1. Introduction
Latent prints or invisible fingerprints left at crime scenes have long been used to convict or exonerate suspects in courtroom proceedings [
1]. Due to their latent nature, fingerprints involved in criminal proceedings must be developed and enhanced to an appreciable clarity for their use in the courtroom. Very often, depending on the nature of the surface (porous or nonporous) on which the prints were deposited, different methods [
2,
3,
4] of development were used.
The two most widely used chemical methods for the development of latent prints on porous surfaces are ninhydrin and 1,8-diazafluoren-9-one (DFO) [
3,
5,
6,
7]. Fingerprints treated with ninhydrin form a purple product [
3,
7], while DFO forms fluorescent species. While Ninhydrin is the most established and often used to analyze prints, DFO [
3] is an alternative. An important advantage of DFO over ninhydrin is the fluorescent species that results from its reaction with latent prints [
3,
7,
8]. Fingerprints developed with DFO are red-colored/pale-pink/pale-purple under daylight [
3,
7,
9]. Under high-intensity light, the prints fluoresce [
3,
10]. This fluorescence enhances the clarity of the fingerprints with ease to analyze and photograph [
11]. In addition, the DFO has higher sensitivity and is estimated to be three to four orders of magnitude [
7].
Common solvents used by police and forensic laboratories for DFO formulation include 1,1,2-trichlorotrifluoroethane (CFC113), HFE-7100, etc. [
7]. However, these solvents are toxic and not environmentally friendly [
12,
13].
In this report, fingerprints were developed on porous surfaces (i.e., Xerox papers, bank checks, black carbon papers, assorted coin wrappers, event tickets, bank deposit slips, etc.) using DFO. The DFO was formulated in environmentally friendlier solvents [
11] such as methanol, glacial acetic acid, and a minimum amount of methylene chloride. The visualization occurred under daylight and UV at 254 nm, 365 nm, and LED at 395–405 nm. The developed prints were photographed using iPhone 11.
2. Materials
1,8-diazafluoren-9-one (Sigma-Aldrich, St Louis, MO, USA), Glycine (Acros), Glacial acetic Acid (Fisher Scientific, Waltham, MA, USA), Methanol (Fisher Scientific), Methylene chloride (Fisher Scientific), UV lamp (UVP) (254 nm, 365 nm), 395–405 nm LED Flashlight 502B UV (UltraFire), Dell laptop computer, LS 45 Luminescence Spectrometer (PerkinElmer, Waltham, MA, USA), Cuvettes (Starna Cells, Inc., Atascadero, CA, USA), Xerox paper Premium Multipurpose, 20lb, 8 1/2 × 11”, Black carbon paper, 310 × 200 mm (Offeara), Assorted coin wrappers (quarter, dime, nickel and penny) (Staples), Event tickets (Blue, Red, White, Yellow, and Green) (Staples), Bank checks (Bank of America, Chase Bank (personal and business), and Wells Fargo), Chase Bank deposit slips, and iPhone 11. Laboratory hot plate and press iron (Black and Decker).
3. Methods
3.1. DFO Stock Solution
The stock solution of 1,8-diazafluoren-9-one was prepared according to a modified procedure of Pounds et al. [
4]. Into a glass vial (3 mL) were added DFO (82 mg), methylene Chloride (500 μL), acetic acid (2 mL), and methanol (500 μL), respectively, and the mixture was homogenized. The vial was caped, hand-shaken to a homogenous solution and used as the stock solution of DFO.
3.2. Latent Fingerprints
The porous materials were cut with scissors (e.g., 50 × 100 mm) and used for fingerprint impressions. The latent prints were prepared by applying the sebum-rubbed fingers on the porous materials. The latent prints were developed immediately after.
3.3. Latent Fingerprints Development
Typically, latent prints were dipped (i.e., 20–30 s) into an aliquot (25 mL) of stock solution of DFO in a beaker (50 mL). They were dry in air (i.e., 60 s), followed by heating using a hot plate (i.e., 70–80 °C) and press iron (i.e., 60 s) until the fingerprints were visible to the naked eye (daylight) or under the UV/LED lamp. The prints were cooled to room temperature and visualized under daylight and UV at 254 nm, 365 nm, and LED at 395–405 nm. The developed fingerprints were photographed using iPhone 11.
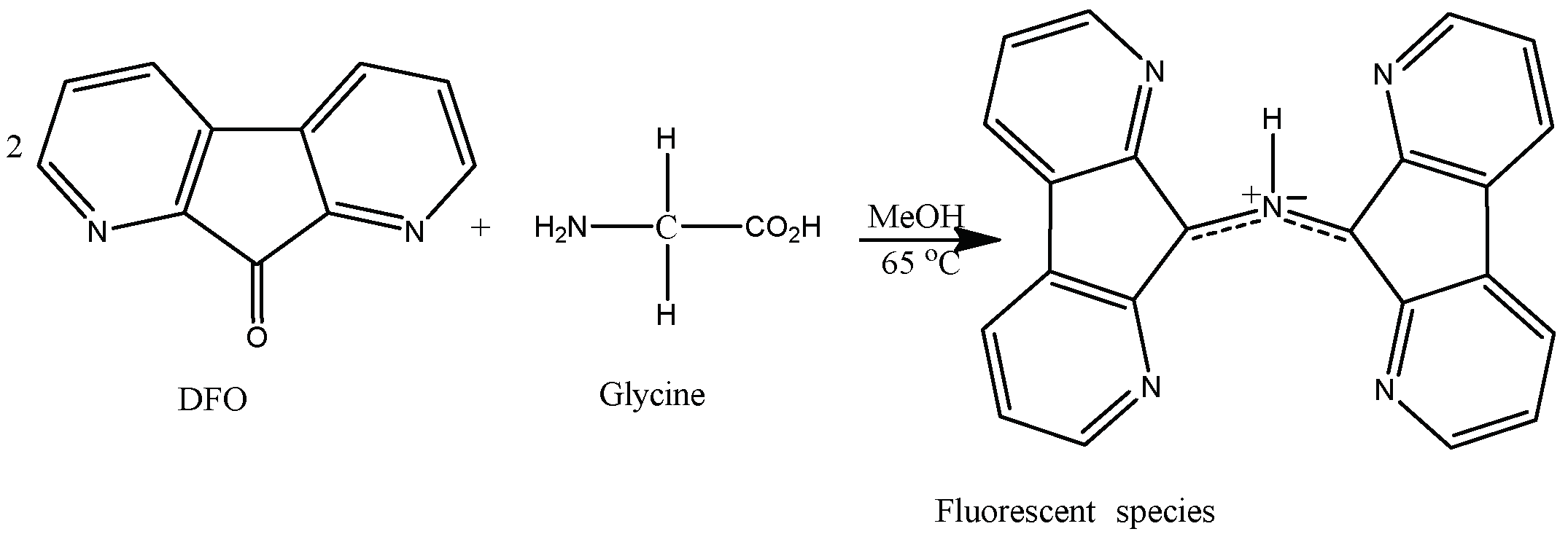
3.4. Reaction of DFO with Glycine
Glycine (50 mg) was added to a glass vial (15 mL) containing a freshly made DFO solution (10 mL) from the stock. Glacial acetic acid (5 mL) was added, and the vial was hand-shaken for homogenization. The glycine solution was used shortly after to acquire the excitation and emission spectra.
4. Results
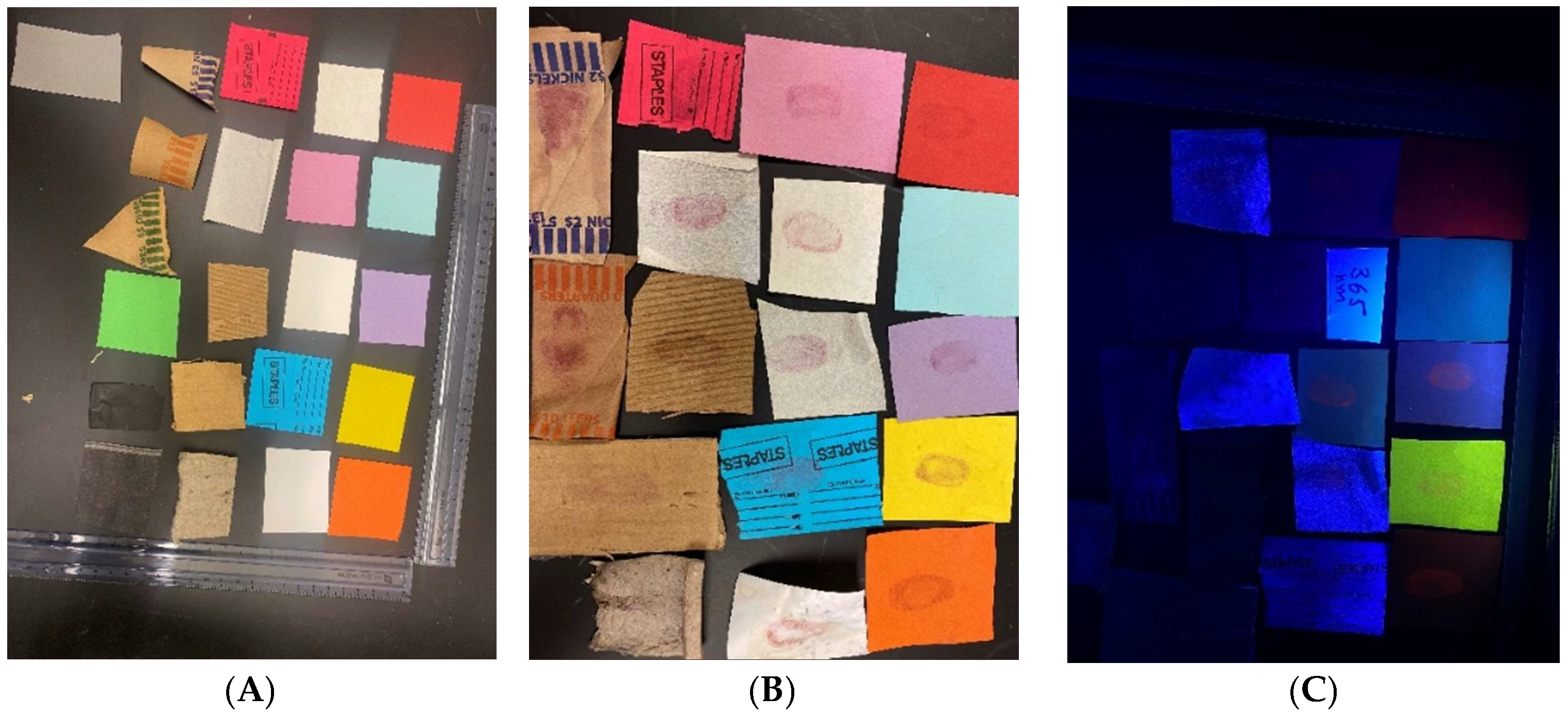
Latent prints were developed on various porous surfaces with DFO and were visualized under a UV lamp (254 and 365 nm) and LED (395–405 nm) after exposure to heat, a hot plate, and pressing iron (70–80 °C), and the results are shown below. The fingers were coated with sebum and applied to the porous surfaces. All the fingers, right hand and left hand, were used to develop the fingerprints. The prints were prepared by dipping the porous surfaces into DFO solution (i.e., 20–30 s), followed by drying them in the air. Shortly after, they were treated to the heat and visualized. Care was taken to flip the porous surface on the hot plate to expose both sides to heat while pressing it with an iron. The prints on Xerox paper, in general, responded well to DFO and fluoresced under UV light (
Figure 1). However, due to the background and colors of certain Xerox paper, prints developed on them were faintly visible under daylight or UV (
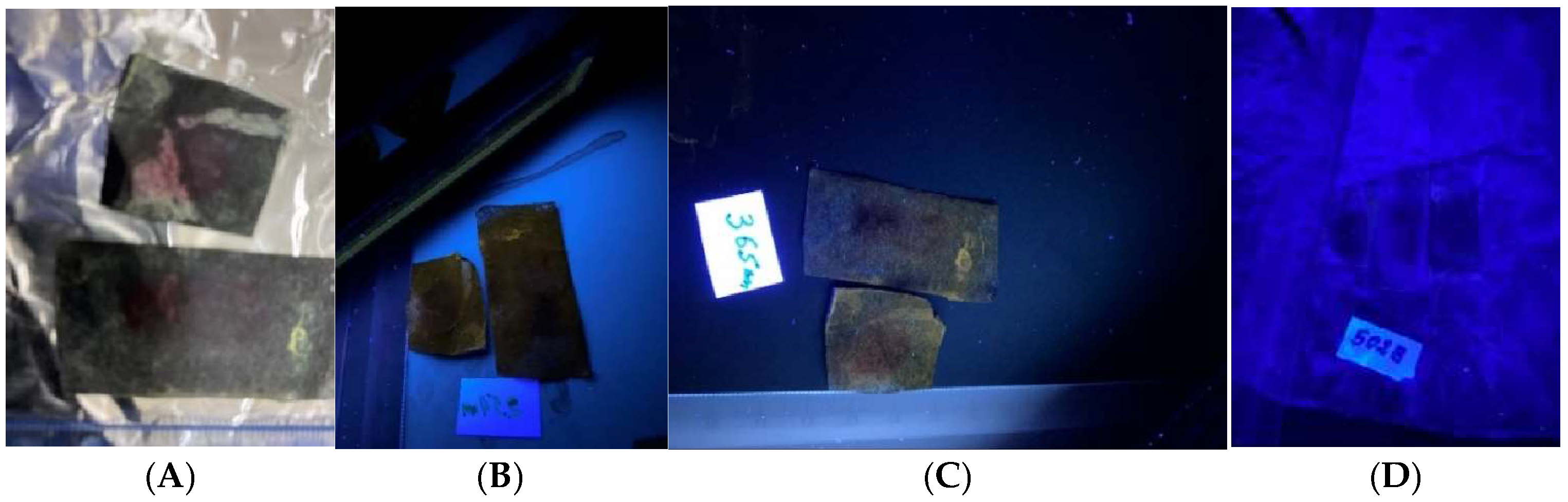
Figure 2). The fingerprints on carbon papers were not visible under daylight or UV (
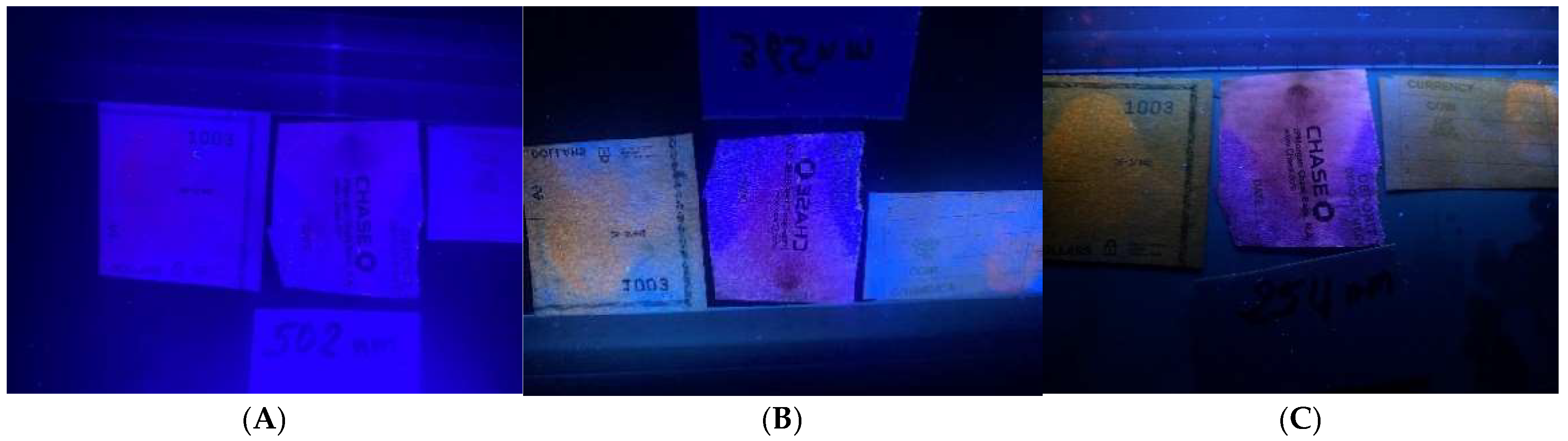
Figure 3). Prolonged heating to enhance the prints overheated the carbon paper. The prints on bank checks and deposit slips reacted successfully to DFO treatment and were visible under daylight (
Figure 4) and UV light at 254 and 365 nm, respectively (
Figure 5). However, they were indistinguishable under LED (
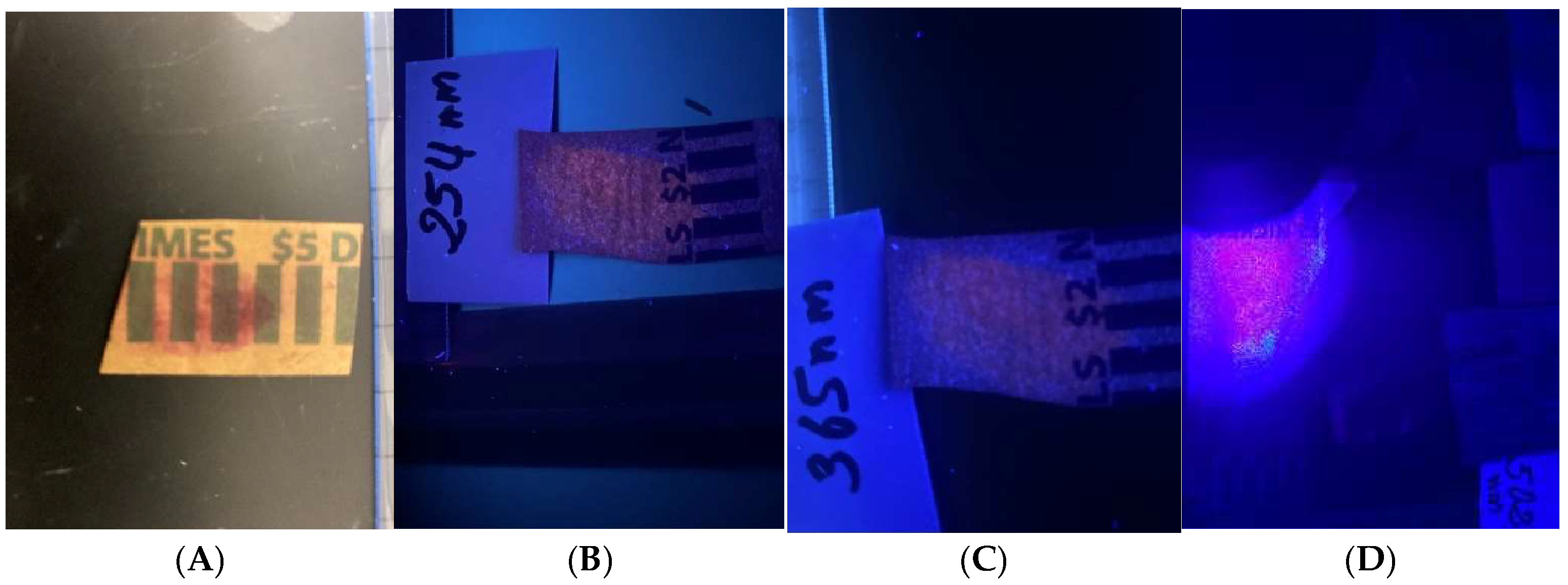
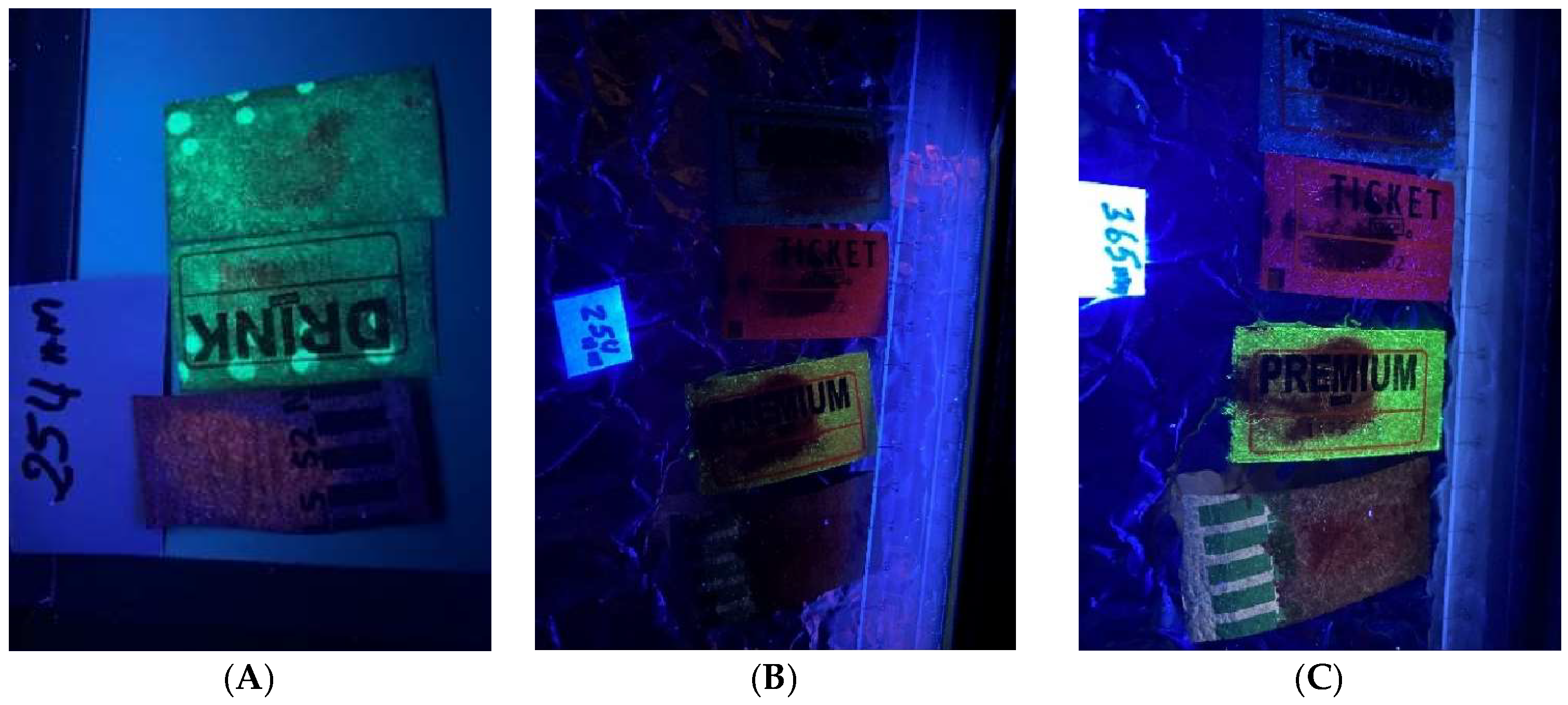
Figure 5A). The prints on the event tickets and assorted coin wrappers (
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11 and
Figure 12) were all visible under all the light sources, although with varying degrees of clarity.
The reaction of DFO with the amino acid glycine allows us to determine the excitation and the emission maxima of the resulting fluorescent species (
Scheme 1) [
7].
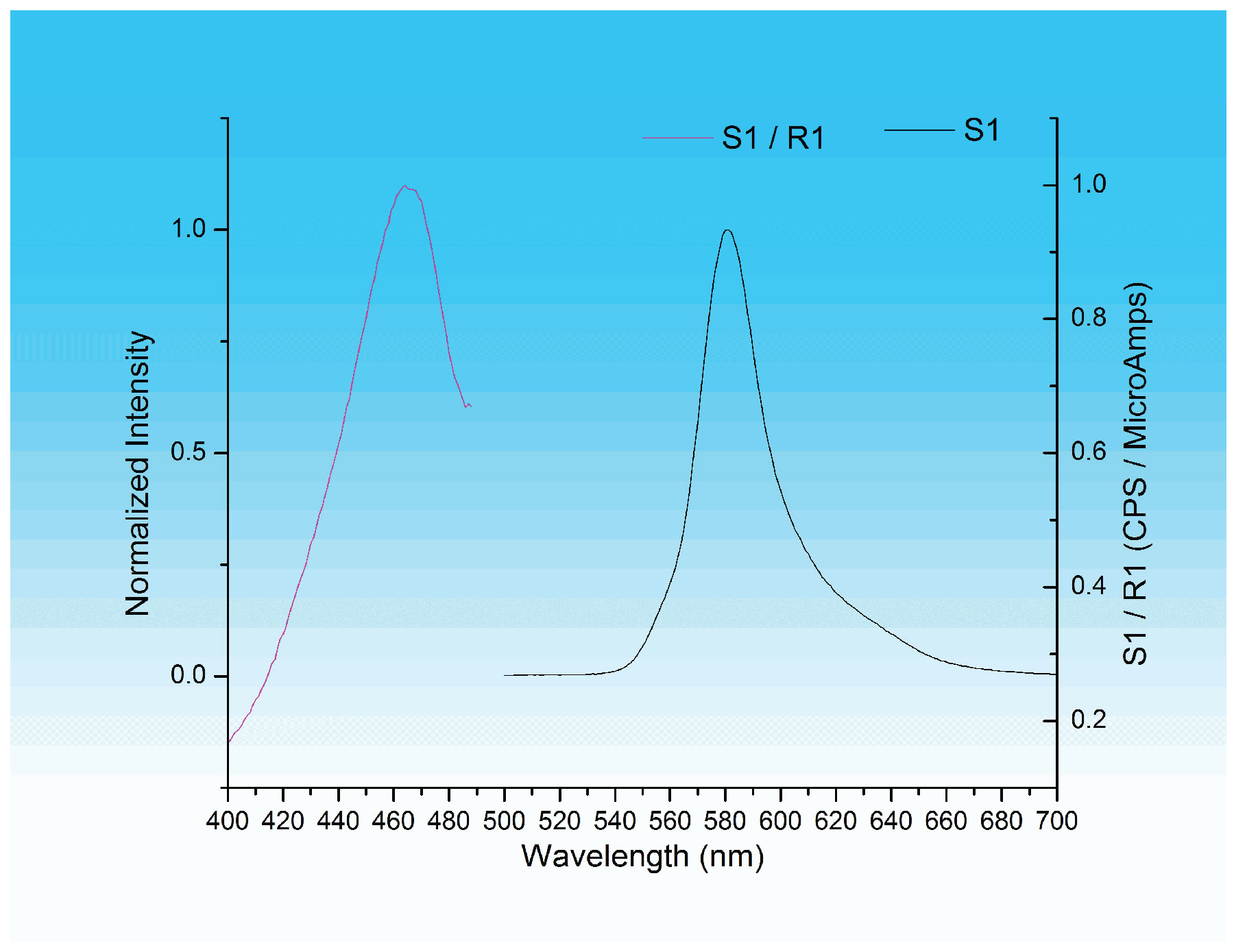
Figure 13 shows the fluorescent spectra of the product of that reaction. The excitation and emission maxima are 470 nm and 585 nm, respectively.
5. Discussion
In this study, we set out to develop and visualize fingerprints on porous surfaces using DFO formulated in environmentally friendlier solvents. The fingerprints were developed using DFO, enhanced with heat, and visualized under a handheld UV lamp at 254 nm and 365 nm, LED at 395–405 nm, and photographed using iPhone 11. The porous surfaces were everyday items such as Xerox papers, bank checks, assorted coin papers, event receipts, bank deposit slips, and black carbon papers. The availability of various porous surfaces, handheld UV lamps, and LEDs in most forensic chemistry laboratories makes this experiment suitable for undergraduate experiments and forensic laboratories. Some porous surfaces (i.e., Xerox papers), used in this experiment show some background [
4,
14] fluorescence when illuminated by UV light (
Figure 2). This fluorescent background did not inhibit, to any significant extent, the visualization of the developed prints. However, some prints were not clearly visible under UV (i.e.,
Figure 2C,
Figure 3,
Figure 5A and
Figure 10B). The light source [
4] commonly used by the police to visualize fingerprints developed on porous surfaces involves, for example, laser or blue-green light with a viewing filter. However, in this work, we used UV light (254 nm and 365 nm), and LED at 395–405 nm without viewing filters.
The fluorescent spectra (
Figure 13) show that the wavelengths of maximum fluorescence for the reaction of DFO and the amino acid glycine are λ
ex = 470 nm for excitation and λ
em = 585 nm for emission. These results are in accord with the maximum excitation and emission wavelengths (λ
ex = 470 nm and λ
em = 570 nm) reported [
3,
4] for such a reaction.
The formulation of 1,8-diazafluoren-9-one used in this work contains methanol, glacial acetic acid, and a minimum amount of methylene chloride, making it environmentally friendlier than comparable solvents used for this purpose [
4,
7,
11]. To our knowledge, the use of a minimum amount of methylene chloride in DFO’s formulation has yet to be reported [
4,
15]. The mixture of the three solvents system was required to solubilize the DFO. The readily available and affordable methylene chloride can replace 1,1,2-trichlorotrifluoroethane (CFC113) and hydrofluoroethane (HFE7100), solvents commonly associated with DFO formulation [
7] and not environmentally friendly [
12,
13]. In addition, methylene chloride’s relatively low boiling point shortens samples’ drying time.
It is believed that DFO formulated in CFC113 or HFE7100 has a shelf life of six months [
4]. Although we did not do a stability study, we were able to develop fingerprints with the same clarity as a freshly made DFO solution one week after the solution was prepared. The solution was kept at 4 °C and caped.
It was reported [
4,
9] that heat plays a catalytic role in the development of fingerprints with DFO, and that the heat source commonly used is an oven at 100 °C with humidity [
4]. This work used a hot plate and a pressing iron as the heat source. After drying them at room temperature, the prints were placed directly on a hot plate at 70–80 °C and pressed on both sides with a hot iron for 60 s. No humidity (except the humidity in the air) was used in this work, and a longer exposure time to heat (i.e., more than 60 s) produced burned prints.
6. Conclusions
Fingerprints were developed on porous surfaces using DFO and visualized under a UV lamp at 254 nm, 365 nm, and LED at 395–405 nm and photographed with iPhone 11. Porous surfaces were made of everyday items such as Xerox papers, bank checks, assorted coin wrappers, black carbon papers, bank deposit slips, and event tickets chosen due to their susceptibility to being found on a crime scene. The formulation of DFO was made of methanol, glacial acetic acid, and a minimum amount of methylene chloride, solvents that are environmentally friendlier compared to those that were commonly used. After exposure to the heat (hot plate and pressing iron) to dry, the developed prints are pale purple under daylight and fluorescent under UV. The reaction of glycine with DFO allowed the determination of the wavelengths for maximum excitation (470 nm) and emission (585 nm) of the fluorescent species.
The easy access to porous surfaces, the availability of UV lamps and LEDs on one hand, and the facile formulation of DFO in methanol, glacial acetic acid, and methylene chloride make this fingerprint development method suitable for forensic laboratories and undergraduate forensic laboratories. The future direction of this project will involve working on the clarity of the friction ridges, the long-term stability study of DFO in methylene chloride, glacial acetic acid, and methanol, and the comparative study of wet fingerprints using both DFO and fluorescent small particle reagents.
Safety: Lab coat, Goggle, and glove must be worn while preparing DFO solutions, and the visualization of latent prints. The formulation of DFO shall be prepared in the fume hood. All chemicals, reagents, and solvents must be treated cautiously and as the MSDS directs. DFO must be treated as a health hazard chemical.