Brain-Eating Amoebae. Antibiotics 2020, 9, 188. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Hameed, A.; Shah, M.R.; Khan, N.A. Synthetic Dihydropyridines as Novel Antiacanthamoebic Agents. Med. Chem. 2020, 16, 841–847. [Google Scholar] [CrossRef]
- Aqeel, Y.; Siddiqui, R.; Anwar, A.; Shah, M.R.; Khoja, S.; Khan, N.A. Photochemotherapeutic strategy against Acanthamoeba infections. Antimicrob. Agents Chemother. 2015, 59, 3031–3041. [Google Scholar] [CrossRef]
- Atalay, H.T.; Dogruman-Al, F.; Sarzhanov, F.; Özmen, M.C.; Tefon, A.B.; Arıbaş, Y.K.; Bilgihan, K. Effect of Riboflavin/Rose Bengal-Mediated PACK-CXL on Acanthamoeba Trophozoites and Cysts in vitro. Curr. Eye Res. 2018, 43, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Bittner Fialová, S.; Kello, M.; Čoma, M.; Slobodníková, L.; Drobná, E.; Holková, I.; Garajová, M.; Mrva, M.; Zachar, V.; Lukáč, M. Derivatization of Rosmarinic Acid Enhances its in vitro Antitumor, Antimicrobial and Antiprotozoal Properties. Molecules 2019, 24, 1078. [Google Scholar] [CrossRef] [PubMed]
- Boonman, N.; Prachya, S.; Boonmee, A.; Kittakoop, P.; Wiyakrutta, S.; Sriubolmas, N.; Warit, S.; Chusattayanond, A.S.-A. In vitro acanthamoebicidal activity of fusaric acid and dehydrofusaric acid from an endophytic fungus Fusarium sp. Tlau3. Planta Med. 2012, 78, 1562–1567. [Google Scholar] [PubMed]
- Borase, H.P.; Patil, C.D.; Sauter, I.P.; Rott, M.B.; Patil, S.V. Amoebicidal activity of phytosynthesized silver nanoparticles and their in vitro cytotoxicity to human cells. FEMS Microbiol. Lett. 2013, 345, 127–131. [Google Scholar] [CrossRef]
- Cariello, A.J.; de Souza, G.F.; Foronda, A.S.; Yu, M.C.; Hofling-Lima, A.L.; de Oliveira, M.G. In vitro amoebicidal activity of S-nitrosoglutathione and S-nitroso-N-acetylcysteine against trophozoites of Acanthamoeba castellanii. J. Antimicrob. Chemother. 2010, 65, 588–591. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Delgado-Hernández, S.; Arberas-Jiménez, I.; Sifaoui, I.; Tejedor, D.; García-Tellado, F.; Piñero, J.E.; Lorenzo-Morales, J. Synthesis and Biological Evaluation of Cyanoacrylamides and 5-Iminopyrrol-2-Ones Against Naegleria fowleri. ACS Infect. Dis. 2024, 10, 3332–3345. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J. Exploring therapeutic approaches against Naegleria fowleri infections through the COVID box. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100545. [Google Scholar] [CrossRef]
- Chen, Z.; Xuguang, S.; Zhiqun, W.; Ran, L. In vitro amoebacidal activity of photodynamic therapy on Acanthamoeba. Br. J. Ophthalmol. 2008, 92, 1283–1286. [Google Scholar] [CrossRef]
- Chuprom, J.; Sangkanu, S.; Mitsuwan, W.; Boonhok, R.; Mahabusarakam, W.; Singh, L.R.; Dumkliang, E.; Jitrangsri, K.; Paul, A.K.; Surinkaew, S.; et al. Anti-Acanthamoeba activity of a semi-synthetic mangostin derivative and its ability in removal of Acanthamoeba triangularis WU19001 on contact lens. PeerJ 2022, 10, e14468. [Google Scholar] [CrossRef]
- Corrêa, T.Q.; Geralde, M.C.; Carvalho, M.T.; Bagnato, V.S.; Kurachi, C.S.; Clovis, W.O. Photodynamic inactivation of Acanthamoeba polyphaga with curcuminoids: An in vitro study. Proc. SPIE 2016, 9694, 969415. [Google Scholar]
- da Silva, A.; Nobre, H., Jr.; Sampaio, L.; Nascimento, B.D.; da Silva, C.; de Andrade Neto, J.B.; Manresa, Á.; Pinazo, A.; Cavalcanti, B.; de Moraes, M.O.; et al. Antifungal and antiprotozoal green amino acid-based rhamnolipids: Mode of action, antibiofilm efficiency and selective activity against resistant Candida spp. strains and Acanthamoeba castellanii. Colloids Surf. B Biointerfaces 2020, 193, 111148. [Google Scholar] [CrossRef]
- Debnath, A.; Tunac, J.B.; Silva-Olivares, A.; Galindo-Gómez, S.; Shibayama, M.; McKerrow, J.H. In vitro efficacy of corifungin against Acanthamoeba castellanii trophozoites and cysts. Antimicrob. Agents Chemother. 2014, 58, 1523–1528. [Google Scholar] [CrossRef]
- Debnath, A.; Nelson, A.T.; Silva-Olivares, A.; Shibayama, M.; Siegel, D.; McKerrow, J.H. In vitro Efficacy of Ebselen and BAY 11-7082 Against Naegleria fowleri. Front. Microbiol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Fabres, L.F.; da Costa Gonçalves, F.; Duarte, E.O.S.; Berté, F.K.; da Conceição, D.K.S.L.; Ferreira, L.A.; Schrekker, H.S.; Rott, M.B. In vitro Amoebicidal Activity of Imidazolium Salts Against Trophozoites. Acta Parasitol. 2020, 65, 317–326. [Google Scholar] [CrossRef]
- Ferrante, A.; Lederer, E. Curative properties of muramyl dipeptide in experimental Naegleria meningoencephalitis. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Abell, T.J.; Robinson, B.; Lederer, E. Effects of sinefungin and difluoromethylornithine on pathogenic free-living amoebae in vitro. FEMS Microbiol. Lett. 1987, 40, 1. [Google Scholar] [CrossRef]
- Ferrins, L.; Buskes, M.J.; Kapteyn, M.M.; Engels, H.N.; Enos, S.E.; Lu, C.; Klug, D.M.; Singh, B.; Quotadamo, A.; Bachovchin, K.; et al. Corrigendum: Identification of novel anti-amoebic pharmacophores from kinase inhibitor chemotypes. Front. Microbiol. 2023, 14, 1304196. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.; Coppellotti, O.; Roncucci, G.; Ben Amor, T.; Jori, G. Photosensitized inactivation of Acanthamoeba palestinensis in the cystic stage. J. Appl. Microbiol. 2006, 101, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Garajová, M.; Mrva, M.; Timko, L.; Lukáč, M.; Ondriska, F. Cytomorphological changes and susceptibility of clinical isolates of Acanthamoeba spp. to heterocyclic alkylphosphocholines. Exp. Parasitol. 2014, 145, S102–S110. [Google Scholar] [CrossRef]
- González-Fernández, S.; Lozano-Iturbe, V.; Menéndez, M.F.; Ordiales, H.; Fernández-Vega, I.; Merayo, J.; Vazquez, F.; Quirós, L.M.; Martín, C. A Promising Antifungal and Antiamoebic Effect of Silver Nanorings, a Novel Type of AgNP. Antibiotics 2022, 11, 1054. [Google Scholar] [CrossRef]
- Hendiger, E.B.; Padzik, M.; Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Rizo-Liendo, A.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Chiboub, O.; Rodríguez-Expósito, R.L.; et al. Silver Nanoparticles as a Novel Potential Preventive Agent against Acanthamoeba Keratitis. Pathogens 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Heredero-Bermejo, I.; Copa-Patiño, J.L.; Soliveri, J.; García-Gallego, S.; Rasines, B.; Gómez, R.; de la Mata, F.J.; Pérez-Serrano, J. In vitro evaluation of the effectiveness of new water-stable cationic carbosilane dendrimers against Acanthamoeba castellanii UAH-T17c3 trophozoites. Parasitol. Res. 2013, 112, 961–969. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Copa-Patiño, J.L.; Soliveri, J.; Fuentes-Paniagua, E.; de la Mata, F.J.; Gomez, R.; Perez-Serrano, J. Evaluation of the activity of new cationic carbosilane dendrimers on trophozoites and cysts of Acanthamoeba polyphaga. Parasitol. Res. 2015, 114, 473–486. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Martín-Pérez, T.; Copa-Patiño, J.L.; Gómez, R.; de la Mata, F.J.; Soliveri, J.; Pérez-Serrano, J. Ultrastructural Study of Acanthamoeba polyphaga Trophozoites and Cysts Treated In vitro with Cationic Carbosilane Dendrimers. Pharmaceutics 2020, 12, 565. [Google Scholar] [CrossRef]
- Hezarjaribi, H.Z.; Toluee, E.; Saberi, R.; Dadi Moghadam, Y.; Fakhar, M.; Akhtari, J. In vitro anti-Acanthamoeba activity of commercial chitosan and nano-chitosan against pathogenic Acanthamoeba genotype T4. J. Parasit. Dis. 2021, 45, 921–929. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Yusof, M.S.; Amin, N.M. Anti-amoebic properties of carbonyl thiourea derivatives. Molecules 2014, 19, 5191–5204. [Google Scholar] [CrossRef] [PubMed]
- Kanwal; Mungroo, M.R.; Anwar, A.; Ali, F.; Khan, S.; Abdullah, M.A.; Siddiqui, R.; Khan, K.M.; Khan, N.A. Synthetic nanoparticle-conjugated bisindoles and hydrazinyl arylthiazole as novel antiamoebic agents against brain-eating amoebae. Exp. Parasitol. 2020, 218, 107979. [Google Scholar] [CrossRef]
- Kassab, K.; Dei, D.; Roncucci, G.; Jori, G.; Coppellotti, O. Phthalocyanine-photosensitized inactivation of a pathogenic protozoan, Acanthamoeba palestinensis. Photochem. Photobiol. Sci. 2003, 2, 668–672. [Google Scholar] [CrossRef]
- Kennedy, S.M.; Deshpande, P.; Gallagher, A.G.; Horsburgh, M.J.; Allison, H.E.; Kaye, S.B.; Wellings, D.A.; Williams, R.L. Amoebicidal Activity of Poly-Epsilon-Lysine Functionalized Hydrogels. Investig. Ophthalmol. Vis. Sci. 2022, 63, 11. [Google Scholar] [CrossRef] [PubMed]
- Khairul, W.M.; Goh, Y.-P.; Daud, A.I.; Nakisah, M.A. Cytotoxicity effects of alkoxy substituted thiourea derivatives towards Acanthamoeba sp. Arab. J. Chem. 2015, 10, 532–538. [Google Scholar]
- Khairul, W.M.; Goh, Y.-P.; Daud, A.I.; Nakisah, M.A. Bringing Forward the New Generation of Alkoxy-Thiourea as Potential Treatment for Acanthamoeba Keratitis. AIP Conf. Proc. 2017, 1817, 030001-1–030001-8. [Google Scholar]
- Kopańska, K.; Najda, A.; Zebrowska, J.; Chomicz, L.; Piekarczyk, J.; Myjak, P.; Bretner, M. Synthesis and activity of 1H-benzimidazole and 1H-benzotriazole derivatives as inhibitors of A. castellanii. Bioorg. Med. Chem. 2004, 12, 2617–2624. [Google Scholar] [CrossRef]
- Kusrini, E.; Hashim, F.; Azmi, W.N.; Amin, N.M.; Estuningtyas, A. A novel antiamoebic agent against Acanthamoeba sp.—A causative agent for eye keratitis infection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 714–721. [Google Scholar] [CrossRef]
- Kusrini, E.; Hashim, F.; Gunawan, C.; Mann, R.; Azmi, W.N.; Amin, N.M. Anti-amoebic activity of acyclic and cyclic-samarium complexes on Acanthamoeba. Parasitol. Res. 2018, 117, 1409–1417. [Google Scholar] [CrossRef]
- Kusrini, E.; Hashim, F.; Saleh, M.I.; Adnan, R.; Usman, A.; Zakaria, I.N.; Prihandini, W.W.; Putra, N.; Prasetyanto, E.A. Monoclinic cerium(III) picrate tetraethylene glycol complex: Design, synthesis and biological evaluation as anti-amoebic activity against Acanthamoeba sp. J. Mater. Sci. 2020, 55, 9795–9811. [Google Scholar] [CrossRef]
- Lê, H.G.; Kang, J.M.; Võ, T.C.; Na, B.K. Kaempferol induces programmed cell death in Naegleria fowleri. Phytomedicine 2023, 119, 154994. [Google Scholar] [CrossRef] [PubMed]
- López-Arencibia, A.; Reyes-Batlle, M.; Freijo, M.B.; McNaughton-Smith, G.; Martín-Rodríguez, P.; Fernández-Pérez, L.; Sifaoui, I.; Wagner, C.; García-Méndez, A.B.; Liendo, A.R.; et al. In vitro activity of 1H-phenalen-1-one derivatives against Acanthamoeba castellanii Neff and their mechanisms of cell death. Exp. Parasitol. 2017, 183, 218–223. [Google Scholar] [CrossRef] [PubMed]
- López-Barona, P.; Verdú-Expósito, C.; Martín-Pérez, T.; Gómez-Casanova, N.; Lozano-Cruz, T.; Ortega, P.; Gómez, R.; Pérez-Serrano, J.; Heredero-Bermejo, I. Amoebicidal activity of cationic carbosilane dendrons derived with 4-phenylbutyric acid against Acanthamoeba griffini and Acanthamoeba polyphaga trophozoites and cysts. Sci. Rep. 2022, 12, 14926. [Google Scholar] [CrossRef]
- Lukáč, M.; Timko, L.; Mrva, M.; Ondriska, F.; Karlovská, J.; Valentová, J.; Lacko, I. Synthesis, aggregation properties, and antiprotozoal activity of heterocyclic heterogemini surfactants. Heteroat. Chem. 2010, 21, 203–209. [Google Scholar] [CrossRef]
- Lukáč, M.; Mrva, M.; Garajová, M.; Mojžišová, G.; Varinská, L.; Mojžiš, J.; Sabol, M.; Kubincová, J.; Haragová, H.; Ondriska, F.; et al. Synthesis, self-aggregation and biological properties of alkylphosphocholine and alkylphosphohomocholine derivatives of cetyltrimethylammonium bromide, cetylpyridinium bromide, benzalkonium bromide (C16) and benzethonium chloride. Eur. J. Med. Chem. 2013, 66, 46–55. [Google Scholar] [CrossRef]
- Lukáč, M.; Garajová, M.; Mrva, M.; Devínsky, F.; Ondriska, F.; Kubincová, J. Novel fluorinated dialkylphosphonatocholines: Synthesis, physicochemical properties and antiprotozoal activities against Acanthamoeba spp. J. Fluor. Chem. 2014, 164, 10–17. [Google Scholar] [CrossRef]
- Lukáč, M.; Pisárčik, M.; Garajová, M.; Mrva, M.; Dušeková, A.; Vrták, A.; Horáková, R.; Horváth, B.; Devínsky, F. Synthesis, Surface Activity, and Biological Activities of Phosphonium and Metronidazole Salts. J. Surfactants Deterg. 2020, 23, 1025–1032. [Google Scholar] [CrossRef]
- Lukáč, M.; Slobodníková, L.; Mrva, M.; Dušeková, A.; Garajová, M.; Kello, M.; Šebová, D.; Pisárčik, M.; Kojnok, M.; Vrták, A.; et al. Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents. Int. J. Mol. Sci. 2024, 25, 1200. [Google Scholar] [CrossRef]
- Mahboob, T.; Nawaz, M.; Tian-Chye, T.; Samudi, C.; Wiart, C.; Nissapatorn, V. Preparation of Poly (dl-Lactide-co-Glycolide) Nanoparticles Encapsulated with Periglaucine A and Betulinic Acid for In Vitro Anti-Acanthamoeba and Cytotoxicity Activities. Pathogens 2018, 7, 62. [Google Scholar] [CrossRef]
- Martín-Navarro, C.M.; López-Arencibia, A.; Lorenzo-Morales, J.; Oramas-Royo, S.; Hernández-Molina, R.; Estévez-Braun, A.; Ravelo, A.G.; Valladares, B.; Piñero, J.E. Acanthamoeba castellanii Neff: In vitro activity against the trophozoite stage of a natural sesquiterpene and a synthetic cobalt(II)-lapachol complex. Exp. Parasitol. 2010, 126, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Castillo, M.; Ramírez-Rico, G.; Shibayama, M.; de la Garza, M.; Serrano-Luna, J. Lactoferrin and Lysozyme Inhibit the Proteolytic Activity and Cytopathic Effect of Naegleria fowleri Enzymes. Pathogens 2024, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pérez, T.; Lozano-Cruz, T.; Criado-Fornelio, A.; Ortega, P.; Gómez, R.; de la Mata, F.J.; Pérez-Serrano, J. Synthesis and in vitro activity of new biguanide-containing dendrimers on pathogenic isolates of Acanthamoeba polyphaga and Acanthamoeba griffini. Parasitol. Res. 2019, 118, 1953–1961. [Google Scholar] [CrossRef]
- Masri, A.; Abdelnasir, S.; Anwar, A.; Iqbal, J.; Numan, A.; Jagadish, P.; Shahabuddin, S.; Khalid, M. Antimicrobial properties of multifunctional polypyrrole-cobalt oxide-silver nanocomposite against pathogenic bacteria and parasite. Appl. Microbiol. Biotechnol. 2021, 105, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Mito, T.; Suzuki, T.; Kobayashi, T.; Zheng, X.; Hayashi, Y.; Shiraishi, A.; Ohashi, Y. Effect of photodynamic therapy with methylene blue on Acanthamoeba in vitro. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6305–6313. [Google Scholar] [CrossRef]
- Osuna, A.; Rodriguez-Santiago, J.I.; Ruiz-Perez, L.M.; Gamarro, F.; Castanys, S.; Giovannangeli, G.; Galy, A.M.; Galy, J.P.; Soyfer, J.C.; Barbe, J. Antiamebic activity of new acridinic derivatives against Naegleria and Acanthamoeba species in vitro. Chemotherapy 1987, 33, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Padzik, M.; Hendiger, E.B.; Chomicz, L.; Grodzik, M.; Szmidt, M.; Grobelny, J.; Lorenzo-Morales, J. Tannic acid-modified silver nanoparticles as a novel therapeutic agent against Acanthamoeba. Parasitol. Res. 2018, 117, 3519–3525. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, C.Y. Effects of In vitro Combination of Nitric Oxide Donors and Hypochlorite on Acanthamoeba castellanii Viability. Transl. Vis. Sci. Technol. 2023, 12, 23. [Google Scholar] [CrossRef]
- Peguda, H.K.; Carnt, N.A.; Gu, Z.; Kumar, N.; Willcox, M.D.P.; Kuppusamy, R. The Anti-Amoebic Activity of a Peptidomimetic against Acanthamoeba castellanii. Microorganisms 2022, 10, 2377. [Google Scholar] [CrossRef]
- Peguda, H.K.; Lakshminarayanan, R.; Carnt, N.A.; Gu, Z.; Willcox, M.D.P. The Activity of Polyhomoarginine against A. castellanii. Biology 2023, 11, 1726. [Google Scholar]
- Polat, Z.A.; Savage, P.B.; Genberg, C. In vitro amoebicidal activity of a ceragenin, cationic steroid antibiotic-13, against Acanthamoeba castellanii and its cytotoxic potential. J. Ocul. Pharmacol. Ther. 2011, 27, 1–5. [Google Scholar] [CrossRef]
- Pomeroy, J.M.; Khalifa, M.M.; Milanes, J.E.; Palmentiero, C.M.; Morris, J.C.; Golden, J.E. Synthesis and Evaluation of Benzylamine Inhibitors of Neuropathogenic Naegleria fowleri “Brain-Eating” Amoeba. ACS Med. Chem. Lett. 2023, 15, 87–92. [Google Scholar] [CrossRef]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Shah, M.R.; Siddiqui, R. trans-Cinnamic Acid Conjugated Gold Nanoparticles as Potent Therapeutics against Brain-Eating Amoeba Naegleria fowleri. ACS Chem. Neurosci. 2019, 10, 2692–2696. [Google Scholar] [CrossRef]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Aslam, Z.; Shah, M.R.; Siddiqui, R. Oleic Acid Coated Silver Nanoparticles Showed Better in vitro Amoebicidal Effects against Naegleria fowleri than Amphotericin B. ACS Chem. Neurosci. 2020, 11, 2431–2437. [Google Scholar] [CrossRef]
- Rajendran, K.; Ahmed, U.; Meunier, A.C.; Shaikh, M.F.; Siddiqui, R.; Anwar, A. Nanoparticle-Terpene Fusion: A Game-Changer in Combating Primary Amoebic Meningoencephalitis Caused by Naegleria fowleri. ACS Omega 2024, 9, 11597–11607. [Google Scholar] [CrossRef]
- Raza, R.; Matin, A.; Sarwar, S.; Barsukova-Stuckart, M.; Ibrahim, M.; Kortz, U.; Iqbal, J. Polyoxometalates as potent and selective inhibitors of alkaline phosphatases with profound anticancer and amoebicidal activities. Dalton Trans. 2012, 41, 14329–14336. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Freijo, M.B.; López-Arencibia, A.; Lorenzo-Morales, J.; McNaughton-Smith, G.; Piñero, J.E.; Abad-Grillo, T. Identification of N-acyl quinolin-2(1H)-ones as new selective agents against clinical isolates of Acanthamoeba keratitis. Bioorg. Chem. 2020, 99, 103791. [Google Scholar] [CrossRef]
- Rice, C.A.; Troth, E.V.; Russell, A.C.; Kyle, D.E. Discovery of Anti-Amoebic Inhibitors from Screening the MMV Pandemic Response Box on Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba castellanii. Pathogens 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Liendo, A.; Sifaoui, I.; Cartuche, L.; Arberas-Jiménez, I.; Reyes-Batlle, M.; Fernández, J.J.; Piñero, J.E.; Díaz-Marrero, A.R.; Lorenzo-Morales, J. Evaluation of Indolocarbazoles from Streptomyces sanyensis as a Novel Source of Therapeutic Agents against the Brain-Eating Amoeba Naegleria fowleri. Microorganisms 2020, 8, 789. [Google Scholar] [CrossRef]
- Rizo-Liendo, A.; Arberas-Jiménez, I.; Sifaoui, I.; Gkolfi, D.; Santana, Y.; Cotos, L.; Tejedor, D.; García-Tellado, F.; Piñero, J.E.; Lorenzo-Morales, J. The therapeutic potential of novel isobenzofuranones against Naegleria fowleri. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Liendo, A.; Arberas-Jiménez, I.; Martin-Encinas, E.; Sifaoui, I.; Reyes-Batlle, M.; Chao-Pellicer, J.; Alonso, C.; Palacios, F.; Piñero, J.E.; Lorenzo-Morales, J. Naphthyridine Derivatives Induce Programmed Cell Death in Naegleria fowleri. Pharmaceuticals 2021, 14, 1013. [Google Scholar] [CrossRef]
- Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; Sifaoui, I.; Tejedor, D.; García-Tellado, F.; Piñero, J.E.; Lorenzo-Morales, J. Isobenzofuran-1(3H)-one derivatives: Amoebicidal activity and program cell death in Acanthamoeba castellanii Neff. Biomed. Pharmacother. 2022, 150, 113062. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.Q.; Hussain, K.; Akbar, N.; Khan, H.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Nanovesicles containing curcumin hold promise in the development of new formulations of anti-Acanthamoebic agents. Mol. Biochem. Parasitol. 2022, 247, 111430. [Google Scholar] [CrossRef]
- Shahbaz, M.S.; Anwar, A.; Saad, S.M.; Kanwal; Anwar, A.; Khan, K.M.; Siddiqui, R.; Khan, N.A. Antiamoebic activity of 3-aryl-6,7-dimethoxyquinazolin-4(3H)-one library against Acanthamoeba castellanii. Parasitol. Res. 2020, 119, 2327–2335. [Google Scholar] [CrossRef]
- Shirai, A.; Endo, T.; Maseda, H.; Omasa, T. Synthesis of thiazole derivatives and evaluation of their antiamoebic activity and cytotoxicity. Biocontrol Sci. 2013, 18, 183–191. [Google Scholar] [CrossRef][Green Version]
- Siddiqui, R.; Khan, N.A. Photochemotherapeutic strategies against Acanthamoeba keratitis. AMB Express 2012, 2, 47. [Google Scholar] [CrossRef]
- Siddiqui, R.; Abjani, F.; Yeo, C.I.; Tiekink, E.R.; Khan, N.A. The effects of phosphanegold(I) thiolates on the biological properties of Acanthamoeba castellanii belonging to the T4 genotype. J. Negat. Results Biomed. 2017, 16, 6. [Google Scholar] [CrossRef]
- Siddiqui, R.; Boghossian, A.; Khatoon, B.; Kawish, M.; Alharbi, A.M.; Shah, M.R.; Alfahemi, H.; Khan, N.A. Antiamoebic Properties of Metabolites against Naegleria fowleri and Balamuthia mandrillaris. Antibiotics 2022, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Boghossian, A.; Akbar, N.; Jabri, T.; Aslam, Z.; Shah, M.R.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. Zinc Oxide Nanoconjugates against Brain-Eating Amoebae. Antibiotics 2022, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Mungroo, M.R.; Anuar, T.S.; Alharbi, A.M.; Alfahemi, H.; Elmoselhi, A.B.; Khan, N.A. Antiamoebic Properties of Laboratory and Clinically Used Drugs against Naegleria fowleri and Balamuthia mandrillaris. Antibiotics 2022, 11, 749. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Akbar, N.; Khamis, M.; Ibrahim, T.; Khan, A.S.; Khan, N.A. Antiamoebic properties of Methyltrioctylammonium chloride based deep eutectic solvents. Cont. Lens Anterior Eye 2023, 46, 101758. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; El-Gamal, M.I.; Sajeev, S.; Zaraei, S.O.; Khan, N.A. Novel anti-Acanthamoebic properties of raloxifene sulfonate/sulfamate derivatives. Mol. Biochem. Parasitol. 2023, 256, 111582. [Google Scholar] [CrossRef]
- Siddiqui, R.; Rawas-Qalaji, M.; El-Gamal, M.I.; Sajeev, S.; Jagal, J.; Zaraei, S.O.; Sbenati, R.M.; Anbar, H.S.; Dohle, W.; Potter, B.V.L.; et al. Novel Anti-Acanthamoebic Activities of Irosustat and STX140 and Their Nanoformulations. Antibiotics 2023, 12, 561. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khatoon, B.; Kawish, M.; Sajeev, S.; Faizi, S.; Shah, M.R.; Alharbi, A.M.; Khan, N.A. The potential of nanocomposites (patuletin-conjugated with gallic acid-coated zinc oxide) against free-living amoebae pathogens. Int. Microbiol. 2024, 28, 929–939. [Google Scholar] [CrossRef]
- Sifaoui, I.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; Rizo-Liendo, A.; Piñero, J.E.; Bazzocchi, I.L.; Lorenzo-Morales, J.; Jiménez, I.A. Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba spp. Pathogens 2019, 8, 130. Pathogens 2019, 8, 130. [Google Scholar] [CrossRef]
- Sifaoui, I.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; Dumpiérrez Ramos, A.; Diana-Rivero, R.; García-Tellado, F.; Tejedor, D.; Piñero, J.E.; Lorenzo-Morales, J. Amoebicidal effect of synthetic indoles against Acanthamoeba spp.: A study of cell death. Antimicrob. Agents Chemother. 2024, 68, e0165123. [Google Scholar] [CrossRef]
- Sohn, H.J.; Park, A.Y.; Lee, J.H.; Yun, K.H.; Song, K.J.; Kim, J.H.; Shin, H.J. Amoebicidal effect of chlorine dioxide gas against pathogenic Naegleria fowleri and Acanthamoeba polyphaga. Parasitol. Res. 2024, 123, 192. [Google Scholar] [CrossRef]
- Souza, G.B.; Santos, T.A.C.; Silva, A.P.S.; Barreiros, A.L.B.S.; Nardelli, V.B.; Siqueira, I.B.; Dolabella, S.S.; Costa, E.V.; Alves, P.B.; Scher, R.; et al. Synthesis of chalcone derivatives by Claisen-Schmidt condensation and in vitro analyses of their antiprotozoal activities. Nat. Prod. Res. 2024, 38, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Timko, L.; Fischer-Fodor, E.; Garajová, M.; Mrva, M.; Chereches, G.; Ondriska, F.; Bukovský, M.; Lukáč, M.; Karlovská, J.; Kubincová, J.; et al. Synthesis of structural analogues of hexadecylphosphocholine and their antineoplastic, antimicrobial and amoebicidal activity. Eur. J. Med. Chem. 2015, 93, 263–273. [Google Scholar] [CrossRef]
- Timko, L.; Pisárčik, M.; Mrva, M.; Garajová, M.; Juhásová, A.; Mojžiš, J.; Mojžišová, G.; Bukovský, M.; Devínsky, F.; Lukáč, M. Synthesis, physicochemical properties and biological activities of novel alkylphosphocholines with foscarnet moiety. Bioorg. Chem. 2020, 104, 104224. [Google Scholar] [CrossRef]
- Vázquez-Ortega, F.; Sifaoui, I.; Reyes-Batlle, M.; Piñero, J.E.; Lagunes, I.; Trigos, Á.; Lorenzo-Morales, J.; Díaz-Marrero, A.R.; Fernández, J.J. Photodynamic treatment induced membrane cell damage in Acanthamoeba castellanii Neff. Dyes Pigment. 2020, 180, 108481. [Google Scholar] [CrossRef]
- Walochnik, J.; Duchêne, M.; Seifert, K.; Obwaller, A.; Hottkowitz, T.; Wiedermann, G.; Eibl, H.; Aspöck, H. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 2002, 46, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Yim, B.; Park, J.H.; Jeong, H.; Hong, J.; Kim, M.; Chang, M.; Chuck, R.S.; Park, C.Y. Effect of Nitric Oxide on Acanthamoeba castellanii. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, Y.D.; Chikama, T.; Sueoka, K.; Ko, J.A.; Kiuchi, Y.; Onodera, M.; Sakaguchi, T. Antimicrobial Photodynamic Therapy with the photosensitizer TONS504 eradicates Acanthamoeba. Photodiagnosis Photodyn. Ther. 2019, 28, 166–171. [Google Scholar] [CrossRef]
- Pertiwi, Y.D.; Chikama, T.; Sueoka, K.; Ko, J.A.; Kiuchi, Y.; Onodera, M.; Sakaguchi, T. Efficacy of Photodynamic Anti-Microbial Chemotherapy for Acanthamoeba Keratitis In vivo. Lasers Surg. Med. 2021, 53, 695–702. [Google Scholar] [CrossRef]
- Büchele, M.L.C.; Nunes, B.F.; Filippin-Monteiro, F.B.; Caumo, K.S. Diagnosis and treatment of Acanthamoeba Keratitis: A scoping review demonstrating unfavorable outcomes. Cont. Lens Anterior Eye 2023, 46, 101844. [Google Scholar] [CrossRef]
- Petrillo, F.; Tortori, A.; Vallino, V.; Galdiero, M.; Fea, A.M.; De Sanctis, U.; Reibaldi, M. Understanding Acanthamoeba Keratitis: An In-Depth Review of a Sight-Threatening Eye Infection. Microorganisms 2024, 12, 758. [Google Scholar] [CrossRef] [PubMed]
- Leal Dos Santos, D.; Chaúque, B.J.M.; Virginio, V.G.; Cossa, V.C.; Pettan-Brewer, C.; Schrekker, H.S.; Rott, M.B. Occurrence of Naegleria fowleri and their implication for health—A look under the One Health approaches. Int. J. Hyg. Environ. Health 2022, 246, 114053. [Google Scholar] [CrossRef]
- Pengsart, W.; Tongkrajang, N.; Whangviboonkij, N.; Sarasombath, P.T.; Kulkeaw, K. Balamuthia mandrillaris trophozoites ingest human neuronal cells via a trogocytosis-independent mechanism. Parasitol. Vectors 2022, 15, 232. [Google Scholar] [CrossRef]
- Xu, C.; Wu, X.; Tan, M.; Wang, D.; Wang, S.; Wu, Y. Subacute Balamuthia mandrillaris encephalitis in an immunocompetent patient diagnosed by next-generation sequencing. J. Int. Med. Res. 2022, 50, 3000605221093217. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, J.; Wei, R.; Feng, X.; Wang, L.; Wang, L.; Zhao, P.; Yu, H.; Gu, Y.; Yao, Z. Facial Balamuthia mandrillaris infection with neurological involvement in an immunocompetent child. Lancet Infect. Dis. 2022, 22, e93–e100. [Google Scholar] [CrossRef]
- Alvarez, P.; Torres-Cabala, C.; Gotuzzo, E.; Bravo, F. Cutaneous balamuthiasis: A clinicopathological study. JAAD Int. 2022, 6, 51–58. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Q.; Meng, Q.; Wang, Z.; Li, S.; Cui, J. The Chemistry and Biological Effects of Thioflavones. Mini Rev. Med. Chem. 2018, 18, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, J.; Pan, G.; Reinke, A.W. Screening of the Pandemic Response Box identifies anti-microsporidia compounds. PLoS Negl. Trop. Dis. 2023, 17, e0011806. [Google Scholar] [CrossRef]
- Reader, J.; van der Watt, M.E.; Taylor, D.; Le Manach, C.; Mittal, N.; Ottilie, S.; Theron, A.; Moyo, P.; Erlank, E.; Nardini, L.; et al. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. Nat. Commun. 2021, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, R.; Bliton, J.; Goodman, A.; Ali, I.K.M.; Yoder, J.; Cope, J.R. Epidemiology and Clinical Characteristics of Primary Amebic Meningoencephalitis Caused by Naegleria fowleri: A Global Review. Clin. Infect. Dis. 2021, 73, e19–e27. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Zhang, Q.; Wen, S.Y.; Chen, F.F.; Zhou, C.Q. Pathogenic free-living amoebic encephalitis from 48 cases in China: A systematic review. Front. Neurol. 2023, 14, 1100785. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.A.; Citron, D.M.; Wang, C.C. Development of Tyrocidine A analogues with improved antibacterial activity. Bioorg. Med. Chem. 2007, 15, 6667–6677. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Omura, S. Chemical biology of natural indolocarbazole products: 30 years since the discovery of staurosporine. J. Antibiot. 2009, 62, 17–26. [Google Scholar] [CrossRef]
- Han, K.L.; Lee, H.J.; Shin, M.H.; Shin, H.J.; Im, K.I.; Park, S.J. The involvement of an integrin-like protein and protein kinase C in amoebic adhesion to fibronectin and amoebic cytotoxicity. Parasitol. Res. 2004, 94, 53–60. [Google Scholar] [CrossRef]
- Lê, H.G.; Kang, J.M.; Võ, T.C.; Na, B.K. Naegleria fowleri Cathepsin B Induces a Pro-Inflammatory Immune Response in BV-2 Microglial Cells via NF-κB and AP-1 Dependent-MAPK Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8388. [Google Scholar] [CrossRef]
- Hussain, T.; Yogavel, M.; Sharma, A. Inhibition of protein synthesis and malaria parasite development by drug targeting of methionyl-tRNA synthetases. Antimicrob. Agents Chemother. 2015, 59, 1856–1867. [Google Scholar] [CrossRef]
- Bickels, N.R.; Feldmesser, E.; Fridmann-Sirkis, Y.; Keren-Shaul, H.; Nevo, R.; Minsky, A.; Reich, Z. Acanthamoeba polyphaga de novo transcriptome and its dynamics during Mimivirus infection. Sci. Rep. 2024, 14, 25894. [Google Scholar] [CrossRef]
- Lazarovici, P.; Rasouly, D.; Friedman, L.; Tabekman, R.; Ovadia, H.; Matsuda, Y. K 252a and staurosporine microbial alkaloid toxins as prototype of neurotropic drugs. Adv. Exp. Med. Biol. 1996, 391, 367–377. [Google Scholar]
- Roux, P.P.; Dorval, G.; Boudreau, M.; Angers-Loustau, A.; Morris, S.J.; Makkerh, J.; Barker, P.A. K252a and CEP1347 are neuroprotective compounds that inhibit mixed-lineage kinase-3 and induce activation of Akt and ERK. J. Biol. Chem. 2002, 277, 49473–49480. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Koshino, H.; Kudo, T.; Onose, R.; Isono, K. A new inhibitor of protein kinase C, RK-1409 (7-oxostaurosporine). I. Taxonomy and biological activity. J. Antibiot. 1992, 45, 189–194. [Google Scholar] [CrossRef]
- Jimenez, P.C.; Wilke, D.V.; Ferreira, E.G.; Takeara, R.; De Moraes, M.O.; Silveira, E.R.; Da Cruz Lotufo, T.M.; Lopes, N.P.; Costa-Lotufo, L.V. Structure elucidation and anticancer activity of 7-oxostaurosporine derivatives from the Brazilian endemic tunicate Eudistoma vannamei. Mar. Drugs 2012, 10, 1092–1102. [Google Scholar] [CrossRef]
- Cartuche, L.; Reyes-Batlle, M.; Sifaoui, I.; Arberas-Jiménez, I.; Piñero, J.E.; Fernández, J.J.; Lorenzo-Morales, J.; Díaz-Marrero, A.R. Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures. Mar. Drugs 2019, 17, 588. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Lamb, D.C.; Wawrzak, Z.; Hull, M.; Kelly, S.L.; Guengerich, F.P.; Lepesheva, G.I. Identification of potent and selective inhibitors of Acanthamoeba: Structural insights into sterol 14α-demethylase as a key drug target. J. Med. Chem. 2024, 67, 7443–7457. [Google Scholar] [CrossRef]
- Kang, S.J.; Lee, J.W.; Song, J.; Park, J.; Choi, J.; Suh, K.H.; Min, K.H. Synthesis and biological activity of 2-cyanoacrylamide derivatives tethered to imidazopyridine as TAK1 inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1928–1936. [Google Scholar] [CrossRef]
- Faridoon Ng, R.; Zhang, G.; Li, J.J. An update on the discovery and development of reversible covalent inhibitors. Med. Chem. Res. 2023, 32, 1039–1062. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Wei, Z.; Cao, K.; Zhang, Z.; Liang, Q. The global epidemiology and clinical diagnosis of Acanthamoeba keratitis. J. Infect. Public Health 2023, 16, 841–852. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological characteristics and pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [Google Scholar] [CrossRef]
- Dias Barroso, F.D.; da Silva, L.J.; Queiroz, H.A.; do Amaral Valente Sá, L.G.; da Silva, A.R.; da Silva, C.R.; de Andrade Neto, J.B.; Cavalcanti, B.C.; de Moraes, M.O.; Pinazo, A.; et al. Biosurfactant complexed with arginine has antibiofilm activity against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2024, 19, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, C.; Legrand, N. Celecoxib analogues for cancer treatment: An update on OSU-03012 and 2,5-dimethyl-celecoxib. Biomolecules 2021, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Koselny, K.; Green, J.; Favazzo, L.; Glazier, V.E.; DiDone, L.; Ransford, S.; Krysan, D.J. Antitumor/antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme acetyl CoA synthetase. ACS Infect. Dis. 2016, 2, 268–280. [Google Scholar] [CrossRef]
- Abt, E.R.; Rosser, E.W.; Durst, M.A.; Lok, V.; Poddar, S.; Le, T.M.; Cho, A.; Kim, W.; Wei, L.; Song, J.; et al. Metabolic modifier screen reveals secondary targets of protein kinase inhibitors within nucleotide metabolism. Cell Chem. Biol. 2020, 27, 197–205.e6. [Google Scholar] [CrossRef]
- Fritz-Laylin, L.K.; Prochnik, S.E.; Ginger, M.L.; Dacks, J.B.; Carpenter, M.L.; Field, M.C.; Kuo, A.; Paredez, A.; Chapman, J.; Pham, J.; et al. The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell 2010, 140, 631–642. [Google Scholar] [CrossRef]
- Won, S.R.; Hong, M.J.; Kim, Y.M.; Li, C.Y.; Kim, J.W.; Rhee, H.I. Oleic acid: An efficient inhibitor of glucosyltransferase. FEBS Lett. 2007, 581, 4999–5002. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, J.; Varghese, R.M.; Subramanian, A.K.; Shanmugam, R. Mechanism of action of green-synthesized silver nanoparticle-incorporated dental varnish against Candida albicans. Cureus 2024, 16, e69353. [Google Scholar] [CrossRef]
- Al-Sawarees, D.K.; Darwish, R.M.; Abu-Zurayk, R.; Masri, M.A. Assessing silver nanoparticle and antimicrobial combinations for antibacterial activity and biofilm prevention on surgical sutures. J. Appl. Microbiol. 2024, 135, lxae063. [Google Scholar] [CrossRef] [PubMed]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver nanoparticles: Review of antiviral properties, mechanism of action and applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, Y.; Alnaggar, M. Silver nanoparticles induce cell death of colon cancer cells through impairing cytoskeleton and membrane nanostructure. Micron 2019, 126, 102750. [Google Scholar] [CrossRef]
- Aguilar-Díaz, H.; Laclette, J.P.; Carrero, J.C. Silencing of Entamoeba histolytica glucosamine 6-phosphate isomerase by RNA interference inhibits the formation of cyst-like structures. BioMed Res. Int. 2013, 2013, 758341. [Google Scholar] [CrossRef]
- Villemez, C.L.; Carlo, P.L. Properties of a soluble polyprenyl phosphate: UDP-D-glucose glucosyltransferase. J. Biol. Chem. 1979, 254, 4814–4819. [Google Scholar] [CrossRef]
- Anwar, A.; Khan, N.A.; Siddiqui, R. Galactose as novel target against Acanthamoeba cysts. PLoS Negl. Trop. Dis. 2019, 13, e0007385. [Google Scholar] [CrossRef]
- Tear, W.F.; Bag, S.; Diaz-Gonzalez, R.; Ceballos-Pérez, G.; Rojas-Barros, D.I.; Cordon-Obras, C.; Pérez-Moreno, G.; García-Hernández, R.; Martinez-Martinez, M.S.; Ruiz-Perez, L.M.; et al. Selectivity and physicochemical optimization of repurposed pyrazolo[1,5-b]pyridazines for the treatment of human African trypanosomiasis. J. Med. Chem. 2020, 63, 756–783. [Google Scholar] [CrossRef] [PubMed]
- Bínová, E.; Bína, D.; Nohýnková, E. DNA content in Acanthamoeba during two stress defense reactions: Encystation, pseudocyst formation and cell cycle. Eur. J. Protistol. 2021, 77, 125745. [Google Scholar] [CrossRef]
- Mochida, K.; Gomyoda, M. Toxicity of ethylene glycol, diethylene glycol, and propylene glycol to human cells in culture. Bull. Environ. Contam. Toxicol. 1987, 38, 151–153. [Google Scholar] [CrossRef]
- Knauf, G.A.; Cunningham, A.L.; Kazi, M.I.; Riddington, I.M.; Crofts, A.A.; Cattoir, V.; Trent, M.S.; Davies, B.W. Exploring the Antimicrobial Action of Quaternary Amines against Acinetobacter baumannii. mBio 2018, 9, e02394-17. [Google Scholar] [CrossRef]
- Shirai, A.; Maeda, T.; Itoh, M.; Kawano, G.; Kourai, H. Control of Legionella Species and Host Amoeba by Bis-quaternary Ammonium Compounds. Biocontrol Sci. 2000, 5, 97–102. [Google Scholar] [CrossRef]
- Babalola, B.A.; Malik, M.; Olowokere, O.; Adebesin, A.; Sharma, L. Indoles in drug design and medicinal chemistry. Eur. J. Med. Chem. Rep. 2025, 13, 100252. [Google Scholar] [CrossRef]
- Kaufman, A.R.; Tu, E.Y. Advances in the management of Acanthamoeba keratitis: A review of the literature and synthesized algorithmic approach. Ocul. Surf. 2022, 25, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, A.; Arboleda, A.; Martinez, J.D.; Durkee, H.; Aguilar, M.C.; Relhan, N.; Nikpoor, N.; Galor, A.; Dubovy, S.R.; Leblanc, R.; et al. Rose Bengal Photodynamic Antimicrobial Therapy for Patients with Progressive Infectious Keratitis: A Pilot Clinical Study. Am. J. Ophthalmol. 2019, 208, 387–396. [Google Scholar] [CrossRef]
- Sepulveda-Beltran, P.A.; Levine, H.; Altamirano, D.S.; Martinez, J.D.; Durkee, H.; Mintz, K.; Leblanc, R.; Tóthová, J.D.; Miller, D.; Parel, J.M.; et al. Rose Bengal Photodynamic Antimicrobial Therapy: A Review of the Intermediate-Term Clinical and Surgical Outcomes. Am. J. Ophthalmol. 2022, 243, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.M.; Chen, J.; Navia, J.C.; Durkee, H.; Gonzalez, A.; Rowaan, C.; Arcari, T.; Aguilar, M.C.; Llanes, K.; Ziebarth, N.; et al. Enhancing Rose Bengal penetration in ex vivo human corneas using iontophoresis. Ther. Deliv. 2024, 15, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Varnado, N.; Abdelrahman, S.; Cavallino, V.; Arnold, B.F.; Lietman, T.M.; Rose-Nussbaumer, J. A double-masked, sham-controlled trial of rose bengal photodynamic therapy for the treatment of fungal and Acanthamoeba keratitis: Rose Bengal Electromagnetic Activation with Green Light for Infection Reduction (REAGIR) study. Trials 2024, 25, 566. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).