Assessment of Trichinella Infection in Animals from Argentina
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction
2.3. PCR Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pozio, E.; Zarlenga, D.S. Taxonomy of the Trichinella genus. In Trichinella and Trichinellosis; Academic Press: Cambridge, MA, USA, 2021; pp. 35–76. [Google Scholar] [CrossRef]
- Malone, C.J.; Oksanen, A.; Mukaratirwa, S.; Sharma, R.; Jenkins, E. From wildlife to humans: The global distribution of Trichinella species and genotypes in wildlife and wildlife-associated human trichinellosis. Int. J. Parasitol. Parasites Wildl. 2024, 24, 100934. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Murrell, K.D. New aspects of human trichinellosis: The impact of new Trichinella species. Postgrad. Med. J. 2002, 78, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bilska-Zajac, E.; Tonanzi, D.; Pozio, E.; Rozycki, M.; Cencek, T.; Thompson, P.C.; Rosenthal, B.M.; La Rosa, G. Genetic evidence substantiates transmission of Trichinella spiralis from one swine farm to another. Parasites Vectors 2021, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Krivokapich, S.J.; Gatti, G.M.; Gonzalez Prous, C.L.; Degese, M.F.; Arbusti, P.A.; Ayesa, G.E.; Bello, G.V.; Salomón, M.C. Detection of Trichinella britovi in pork sausage suspected to be implicated in a human outbreak in Mendoza, Argentina. Parasitol. Int. 2019, 71, 53–55. [Google Scholar] [CrossRef]
- Fariña, F.A.; Krivokapich, S.J.; Pasqualetti, M.I.; Gatti, G.; Aronowicz, T.; Betti, A.; Laurito, F.J.; Lopez, L.; Bessi, C.; Montalvo, F.; et al. New records of Trichinella patagoniensis from Argentina. Vet. Parasitol. 2025, 333, 110198. [Google Scholar] [CrossRef]
- Ribicich, M.M.; Fariña, F.A.; Aronowicz, T.; Ercole, M.E.; Bessi, C.; Winter, M.; Pasqualetti, M.I. Reprint of: A review on Trichinella infection in South America. Vet. Parasitol. 2021, 297, 109540. [Google Scholar] [CrossRef]
- SENASA. Boletin Oficial Republica Argentina—Servicio Nacional de Sanidad y Calidad Agroalimentaria—Resolución 1035/2024. 2024. Available online: https://www.boletinoficial.gob.ar/detalleAviso/primera/313307/20240904 (accessed on 17 October 2024).
- SENASA. Boletín Epidemiológico Nacional N°679, SE 46. 2023; Ministerio de Salud: Buenos Aires, Argentina, 2024. [Google Scholar]
- Ministerio de Salud de la República Argentina; Dirección de Epidemiologia. Boletín Epidemiológico Nacional N°723, SE 38; Minnisterio de Salud: Buenos Aires, Argentina, 2024. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 10 January 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. Available online: http://journal.r-project.org/archive/2013-1/kahle-wickham.pdf (accessed on 10 January 2025). [CrossRef]
- Pebesma, E.; Bivand, R. Spatial Data Science: With Applications in R; Chapman and Hall: Boca Raton, FL, USA; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Dunnington, D. ggspatial: Spatial Data Framework for ggplot2. R Package Version 1.1.9. 2023. Available online: https://CRAN.R-project.org/package=ggspatial (accessed on 10 January 2025).
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package Version 1.4.3. 2023. Available online: https://CRAN.R-project.org/package=readxl (accessed on 10 January 2025).
- Ruiz Nicolini, J.; Del Boca, P.; Juara, J. geoAr: Argentina’s Spatial Data Toolbox. R Package Version 1.0.0. 2024. Available online: https://github.com/PoliticaArgentina/geoAr (accessed on 10 January 2025).
- Ribicich, M.; Gamble, H.R.; Rosa, A.; Bolpe, J.; Franco, A. Trichinellosis in Argentina: An historical review. Vet. Parasitol. 2005, 132, 137–142. [Google Scholar] [CrossRef]
- Anonymous. Guía para la Prevención y el Control de la Triquinosis/Trichinellosis en la República Argentina; Ministerio de Salud Argentina: Buenos Aires, Argentina, 2021. [Google Scholar]
- Landaeta-Aqueveque, C.; Ayala, S.; Poblete-Toledo, D.; Canals, M. Temporal and geographic analysis of trichinellosis incidence in Chile with risk assessment. Parasites Vectors 2021, 14, 282. [Google Scholar] [CrossRef]
- Bruschi, F. Trichinella and Trichinellosis; Academic Press: Cambridge, MA, USA, 2021; 558p. [Google Scholar]
- Pozio, E. The impact of globalization and climate change on Trichinella spp. epidemiology. Food Waterborne Parasitol. 2022, 27, e00154. [Google Scholar] [CrossRef] [PubMed]
- Bilska-Zając, E.; Thompson, P.; Rosenthal, B.; Różycki, M.; Cencek, T. Infection, genetics, and evolution of Trichinella: Historical insights and applications to molecular epidemiology. Infect. Genet. Evol. 2021, 95, 105080. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Pasqualetti, M.; Fariña, F.; Ercole, M.; Failla, M.; Perello, M.; Birochio, D.; Abate, S.; Soricetti, M.; Ribicich, M. Trichinellosis surveillance in wildlife in northeastern argentine patagonia. Vet. Parasitol. Reg. Stud. Rep. 2018, 11, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, M.I.; Fariña, F.A.; Krivokapich, S.J.; Gatti, G.M.; Daneri, G.A.; Varela, E.A.; Lucero, S.; Ercole, M.E.; Bessi, C.; Winter, M.; et al. Trichinella spiralis in a South American sea lion (Otaria flavescens) from Patagonia, Argentina. Parasitol. Res. 2018, 117, 4033–4036. [Google Scholar] [CrossRef]
- Ballari, S.A.; La Sala, L.F.; Merino, M.L.; Carpinetti, B.; Winter, M.; Gürtler, R.E.; Barandiaran, S.; Cuevas, M.F.; Condori, W.E.; Tammone, A.; et al. El jabalí y el cerdo silvestre (Sus scrofa) en la Argentina. Ecol. Austral 2024, 34, 401–421. [Google Scholar] [CrossRef]
- Pozio, E.; Zarlenga, D.S. New pieces of the Trichinella puzzle. Int. J. Parasitol. 2013, 43, 983–997. [Google Scholar] [CrossRef]
- Krivokapich, S.J.; Gonzalez Prous, C.L.; Gatti, G.M.; Saldía, L. First finding of Trichinella pseudospiralis in the Neotropical region. Vet. Parasitol. 2015, 208, 268–271. [Google Scholar] [CrossRef]
- Anonymous. Trichinella Database. 2024. Available online: https://trichinella.iss.it/Database.aspx (accessed on 18 December 2024).
- Cohen, M.; Costantino, S.N.; Calcagno, M.A.; Blanco, G.A.; Pozio, E.; Venturiello, S.M. Trichinella infection in wild boars (Sus scrofa) from a protected area of Argentina and its relationship with the presence of humans. Vet. Parasitol. 2010, 169, 362–366. [Google Scholar] [CrossRef]
- Krivokapich, S.J.; Pozio, E.; Gatti, G.M.; Gonzalez Prous, C.L.; Ribicich, M.; Marucci, G.; La Rosa, G.; Confalonieri, V. Trichinella patagoniensis n. sp. (Nematoda), a new encapsulated species infecting carnivorous mammals in South America. Int. J. Parasitol. 2012, 42, 903–910. [Google Scholar] [CrossRef]
- Krivokapich, S.J.; Molina, V.; Bergagna, H.F.J.; Guarnera, E.A. Epidemiological survey of Trichinella infection in domestic, synanthropic and sylvatic animals from Argentina. J. Helminthol. 2006, 80, 267–269. [Google Scholar] [CrossRef]
- Ribicich, M.; Gamble, H.R.; Bolpe, J.; Scialfa, E.; Krivokapich, S.; Cardillo, N.; Betti, A.; Cambiaggi Holzmann, M.L.; Pasqualetti, M.; Fariña, F.; et al. Trichinella infection in wild animals from endemic regions of Argentina. Parasitol. Res. 2010, 107, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, D.P.; Domínguez, M.G.; Morici, G.; Lammel, M.N.; Cavia, R. Natural Trichinella spiralis infection in wild and domestic vertebrates in a trichinellosis endemic area from Argentina. Mastozool. Neotrop. 2024, 31, e0993. [Google Scholar] [CrossRef]
- Hidalgo, A.; Villanueva, J.; Becerra, V.; Soriano, C.; Melo, A.; Fonseca-Salamanca, F. Trichinella spiralis Infecting Wild Boars in Southern Chile: Evidence of an Underrated Risk. Vector-Borne Zoonotic Dis. 2019, 19, 625–629. [Google Scholar] [CrossRef]
- Echeverry, D.M.; Santodomingo, A.M.S.; Thomas, R.S.; González-Ugás, J.; Oyarzún-Ruiz, P.; Silva-De la Fuente, M.C.; Landaeta-Aqueveque, C. Trichinella spiralis in a cougar (Puma concolor) hunted by poachers in Chile. Rev. Bras. Parasitol. Vet. 2021, 30, e002821. [Google Scholar] [CrossRef]
- Espinoza-Rojas, H.; Lobos-Chávez, F.; Silva-de la Fuente, M.C.; Echeverry, D.M.; Muñoz-Galaz, J.; Yáñez-Crisóstomo, C.; Oyarzún-Ruiz, P.; Ortega, R.; Sandoval, D.; Henríquez, A.L.; et al. Survey of Trichinella in American minks (Neovison vison Schreber, 1777) and wild rodents (Muridae and Cricetidae) in Chile. Zoonoses Public Health 2021, 68, 842–848. [Google Scholar] [CrossRef]
- Echeverry, D.M.; Henríquez, A.L.; Oyarzún-Ruiz, P.; Silva-De la Fuente, M.C.; Ortega, R.; Sandoval, D.; Landaeta-Aqueveque, C. First record of Trichinella in Leopardus guigna (Carnivora, Felidae) and Galictis cuja (Carnivora, Mustelidae): New hosts in Chile. PeerJ 2021, 9, e11601. [Google Scholar] [CrossRef]
- Silva, C.S.; Mendonça, T.O.; Machado, D.M.R.; Arias-Pacheco, C.A.; Oliveira, W.J.; Perin, P.P.; Werther, K.; Carraro, P.E.; Trevisol, I.M.; Kramer, B.; et al. Seropositive Wild Boars Suggesting the Occurrence of a Wild Cycle of Trichinella spp. in Brazil. Animals 2022, 12, 462. [Google Scholar] [CrossRef]
- Santiago, V. Triquinelose em javalis no Brasil. In Proceedings of the Encontro Nacional de Defesa Sanitária Animal—ENDESA, Belem, Brasil, 4–8 December 2017. [Google Scholar]
- Bartoloni, A.; Cancrini, G.; Bartalesi, F.; Nicoletti, A.; Prado, G.M.; Rosado, J.; Roselli, M.; Paradisi, F. Anticuerpos contra Trichinella spiralis en la población rural de la provincia Cordillera, Bolivia. Rev. Panam. Salud Pública 1999, 5, 97–99. [Google Scholar] [CrossRef]
- Macchioni, F.; Magi, M.; Guardone, L.; Tolari, F.; Bruschi, F.; Gabrielli, S.; Lopez Ramos, R.; Guzman Rios, R.; Guzman Jos, L.R.; Quiroga Civera, L. Investigation on Trichinella spp. in Swine in Eastern Bolivia. Una Salud Rev. Sapuvet Salud Pública 2012, 3, 37–43. [Google Scholar]
- Bjorland, J.; Brown, D.; Ray Gamble, H.; McAuley, J.B. Trichinella spiralis infection in pigs in the Bolivian Altiplano. Vet. Parasitol. 1993, 47, 349–354. [Google Scholar] [CrossRef]
- Altuna, M.; Castro, G.; Lozano, A.; Gayo, V.; Silva, M.; Mautone, E.; Birriel, S. Surveillance of brucelosis and trichinellosis in feral swine in Uruguay. In Proceedings of the International Congress on Tropical Veterinary Medicine, Buenos Aires, Argentina, 23–28 September 2018. [Google Scholar]
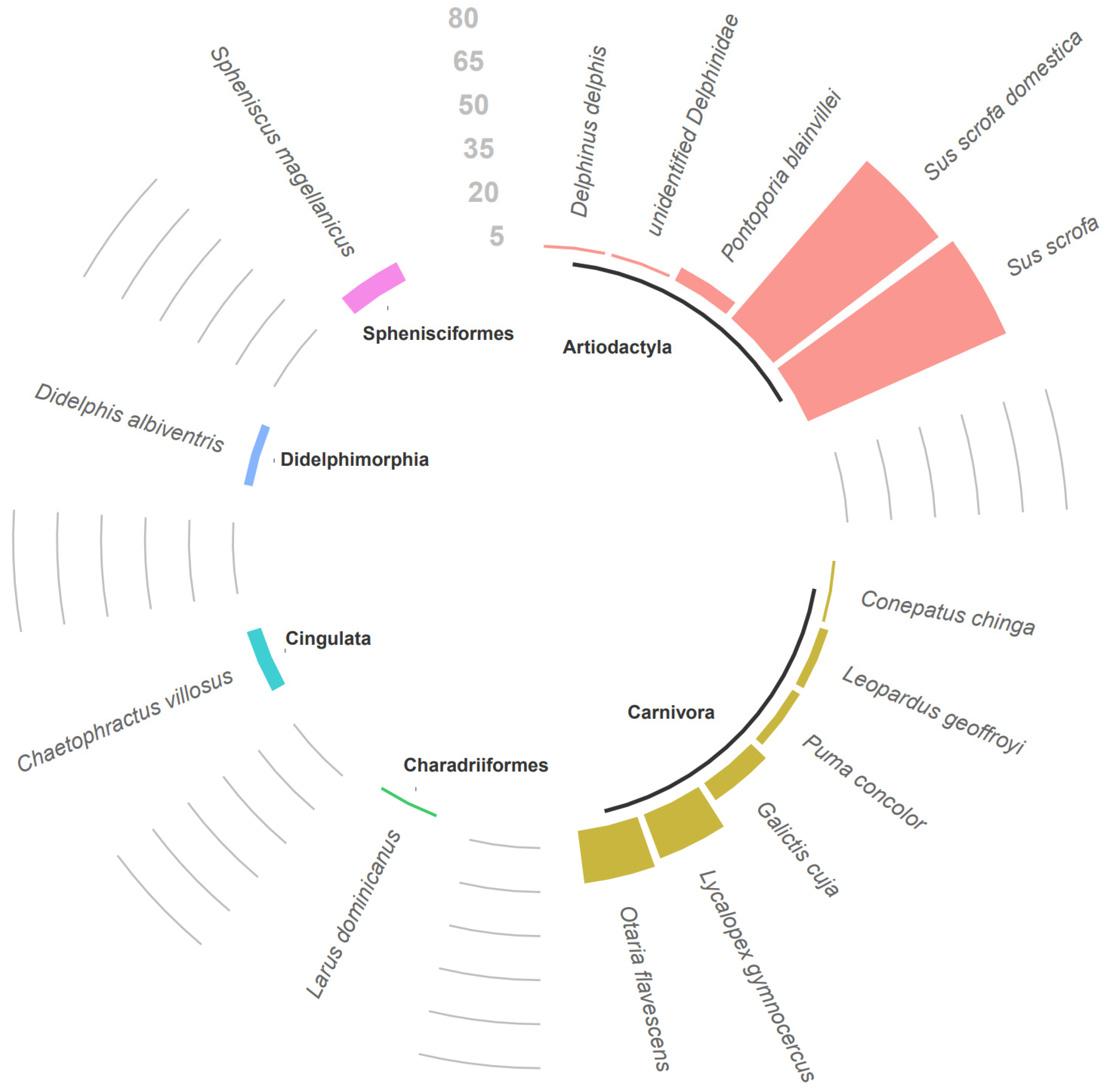
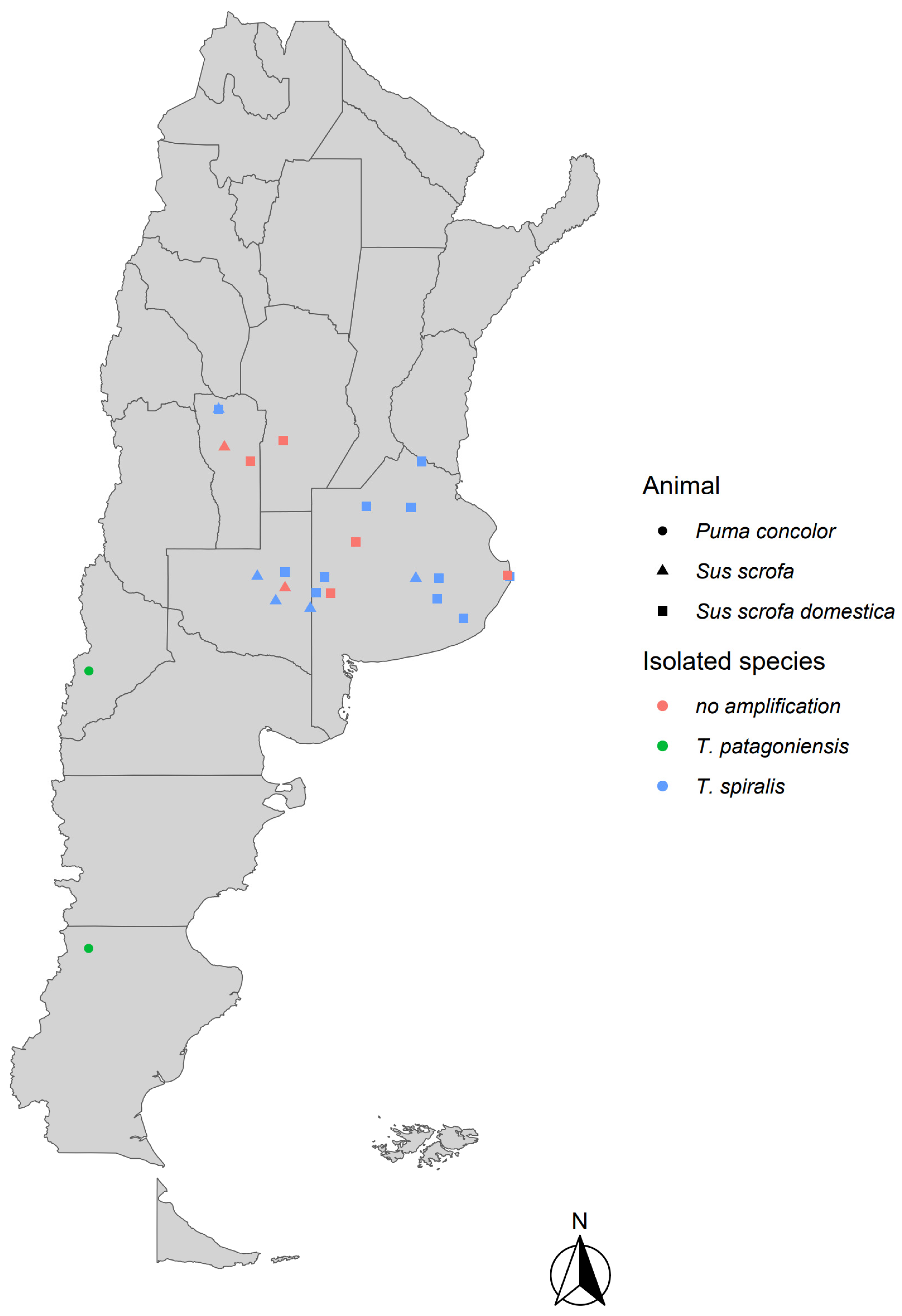
| Animal Species | Number of Animals | Location | Muscle Samples | Results |
|---|---|---|---|---|
| Sus scrofa | 2 | Buenos Aires | diaphragm, masseters, and intercostal muscles | T. spiralis |
| Sus scrofa domestica | 32 | Buenos Aires | diaphragm, masseters, and intercostal muscles | T. spiralis |
| Sus scrofa domestica | 14 | Buenos Aires | diaphragm, masseters, and intercostal muscles | negative |
| Sus scrofa domestica | 4 | Buenos Aires | diaphragm, masseters, and intercostal muscles | unidentifiable species |
| Otaria flavescens | 2 | Buenos Aires | diaphragm, masseters, and tongue | negative |
| Sus scrofa domestica | 1 | Córdoba | diaphragm, masseters, and intercostal muscles | T. spiralis |
| Sus scrofa domestica | 13 | Córdoba | diaphragm, masseters, and intercostal muscles | negative |
| Sus scrofa domestica | 2 | Córdoba | diaphragm, masseters, and intercostal muscles | unidentifiable species |
| Sus scrofa domestica | 1 | Entre Ríos | diaphragm, masseters, and intercostal muscles | negative |
| Sus scrofa | 3 | La Pampa | diaphragm, masseters, and intercostal muscles | T. spiralis |
| Sus scrofa domestica | 1 | La Pampa | diaphragm, masseters, and intercostal muscles | T. spiralis |
| Sus scrofa | 66 | La Pampa | diaphragm, masseters, and intercostal muscles | negative |
| Sus scrofa | 1 | La Pampa | diaphragm, masseters, and intercostal muscles | unidentifiable species |
| Puma concolor * | 1 | Neuquén | diaphragm and masseters | T. patagoniensis |
| Chaetophractus villosus | 5 | Río Negro | diaphragm and hind limbs | negative |
| Conepatus chinga | 1 | Río Negro | diaphragm and hind limbs | negative |
| Delphinus delphis | 1 | Río Negro | diaphragm and hind limbs | negative |
| Didelphis albiventris | 3 | Río Negro | diaphragm and hind limbs | negative |
| Galictis cuja | 7 | Río Negro | diaphragm and hind limbs | negative |
| Larus dominicanus | 1 | Río Negro | pectoral muscles | negative |
| Leopardus geoffroyi | 3 | Río Negro | diaphragm and hind limbs | negative |
| Lycalopex gymnocercus | 15 | Río Negro | diaphragm and hind limbs | negative |
| Lycalopex gymnocercus | 1 | Río Negro | diaphragm and hind limbs | negative |
| Otaria flavescens | 16 | Río Negro | diaphragm, masseters, and tongue | negative |
| Pontoporia blainvillei | 5 | Río Negro | axial muscles | negative |
| Puma concolor | 1 | Río Negro | diaphragm and masseters | negative |
| Spheniscus magellanicus | 7 | Río Negro | pectoral muscles | negative |
| unidentified Delphinidae | 1 | Río Negro | axial muscles | negative |
| Sus scrofa | 1 | San Luis | diaphragm, masseters, and intercostal muscles | negative |
| Sus scrofa | 1 | San Luis | diaphragm, masseters, and intercostal muscles | unidentifiable species |
| Sus scrofa domestica | 3 | San Luis | diaphragm, masseters, and intercostal muscles | unidentifiable species |
| Puma concolor * | 1 | Santa Cruz | diaphragm and masseters | T. patagoniensis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fariña, F.A.; Pasqualetti, M.I.; Winter, M.; Abate, S.; Daneri, G.; Harrington, A.; Aronowicz, T.; Calvo, C.; Lapuyade, C.; D’Francisco, F.A.; et al. Assessment of Trichinella Infection in Animals from Argentina. Parasitologia 2025, 5, 21. https://doi.org/10.3390/parasitologia5020021
Fariña FA, Pasqualetti MI, Winter M, Abate S, Daneri G, Harrington A, Aronowicz T, Calvo C, Lapuyade C, D’Francisco FA, et al. Assessment of Trichinella Infection in Animals from Argentina. Parasitologia. 2025; 5(2):21. https://doi.org/10.3390/parasitologia5020021
Chicago/Turabian StyleFariña, Fernando A., Mariana I. Pasqualetti, Marina Winter, Sergio Abate, Gustavo Daneri, Ana Harrington, Tatiana Aronowicz, Claudio Calvo, Cecilia Lapuyade, Florencia A. D’Francisco, and et al. 2025. "Assessment of Trichinella Infection in Animals from Argentina" Parasitologia 5, no. 2: 21. https://doi.org/10.3390/parasitologia5020021
APA StyleFariña, F. A., Pasqualetti, M. I., Winter, M., Abate, S., Daneri, G., Harrington, A., Aronowicz, T., Calvo, C., Lapuyade, C., D’Francisco, F. A., & Ribicich, M. M. (2025). Assessment of Trichinella Infection in Animals from Argentina. Parasitologia, 5(2), 21. https://doi.org/10.3390/parasitologia5020021






