Vector-Borne Pathogens in Ticks and Fleas of Client-Owned Dogs in Metro Manila, Philippines
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
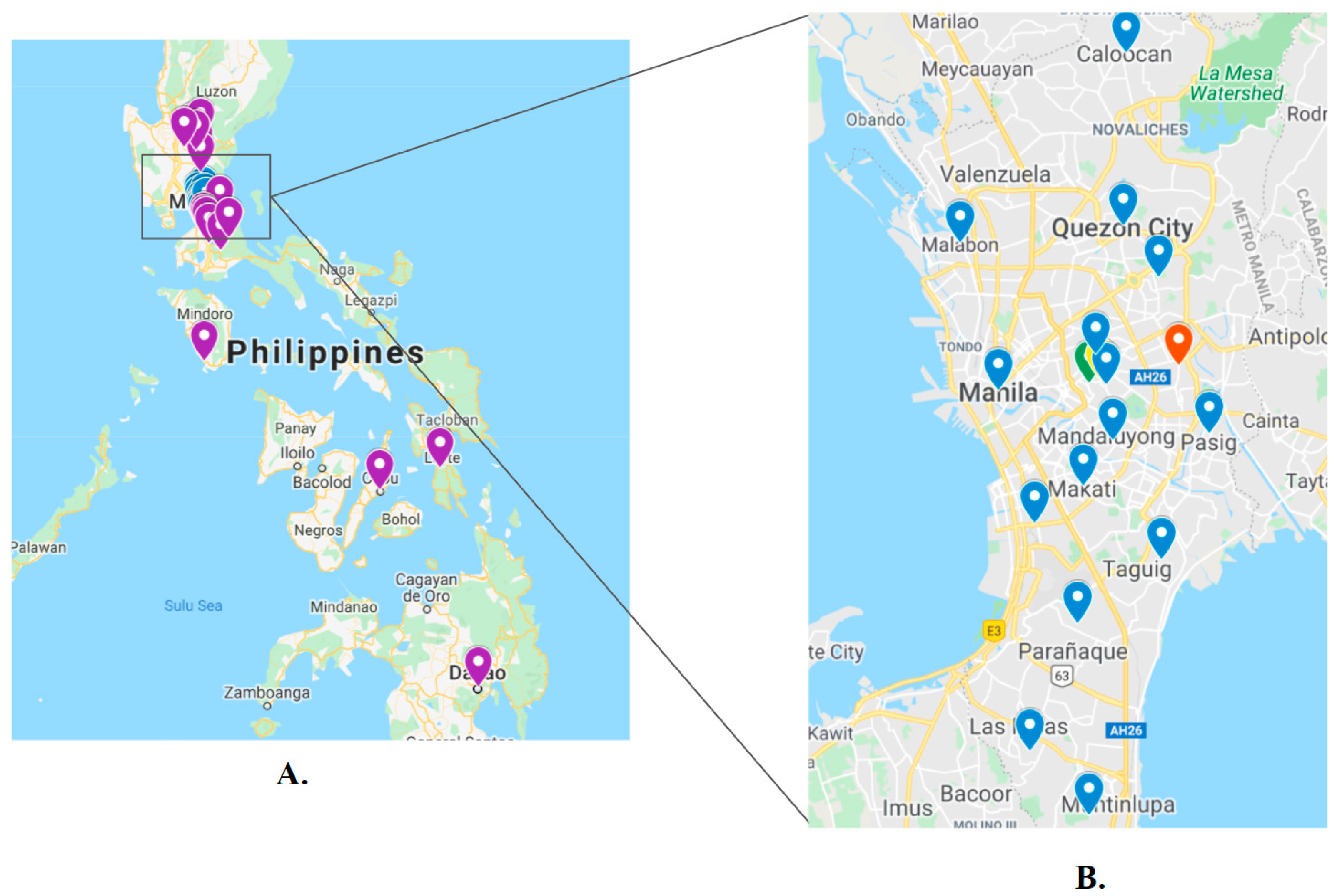
4.1. Collection and Morphological Identification of Ticks and Fleas from Dogs in Metro Manila, Philippines
4.2. Molecular Characterisation of Ticks and Gleas at Cytochrome C Oxidase Subunit I (cox1)
4.3. Molecular Detection of Vector-Borne Pathogens in Ticks and Fleas
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Authors | Number of Dogs Examined | Ticks/Fleas on Dogs (no#) | VBP’s (Prevalence) | Location | ||
|---|---|---|---|---|---|---|
| Molecular Analysis | Serology-based | |||||
| Blood PCR | Ectoparasite PCR | |||||
| Baron and Ruaes [18] | 16 | R. sanguineus s.l (44) C. felis (96) | n/a | n/a | n/a | Davao City |
| Bartolome-Cruz [23] | 97 | R. sanguineus s.l (2498) | n/a | n/a | n/a | Quezon City, Pasay City, Laguna City |
| Bartolome-Cruz [25] | 953 | R. sanguineus s.l (52) | n/a | Babesia spp. (2.08%), Hepatozoon spp. (2.08%), Ehrlichia spp. (8.33%) | n/a | Quezon City, Pasay City, Laguna City |
| Baticados and Baticados [34] | 169 | R. sanguineus s.l | n/a | n/a | E. canis (95.3%) | Malabon, Quezon City, Manila, San Juan City, Pasig City, Makati City, Mandaluyong City, Taguig City, Parañaque City, Muntinlupa City, Las Pinas, Caloocan, Rizal |
| Colella, Nguyen, Tan, Lu, Fang, Zhijuan, Wang, Liu, Chen, Dong, Nurcahyo, Hadi, Venturina, Tong, Tsai, Taweethavonsawat, Tiwananthagorn, Le, Bui, Watanabe, Rani, Annoscia, Beugnet, Otranto, and Halos [10] | 120 | R. linniae, C. felis, C. canis, C. orientis | H. canis (9.7%), B. gibsoni (0%) | n/a | E. canis (33.0%), A. platys (17.0%), D. immitis (29.8%) | San Jose City (Occidental Mindoro), Cabanatuan and Muñoz (Nueva Ecija) |
| Corales, Viloria, Venturina, and Mingala [19] | 70 | n/a | E. canis (2.85%), Babesia spp. (7.14%), A. platys (0%) | n/a | n/a | San Jose City, Muñoz, Talavera, Guimba, Sta. Rosa, Cabanatuan City, Gapan City (Nueva Ecija) |
| Galay, Manalo, Dolores, Aguilar, Sandalo, Cruz, Divina, Andoh, Masatani, and Tanaka [3] | 248 | R. sanguineus s.l (157) | E. canis (19.8%), A. platys (6.0%), Rickettsia spp. (2.4%), B. vogeli (6.8%), H. canis (2.4%) | E. canis (3.2%), A. platys (0.6%), B. vogeli (0.6%), H. canis (0.6%), Rickettsia spp. (0%) | n/a | Pasay, Taguig City, Parañaque City, Las Piñas, Muntinlupa City, Laguna (San Pedro, Biñan, Santa Rosa, Calamba, Los Baños, San Pablo, Pagsanjan) |
| Nguyen, Colella, Greco, Fang, Nurcahyo, Hadi, Venturina, Tong, Tsai, Taweethavonsawat, Tiwananthagorn, Tangtrongsup, Le, Bui, Do, Watanabe, Rani, Dantas-Torres, Halos, Beugnet, and Otranto [6] | 120 | R. linniae (63), C. felis (47), C. orientis (10) | n/a | R. sanguineus s.l: R. felis (1.6%), H. canis (15.9%), A. platys (0%), E. canis (0%), R. asembonensis (0%), Babesia vogeli (0%) C. felis: R. felis (27.7%), R. asembonensis (2.1%), Bartonella spp. (0%) C. orientis: R. asembonensis (80%), R. felis (0%), Bartonella spp. (0%) | n/a | Philippines |
| Portugaliza and Bagot [13] | Not specified | C. felis (70), Pulex irritans (1), R. sanguineus s.l (93) | n/a | n/a | n/a | Baybay (Leyte) |
| Singer, Loya, Lapsley, Tobar, Carlos, Carlos, Carlos, Adao, Rivera, Jaffe, Mazet, and Chomel [1] | 116 | n/a | B. henselae (11.2%) | n/a | B. henselae (2.6%) | Makati City, Parañaque City |
| Ybañez, Julian, and Carlos [9] | 68 | n/a | n/a | n/a | E. canis (86.7%) | Makati City |
| Ybanez, Ybanez, Arnado, Belarmino, Malingin, Cabilete, Amores, Talle, Liu, and Xuan [20] | 100 | n/a | Ehrlichia/Anaplasma spp. (10%), Babesisa spp. (18%) | n/a | n/a | Cebu |
References
- Singer, G.A.; Loya, F.P.; Lapsley, W.D.; Tobar, B.Z.; Carlos, S.; Carlos, R.S.; Carlos, E.T.; Adao, D.E.V.; Rivera, W.L.; Jaffe, D.A.; et al. Detection of Bartonella infection in pet dogs from Manila, the Philippines. Acta Trop. 2020, 205, 105277. [Google Scholar] [CrossRef] [PubMed]
- Brites-Neto, J.; Duarte, K.M.; Martins, T.F. Tick-borne infections in human and animal population worldwide. Vet. World 2015, 8, 301–315. [Google Scholar] [CrossRef]
- Galay, R.L.; Manalo, A.A.L.; Dolores, S.L.D.; Aguilar, I.P.M.; Sandalo, K.A.C.; Cruz, K.B.; Divina, B.P.; Andoh, M.; Masatani, T.; Tanaka, T. Molecular detection of tick-borne pathogens in canine population and Rhipicephalus sanguineus (sensu lato) ticks from southern Metro Manila and Laguna, Philippines. Parasit Vectors 2018, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Carlos, E.T.; Kasten, R.W.; Yamamoto, K.; Chang, C.C.; Carlos, R.S.; Abenes, M.V.; Pajares, C.M. Bartonella henselae and Bartonella clarridgeiae infection in domestic cats from The Philippines. Am. J. Trop. Med. Hyg. 1999, 60, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Fédération Cynologique Internationale. Statistics: Philippine Canine Club, Inc. (Philippines). Available online: http://www.fci.be/en/statistics/ByNco.aspx?iso=PH (accessed on 7 July 2021).
- Nguyen, V.L.; Colella, V.; Greco, G.; Fang, F.; Nurcahyo, W.; Hadi, U.K.; Venturina, V.; Tong, K.B.Y.; Tsai, Y.L.; Taweethavonsawat, P.; et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasit Vectors 2020, 13, 420. [Google Scholar] [CrossRef]
- Low, V.L.; Prakash, B.K. First genetic characterization of the brown dog tick Rhipicephalus sanguineus sensu lato in Peninsular Malaysia. Exp. Appl. Acarol. 2018, 75, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Šlapeta, J.; Chandra, S.; Halliday, B. The “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826). Int. J. Parasitol. 2021, 51, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, A.P.; Julian, K.J.V.; Carlos, S.M.A.S. Clinical observation of dogs serologically positive for the potentially zoonotic Ehrlichia canis in the Philippines. Univ. Vis. J. Res. 2016, 10, 1–6. [Google Scholar]
- Colella, V.; Nguyen, V.L.; Tan, D.Y.; Lu, N.; Fang, F.; Zhijuan, Y.; Wang, J.; Liu, X.; Chen, X.; Dong, J.; et al. Zoonotic Vectorborne Pathogens and Ectoparasites of Dogs and Cats in Eastern and Southeast Asia. Emerg. Infect. Dis. 2020, 26, 1221–1233. [Google Scholar] [CrossRef]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Soneshine, D.W.; Brown, R.N. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G., Durden, L., Eds.; Elsevier Inc.: Oxford, UK, 2019; pp. 603–672. [Google Scholar]
- Portugaliza, H.P.; Bagot, M.A. Different species of lice (Phthiraptera), fleas (Siphonaptera), and ticks (Ixodida) collected from livestock, poultry, reptile, and companion animal in Leyte Island, Philippines. Livest. Res. Rural Dev. 2015, 27, 1–10. [Google Scholar]
- Lawrence, A.L.; Webb, C.E.; Clark, N.J.; Halajian, A.; Mihalca, A.D.; Miret, J.; D’Amico, G.; Brown, G.; Kumsa, B.; Modry, D.; et al. Out-of-Africa, human-mediated dispersal of the common cat flea, Ctenocephalides felis: The hitchhiker’s guide to world domination. Int. J. Parasitol. 2019, 49, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Crkvencic, N.; Šlapeta, J. Climate change models predict southerly shift of the cat flea (Ctenocephalides felis) distribution in Australia. Parasit Vectors 2019, 12, 137. [Google Scholar] [CrossRef]
- Ng-Nguyen, D.; Hii, S.F.; Hoang, M.T.; Nguyen, V.T.; Rees, R.; Stenos, J.; Traub, R.J. Domestic dogs are mammalian reservoirs for the emerging zoonosis flea-borne spotted fever, caused by Rickettsia felis. Sci. Rep. 2020, 10, 4151. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Boulouis, H.J.; Maruyama, S.; Breitschwerdt, E.B. Bartonella spp. in pets and effect on human health. Emerg. Infect. Dis. 2006, 12, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.M.; Ruaes, C.A.S. Ectoparasites of domesticated animals in a rural barangay, Davao city, Mindanao island, Philippines. Serangga 2020, 1, 118–130. [Google Scholar]
- Corales, J.M.; Viloria, V.V.; Venturina, V.M.; Mingala, C.N. The prevalence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs in Nueva Ecija, Philippines based on multiplex polymerase chain reaction (mPCR) assay. Ann. Parasitol. 2014, 60, 267–272. [Google Scholar]
- Ybanez, R.H.D.; Ybanez, A.P.; Arnado, L.L.A.; Belarmino, L.M.P.; Malingin, K.G.F.; Cabilete, P.B.C.; Amores, Z.R.O.; Talle, M.G.; Liu, M.; Xuan, X. Detection of Ehrlichia, Anaplasma, and Babesia spp. in dogs of Cebu, Philippines. Vet. World 2018, 11, 14–19. [Google Scholar] [CrossRef]
- Šlapeta, S.; Šlapeta, J. Molecular identity of cat fleas (Ctenocephalides felis) from cats in Georgia, USA carrying Bartonella clarridgeiae, Bartonella henselae and Rickettsia sp. RF2125. Vet. Parasitol. Reg. Stud. Rep. 2016, 3–4, 36–40. [Google Scholar] [CrossRef]
- Mapa, D.S. 2020 Census of Population and Housing (2020 CPH) Populaiton Counts Declared Official by the President. 2021. Available online: https://psa.gov.ph/content/2020-census-population-and-housing-2020-cph-population-counts-declared-official-president (accessed on 14 July 2021).
- Bartolome-Cruz, K. Prevalence and intensity of infestation of the brown dog tick, Rhipicephalus sanguineus (Latreille) (Arachnida: Acari: Ixodidae) in three veterinary facilities. Philipp. J. Vet. Med. 2018, 55, 107–114. [Google Scholar]
- Dantas-Torres, F.; da Silva, Y.Y.; de Oliveira Miranda, D.E.; da Silva Sales, K.G.; Figueredo, L.A.; Otranto, D. Ehrlichia spp. infection in rural dogs from remote indigenous villages in north-eastern Brazil. Parasit Vectors 2018, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolome-Cruz, K. Detection of pathogens on the brown dog tick, Rhipicephalus sanguineus sensu lato (s.l.) (Arachnida: Acari: Ixodidae) in the Philippines. Philipp. J. Sci. 2018, 147, 741–751. [Google Scholar]
- Harrus, S.; Waner, T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): An overview. Vet. J. 2011, 187, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Calvani, N.E.D.; Bell, L.; Carney, A.; De La Fuente, C.; Stragliotto, T.; Tunstall, M.; Šlapeta, J. The molecular identity of fleas (Siphonaptera) carrying Rickettsia felis, Bartonella clarridgeiae and Bartonella rochalimae from dogs and cats in Northern Laos. Heliyon 2020, 6, e04385. [Google Scholar] [CrossRef]
- The Philippine Atmospheric Geophysical and Astronomical Services Administration. Climate of the Philippines. Available online: http://bagong.pagasa.dost.gov.ph/information/climate-philippines (accessed on 14 July 2021).
- Nguyen, V.L.; Dantas-Torres, F.; Otranto, D. Canine and feline vector-borne diseases of zoonotic concern in Southeast Asia. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Chomel, B.B.; Kasten, R.W. Bartonellosis, an increasingly recognized zoonosis. J. Appl. Microbiol. 2010, 109, 743–750. [Google Scholar] [CrossRef]
- Perez-Osorio, C.E.; Zavala-Velazquez, J.E.; Arias Leon, J.J.; Zavala-Castro, J.E. Rickettsia felis as emergent global threat for humans. Emerg. Infect. Dis. 2008, 14, 1019–1023. [Google Scholar] [CrossRef]
- Wolf, S.P.; Reeves, W.K. Rickettsia felis (Rickettsiales: Rickettsiaceae) Discovered in Cat Fleas (Siphonaptera: Pulicidae) in the Philippines. J. Entomol. Sci. 2012, 47, 95–96. [Google Scholar] [CrossRef]
- Tsai, K.H.; Huang, C.G.; Fang, C.T.; Shu, P.Y.; Huang, J.H.; Wu, W.J. Prevalence of Rickettsia felis and the first identification of Bartonella henselae Fizz/CAL-1 in cat fleas (Siphonaptera: Pulicidae) from Taiwan. J. Med. Entomol. 2011, 48, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Baticados, A.M.; Baticados, W.N. Serological Evidence for Ehrlichia canis Exposure in Military Dogs and Other Canines in Metropolitan Manila, Philippines. Isr. J. Vet. Med. 2011, 66, 151–156. [Google Scholar]
- Cruz-Flores, M.J.; Garcia Claveria, F.; Verdida, R.; Xuan, X.; Igarashi, I. First detection of Babesia gibsoni infection in Philippine stray dogs by immunochromatographic test (ICT). Vet. Arh. 2008, 78, 149–157. [Google Scholar]
- Irwin, P.J.; Jefferies, R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. 2004, 20, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.H.E.; Rothschild, M. An Illustrated Catalogue of the Rothschild Collection of Fleas (Siphonaptera) in the British Museum (Natural History) with Keys and Short Descriptions for the Identification of Families, Genera, Species and Subspecies. Vol I. Tungidae and Pulicidae; British Museum: London, UK, 1953. [Google Scholar]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari, Ixodidae) a Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK, 2005; p. 656. [Google Scholar]
- Lawrence, A.L.; Hii, S.F.; Jirsova, D.; Panakova, L.; Ionica, A.M.; Gilchrist, K.; Modry, D.; Mihalca, A.D.; Webb, C.E.; Traub, R.J.; et al. Integrated morphological and molecular identification of cat fleas (Ctenocephalides felis) and dog fleas (Ctenocephalides canis) vectoring Rickettsia felis in central Europe. Vet. Parasitol. 2015, 210, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Ma, G.C.; Burleigh, A.; Brown, G.; Norris, J.M.; Ward, M.P.; Emery, D.; Šlapeta, J. The brown dog tick Rhipicephalus sanguineus sensu Roberts, 1965 across Australia: Morphological and molecular identification of R. sanguineus s.l. tropical lineage. Ticks Tick Borne Dis. 2020, 11, 101305. [Google Scholar] [CrossRef]
- da Silva, C.B.; Pires, M.S.; Vilela, J.A.; Peckle, M.; da Costa, R.L.; Vitari, G.L.; Santos, L.A.; Santos, H.A.; Massard, C.L. A new quantitative PCR method for the detection of Anaplasma platys in dogs based on the citrate synthase gene. J. Vet. Diagn. Investig. 2016, 28, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Baneth, G.; Harrus, S.; Ohnona, F.S.; Schlesinger, Y. Longitudinal quantification of Ehrlichia canis in experimental infection with comparison to natural infection. Vet. Microbiol. 2009, 136, 321–325. [Google Scholar] [CrossRef]
- Diaz, M.H.; Bai, Y.; Malania, L.; Winchell, J.M.; Kosoy, M.Y. Development of a novel genus-specific real-time PCR assay for detection and differentiation of Bartonella species and genotypes. J. Clin. Microbiol. 2012, 50, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Stenos, J.; Graves, S.R.; Unsworth, N.B. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am. J. Trop. Med. Hyg. 2005, 73, 1083–1085. [Google Scholar] [CrossRef]
- Hii, S.F.; Lawrence, A.L.; Cuttell, L.; Tynas, R.; Abd Rani, P.A.; Šlapeta, J.; Traub, R.J. Evidence for a specific host-endosymbiont relationship between ‘Rickettsia sp. genotype RF2125’ and Ctenocephalides felis orientis infesting dogs in India. Parasit Vectors 2015, 8, 169. [Google Scholar] [CrossRef] [Green Version]
| Demographic | Sex | Housing | Age, Y | Ectoparasite | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinic | M | F | Indoor | Outdoor | Both | ≤4 | >4 | R. linnaei | C. felis | Both |
| Clinic 1 | 9 | 11 | 8 | 4 | 8 | 14 | 6 | 6 | 13 | 1 |
| Clinic 2 | 8 | 11 | 9 | 2 | 8 | 8 | 9 | 12 | 5 | 2 |
| Clinic 3 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 3 | 0 | 0 |
| Total | 18 | 24 | 19 | 6 | 17 | 22 | 16 | 21 | 18 | 3 |
| Clinic | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| Pathogen | R. linnaei | C. felis | R. linnaei | C. felis | R. linnaei | C. felis |
| B. clarridgeiae | 0 | 0 | 0 | 2 | 0 | 0 |
| R. felis | 1 | 2 | 0 | 0 | 0 | 0 |
| B. clarridgeiae and R. felis | 0 | 0 | 0 | 1 | 0 | 0 |
| E. canis | 0 | 0 | 0 | 0 | 0 | 0 |
| A. platys | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez, A.R.A.; Eamens, K.; Westman, M.; Šlapeta, J. Vector-Borne Pathogens in Ticks and Fleas of Client-Owned Dogs in Metro Manila, Philippines. Parasitologia 2021, 1, 247-256. https://doi.org/10.3390/parasitologia1040026
Marquez ARA, Eamens K, Westman M, Šlapeta J. Vector-Borne Pathogens in Ticks and Fleas of Client-Owned Dogs in Metro Manila, Philippines. Parasitologia. 2021; 1(4):247-256. https://doi.org/10.3390/parasitologia1040026
Chicago/Turabian StyleMarquez, Anna Regina Angela, Kieran Eamens, Mark Westman, and Jan Šlapeta. 2021. "Vector-Borne Pathogens in Ticks and Fleas of Client-Owned Dogs in Metro Manila, Philippines" Parasitologia 1, no. 4: 247-256. https://doi.org/10.3390/parasitologia1040026
APA StyleMarquez, A. R. A., Eamens, K., Westman, M., & Šlapeta, J. (2021). Vector-Borne Pathogens in Ticks and Fleas of Client-Owned Dogs in Metro Manila, Philippines. Parasitologia, 1(4), 247-256. https://doi.org/10.3390/parasitologia1040026







