Investigation of Causative Agents of Vaginitis in Symptomatic and Asymptomatic Women in Konya, Turkey
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Patients
2.2. Collection and Storage of Samples
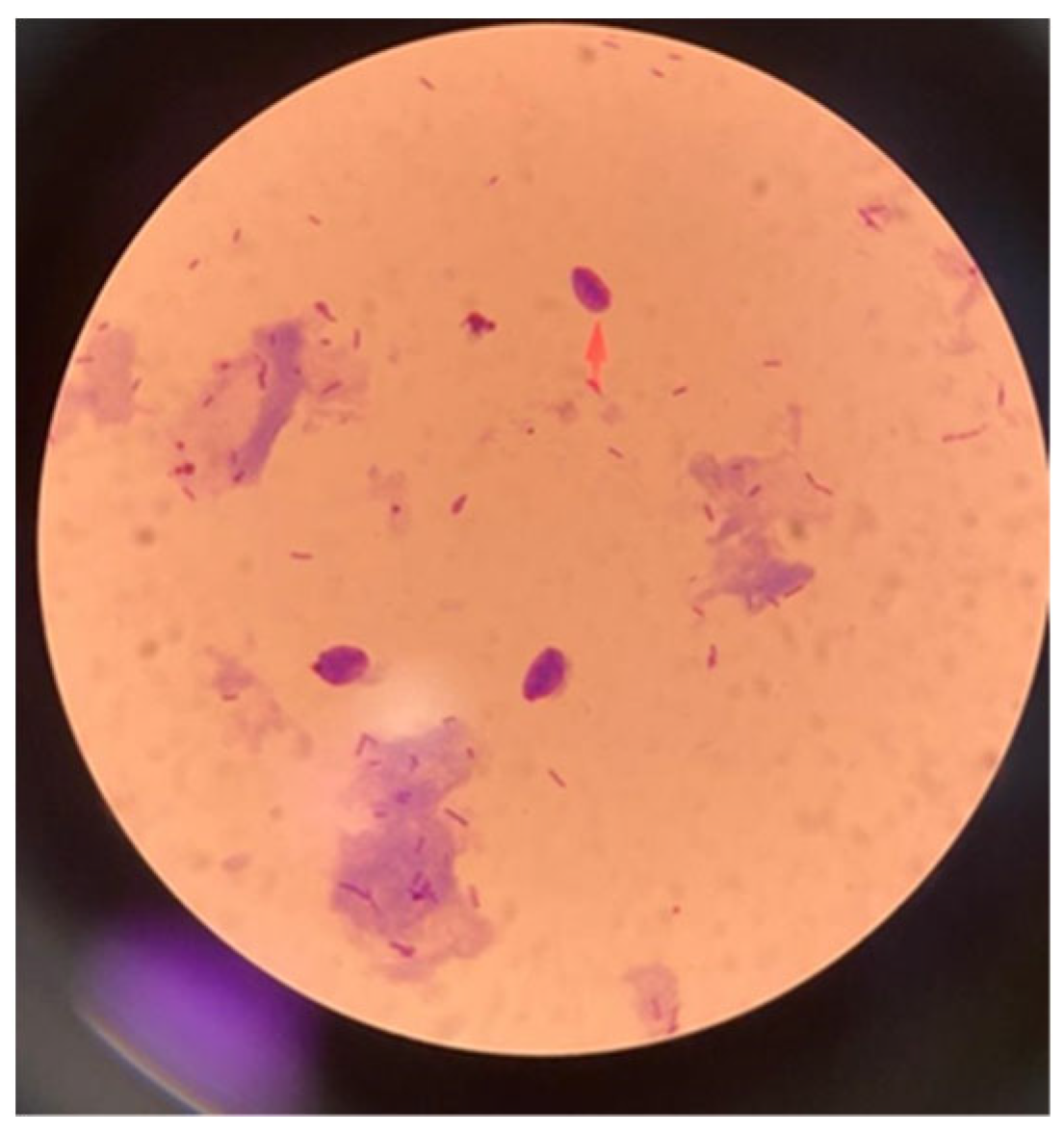
2.3. Stained Microscopic Examination
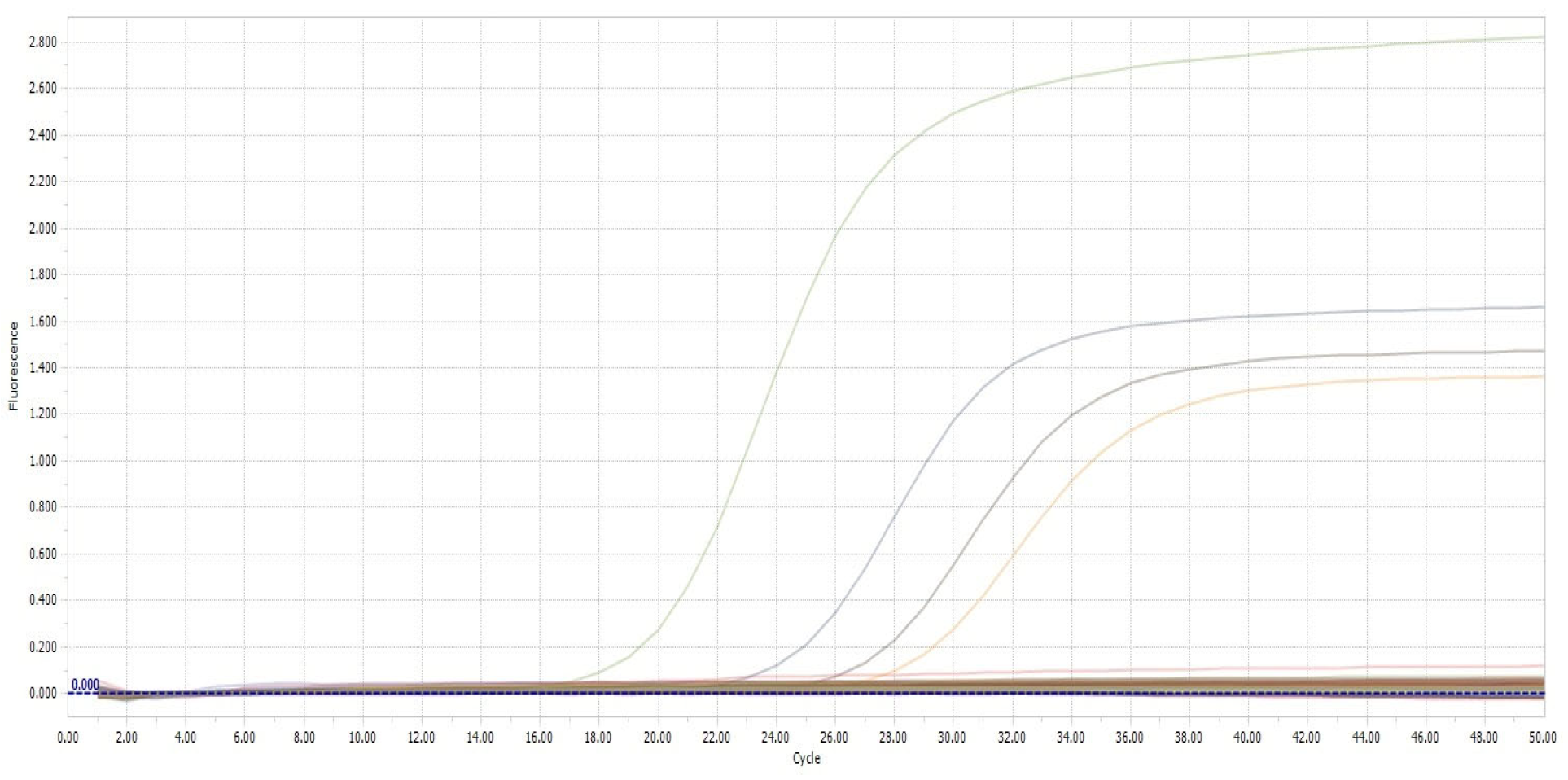
2.4. Polymerase Chain Reaction (PCR)
2.5. Culture
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Van de Wijgert, J.H.H.M.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef] [PubMed]
- Mendling, W. Vaginal microbiota. In Microbiota of the Human Body: Implications in Health and Disease; Schwiertz, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 83–93. [Google Scholar]
- Dallabetta, G.; Wi, T.E.C.; Nielsen, G. Prevention and control of STD and HIV infection in developing countries. In Sexually Transmitted Diseases, 4th ed.; Holmes, K.K., Sparling, P.F., Stamm, W., Piot, P., Wasserheit, J., Corey, L., Eds.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Savini, V.; Marrollo, R.; D’Antonio, M.; D’Amario, C.; Fazii, P.; D’Antonio, D. Streptococcus agalactiae vaginitis: Nonhemolytic variant on the Liofilchem® Chromatic StreptoB. Int. J. Clin. Exp. Pathol. 2013, 6, 1693–1695. [Google Scholar] [PubMed]
- Acarkan, T. Vajinal flora dysfunction and vaginitis. J. Complement. Med. Reg. Neural. Ther. 2016, 10, 8–12. [Google Scholar]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Karakoç, Z.Ç. Does the Etiology Change in Vaginitis? Data Results of Samples from a Single Center. Med. J. Mugla Sitki Kocman Univ. 2021, 8, 18–22. [Google Scholar] [CrossRef]
- Aksakal, S.E.; Cırık, D.A.; Ensari, T.; Coskun, B.; Aksu, G.; Mollamahmutoglu, L.; Tapısız, O.L. Comparison of the Effectiveness of VGTest™ and Standard Microbiological Tests in the Diagnosis of the Vaginal Infections. Turk. J. Womens. Health. Neonatol. 2020, 2, 33–40. [Google Scholar]
- Coudray, M.S.; Madhivanan, P. Bacterial vaginosis-A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef]
- Bansal, R.; Garg, P.; Garg, A. Comparison of Amsel’s criteria and Nugent’s criteria for diagnosis of bacterial vaginosis in tertiary care centre. Int. J. Reprod. Contracept. Obstet. Gynecol. 2019, 8, 637–640. [Google Scholar] [CrossRef]
- Gibbs, R.S. Asymptomatic bacterial vaginosis: Is it time to treat? Am. J. Obstet. Gynecol. 2007, 196, 495–496. [Google Scholar] [CrossRef]
- Swidsinski, S.; Moll, W.M.; Swidsinski, A. Bacterial vaginosis-vaginal polymicrobial biofilms and dysbiosis. Dtsch. Arztebl. Int. 2023, 120, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Sexually Transmitted Infections Treatment Guidelines; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/std/treatment-guidelines/bv.htm (accessed on 13 December 2024).
- Mikamo, H.; Sato, Y.; Hayasaki, Y.; Hua, Y.X.; Tamaya, T. Vaginal microflora in healthy women with Gardnerella vaginalis. J. Infect. Chemother. 2000, 6, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspõllu, A.; Väin, E.; Saarma, I.; Salumets, A.; Donders, G.; Metsis, M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef]
- Ünlü, Ö.; Çağlar, T.; Erdem, M.G.; Ekici, S.; Demirci, M. A Three Year Retrospective Analysis of Vaginitis Agents in Patients Admitted to a Private Hospital. Kırıkkale Üniv. Tıp Fak. Derg. 2022, 24, 181–186. [Google Scholar] [CrossRef]
- Kanbur, S. Comparison of Screening And Treatment Protocols of Sexually Transmitted Diseases. Zeynep Kamil Tıp Bül. 2018, 49, 15–20. [Google Scholar] [CrossRef][Green Version]
- Pramanick, R.; Mayadeo, N.; Warke, H.; Begum, S.; Aich, P.; Aranha, C. Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: Are they different from normal microbiota? Microb. Pathog. 2019, 134, 103599. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually transmitted infections treatment guidelines. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef]
- Farr, A.; Effendy, I.; Tirri, B.F.; Hof, H.; Mayser, P.; Petricevic, L.; Ruhnke, M.; Schaller, M.; Schaefer, A.P.A.; Sustr, V.; et al. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses 2021, 64, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Okungbowa, F.I.; Isikhuemhen, O.S.; Dede, A.P.O. The distribution frequency of Candida species in the genitourinary tract among symptomatic individuals in Nigerian cities. Rev. Iberoam. Micol. 2003, 20, 60–63. [Google Scholar] [PubMed]
- Amouri, I.; Sellami, H.; Borji, N.; Abbes, S.; Sellami, A.; Cheikhrouhou, F.; Maazoun, L.; Khaled, S.; Khrouf, S.; Boujelben, Y.; et al. Epidemiological survey of vulvovaginal candidosis in Sfax, Tunisia. Mycoses 2011, 54, e499–e505. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, O.; Baka, S.; Makrakis, E.; Hassiakos, D.; Kapparos, G.; Kouskouni, E. Prevalence of clinical vaginal candidiasis in a university hospital and possible risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 126, 121–125. [Google Scholar] [CrossRef]
- Tibaldi, C.; Cappello, N.; Latino, M.A.; Masuelli, G.; Marini, S.; Benedetto, C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: Risk factors and rates of occurrence. Clin. Microbiol. Infect. 2009, 15, 670–679. [Google Scholar] [CrossRef][Green Version]
- Mills, B.B. Vaginitis: Beyond the Basics. Obstet. Gynecol. Clin. N. Am. 2017, 44, 159–177. [Google Scholar] [CrossRef]
- Chatzivasileiou, P.; Vyzantiadis, T.A. Vaginal yeast colonisation: From a potential harmless condition to clinical implications and management approaches-A literature review. Mycoses 2019, 62, 638–650. [Google Scholar] [CrossRef]
- Sobel, J.D. Pathogenesis of Candida vulvovaginitis. Curr. Top. Med. Mycol. 1989, 3, 86–108. [Google Scholar] [CrossRef]
- Doni, N.Y.; Aksoy, M.; Şimşek, Z.; Gürses, G.; Hilali, N.G.; Zeyrek, F.Y.; Özek, B.; Yıldırımkaya, G. Vajinit yakınmaları olan 15–49 yaş arasındaki suriyeli mülteci kadınlarda Trichomonas vaginalis sıklığının araştırılması. Mikrobiyol. Bul. 2016, 50, 590–597. [Google Scholar] [CrossRef]
- Bruni, M.P.; Freitas da Silveira, M.; Stauffert, D.; Bicca, G.L.O.; Caetano Dos Santos, C.; Rosa Farias, N.A.; Golparian, D.; Unemo, M. Aptima Trichomonas vaginalis assay elucidates significant underdiagnosis of trichomoniasis among women in Brazil according to an observational study. Sex. Transm. Infect. 2019, 95, 129–132. [Google Scholar] [CrossRef]
- Meites, E.; Gaydos, C.A.; Hobbs, M.M.; Kissinger, P.; Nyirjesy, P.; Schwebke, J.R.; Secor, W.E.; Sobel, J.D.; Workowski, K.A. A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin. Infect. Dis. 2015, 61, S837–S848. [Google Scholar] [CrossRef] [PubMed]
- Van Gerwen, O.T.; Muzny, C.A. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Research 2019, 8, 1666. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Hobbs, M.M.; Taylor, S.N.; Sena, A.C.; Catania, M.G.; Weinbaum, B.S.; Johnson, A.D.; Getman, D.K.; Gaydos, C.A. Molecular testing for Trichomonas vaginalis in women: Results from a prospective U.S. clinical trial. J. Clin. Microbiol. 2011, 49, 4106–4111. [Google Scholar] [CrossRef]
- Yazısız, H.; Özyurt, Ö.; Eryiğit, F.; Özhak, B.; Öngüt, G.; Özekinci, M.; Dönmez, D.; Çolak, D.; Gümüş, S.; Öğünç, D. Evaluation of Microscopic Examination, Culture and Polymerase Chain Reaction Tests in the Diagnosis of Trichomonas vaginalis Infection. Mikrobiyol. Bul. 2020, 54, 135–143. [Google Scholar] [CrossRef]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.S. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front. Public Health 2018, 6, 78. [Google Scholar] [CrossRef]
- Donders, G.; Bellen, G.; Rezeberga, D. Aerobic vaginitis in pregnancy. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
| Age < 45 Years (n/N) | Age ≥ 45 (n/N) | |
|---|---|---|
| Number of samples | 160 | 40 |
| Bacterial vaginosis | 27/160 (%16.9) | 12/40 (%30) |
| Trichomoniasis | 5/160 (%3.1) | 2/40 (%5) |
| Candidiasis | 59/160 (%36.9) | 3/40 (%7.5) |
| Aerobic vaginitis | 19/160 (%11.9) | 4/40 (%10) |
| Symptomatic (n/N) | Asymptomatic (n/N) | |
|---|---|---|
| Number of samples | 148 | 52 |
| Bacterial vaginosis | 32/148 (%21.6) | 7/52 (%13.5) |
| Trichomoniasis | 5/148 (%3.4) | 2/52 (%3.8) |
| Candidiasis | 51/148 (%34.5) | 11/52 (%21.2) |
| Aerobic vaginitis | 19/148 (%12.8) | 4/52 (%7.7) |
| Variable | Count (n) | Number of Sample | Percentage (%) | p | |
|---|---|---|---|---|---|
| Education | Primary school | 5 | 3 | 60 | 0.946 |
| Middle school | 99 | 55 | 55.6 | ||
| High school | 42 | 23 | 54.8 | ||
| University | 51 | 30 | 58.8 | ||
| Postgraduate | 3 | 1 | 33.3 | ||
| Profession | Housewife | 169 | 96 | 56.8 | 0.436 |
| Employed | 29 | 14 | 48.3 | ||
| Student | 2 | 2 | 100 | ||
| Chronic disease | No | 158 | 94 | 59.5 | 0.079 |
| Yes | 42 | 18 | 42.9 | ||
| Hemorrhoid | No | 194 | 109 | 56.2 | 1.000 |
| Yes | 6 | 3 | 50 | ||
| Antibiotic use | No | 178 | 95 | 53.4 | 0.057 |
| Yes | 22 | 17 | 77.3 | ||
| Age at first sexual intercourse | ≤18 years | 41 | 22 | 53.7 | 0.753 |
| 18–25 years old | 137 | 79 | 57.7 | ||
| ≥25 years | 22 | 11 | 50 | ||
| Number of partners | 0 | 8 | 4 | 50 | 0.362 |
| 1 | 189 | 105 | 55.6 | ||
| ≥2 | 3 | 3 | 100 | ||
| Contraceptive method | Birth control pills | 9 | 5 | 55.6 | 0.254 |
| Condom | 38 | 20 | 52.6 | ||
| Spiral (RIA) | 24 | 18 | 75 | ||
| Other | 129 | 69 | 53.5 | ||
| Number of births | 0 | 38 | 28 | 73.7 | 0.146 |
| 1 | 33 | 15 | 45.5 | ||
| 2 | 60 | 33 | 55 | ||
| 3 | 47 | 24 | 51.1 | ||
| ≥4 | 22 | 12 | 54.5 | ||
| Mode of delivery | Normal | 103 | 54 | 52.4 | 0.776 |
| Cesarean | 45 | 24 | 53.3 | ||
| Both | 14 | 6 | 42.9 | ||
| Discharge | No | 70 | 34 | 48.5 | 0.120 |
| Yes | 130 | 78 | 60 | ||
| Redness | No | 152 | 82 | 53.9 | 0.382 |
| Yes | 48 | 30 | 62.5 | ||
| Burning | No | 104 | 57 | 54.8 | 0.724 |
| Yes | 96 | 55 | 57.3 | ||
| Itching | No | 77 | 39 | 50.6 | 0.228 |
| Yes | 123 | 73 | 59.3 | ||
| Frequency of changing underwear | Every day | 167 | 98 | 58.7 | 0.127 |
| Every few days | 33 | 14 | 42.4 | ||
| Daily pad | No | 148 | 85 | 57.4 | 0.599 |
| Yes | 52 | 27 | 51.9 | ||
| Toilet type | Squat toilet | 112 | 61 | 54.5 | 0.873 |
| Western-style toilet | 61 | 35 | 57.4 | ||
| Squat/Western style | 27 | 16 | 59.3 | ||
| Frequency of bathing | 1 | 8 | 4 | 50 | 0.318 |
| 2 | 41 | 19 | 46.3 | ||
| ≥3 | 151 | 89 | 58.9 | ||
| Frequency of changing pads during menstruation | 1 | 20 | 12 | 60 | 0.848 |
| 2 | 58 | 31 | 53.4 | ||
| ≥3 | 121 | 69 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beder, D.; Esenkaya Taşbent, F.; Kılıç Hamzaoğlu, F.; Türen Demir, E.; Özdemir, M.; Arslan, G.K. Investigation of Causative Agents of Vaginitis in Symptomatic and Asymptomatic Women in Konya, Turkey. Parasitologia 2025, 5, 15. https://doi.org/10.3390/parasitologia5020015
Beder D, Esenkaya Taşbent F, Kılıç Hamzaoğlu F, Türen Demir E, Özdemir M, Arslan GK. Investigation of Causative Agents of Vaginitis in Symptomatic and Asymptomatic Women in Konya, Turkey. Parasitologia. 2025; 5(2):15. https://doi.org/10.3390/parasitologia5020015
Chicago/Turabian StyleBeder, Duygu, Fatma Esenkaya Taşbent, Fatma Kılıç Hamzaoğlu, Emine Türen Demir, Mehmet Özdemir, and Gökçe Kader Arslan. 2025. "Investigation of Causative Agents of Vaginitis in Symptomatic and Asymptomatic Women in Konya, Turkey" Parasitologia 5, no. 2: 15. https://doi.org/10.3390/parasitologia5020015
APA StyleBeder, D., Esenkaya Taşbent, F., Kılıç Hamzaoğlu, F., Türen Demir, E., Özdemir, M., & Arslan, G. K. (2025). Investigation of Causative Agents of Vaginitis in Symptomatic and Asymptomatic Women in Konya, Turkey. Parasitologia, 5(2), 15. https://doi.org/10.3390/parasitologia5020015






