Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA
Abstract
:1. Introduction
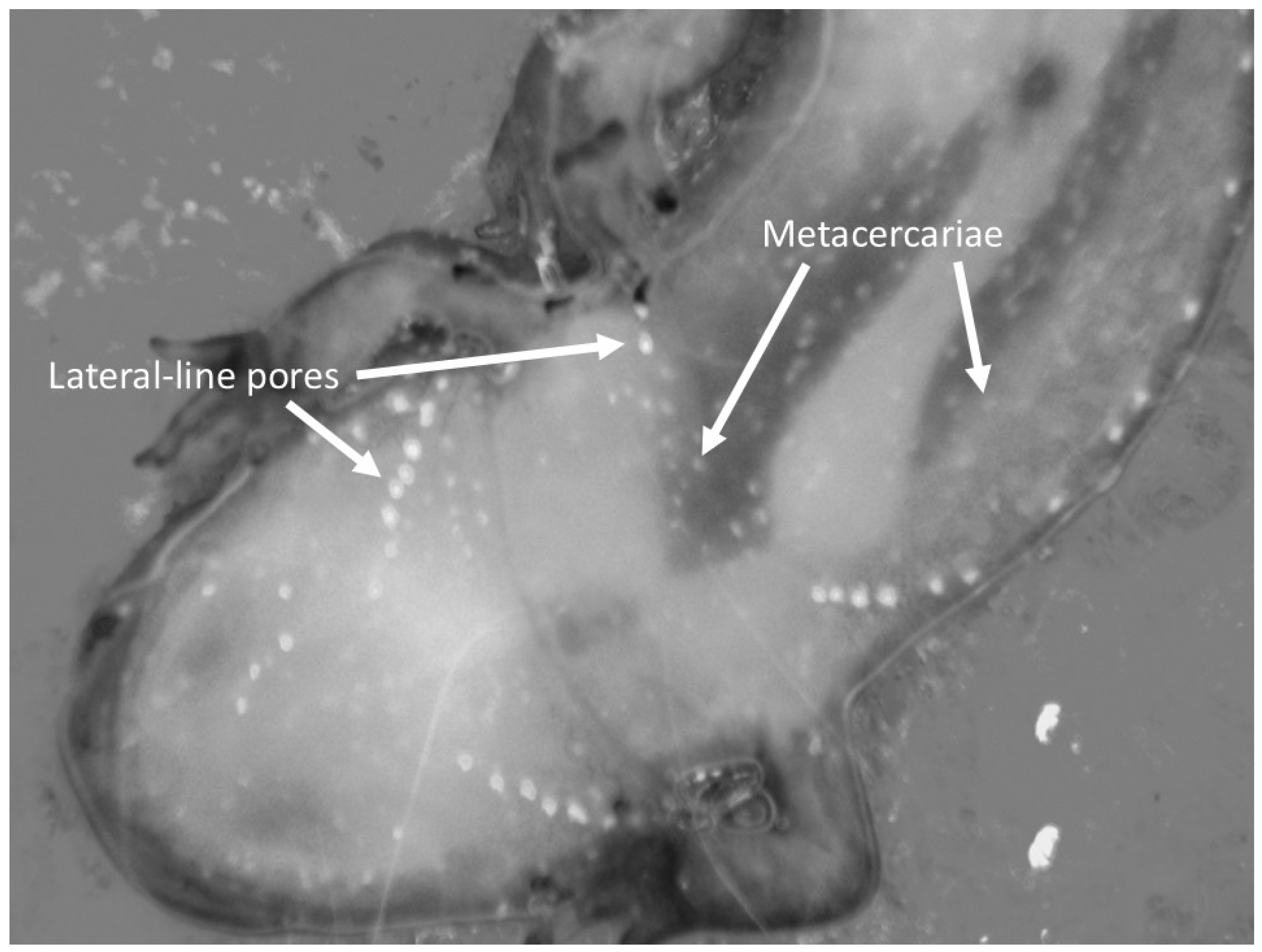
2. Materials and Methods
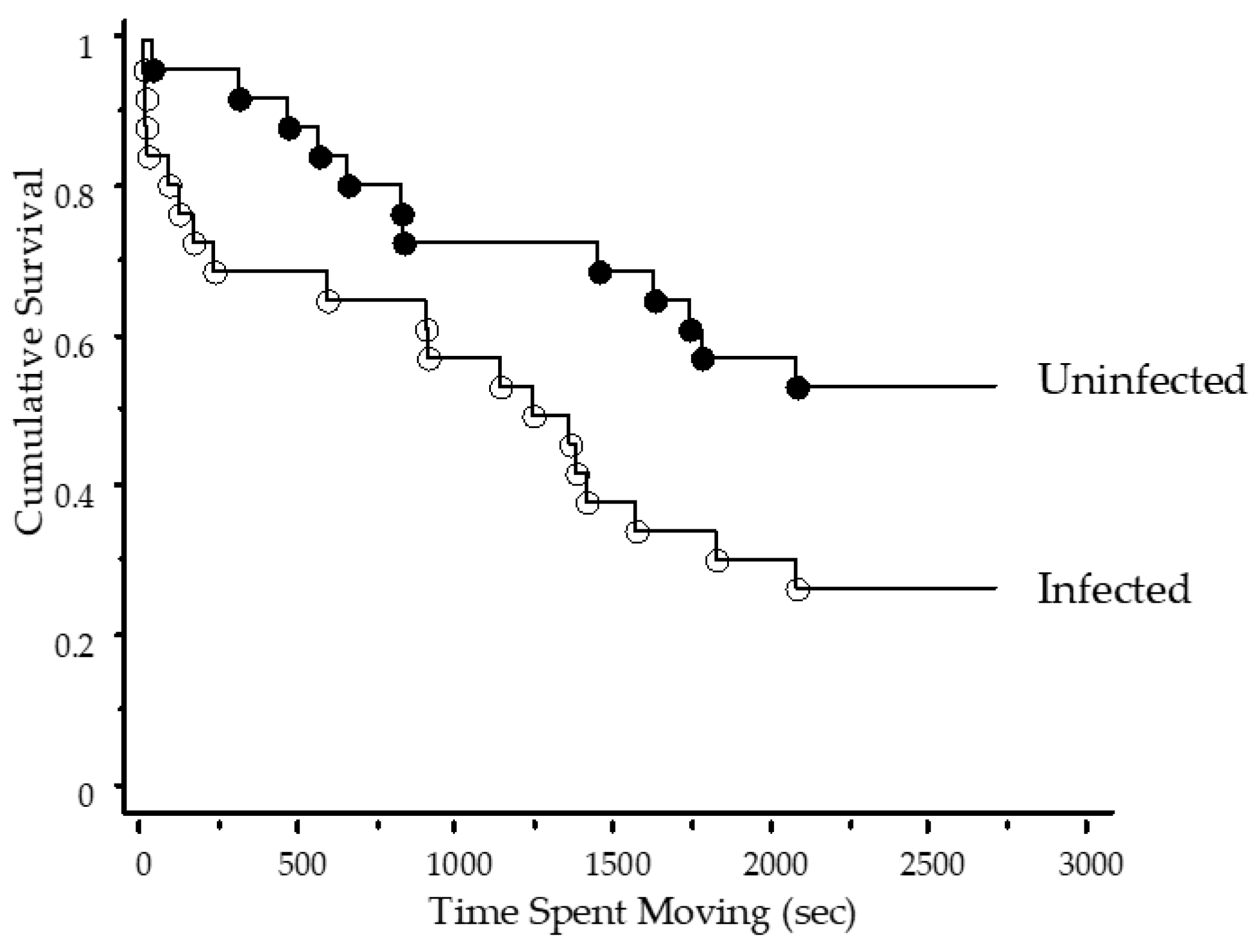
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez-Caballero, I.; Garcia-Longoria, L.; Gomez-Mestre, I.; Marzal, A. The Adaptive Host Manipulation Hypothesis: Parasites Modify the Behaviour, Morphology, and Physiology of Amphibians. Diversity 2022, 14, 739. [Google Scholar] [CrossRef]
- Goodman, B.A.; Johnson, P.T.J. Disease and the Extended Phenotype: Parasites Control Host Performance and Survival through Induced Changes in Body Plan. PLoS ONE 2011, 6, e20193. [Google Scholar] [CrossRef]
- Binning, S.A.; Shaw, A.K.; Roche, D.G. Parasites and Host Performance: Incorporating Infection into Our Understanding of Animal Movement. Integr. Comp. Biol. 2017, 57, 267–280. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and Trends of Amphibian Declines and Extinctions Worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological Annihilation via the Ongoing Sixth Mass Extinction Signaled by Vertebrate Population Losses and Declines. Proc. Natl. Acad. Sci. USA 2017, 114, 6089–6096. [Google Scholar] [CrossRef]
- Hussain, Q.A.; Pandit, A.K. Global Amphibian Declines: A Review. Int. J. Biodivers. Conserv. 2012, 4, 348–357. [Google Scholar]
- Stopper, G.F.; Hecker, L.; Franssen, R.A.; Sessions, S.K. How Trematodes Cause Limb Deformities in Amphibians. J. Exp. Zool. (Mol. Dev. Evol.) 2002, 294, 252–263. [Google Scholar] [CrossRef]
- Dubois, A. Rostand’s Anomaly P in Palaearctic Green Frogs (Pelophylax) and Similar Anomalies in Amphibians. Mertensiella 2017, 25, 49–56. [Google Scholar]
- Henle, K.; Dubois, A.; Vershinin, V. A Review of Anomalies in Natural Populations of Amphibians and Their Potential Causes. Studies on Anomalies in Natural Populations of Amphibians. Mertensiella 2017, 25, 57–164. [Google Scholar]
- Svinin, A.O.; Bashinskiy, I.W.; Litvinchuk, S.N.; Ermakov, O.A.; Ivanov, A.Y.; Neymark, L.A.; Vedernikov, A.A.; Osipov, V.V.; Drobot, G.P.; Dubois, A. Strigea robusta Causes Polydactyly and Severe Forms of Rostand’s Anomaly P in Water Frogs. Parasit. Vectors 2020, 13, 381. [Google Scholar] [CrossRef]
- Svinin, A.O.; Matushkina, K.A.; Dedukh, D.V.; Bashinskiy, I.V.; Ermakov, O.A.; Litvinchuk, S.N. Strigea robusta (Digenea: Strigeidae) Infection Effects on the Gonadal Structure and Limb Malformation in Toad Early Development. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 675–686. [Google Scholar] [CrossRef]
- Koprivnikar, J.; Marcogliese, D.J.; Rohr, J.R.; Orlofske, S.A.; Raffel, T.R.; Johnson, P.T.J. Macroparasite Infections of Amphibians: What Can They Tell Us? Ecohealth 2012, 9, 342–360. [Google Scholar] [CrossRef]
- Bower, D.S.; Brannelly, L.A.; McDonald, C.A.; Webb, R.J.; Greenspan, S.E.; Vickers, M.; Gardner, M.G.; Greenlees, M.J. A Review of the Role of Parasites in the Ecology of Reptiles and Amphibians. Austral Ecol. 2018, 44, 433–448. [Google Scholar] [CrossRef]
- Shen, X.; Liang, D.; Chen, M.; Mao, R.; Wake, D.B.; Zhang, P. Enlarged Multilocus Data Set Provides Surprisingly Younger Time of Origin for the Plethodontidae, the Largest Family of Salamanders. Syst. Biol. 2016, 65, 66–81. [Google Scholar] [CrossRef]
- Feder, M.E. Integrating the Ecology and Physiology of Plethodontid Salamanders. Herpetologica 1983, 39, 291–310. [Google Scholar]
- Falcón-Ordaz, J.; Windfield-Pérez, J.C.; Mendoza-Garfias, B.; Parra-Olea, G.; de León, P.-P. Cosmocerca acanthurum n. sp. (Nematoda: Cosmocercidae) in Pseudoeurycea leprosa and Chiropterotriton orculus from the Transmexican Volcanic Belt, Central Mexico, with a Checklist of the Helminth Parasites of Plethodontid Salamanders. Zootaxa 2007, 1434, 27–49. [Google Scholar] [CrossRef]
- Pyron, R.A.; Beamer, D.A. Nomenclatural Solutions for Diagnosing ‘Cryptic’ Species using Molecular and Morphological Data Facilitate a Taxonomic Revision of the Black-bellied Salamanders (Urodela, Desmognathus ‘quadramaculatus’) from the Southern Appalachian Mountains. Bionomina 2022, 27, 1–43. [Google Scholar] [CrossRef]
- Austin, R.M., Jr.; Camp, C.D. Larval Development of Black-Bellied Salamanders, Desmognathus quadramaculatus, in Northeastern Georgia. Herpetologica 1992, 48, 313–317. [Google Scholar]
- Bruce, R.C. Life History Variation in the Salamander Desmognathus quadramaculatus. Herpetologica 1988, 44, 218–227. [Google Scholar]
- Bruce, R.C.; Castanet, J.; Francillon-Vieillot, H. Skeletochronological Analysis of Variation in Age Structure, Body Size, and Life History in Three Species of Desmognathine Salamanders. Herpetologica 2002, 58, 181–193. [Google Scholar] [CrossRef]
- Belden, L.K.; Peterman, W.E.; Smith, S.A.; Brooks, L.R.; Benfield, E.F.; Black, W.P.; Yang, Z.; Wojdak, J.M. Metagonimoides oregonensis (Heterophyidae: Digenea) Infection in Pleurocerid Snails and Desmognathus quadramaculatus Salamander Larvae in Southern Appalachian Streams. J. Parasitol. 2012, 98, 760–767. [Google Scholar] [CrossRef]
- Burns, W.C.; Pratt, I. The Life Cycle of Metagonimoides oregonensis Price (Trematoda: Heterophyidae). J. Parasitol. 1953, 39, 60–69. [Google Scholar] [CrossRef]
- Wyderko, J.A.; Benfield, E.F.; Maerz, J.C.; Cecala, K.C.; Belden, L.K. Variable Infection of Stream Salamanders in the Southern Appalachians by the Trematode Metagonimoides oregonensis (family: Heterophyidae). Parasitol. Res. 2015, 114, 3159–3165. [Google Scholar] [CrossRef]
- Goater, T.M.; Esch, G.W.; Bush, A.O. Helminth Parasites of Sympatric Salamanders: Ecological Concepts at Infracommunity, Component and Compound Community Levels. Am. Midl. Nat. 1987, 118, 289–300. [Google Scholar] [CrossRef]
- Camp, C.D.; Jones, D.; Phillips, J.; Brock, T.L.; Wooten, J.A. Differential Infection of Two Sympatric, Cryptic Species of Appalachian Salamander (Genus Desmognathus) by the Trematode Metagonimoides oregonensis. Comp. Parasitol. 2021, 88, 183–186. [Google Scholar] [CrossRef]
- Ward, J.K.; Hoare, D.J.; Couzin, I.D.; Broom, M.; Krause, J. The Effects of Parasitism and Body Length on Positioning within Wild Fish Shoals. J. Anim. Ecol. 2002, 71, 10–14. [Google Scholar] [CrossRef]
- Kework, C.A.; Ash, J.A.; Camp, C.D. Factors Affecting Infection Levels in the Salamander Host Desmognathus amphileucus by a Digenetic Trematode Within Appalachian Headwater Streams. Herpetologica 2024, 80, 112–116. [Google Scholar] [CrossRef]
- Camp, C.D. The Status of the Black-Bellied Salamander (Desmognathus quadramaculatus) as a Predator of Heterospecific Salamanders in Appalachian Streams. J. Herpetol. 1997, 31, 613–616. [Google Scholar] [CrossRef]
- Marsh, D.M.; Thakur, K.A.; Bulka, K.C.; Clarke, L.B. Dispersal and Colonization through Open Fields by a Terrestrial, Woodland Salamander. Ecology 2004, 85, 3396–3405. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.; Ly, A.; Gronau, Q.F.; Šmíra, M.; Epskamp, S.; et al. JASP: Graphical Statistical Software for Common Statistical Designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Etikan, İ.; Abubakar, S.; Alkassim, R. The Kaplan Meier Estimate in Survival Analysis. Biom. Biostat. Int. J. 2017, 5, 00128. [Google Scholar] [CrossRef]
- Muñoz, G.B.; Rebolledo, M.; Landaeta, M.F. Effects of Metacercariae of Prosorhynchoides sp. (Trematoda: Bucephalidae) on the Swimming Ability and Blood Parameters of the Intertidal Fish Girella laevifrons (Osteichthyes: Kyphosidae). Exp. Parasitol. 2023, 246, 108473. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.D.; Dickinson, T.E. Ribeiroia ondatrae Causes Limb Abnormalities in a Canadian Amphibian Community. Can. J. Zool. 2012, 90, 808–814. [Google Scholar] [CrossRef]
- Marvin, G.A. Effect of Caudal Autotomy on Aquatic and Terrestrial Locomotor Performance in Two Desmognathine Salamander Species. Copeia 2010, 2010, 468–474. [Google Scholar] [CrossRef]
- Landberg, T.; Azizi, E. Ontogeny of Escape Swimming Performance in the Spotted Salamander. Funct. Ecol. 2010, 24, 576–587. [Google Scholar] [CrossRef]
- Ijspeert, A.J.; Crespi, A.; Ryczko, D.; Cabelguen, J. From Swimming to Walking with a Salamander Robot Driven by a Spinal Cord Model. Science 2007, 315, 1416–1420. [Google Scholar] [CrossRef]
- Wright, S. Isolation by Distance. Genetics 1942, 38, 114–138. [Google Scholar] [CrossRef]
- Slatkin, M. Isolation by Distance in Equilibrium and Non-equilibrium populations. Evolution 1993, 47, 264–279. [Google Scholar] [CrossRef]
- Baptestini, E.M.; de Aguiar, M.A.M.; Bar-Yam, Y. Conditions for Neutral Speciation via Isolation by Distance. J. Theor. Biol. 2013, 335, 51–56. [Google Scholar] [CrossRef]
- Cabe, P.R.; Page, R.B.; Hanlon, T.J.; Aldrich, M.E.; Connors, L.; Marsh, D.M. Fine-scale Population Differentiation and Gene Flow in a Terrestrial Salamander (Plethodon cinereus) Living in Continuous Habitat. Heredity 2007, 98, 53–60. [Google Scholar] [CrossRef]
- Staub, N.L.; Brown, C.W.; Wake, D.B. Patterns of Growth and Movements in a population of Ensatina eschscholtzii platensis (Caudata: Plethodontidae) in the Sierra Nevada. California. J. Herpetol. 1995, 29, 593–599. [Google Scholar] [CrossRef]
- Bonett, R.M.; Kozak, K.H.; Vieites, D.R.; Bare, A.; Wooten, J.A.; Trauth, S.E. The Importance of Comparative Phylogeography in Diagnosing Introduced Species: A Lesson from the Seal Salamander, Desmognathus monticola. BMC Ecol. 2007, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Solano, I.; Jockusch, E.L.; Wake, D.B. Extreme Population Subdivision Throughout a Continuous Range: Phylogeography of Batrachoseps attenuatus (Caudata: Plethodontidae) in Western North America. Mol. Ecol. 2007, 16, 4335–4355. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Snodgrass, J.W.; Gasparich, G.E. The Importance of Terrestrial Dispersal for Connectivity among Headwater Salamander Populations. Ecosphere 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Radomski, T.; Hantak, M.M.; Brown, A.D.; Kuchta, S.R. Multilocus Phylogeography of Eastern Red-Backed Salamanders (Plethodon cinereus): Cryptic Appalachian Diversity and Postglacial Range Expansion. Herpetologica 2020, 76, 61–73. [Google Scholar] [CrossRef]
- Dillon, R.T., Jr.; Robinson, J.D. The Snails the Dinosaurs Saw: Are the Pleurocerid Populations of the Older Appalachians a Relict of the Paleozoic Era? J. N. Am. Benthol. Soc. 2009, 28, 1–11. [Google Scholar] [CrossRef]
- Zemmer, S.A.; Wyderko, J.; Da Silva Neto, J.; Cedillos, I.; Clay, L.; Benfield, E.F.; Belden, L.K. Seasonal and Annual Variation in Trematode Infection of Stream Snail Elimia proxima in the Southern Appalachian Mountains of Virginia. J. Parasitol. 2017, 103, 213–220. [Google Scholar] [CrossRef]
- Karvonen, A.; Seehausen, O. The Role of Parasitism in Adaptive Radiations—When Might Parasites Promote and When Might They Constrain Ecological Speciation? Int. J. Ecol. 2012, 2012, 280169. [Google Scholar] [CrossRef]
- Bracamonte, S.E.; Hofmann, M.J.; Lozano-Martín, C.; Eizaguirre, C.; Barluenga, M. Divergent and Non-parallel Evolution of MHC IIB in the Neotropical Midas Cichlid Species Complex. BMC Ecol. Evol. 2022, 22, 41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camp, C.; Vaca-Nava, A.; Bowen, A. Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA. Parasitologia 2024, 4, 375-381. https://doi.org/10.3390/parasitologia4040033
Camp C, Vaca-Nava A, Bowen A. Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA. Parasitologia. 2024; 4(4):375-381. https://doi.org/10.3390/parasitologia4040033
Chicago/Turabian StyleCamp, Carlos, Alexia Vaca-Nava, and Addison Bowen. 2024. "Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA" Parasitologia 4, no. 4: 375-381. https://doi.org/10.3390/parasitologia4040033
APA StyleCamp, C., Vaca-Nava, A., & Bowen, A. (2024). Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA. Parasitologia, 4(4), 375-381. https://doi.org/10.3390/parasitologia4040033




