Sand Flies and Their Microbiota
Abstract
1. Introduction
2. Methodological Approaches to Study the Microbiota of Sand Flies
3. Diversity and Composition of the Microbiome in Sand Flies
3.1. Microbiota of Different Sand Fly Developmental Stages
3.2. Microbiota of Wild and Laboratory Sand Flies
3.2.1. New World Sand Flies
3.2.2. Old World Sand Flies
3.3. Microbiota and Sand Fly Species
3.3.1. New World Sand Fly Species
3.3.2. Old World Sand Fly Species
4. Gut Microbiota Alterations and Their Impact on Flies’ Life Traits and Leishmania Infection
4.1. Gut Microbiota Alterations and Their Impact on Flies’ Life Traits
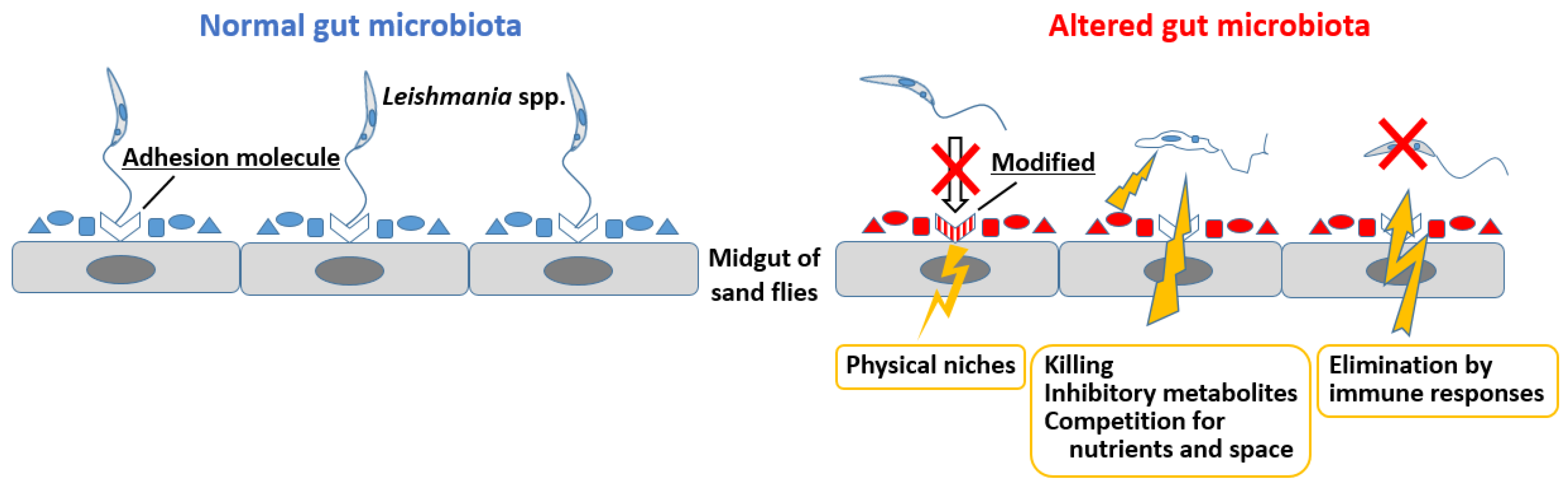
4.2. Gut Microbiota Alterations and Their Impact on Leishmania Infection
5. Microbiota-Driven Mechanisms Affecting Vector Competence
5.1. Genetic Determinants of Sand Fly Competence for Leishmania Parasites
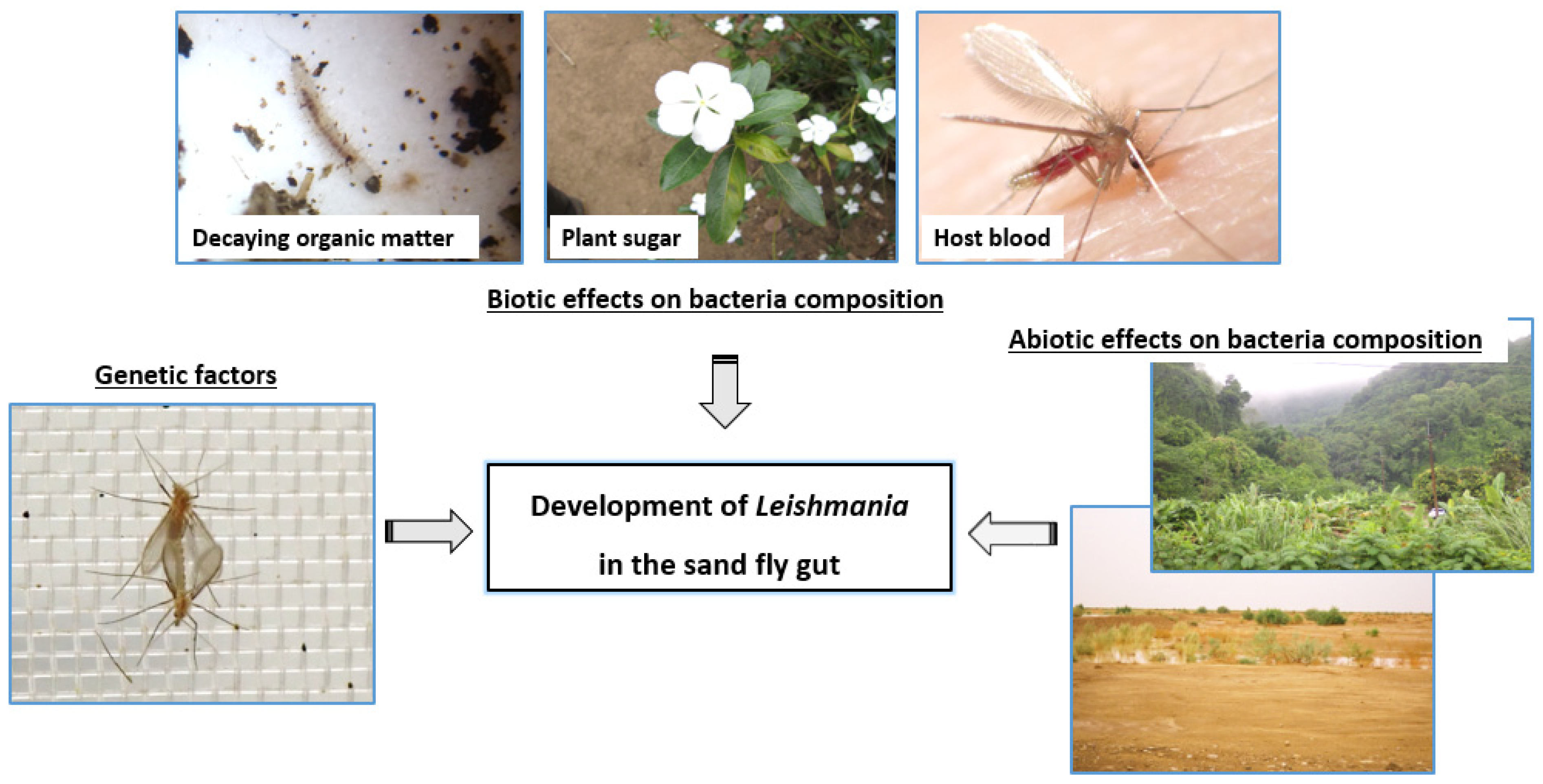
5.2. Non-Genetic Determinants of Sand Fly Competence for Leishmania Parasites
6. Fungi Associated with the Midgut of Sand Flies
7. Microbiota as a Target for Novel Vector Control Strategies
7.1. Introduction of Symbionts to Manipulate Host Life Traits
7.2. Exploitation of Endosymbionts with Antipathogen Effects
7.3. Paratransgenesis Approaches
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votypka, J.; Marty, P.; Delaunay, P.; Sereno, D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef] [PubMed]
- Alidosti, M.; Heidari, Z.; Shahnazi, H.; Zamani-Alavijeh, F. Behaviors and perceptions related to cutaneous leishmaniasis in endemic areas of the world: A review. Acta Trop. 2021, 223, 106090. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Leishmania Fact Sheet Number 375. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 3 February 2022).
- Sereno, D.; Maia, C.; Aït-Oudhia, K. Antimony resistance and environment: Elusive links to explore during Leishmania life cycle. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 200–203. [Google Scholar] [CrossRef]
- Seblova, V.; Oury, B.; Eddaikra, N.; Ait-Oudhia, K.; Pratlong, F.; Gazanion, E.; Maia, C.; Volf, P.; Sereno, D. Transmission potential of antimony-resistant Leishmania field isolates. Antimicrob. Agents Chemother. 2014, 58, 6273–6276. [Google Scholar] [CrossRef]
- Louradour, I.; Monteiro, C.C.; Inbar, E.; Ghosh, K.; Merkhofer, R.; Lawyer, P.; Paun, A.; Smelkinson, M.; Secundino, N.; Lewis, M.; et al. The midgut microbiota plays an essential role in sand fly vector competence for Leishmania major. Cell. Microbiol. 2017, 19, e12755. [Google Scholar] [CrossRef]
- Li, K.; Chen, H.; Jiang, J.; Li, X.; Xu, J.; Ma, Y. Diversity of bacteriome associated with Phlebotomus chinensis (Diptera: Psychodidae) sand flies in two wild populations from China. Sci. Rep. 2016, 6, 36406. [Google Scholar] [CrossRef]
- Vivero, R.J.; Villegas-Plazas, M.; Cadavid-Restrepo, G.E.; Herrera, C.X.M.; Uribe, S.I.; Junca, H. Wild specimens of sand fly phlebotomine Lutzomyia evansi, vector of leishmaniasis, show high abundance of Methylobacterium and natural carriage of Wolbachia and Cardinium types in the midgut microbiome. Sci. Rep. 2019, 9, 17746. [Google Scholar] [CrossRef]
- Fraihi, W.; Fares, W.; Perrin, P.; Dorkeld, F.; Sereno, D.; Barhoumi, W.; Sbissi, I.; Cherni, S.; Chelbi, I.; Durvasula, R.; et al. An integrated overview of the midgut bacterial flora composition of Phlebotomus perniciosus, a vector of zoonotic visceral leishmaniasis in the Western Mediterranean Basin. PLOS Negl. Trop. Dis. 2017, 11, e0005484. [Google Scholar] [CrossRef]
- Karimian, F.; Vatandoost, H.; Rassi, Y.; Maleki-Ravasan, N.; Mohebali, M.; Shirazi, M.H.; Koosha, M.; Choubdar, N.; Oshaghi, M.A. Aerobic midgut microbiota of sand fly vectors of zoonotic visceral leishmaniasis from northern Iran, a step toward finding potential paratransgenic candidates. Paras. Vector 2019, 12, 10. [Google Scholar] [CrossRef]
- Guernaoui, S.; Garcia, D.; Gazanion, E.; Ouhdouch, Y.; Boumezzough, A.; Pesson, B.; Fontenille, D.; Sereno, D. Bacterial flora as indicated by PCR-temperature gradient gel electrophoresis (TGGE) of 16S rDNA gene fragments from isolated guts of phlebotomine sand flies (Diptera: Psychodidae). J. Vector Ecol. 2011, 36, S144–S147. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.H.; Bahr, S.M.; Serafim, T.D.; Ajami, N.J.; Petrosino, J.F.; Meneses, C.; Kirby, J.R.; Valenzuela, J.G.; Kamhawi, S.; Wilson, M.E. The Gut Microbiome of the Vector Lutzomyia longipalpis Is Essential for Survival of Leishmania infantum. Mbio 2017, 8, e01121-16. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, M.R.V.; Darby, A.C.; Brazil, R.P.; Montoya-Lerma, J.; Dillon, V.M.; Bates, P.A.; Dillon, R.J. Investigation of the Bacterial Communities Associated with Females of Lutzomyia Sand Fly Species from South America. PLoS ONE 2012, 7, e42531. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Muturi, E.J.; Lagos-Kutz, D.; Dunlap, C.; Ramirez, J.L.; Rooney, A.P.; Hartman, G.L.; Fields, C.J.; Rendon, G.; Kim, C.-H. Mosquito microbiota cluster by host sampling location. Paras. Vector 2018, 11, 468. [Google Scholar] [CrossRef]
- Sanders, J.G.; Powell, S.; Kronauer, D.J.; Vasconcelos, H.L.; Frederickson, M.E.; Pierce, N.E. Stability and phylogenetic correlation in gut microbiota: Lessons from ants and apes. Molec. Ecol. 2014, 23, 1268–1283. [Google Scholar] [CrossRef]
- Wang, S.; Jacobs-Lorena, M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends. Biotechnol. 2013, 31, 185–193. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Ye, Y.H.; Carrasco, A.M.; Frentiu, F.D.; Chenoweth, S.F.; Beebe, N.W.; van den Hurk, A.F.; Simmons, C.P.; O’Neill, S.L.; McGraw, E.A. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLOS Negl. Trop. Dis. 2015, 9, e0003894. [Google Scholar] [CrossRef]
- Joshi, D.; Pan, X.; McFadden, M.J.; Bevins, D.; Liang, X.; Lu, P.; Thiem, S.; Xi, Z. The maternally inheritable Wolbachia wAlbB induces refractoriness to Plasmodium berghei in Anopheles stephensi. Front. Microbiol. 2017, 8, 366. [Google Scholar] [CrossRef]
- Coutinho-Abreu, I.V.; Zhu, K.Y.; Ramalho-Ortigao, M. Transgenesis and paratransgenesis to control insect-borne diseases: Current status and future challenges. Parasitol. Int. 2010, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, I.; Fieck, A.; Read, A.; Hillesland, H.; Klein, N.; Kang, A.; Durvasula, R. Paratransgenic control of vector borne diseases. Int. J. Biol. Sci. 2011, 7, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Hillesland, H.; Read, A.; Subhadra, B.; Hurwitz, I.; McKelvey, R.; Ghosh, K.; Das, P.; Durvasula, R. Identification of aerobic gut bacteria from the kala azar vector, Phlebotomus argentipes: A platform for potential paratransgenic manipulation of sand flies. Am. J. Trop. Med. Hyg. 2008, 79, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, I.; Hillesland, H.; Fieck, A.; Das, P.; Durvasula, R. The paratransgenic sand fly: A platform for control of Leishmania transmission. Parasit. Vectors 2011, 4, 82. [Google Scholar] [CrossRef]
- Dillon, R.J.; Kordy, E.E.; Shehata, M.; Lane, R.P. The prevalence of a microbiota in the digestive tract of Phlebotomus papatasi. Ann. Trop. Med. Parasitol. 1996, 90, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.G. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Baltimore: Wantage, UK, 1993. [Google Scholar]
- Oliveira, S.M.; Moraes, B.A.; Goncalves, C.A.; Giordano-Dias, C.M.; D’Almeida, J.M.; Asensi, M.D.; Mello, R.P.; Brazil, R.P. Prevalence of microbiota in the digestive tract of wild females of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae). Rev. Soc. Bras. Med. Trop. 2000, 33, 319–322. [Google Scholar]
- Perira de Oliveira, S.M.; de Morais, B.A.; Goncalves, C.A.; Giordano-Dias, C.M.; Vilela, M.L.; Brazil, R.P.; D’Almeida, J.M.; Asensi, M.D.; Mello, R.P. Digestive tract microbiota in female Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae) from colonies feeding on blood meal and sucrose plus blood meal. Cad. Saude Publica. 2001, 17, 229–232. [Google Scholar]
- Volf, P.; Kiewegova, A.; Nemec, A. Bacterial colonisation in the gut of Phlebotomus duboseqi (Diptera: Psychodidae): Transtadial passage and the role of female diet. Folia. Parasitol. 2002, 49, 73–77. [Google Scholar] [CrossRef]
- Akhoundi, M.; Bakhtiari, R.; Guillard, T.; Baghaei, A.; Tolouei, R.; Sereno, D.; Toubas, D.; Depaquit, J.; Abyaneh, M.R. Diversity of the bacterial and fungal microflora from the midgut and cuticle of phlebotomine sand flies collected in North-Western Iran. PLoS ONE 2012, 7, e50259. [Google Scholar] [CrossRef]
- Campolina, T.B.; Villegas, L.E.M.; Monteiro, C.C.; Pimenta, P.F.P.; Secundino, N.F.C. Tripartite interactions: Leishmania, microbiota and Lutzomyia longipalpis. PLoS Negl. Trop. Dis. 2020, 14, e0008666. [Google Scholar] [CrossRef]
- Tabbabi, A.; Watanabe, S.; Mizushima, D.; Caceres, A.G.; Gomez, E.A.; Yamamoto, D.S.; Cui, L.; Hashiguchi, Y.; Kato, H. Comparative Analysis of Bacterial Communities in Lutzomyia ayacuchensis Populations with Different Vector Competence to Leishmania Parasites in Ecuador and Peru. Microorganisms 2020, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Vivero, R.J.; Castañeda-Monsalve, V.A.; Romero, L.R.D.; Hurst, G.; Cadavid-Restrepo, G.; Moreno-Herrera, C.X. Gut Microbiota Dynamics in Natural Populations of Pintomyia evansi under Experimental Infection with Leishmania infantum. Microorganisms 2021, 9, 1214. [Google Scholar] [CrossRef] [PubMed]
- Depaquit, J.; Grandadam, M.; Fouque, F.; Andry, P.E.; Peyrefitte, C. Arthropod-borne viruses transmitted by Phlebotomine sandflies in Europe: A review. Euro Surveill. 2010, 15, 19507. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.B.; Diambra, L.A.; Rivera Pomar, R.V. Metagenomic analysis of taxa associated with Lutzomyia longipalpis, vector of visceral leishmaniasis, using an unbiased high-throughput approach. PLoS Negl. Trop. Dis. 2011, 5, e1304. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.C.; Armougom, F.; Million, M.; Hugon, P.; Pagnier, I.; Robert, C.; Bittar, F.; Fournous, G.; Gimenez, G.; Maraninchi, M.; et al. Microbial culturomics: Paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 2012, 18, 1185–1193. [Google Scholar] [CrossRef]
- Lagier, J.C.; Hugon, P.; Khelaifia, S.; Fournier, P.E.; La Scola, B.; Raoult, D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin. Microbiol. Rev. 2015, 28, 237–264. [Google Scholar] [CrossRef]
- Abdallah, R.A.; Beye, M.; Diop, A.; Bakour, S.; Raoult, D.; Fournier, P.E. The impact of culturomics on taxonomy in clinical microbiology. Antonie Leeuwenhoek 2017, 110, 1327–1337. [Google Scholar] [CrossRef]
- Amrane, S.; Lagier, J.C. Metagenomic and clinical microbiology. Hum. Microbiome J. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Tandina, F.; Almeras, L.; Koné, A.K.; Doumbo, O.K.; Raoult, D.; Parola, P. Use of MALDI-TOF MS and culturomics to identify mosquitoes and their midgut microbiota. Parasit. Vector 2016, 9, 495. [Google Scholar] [CrossRef]
- Greub, G. Culturomics: A new approach to study the human microbiome. Clin. Microbiol. Infect. 2012, 18, 1157–1159. [Google Scholar] [CrossRef]
- Moraes, C.S.; Lucena, S.A.; Moreira, B.H.; Brazil, R.P.; Gontijo, N.F.; Genta, F.A. Relationship between digestive enzymes and food habit of Lutzomyia longipalpis (Diptera: Psychodidae) larvae: Characterization of carbohydrases and digestion of microorganisms. J. Insect Physiol. 2012, 58, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, M.L.; Marayati, B.F.; Wada-Katsumata, A.; Wasserberg, G.; Schal, C.; Apperson, C.S.; Ponnusamy, L. Sphingobacterium phlebotomi sp. nov., a new member of family Sphingobacteriaceae isolated from sand fly rearing substrate. Int J. Syst Evol. Microbiol. 2021, 71, 004809. [Google Scholar] [CrossRef]
- Zhang, H.B.; Shi, W.; Yang, M.X.; Sha, T.; Zhao, Z.W. Bacterial diversity at different depths in lead-zinc mine tailings as revealed by 16S rRNA gene libraries. J. Microbiol. 2007, 45, 479–484. [Google Scholar] [PubMed]
- Vivero, R.J.; Jaramillo, N.G.; Cadavid-Restrepo, G.; Soto, S.I.; Herrera, C.X. Structural differences in gut bacteria communities in developmental stages of natural populations of Lutzomyia evansi from Colombia’s Caribbean coast. Parasit. Vector 2016, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, C.; Asensi, M.D.; Zahner, V.; Rangel, E.F.; Oliveira, S.M. Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae). Neotrop. Entomol. 2008, 37, 597–601. [Google Scholar]
- Pires, A.; Villegas, L.E.M.; Campolina, T.B.; Orfano, A.S.; Pimenta, P.F.P.; Secundino, N.F.C. Bacterial diversity of wild-caught Lutzomyia longipalpis (a vector of zoonotic visceral leishmaniasis in Brazil) under distinct physiological conditions by metagenomics analysis. Parasit. Vector 2017, 10, 627. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rastogi, G.; Nayduch, D.; Sawant, S.S.; Bhonde, R.R.; Shouche, Y.S. Molecular phylogenetic profiling of gut-associated bacteria in larvae and adults of flesh flies. Med. Vet. Entomol. 2014, 28, 345–354. [Google Scholar] [CrossRef]
- Ngo, C.T.; Romano-Bertrand, S.; Manguin, S.; Jumas-Bilak, E. Diversity of the bacterial microbiota of anopheles’ mosquitoes from Binh Phuoc Province, Vietnam. Front. Microbiol. 2016, 7, 2095. [Google Scholar] [CrossRef][Green Version]
- Garofalo, C.; Osimani, A.; Milanovic, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Robert, L.L.; Perich, M.J.; Schlein, Y.; Jacobson, J.L. Bacillus sphaericus inhibits hatching of phlebotomine sand fly eggs. J. Am. Mosq. Control Assoc. 1998, 14, 351–352. [Google Scholar]
- Wahba, M.M.; Labib, I.M.; el Hamshary, E.M. Bacillus thuringiensis var. israelensis as a microbial control agent against adult and immature stages of the sandfly, Phlebotomus papatasi under laboratory conditions. J. Egypt. Soc. Parasitol. 1999, 29, 587–597. [Google Scholar] [PubMed]
- Monteiro, C.C.; Villegas, L.E.; Campolina, T.B.; Pires, A.C.; Miranda, J.C.; Pimenta, P.F.; Secundino, N.F. Bacterial diversity of the American sand fly Lutzomyia intermedia using high-throughput metagenomics sequencing. Parasit. Vector 2016, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.E.; Martins, P.M.; Ferreira, H.; Ferro, M.; Bacci, M.; Pinto, M.C. Bacterial groups associated with Nyssomyia neivai (Diptera: Psychodidae) sand flies. J. Vector Borne Dis. 2014, 51, 137–139. [Google Scholar] [PubMed]
- Poulsen, L.L.; Thofner, I.; Bisgaard, M.; Olsen, R.H.; Christensen, J.P.; Christensen, H. Staphylococcus agnetis, a potential pathogen in broiler breeders. Vet. Microbiol. 2017, 212, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.C.; Brito, M.A.; Reis, D.R.; Machado, M.A.; Guimaraes, A.S.; Azevedo, A.L.; Salles, E.B.; Alvim, M.C.; Silva, F.S.; Meurer, I.R. Species-level identification of staphylococci isolated from Bovine mastitis in Brazil using partial 16S rRNA sequencing. Vet. Microbiol. 2015, 176, 382–388. [Google Scholar] [CrossRef]
- Montoya-Porras, L.M.; Omar, T.C.; Alzate, J.F.; Moreno-Herrera, C.X.; Cadavid-Restrepo, G.E. 16S rRNA gene amplicon sequencing reveals dominance of Actinobacteria in Rhodnius pallescens compared to Triatoma maculata midgut microbiota in natural populations of vector insects from Colombia. Acta Trop. 2018, 178, 327–332. [Google Scholar] [CrossRef]
- Kaaya, G.P.; Otieno, L.H.; Darji, N.; Alemu, P. Defence reactions of Glossina morsitans morsitans against different species of bacteria and Trypanosoma brucei brucei. Acta Trop. 1986, 43, 31–42. [Google Scholar]
- Memona, H.; Manzoor, F.; Anjum, A.A. Cockroaches (Blattodea: Blattidae): A reservoir of pathogenic microbes in human-dwelling localities in Lahore. J. Med. Entomol. 2017, 54, 435–440. [Google Scholar] [CrossRef]
- Nicoletti, G.; Corbella, M.; Jaber, O.; Marone, P.; Scevola, D.; Faga, A. Non-pathogenic microflora of a spring water with regenerative properties. Biomed. Rep. 2015, 3, 758–762. [Google Scholar] [CrossRef]
- Colauto, N.B.; Fermor, T.R.; Eira, A.F.; Linde, G.A. Pseudomonas putida Stimulates Primordia on Agaricus bitorquis. Curr. Microbiol. 2016, 72, 482–488. [Google Scholar] [CrossRef]
- McMullen, A.R.; Anderson, N.; Wallace, M.A.; Shupe, A.; Burnham, C.A. When Good Bugs Go Bad: Epidemiology and Antimicrobial Resistance Profiles of Corynebacterium striatum, an Emerging Multidrug-Resistant, Opportunistic Pathogen. Antimicrob. Agents Chemother. 2017, 61, e01111-17. [Google Scholar] [CrossRef] [PubMed]
- Panteli, N.; Mastoraki, M.; Lazarina, M.; Chatzifotis, S.; Mente, E.; Kormas, K.A.; Antonopoulou, E. Configuration of Gut Microbiota Structure and Potential Functionality in Two Teleosts under the Influence of Dietary Insect Meals. Microorganisms 2021, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Pragai, Z.; Koczian, Z.; Hajdu, E.; Fodor, E. Investigation of the presence of different broad-spectrum beta-lactamases among clinical isolates of Enterobacteriacae. Acta Microbiol. Immunol. Hung. 1998, 45, 433–446. [Google Scholar] [PubMed]
- Thakur, A.; Dhammi, P.; Saini, H.S.; Kaur, S. Pathogenicity of bacteria isolated from gut of Spodoptera litura (Lepidoptera: Noctuidae) and fitness costs of insect associated with consumption of bacteria. J. Invertebr. Pathol. 2015, 127, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, J.; Braig, H.R.; Rowton, E.D.; Ghosh, K. Naturally occurring culturable aerobic gut flora of adult Phlebotomus papatasi, vector of Leishmania major in the Old World. PLoS ONE 2012, 7, e35748. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.S.; Seabra, S.H.; Castro, D.P.; Brazil, R.P.; de Souza, W.; Garcia, E.S.; Azambuja, P. Leishmania (Leishmania) chagasi interactions with Serratia marcescens: Ultrastructural studies, lysis and carbohydrate effects. Exp. Parasitol. 2008, 118, 561–568. [Google Scholar] [CrossRef]
- Maleki-Ravasan, N.; Oshaghi, M.A.; Afshar, D.; Arandian, M.H.; Hajikhani, S.; Akhavan, A.A.; Yakhchali, B.; Shirazi, M.H.; Rassi, Y.; Jafari, R.; et al. Aerobic bacterial flora of biotic and abiotic compartments of a hyperendemic Zoonotic Cutaneous Leishmaniasis (ZCL) focus. Parasit. Vectors 2015, 8, 63. [Google Scholar] [CrossRef]
- Gunathilaka, N.; Perera, H.; Wijerathna, T.; Rodrigo, W.; Wijegunawardana, N.D.A.D. The Diversity of Midgut Bacteria among Wild-Caught Phlebotomus argentipes (Psychodidae: Phlebotominae), the Vector of Leishmaniasis in Sri Lanka. Biomed. Res. Int. 2020, 2020, 5458063. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Karas, P.A.; Vasileiadis, S.; Ligda, P.; Saratsis, A.; Sotiraki, S.; Karpouzas, D.G. Host Species Determines the Composition of the Prokaryotic Microbiota in Phlebotomus Sandflies. Pathogens 2020, 9, 428. [Google Scholar] [CrossRef]
- Peterkova-Koci, K.; Robles-Murguia, M.; Ramalho-Ortigao, M.; Zurek, L. Significance of bacteria in oviposition and larval development of the sand fly Lutzomyia longipalpis. Parasites and Vectors. Parasit Vectors 2012, 5, 145. [Google Scholar] [CrossRef]
- Aguiar Martins, K.; Meirelles, M.H.A.; Mota, T.F.; Abbasi, I.; de Queiroz, A.T.L.; Brodskyn, C.I.; Veras, P.S.T.; Mothé Fraga, D.B.; Warburg, A. Effects of larval rearing substrates on some life-table parameters of Lutzomyia longipalpis sand flies. PLoS Negl. Trop. Dis. 2021, 15, e0009034. [Google Scholar] [CrossRef] [PubMed]
- Grimont, P.A.; Grimont, F.; Le Minor, S.; Davis, B.; Pigache, F. Compatible results obtained from biotyping and serotyping in Serratia marcescens. J. Clin. Microbiol. 1979, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.S.; Seabra, S.H.; Albuquerque-Cunha, J.M.; Castro, D.P.; Genta, F.A.; de Souza, W.; Brazil, R.P.; Garcia, E.S.; Azambuja, P. Prodigiosin is not a determinant factor in lysis of Leishmania (Viannia) braziliensis after interaction with Serratia marcescens D-mannose sensitive fimbriae. Exp. Parasitol. 2009, 122, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Sant’anna, M.R.; Diaz-Albiter, H.; Aguiar-Martins, K.; Salem, A.S.W.; Cavalcante, R.R.; Dillon, M.V.; Bates, P.A.; Genta, F.A.; Dillon, R.J. Colonisation resistance in the sandfly gut: Leishmania protects Lutzomyia longipalpis from bacterial infection. Parasit. Vectors 2014, 1, 329–339. [Google Scholar] [CrossRef]
- Simões, M.L.; Dimopoulos, G. A mosquito mediator of parasite-induced immune priming. Trends Parasitol. 2015, 31, 402–404. [Google Scholar] [CrossRef]
- Hassan, M.I.; Al-Sawaf, B.M.; Fouda, M.A.; Al-Hosry, S.; Hammad, K.M. A Recent Evaluation of the Sandfly, Phlepotomus Papatasi Midgut Symbiotic Bacteria Effect on the Survivorship of Leishmania Major. J. Anc. Dis. Prev. Rem. 2014, 2, 110. [Google Scholar] [CrossRef]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Herren, J.K.; Mbaisi, L.; Mararo, E.; Makhulu, E.E.; Mobegi, V.A.; Butungi, H.; Mancini, M.; Oundo, J.W.; Teal, E.T.; Pinaud, S.; et al. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat. Commun. 2020, 11, 2187. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- McLean, A.H.C.; Godfrey, H.C.J.; Ellers, J.; Henry, L.M. Host relatedness influences the composition of aphid Microbiomes. Environ. Microbiol. Rep. 2019, 11, 808–816. [Google Scholar] [CrossRef]
- Lanzaro, G.C.; Ostrovska, K.; Herrero, M.V.; Lawyer, P.G.; Warburg, A. Lutzomyia longipalpis is a species complex: Genetic divergence and interspecific hybrid sterility among three populations. Am. J. Trop. Med. Hyg. 1993, 48, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, O.; Presber, W.; Al-Jawabreh, A.; Abdeen, Z.; Amro, A.; Schonian, G. Molecular markers for Phlebotomus papatasi (Diptera: Psychodidae) and their usefulness for population genetic analysis. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Kato, H.; Cáceres, A.G.; Gomez, E.A.; Mimori, T.; Uezato, H.; Hashiguchi, Y. Genetic divergence in populations of Lutzomyia ayacuchensis, a vector of Andean-type cutaneous leishmaniasis, in Ecuador and Peru. Acta Trop. 2015, 141, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mitri, C.; Jacques, J.C.; Thiery, I.; Riehle, M.M.; Xu, J.; Bischoff, E.; Morlais, I.; Nsango, S.E.; Vernick, K.D.; Bourgouin, C. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 2009, 5, e1000576. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Lambrechts, L.; Rousset, F.; Abate, L.; Nsango, S.E.; Fontenille, D.; Morlais, I.; Cohuet, A. Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum. PLoS Pathog. 2010, 6, e1001112. [Google Scholar] [CrossRef]
- Wolinska, J.; King, K.C. Environment can alter selection in host-parasite interactions. Trends Parasitol. 2009, 25, 236–244. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Little, T.J. Immunity in a variable world. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 15–26. [Google Scholar] [CrossRef]
- Pultz, N.J.; Stiefel, U.; Subramanyan, S.; Helfand, M.S.; Donskey, C.J. Mechanisms by which anaerobic microbiota inhibit the establishment in mice of intestinal colonization by vancomycin-resistant Enterococcus. J. Infect. Dis. 2005, 191, 949–956. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Cherrington, C.A.; Hinton, M.; Pearson, G.R.; Chopra, I. Short-chain organic acids at ph 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J. Appl. Microbiol. 1991, 70, 161–165. [Google Scholar]
- Shin, R.; Suzuki, M.; Morishita, Y. Influence of intestinal anaerobes and organic acids on the growth of enterohaemorrhagic Escherichia coli O157:H7. J. Med. Microbiol. 2002, 51, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Guiot, H.F. Role of competition for substrate in bacterial antagonism in the gut. Infect. Immun. 1982, 38, 887–892. [Google Scholar] [CrossRef]
- Wilson, K.H.; Perini, F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect. Immun. 1988, 56, 2610–2614. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Z.; Marquardt, R.R.; Zhao, X. A strain of Enterococcus faecium (18C23) inhibits adhesion of enterotoxigenic Escherichia coli K88 to porcine small intestine mucus. Appl. Environ. Microbiol. 2000, 66, 4200–4204. [Google Scholar] [CrossRef] [PubMed]
- Telleria, E.L.; Martins-Da-Silva, A.; Tempone, A.J.; Traub-Cseko, Y.M. Leishmania, Microbiota and Sand Fly Immunity. Parasitology 2018, 145, 1336–1353. [Google Scholar] [CrossRef]
- Alcolea, P.J.; Alonso, A.; Molina, R.; Jiménez, M.; Myler, P.J.; Larraga, V. Functional Genomics in Sand Fly–Derived Leishmania Promastigotes. PloS Negl. Trop. Dis. 2019, 13, e0007288. [Google Scholar] [CrossRef]
- Boulanger, N.; Lowenberger, C.; Volf, P.; Ursic, R.; Sigutova, L.; Sabatier, L.; Svobodova, M.; Beverley, S.M.; Spath, G.; Brun, R.; et al. Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect. Immun. 2004, 72, 7140–7146. [Google Scholar] [CrossRef]
- Telleria, E.L.; Sant’Anna, M.R.V.; Ortigão-Farias, J.R.; Pitaluga, A.N.; Dillon, V.M.; Bates, P.A.; Traub-Csekö, Y.M.; Dillon, R.J. Caspar-like gene depletion reduces leishmania infection in sand fly host Lutzomyia longipalpis. J. Biol. Chem. 2012, 287, 12985–12993. [Google Scholar] [CrossRef]
- Louradour, I.; Ghosh, K.; Inbar, E.; Sacks, D.L. CRISPR/Cas9 mutagenesis in Phlebotomus papatasi: The immune deficiency pathway impacts vector competence for Leishmania major. Mbio 2019, 10, e01941-19. [Google Scholar] [CrossRef]
- Kykalová, B.; Tichá, L.; Volf, P.; Loza Telleria, E. Phlebotomus papatasi Antimicrobial Peptides in Larvae and Females and a Gut-Specific Defensin Upregulated by Leishmania major Infection. Microorganisms 2021, 9, 2307. [Google Scholar] [CrossRef] [PubMed]
- Telleria, E.L.; Tinoco-Nunes, B.; Leštinová, T.; de Avellar, L.M.; Tempone, A.J.; Pitaluga, A.N.; Volf, P.; Traub-Csekö, Y.M. Lutzomyia longipalpis Antimicrobial Peptides: Differential Expression during Development and Potential Involvement in Vector Interaction with Microbiota and Leishmania. Microorganisms 2021, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Telleria, E.L.; Azevedo-Brito, D.A.; Kykalova, B.; Tinoco-Nunes, B.; Pitaluga, A.N.; Volf, P.; Traub-Csekö, Y.M. Leishmania infantum Infection Modulates the Jak-STAT Pathway in Lutzomyia longipalpis LL5 Embryonic Cells and Adult Females, and Affects Parasite Growth in the Sand Fly. Front. Trop. Dis. 2021, 2, 747820. [Google Scholar] [CrossRef]
- Lefèvre, T.; Vantaux, A.; Dabiré, K.R.; Mouline, K.; Cohuet, A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013, 9, e1003365. [Google Scholar] [CrossRef]
- Lefevre, T.; Oliver, L.; Hunter, M.D.; De Roode, J.C.; Letters, E. Evidence for trans-generational medication in nature. Ecol. Lett. 2010, 13, 1485–1493. [Google Scholar] [CrossRef]
- Lefevre, T.; Chiang, A.; Kelavkar, M.; Li, H.; Li, J.; de Castillejo, C.L.; Oliver, L.; Potini, Y.; Hunter, M.D.; de Roode, J.C. Behavioural resistance against a protozoan parasite in the monarch butterfly. J. Anim. Ecol. 2011, 81, 70–79. [Google Scholar] [CrossRef]
- Lefevre, T.; De Roode, J.C.; Kacsoh, B.Z.; Schlenke, T.A. Defence strategies against a parasitoid wasp in Drosophila: Fight or flight? Biol. Lett. 2011, 8, 230–233. [Google Scholar] [CrossRef]
- De Roode, J.C.; Lefevre, T. Behavioral immunity in insects. Insects 2012, 3, 789–820. [Google Scholar] [CrossRef]
- Meister, S.; Agianian, B.; Turlure, F.; Relo´gio, A.; Morlais, I.; Kafatos, F.C.; Christophides, K. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog. 2009, 5, e1000542. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef]
- Schlein, Y.; Polacheck, I.; Yuval, B. Mycoses, bacterial infections and antibacterial activity in sand flies (Psychodidae) and their possible role in the transmission of leishmaniasis. Parasitology 1985, 90, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Shililu, J.; Githure, J.; Novak, R.; Raskin, L. Thorsellia anopheles is the dominant bacterium in a Kenyan population of adult Anopheles gambiae mosquitoes. ISME J. 2008, 2, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Terenius, O.; Oliveira, C.D.; Pinheiro, W.D.; Tadei, W.P.; James, A.A.; Marinotti, O. 16S rRNA gene sequences from bacteria associated with adult Anopheles darlingi (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2008, 45, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Baldini, F.; Pompon, J.; Garrett, W.S.; Truong, D.T.; Dabiré, R.K.; Diabaté, A.; Levashina, E.A.; Catteruccia, F. The reproductive tracts of two malaria vectors are populated by a core microbiome and by gender- and swarm-enriched microbial biomarkers. Sci. Rep. 2016, 6, 24207. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.; Rainey, S.M.; Mcfarlane, M.; Donald, C.L.; Schnettler, E.; Kohl, A.; Pondeville, E. Fighting arbovirus transmission: Natural and engineered control of vector competence in Aedes mosquitoes. Insects 2015, 6, 236–278. [Google Scholar] [CrossRef]
- Durvasula, R.V.; Gumbs, A.; Panackal, A.; Kruglov, O.; Aksoy, S.; Merrifield, R.B.; Richards, F.F.; Beard, C.B. Prevention of in-sect-borne disease: An approach using transgenic symbiotic bacteria. Proc. Natl. Acad. Sci. USA 1997, 94, 3274–3278. [Google Scholar] [CrossRef]
- Beard, C.B.; Durvasula, R.V.; Richards, F.F. Bacterial symbiosis in arthropods and the control of disease transmission. Emerg. Infect. Dis. 1998, 4, 581–591. [Google Scholar] [CrossRef]
- Feliciangeli, M.D. Natural breeding places of phlebotomine sandflies. Med. Vet. Entomol. 2004, 18, 71–80. [Google Scholar] [CrossRef]
- Singh, R.; Lal, S.; Saxena, V.K. Breeding ecology of visceral leishmaniasis vector sand fly in Bihar state of India. Acta Trop. 2008, 107, 117–120. [Google Scholar] [CrossRef]
- Hanson, W. The Breeding Places of Phlebotomus in Panama (Diptera, Psychodidae). Ann. Entomol. Soc. Am. 1961, 54, 317–322. [Google Scholar] [CrossRef]
- Bettini, S.; Contini, C.; Atzeni, M.C.; Tocco, G. Leishmaniasis in Sardinia. I. Observations on a larval breeding site of Phlebotomus perniciosus, Phlebotomus perfiliewi perfiliewi and Sergentomyia minuta (Diptera: Psychodidae) in the canine leishmaniasis focus of Soleminis (Cagliari). Ann. Trop. Med. Parasitol. 1986, 80, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Melis, P. Leishmaniasis in Sardinia. III. Soil analysis of a breeding site of three species of sandflies. Med. Vet. Entomol. 1988, 2, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S. Sandfly breeding-sites. Life Sci. 1989, 163, 179–188. [Google Scholar]
- Mancini, M.V.; Damiani, C.; Accoti, A.; Tallarita, M.; Nunzi, E.; Capelli, A.; Bozic, J.; Catanzani, R.; Rossi, P.; Valzano, M.; et al. Estimating bacteria diversity in different organs of nine species of mosquito by next generation sequencing. BMC Microbiol. 2018, 18, 126. [Google Scholar] [CrossRef]
- Lantova, L.; Volf, P. The development of Psychodiella sergenti (Apicomplexa: Eugregarinorida) in Phlebotomus sergenti (Diptera: Psychodidae). Parasitology 2012, 139, 726–734. [Google Scholar] [CrossRef][Green Version]
- Wilke, A.B.B.; Marrelli, M.T. Paratransgenesis: A promising new strategy for mosquito vector control. Parasit. Vectors 2015, 8, 342. [Google Scholar] [CrossRef]
- Favia, G.; Ricci, I.; Damiani, C.; Raddadi, N.; Crotti, E.; Marzorati, M.; Rizzi, A.; Urso, R.; Brusetti, L.; Borin, S.; et al. Bacteria of the genus Asaiastably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. USA 2007, 104, 9047–9051. [Google Scholar] [CrossRef]
- Aksoy, S.; Weiss, B.; Attardo, G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Adv. Exp. Med. Biol. 2008, 627, 35–48. [Google Scholar]
- Chavshin, A.R.; Oshaghi, M.A.; Vatandoost, H.; Pourmand, M.R.; Raeisi, A.; Enayati, A.A.; Mardani, N.; Ghoorchian, S. Identification of bacterial microflora in the midgut of the larvae and adult of wild caught Anopheles stephensi: A step toward finding suitable paratransgenesis candidates. Acta Trop. 2012, 121, 129–134. [Google Scholar] [CrossRef]
| Sand Fly Species | Sand Fly Origin [Reference] | Source | Developmental Stage | Tissue | Methodological Approach |
|---|---|---|---|---|---|
| Ph. duboscqi | Senegal [30] | Colony | Larvae, pupae, and adults | Guts | Culturing |
| Ph. duboscqi | Senegal [12] | Colony | Pupae and adults | Guts | DNA sequencing V6–V8 of 16S rDNA gene |
| Ph. papatasi | Egypt [26] | Field | Adults | Guts | Culturing |
| Ph. papatasi | Morocco [12] | Field | Adults | Guts | DNA sequencing V6–V8 of 16S rDNA gene |
| Ph. papatasi | Iran [31] | Field | Adults | Guts | Culturing |
| Ph. papatasi | Egypt, India, Tunisia, and Turkey [67] | Colony and field | Adults | Guts | Culturing and DNA Sequencing V1–V9 of 16S rRNA gene |
| Ph. papatasi | Iran [69] | Field | Adults | Guts | DNA sequencing V1–V9 of 16S rRNA gene |
| Ph. papatasi | Iran [11] | Field | Adults | Guts | Culturing and DNA Sequencing V1–V2 and V3–V5 of 16S rRNA gene |
| Ph. papatasi | Greece [71] | Field | Adults | Guts | Illumina MiSeq V4 of 16S rRNA gene |
| Ph. argentipes | India [24] | Field | Adults | Guts | Culturing and DNA sequencing 16S rDNA gene |
| Ph. halepensis | Iran [31] | Field | Adults | Guts | Culturing |
| Ph. halepensis | Iran [11] | Field | Adults | Guts | Culturing and DNA Sequencing V1–V2 and V3–V5 of 16S rRNA gene |
| Ph. kandelakii | Iran [31] | Field | Adults | Guts | Culturing |
| Ph. kandelakii | Iran [11] | Field | Adults | Guts | Culturing and DNA Sequencing V1–V2 and V3–V5 of 16S rRNA gene |
| Ph. perfiliewi | Iran [31] | Field | Adults | Guts | Culturing |
| Ph. sergenti | Iran [31] | Field | Adults | Guts | Culturing |
| Ph. chinensis | China [8] | Field | Adults | Whole body | Culturing and DNA Sequencing V1–V9 of 16S rRNA gene |
| Ph. perniciosus | Tunisia [10] | Colony and field | Adults | Guts | Culturing and DNA Sequencing V3–V5 of 16S rRNA gene and ITS (16S–23S rRNA) |
| Ph. argentipes | Sri Lanka [70] | Field | Adults | Guts | Culturing and DNA Sequencing V1–V9 of 16S rRNA gene |
| Ph. neglectus | Greece [71] | Field | Adults | Guts | Illumina MiSeq V4 of 16S rRNA gene |
| Ph. tobbi | Greece [71] | Field | Adults | Guts | Illumina MiSeq V4 of 16S rRNA gene |
| Ph. similis | Greece [71] | Field | Adults | Guts | Illumina MiSeq V4 of 16S rRNA gene |
| Lu. evansi | Colombia [46] | Field | Larvae, pupae, and adults | Guts | Culturing and DNA Sequencing V1–V9 of 16S rRNA gene and ITS (16S–23S rRNA) |
| Lu. evansi | Colombia [9] | Field | Adults | Guts | Illumina MiSeq V4 of 16S rRNA gene |
| Lu. longipalpis | Brazil [28] | Field | Adults | Guts | Culturing |
| Lu. longipalpis | Brazil [29] | Colony | Adults | Guts | Culturing |
| Lu. longipalpis | Brazil [47] | Field | Adults | Guts | Culturing and DNA Sequencing 16S rDNA gene |
| Lu. longipalpis | Argentina and Brazil [36] | Field | Adults | Whole body | High-throughput pyrosequencing Total RNA |
| Lu. longipalpis | Brazil and Colombia [14] | Field | Adults | Whole body | DNA sequencing V1–V9 of 16S rRNA gene |
| Lu. longipalpis | Brazil [13] | Colony | Adults | Guts | Illumina MiSeq V4 of 16S rRNA gene and 18S rDNA |
| Lu. longipalpis | Brazil [48] | Field | Adults | Guts | Illumina MiSeq V3–V4 of 16S rRNA gene |
| Lu. longipalpis | Brazil [32] | Field | Larvae, pupae, and adults | Whole body and guts | Culturing and DNA Sequencing V1–V9 of 16S rRNA gene |
| Lu. cruzi | Brazil [14] | Field | Adults | Whole body | DNA sequencing V1–V9 of 16S rRNA gene |
| Lu. intermedia | Brazil [54] | Field | Adults | Guts | Illumina MiSeq V1–V3 16S rDNA |
| Nyssomyia neivai (syn. Lu. neivai) | Brazil [55] | Field | Adults | Whole body | DNA sequencing 16S rDNA |
| Lu. ayacuchensis | Ecuador and Peru [33] | Field | Adults | Whole body | Illumina MiSeq V3–V4 16S rDNA gene |
| Pintomyia evansi | Colombia [34] | Field | Adults | Guts | Illumina MiSeq V4 of 16S rDNA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabbabi, A.; Mizushima, D.; Yamamoto, D.S.; Kato, H. Sand Flies and Their Microbiota. Parasitologia 2022, 2, 71-87. https://doi.org/10.3390/parasitologia2020008
Tabbabi A, Mizushima D, Yamamoto DS, Kato H. Sand Flies and Their Microbiota. Parasitologia. 2022; 2(2):71-87. https://doi.org/10.3390/parasitologia2020008
Chicago/Turabian StyleTabbabi, Ahmed, Daiki Mizushima, Daisuke S. Yamamoto, and Hirotomo Kato. 2022. "Sand Flies and Their Microbiota" Parasitologia 2, no. 2: 71-87. https://doi.org/10.3390/parasitologia2020008
APA StyleTabbabi, A., Mizushima, D., Yamamoto, D. S., & Kato, H. (2022). Sand Flies and Their Microbiota. Parasitologia, 2(2), 71-87. https://doi.org/10.3390/parasitologia2020008






