Advances in Understanding Chronic Traumatic Encephalopathy: A Systematic Review of Clinical and Pathological Evidence
Abstract
1. Introduction
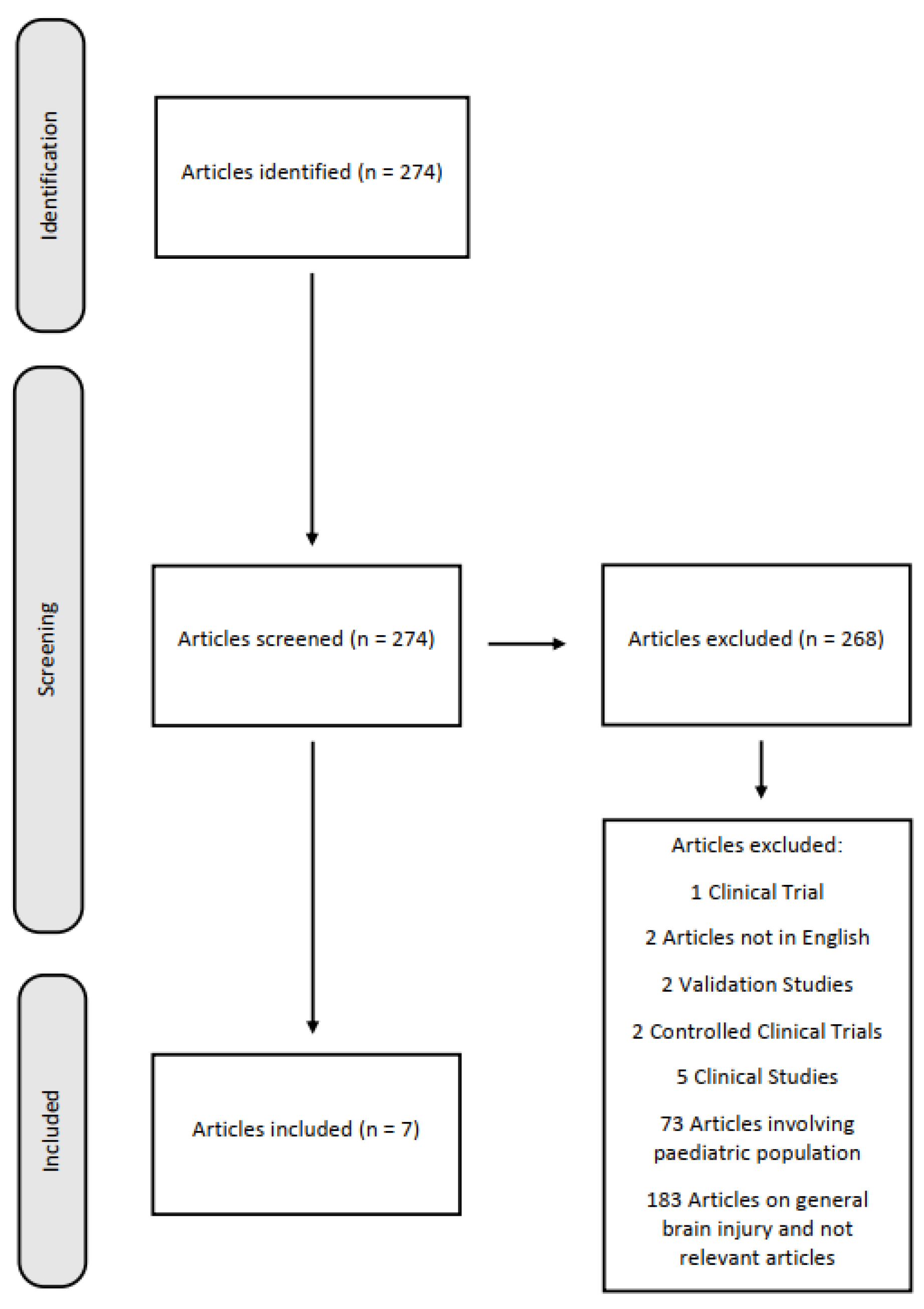
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBI | Traumatic Brain Injury |
| CTE | Chronic Traumatic Encephalopathy |
| NFTs | Neurofibrillary Tangles |
| NINDS | National Institute of Neurological Disorders and Stroke |
| NIBIB | National Institute of Biomedical Imaging and Bioengineering |
| TES | Traumatic Encephalopathy Syndrome |
| TDP-43 | Transactive response DNA-binding protein 43 |
References
- Pinchi, E.; Frati, A.; Cipolloni, L.; Aromatario, M.; Gatto, V.; La Russa, R.; Pesce, A.; Santurro, A.; Fraschetti, F.; Frati, P.; et al. Clinical-Pathological Study on β-APP, IL-1β, GFAP, NFL, Spectrin II, 8OHdG, TUNEL, MiR-21, MiR-16, MiR-92 Expressions to Verify DAI-Diagnosis, Grade and Prognosis. Sci. Rep. 2018, 8, 2387. [Google Scholar] [CrossRef]
- Benevento, M.; d’Amati, A.; Nicolì, S.; Ambrosi, L.; Baj, J.; Ferorelli, D.; Ingravallo, G.; Solarino, B. Dura Mater and Survival Time Determination in Individuals Who Died after Traumatic Brain Injury: A Preliminary Study. Forensic Sci. Med. Pathol. 2025, 21, 107–114. [Google Scholar] [CrossRef]
- Omalu, B.I.; Fitzsimmons, R.P.; Hammers, J.; Bailes, J. Chronic Traumatic Encephalopathy in a Professional American Wrestler. J. Forensic Nurs. 2010, 6, 130–136. [Google Scholar] [CrossRef]
- Omalu, B.I.; Bailes, J.; Hammers, J.L.; Fitzsimmons, R.P. Chronic Traumatic Encephalopathy, Suicides and Parasuicides in Professional American Athletes: The Role of the Forensic Pathologist. Am. J. Forensic Med. Pathol. 2010, 31, 130–132. [Google Scholar] [CrossRef]
- Scanlon, M.M.; Shields, M.M.; Perl, D.P.; Priemer, D.S. Chronic Traumatic Encephalopathy Pathognomonic Lesions Occurring in Isolation Adjacent to Infiltrative and Non-Infiltrative White Matter Lesions. J. Neuropathol. Exp. Neurol. 2024, 83, 695–700. [Google Scholar] [CrossRef]
- Turk, K.W.; Budson, A.E. Chronic Traumatic Encephalopathy. Continuum 2019, 25, 187–207. [Google Scholar] [CrossRef]
- Byard, R.; Tiemensma, M.; Buckland, M.E.; Vink, R. Chronic Traumatic Encephalopathy (CTE)-Features and Forensic Considerations. Forensic Sci. Med. Pathol. 2023, 19, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Tiemensma, M.; Byard, R.W.; Vink, R.; Affleck, A.J.; Blumbergs, P.; Buckland, M.E. Chronic Traumatic Encephalopathy (CTE) in the Context of Longstanding Intimate Partner Violence. Acta Neuropathol. 2024, 148, 1. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.I.; Bernick, C.; Dodick, D.W.; Mez, J.; Mariani, M.L.; Adler, C.H.; Alosco, M.L.; Balcer, L.J.; Banks, S.J.; Barr, W.B.; et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology 2021, 96, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Eaton, R.G.; Lonser, R.R. History of Biological, Mechanistic, and Clinical Understanding of Concussion. Neurosurg. Focus 2024, 57, E2. [Google Scholar] [CrossRef] [PubMed]
- De Simone, S.; Vittorio, S.; Cipolloni, L.; Bibbò, R.; Gurgoglione, G.; Fazio, N.D.; Bosco, M.A. Men’s Suicide by Self-Abdominal Cut and Disembowelment: A Literature Review and Analysis of Three Cases. Available online: https://www.jomh.org/articles/10.31083/j.jomh1807158 (accessed on 17 June 2025).
- McKee, A.C.; Stein, T.D.; Huber, B.R.; Crary, J.F.; Bieniek, K.; Dickson, D.; Alvarez, V.E.; Cherry, J.D.; Farrell, K.; Butler, M.; et al. Chronic Traumatic Encephalopathy (CTE): Criteria for Neuropathological Diagnosis and Relationship to Repetitive Head Impacts. Acta Neuropathol. 2023, 145, 371–394. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A. Navigating the Complexities of Traumatic Encephalopathy Syndrome (TES): Current State and Future Challenges. Biomedicines 2023, 11, 3158. [Google Scholar] [CrossRef]
- Saulle, M.; Greenwald, B.D. Chronic Traumatic Encephalopathy: A Review. Rehabil. Res. Pract. 2012, 2012, 816069. [Google Scholar] [CrossRef]
- Peacock, W.F.; Kuehl, D.; Bazarian, J.; Singer, A.J.; Cannon, C.; Rafique, Z.; d’Etienne, J.P.; Welch, R.; Clark, C.; Diaz-Arrastia, R. Defining Acute Traumatic Encephalopathy: Methods of the “HEAD Injury Serum Markers and Multi-Modalities for Assessing Response to Trauma” (HeadSMART II) Study. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R.A. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy after Repetitive Head Injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef] [PubMed]
- Tharmaratnam, T.; Iskandar, M.A.; Tabobondung, T.C.; Tobbia, I.; Gopee-Ramanan, P.; Tabobondung, T.A. Chronic Traumatic Encephalopathy in Professional American Football Players: Where Are We Now? Front. Neurol. 2018, 9, 445. [Google Scholar] [CrossRef]
- Arciniega, H.; Baucom, Z.H.; Tuz-Zahra, F.; Tripodis, Y.; John, O.; Carrington, H.; Kim, N.; Knyazhanskaya, E.E.; Jung, L.B.; Breedlove, K.; et al. Brain Morphometry in Former American Football Players: Findings from the DIAGNOSE CTE Research Project. Brain 2024, 147, 3596–3610. [Google Scholar] [CrossRef]
- Omalu, B.; Bailes, J.; Hamilton, R.L.; Kamboh, M.I.; Hammers, J.; Case, M.; Fitzsimmons, R. Emerging Histomorphologic Phenotypes of Chronic Traumatic Encephalopathy in American Athletes. Neurosurgery 2011, 69, 173–183. [Google Scholar] [CrossRef]
- van Amerongen, S.; Kamps, S.; Kaijser, K.K.M.; Pijnenburg, Y.A.L.; Scheltens, P.; Teunissen, C.E.; Barkhof, F.; Ossenkoppele, R.; Rozemuller, A.J.M.; Stern, R.A.; et al. Severe CTE and TDP-43 Pathology in a Former Professional Soccer Player with Dementia: A Clinicopathological Case Report and Review of the Literature. Acta Neuropathol. Commun. 2023, 11, 77. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G. Tau Biology, Tauopathy, Traumatic Brain Injury, and Diagnostic Challenges. J. Alzheimer’s Dis. 2019, 67, 447–467. [Google Scholar] [CrossRef]
- Ruchika, F.; Shah, S.; Neupane, D.; Vijay, R.; Mehkri, Y.; Lucke-Wold, B. Understanding the Molecular Progression of Chronic Traumatic Encephalopathy in Traumatic Brain Injury, Aging and Neurodegenerative Disease. Int. J. Mol. Sci. 2023, 24, 1847. [Google Scholar] [CrossRef]
- Blaylock, R.L.; Maroon, J. Immunoexcitotoxicity as a Central Mechanism in Chronic Traumatic Encephalopathy—A Unifying Hypothesis. Surg. Neurol. Int. 2011, 2, 107. Available online: https://www.semanticscholar.org/paper/Immunoexcitotoxicity-as-a-central-mechanism-in-Blaylock-Maroon/83d4fb51df4b528715345e2d7d819f98c5bbcfcb (accessed on 27 July 2025). [CrossRef] [PubMed]
- Falcon, B.; Zivanov, J.; Zhang, W.; Murzin, A.G.; Garringer, H.J.; Vidal, R.; Crowther, R.A.; Newell, K.L.; Ghetti, B.; Goedert, M.; et al. Novel Tau Filament Fold in Chronic Traumatic Encephalopathy Encloses Hydrophobic Molecules. Nature 2019, 568, 420–423. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.J.; Sy, J.; Lee, M.; Harding, A.; Mobbs, R.; Batchelor, J.; Suter, C.M.; Buckland, M.E. Chronic Traumatic Encephalopathy in a Former Australian Rules Football Player Diagnosed with Alzheimer’s Disease. Acta Neuropathol. Commun. 2020, 8, 23. [Google Scholar] [CrossRef]
- Del Bigio, M.R.; Krawitz, S.; Sinha, N. Chronic Traumatic Encephalopathy-Neuropathologic Change in a Routine Neuropathology Service: 7-Year Follow-Up. J. Neuropathol. Exp. Neurol. 2023, 82, 948–957. [Google Scholar] [CrossRef]
- De Paola, L.; Tripi, D.; Napoletano, G.; Marinelli, E.; Montanari Vergallo, G.; Zaami, S. Violence against Women within Italian and European Context: Italian “Pink Code”—Major Injuries and Forensic Expertise of a Socio-Cultural Problem: A Narrative Review. Forensic Sci. 2024, 4, 264–276. [Google Scholar] [CrossRef]
- Mez, J.; Daneshvar, D.H.; Kiernan, P.T.; Abdolmohammadi, B.; Alvarez, V.E.; Huber, B.R.; Alosco, M.L.; Solomon, T.M.; Nowinski, C.J.; McHale, L.; et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA 2017, 318, 360–370. [Google Scholar] [CrossRef]
- Brenner, L.A.; Ignacio, R.V.; Blow, F.C. Suicide and Traumatic Brain Injury among Individuals Seeking Veterans Health Administration Services. J. Head Trauma Rehabil. 2011, 26, 257–264. [Google Scholar] [CrossRef]
- Wortzel, H.S.; Shura, R.D.; Brenner, L.A. Chronic Traumatic Encephalopathy and Suicide: A Systematic Review. BioMed Res. Int. 2013, 2013, 424280. [Google Scholar] [CrossRef] [PubMed]
- Piersanti, V.; Napoletano, G.; David, M.C.; Umani Ronchi, F.; Marinelli, E.; De Paola, L.; Zaami, S. Sudden Death Due to Butane Abuse—An Overview. J. Forensic Leg. Med. 2024, 103, 102662. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Chronic Traumatic Encephalopathy | Alzheimer’s Disease |
|---|---|---|
| p-tau Aggregates | Aggregates of hyperphosphorylated tau (p-tau) in glial cells (e.g., astrocytes), neurons, and cell processes, within the vicinity of small vasculature and in the cortical sulci. Typically presents in an irregular, “spot-like” pattern in perivascular spaces. Also found in the superficial layer (II-III) of the cerebral cortex and in areas 2 and 4 of the hippocampus. | The locations of p-tau NFTs should largely overlap with those of Aβ amyloid, as well as their presence in the hippocampus. |
| Neurofibrillary Tangles Dimensions | Neurofibrillary Tangles are usually larger in size. | Neurofibrillary Tangles are generally smaller in size. |
| Amyloid-Beta Plaques | Amyloid-Beta plaques may be found in a diffuse pattern in sporadic loci, or may be absent. | The primary diagnostic criterion is the post-mortem finding of amyloid-beta plaques and neuritic amyloid plaques in a laminar distribution (in the middle frontal gyrus, the superior and middle temporal gyri, and the inferior parietal lobule). Secondary depositions may also be found in the cerebellum and basal ganglia. |
| TDP-43 Aggregates | Transactive response DNA-binding protein 43 (TDP-43) aggregates are a common finding in the vast majority of Chronic Traumatic Encephalopathy cases, co-localized with p-tau neurofibrillary tangles. Sampling should first be taken from the amygdala and hippocampus. | TDP-43 aggregates are present, but are not co-localized with neurofibrillary tangles. |
| Authors | N. of Cases | Year of Publication | Sex | Age of Death | Profession | Neurological Symptoms and Cognitive and Neuropsychiatric Impairments | Cause of Death | Neuropathology Findings | Toxicological Analysis | History of Traumatic Brain Injury/Clinical History |
|---|---|---|---|---|---|---|---|---|---|---|
| Omalu et al. [3] | 1 | 2010 | male | 40 years old | Professional Wrestler | Reported | Suicide by hanging after killing his wife and son | Neurofibrillary Tangles and Ghost Tangles in the neocortex, subcortical ganglia, and substantia nigra. | Positive for Alprazolam (blood), Hydrocodone (blood), and Testosterone (urine) | Reported |
| Omalu et al. [4] | 5 | 2010 | 5 male | 50 years old 45 years old 44 years old 36 years old 40 years old | 4 professional American football players 1 professional American wrestler | 5 reported | 1 myocardial infarction 1 suicide by ingesting ethylene glycol 1 suicide by a gunshot wound of the head 1 died in traffic accident during a 40-mile-per-hour high-speed police chase 1 suicide by hanging | 1 tau-immunopositive Neurofibrillary Tangles and neuritic threads in the neocortex, subcortical ganglia, and brainstem nuclei, accompanied by amyloid plaques in the neocortex; 4 tau-immunopositive Neurofibrillary Tangles and neuritic threads in the neocortex, subcortical ganglia, and brainstem nuclei without amyloid plaques. | Not reported | Reported |
| Scanlon et al. [5] | 3 | 2024 | 3 male | 41 years old 46 years old 52 years old | 1 former service member of the Navy 1 Service member in the Special Forces 1 Military veteran | Not reported | 1 acute pulmonary embolism 1 glioblastoma 1 acute myocardial infarction | 3 isolated Chronic Traumatic Encephalopathy pathognomonic lesions adjacent to the underlying white matter | Not reported | 1 motor vehicle accident and sustained a traumatic brain injury associated with loss of consciousness 1 combat deployment, participation in boxing 1 participation in wrestling and American Football in High School |
| Tiemensma et al. [8] | 2 | 2024 | 2 female | Fourth decade of life Fifth decade of life | Not reported | Not reported | 1 alleged assault 1 struck by motor vehicle | 1 perivascular foci of neuronal p-tau immunoreactivity at sulcal depths; 1 perivascular foci of neuronal p-tau immunoreactivity in left dorsolateral frontal cortex and at sulcal depths. | Not reported | 2 repeated head injury in the context of longstanding Intimate Partner Violence |
| Van Amerongen et al. [20] | 1 | 2023 | male | 63 years old | Professional soccer player | Reported | COVID-19 infection | 1 frontal and parietal cortex, neuronal tau pathology was found with predilection of sulcal depths and perivascular regions | Not reported | Decent had experienced multiple collisions that involved head impact playing soccer, at least once leading to loss of consciousness. |
| Pearce et al. [26] | 1 | 2020 | male | Ninth decade of life | Australian rules football player | Reported | Not reported | 1 Tau pathology was found with predilection of sulcal depths and perivascular regions; Beta-amyloid and neuritic plaques | Not reported | The decedent had played more than 350 first-grade matches of Australian Rule Football over 19 years. |
| Del Bigio et al. [27] | 16 | 2023 | 14 male 2 female | 2 35-year-olds 37 years old 42 years old 2 44-year-olds 2 46 years old 47 years old 2 50-year-old 51 years old 2 52-year-olds 54 years old 61 years old | Not reported | Reported | 4 Homicide 1 Sudden death, complications of chronic alcoholism 1 Smoke inalation 1 Hypertrophic cardiomiopaty and congestive heart failure 2 Undetermined 1 Choked on food 1 Probable seizure + dilated cardiomyopathy 1 Acute subdural hematoma with minimal external evidence of head trauma 1 Sepsis secondary to scalp wound infection 1 Alcohol toxicity/hypothermia 1 Accidental head trauma + cardiac arrest + hypothermia 1 Ischemic heart disease + metastatic carcinoma in mediastinum | 16 Chronic Traumatic Encephalopathy p-tau immunoreactivity ranging from a single small focus to widespread abnormalities (7 high Chronic Traumatic Encephalopathy, 9 low Chronic Traumatic Encephalopathy) | Not reported | 5 Alcohol and drug abuse 1 Alcohol abuse, alcohol withdrawal seizures/post-traumatic epilepsy 3 Alcohol abuse 1 Short limb dwarfism, cognitive delay, blind from ret- inal detachments, seizures 1 Alcohol, cocaine, methamphetamine, and marijuana use; schizophrenia 1 Cerebral palsy with spastic diplegia and cognitive delay sec- ondary to perinatal hypoxia, suspected fetal alcohol spectrum disorder 1 Seizure disorder since infancy, long-time abuse of solvents and alcohol, Wer- nicke-Korsakoff syndrome 1 Hypoxic brain damage at birth (breech pre- sentation and cord prolapse), cognitive delay, seizure disor- der; chronic alcohol abuse; diabetes mel- litus type 2 1 Headaches and several Assaults 1 Abusive head trauma at 10 months age, mild hydrocephalus (shunt nonfunction- ing), cognitive delay, chronic alcohol abuse |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsini, F.; Pollice, G.; Carpano, F.; Cipolloni, L.; Cioffi, A.; Cecannecchia, C.; Bibbò, R.; De Simone, S. Advances in Understanding Chronic Traumatic Encephalopathy: A Systematic Review of Clinical and Pathological Evidence. Forensic Sci. 2025, 5, 33. https://doi.org/10.3390/forensicsci5030033
Orsini F, Pollice G, Carpano F, Cipolloni L, Cioffi A, Cecannecchia C, Bibbò R, De Simone S. Advances in Understanding Chronic Traumatic Encephalopathy: A Systematic Review of Clinical and Pathological Evidence. Forensic Sciences. 2025; 5(3):33. https://doi.org/10.3390/forensicsci5030033
Chicago/Turabian StyleOrsini, Francesco, Giovanni Pollice, Francesco Carpano, Luigi Cipolloni, Andrea Cioffi, Camilla Cecannecchia, Roberta Bibbò, and Stefania De Simone. 2025. "Advances in Understanding Chronic Traumatic Encephalopathy: A Systematic Review of Clinical and Pathological Evidence" Forensic Sciences 5, no. 3: 33. https://doi.org/10.3390/forensicsci5030033
APA StyleOrsini, F., Pollice, G., Carpano, F., Cipolloni, L., Cioffi, A., Cecannecchia, C., Bibbò, R., & De Simone, S. (2025). Advances in Understanding Chronic Traumatic Encephalopathy: A Systematic Review of Clinical and Pathological Evidence. Forensic Sciences, 5(3), 33. https://doi.org/10.3390/forensicsci5030033






