Migratory Flows and Endomyocardial Fibrosis: A Mysterious Disease in Western Countries
Abstract
1. Introduction
2. Case
2.1. Patient History
2.2. Autopsy Finding
2.3. Macroscopic Examination of the Heart
- -
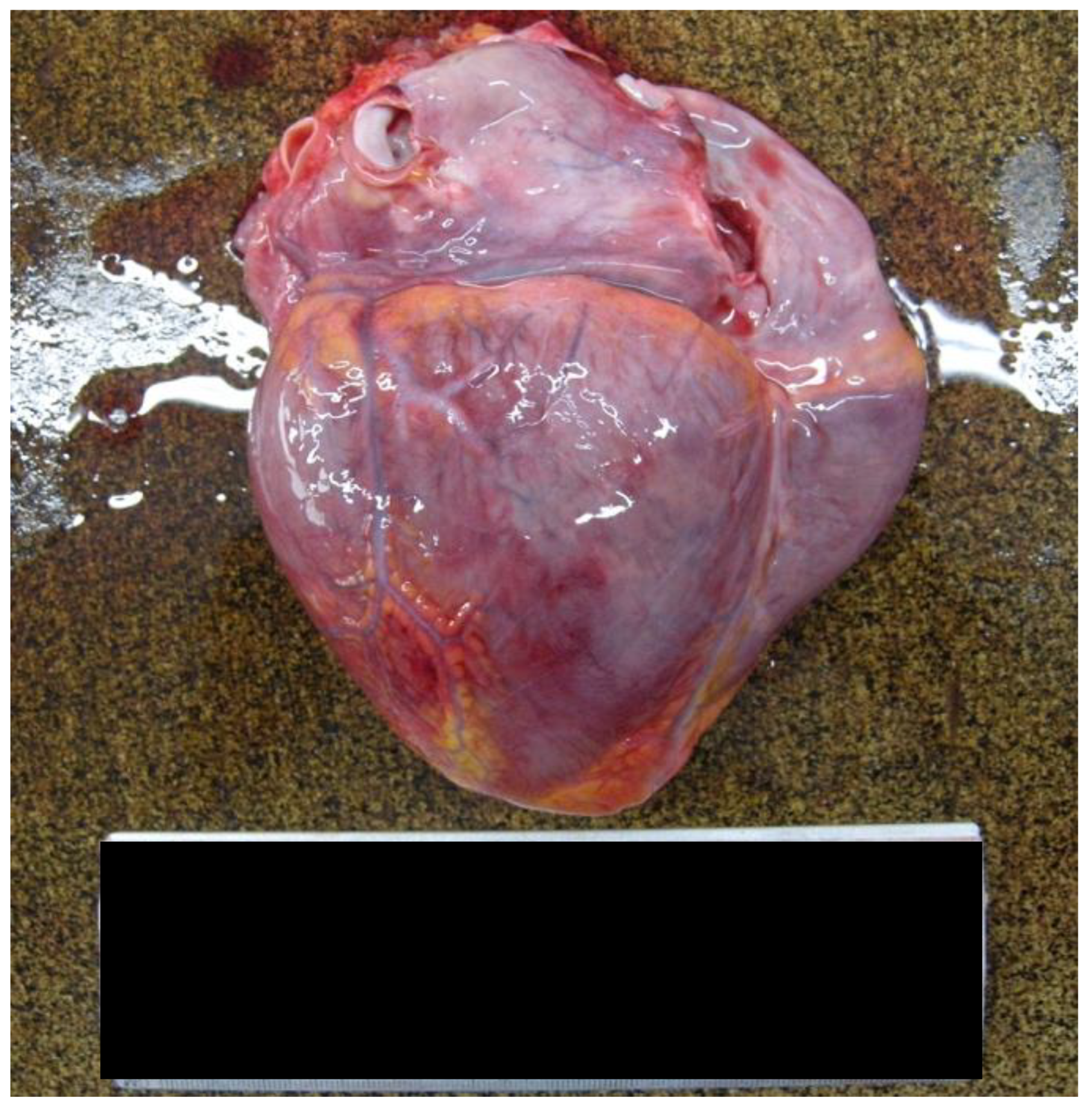
- The heart weighed 350 g, exhibiting a slightly increased size and volume, with dimensions of 9 cm longitudinally, 11.5 cm transversely, and 3 cm anteroposteriorly [Figure 1].
- -
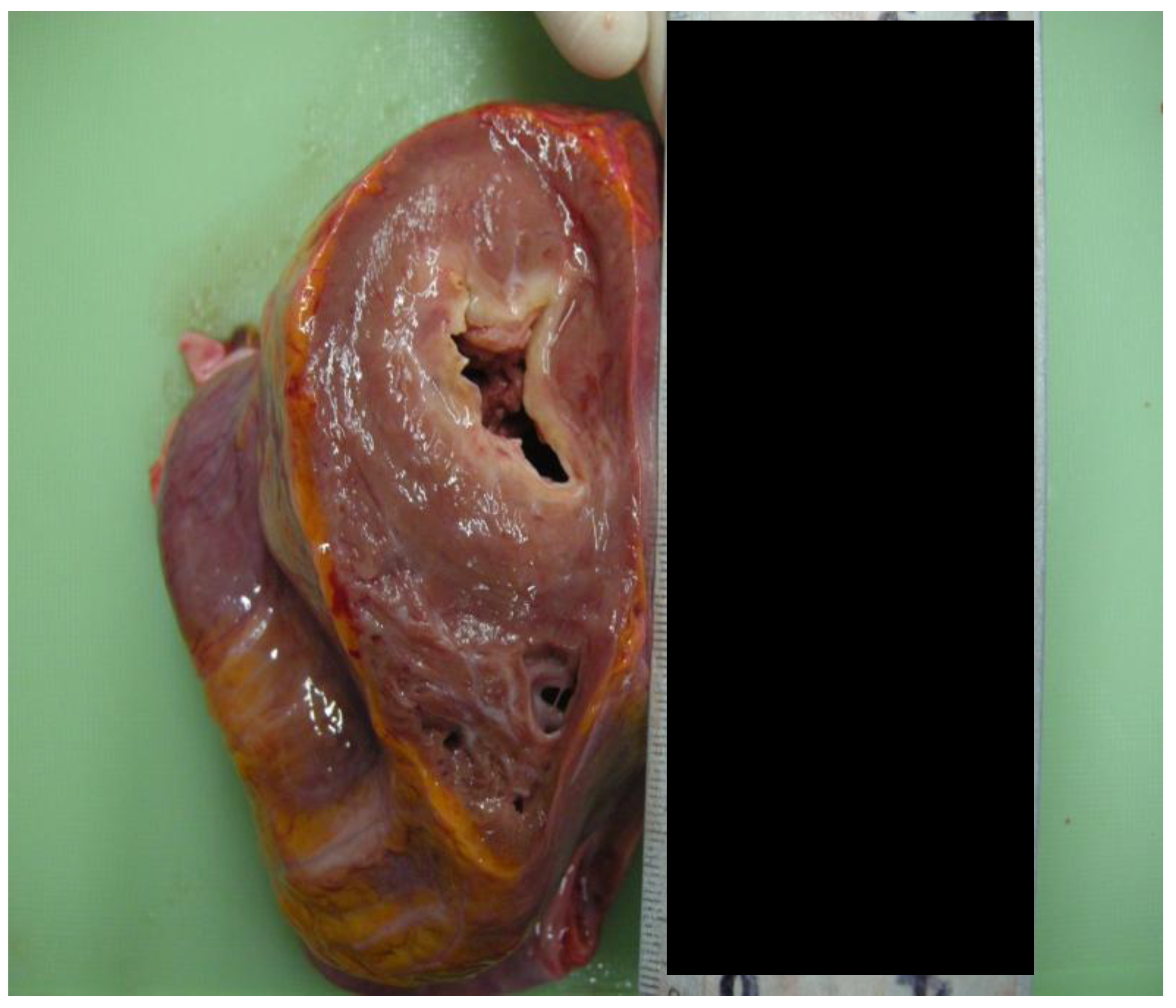
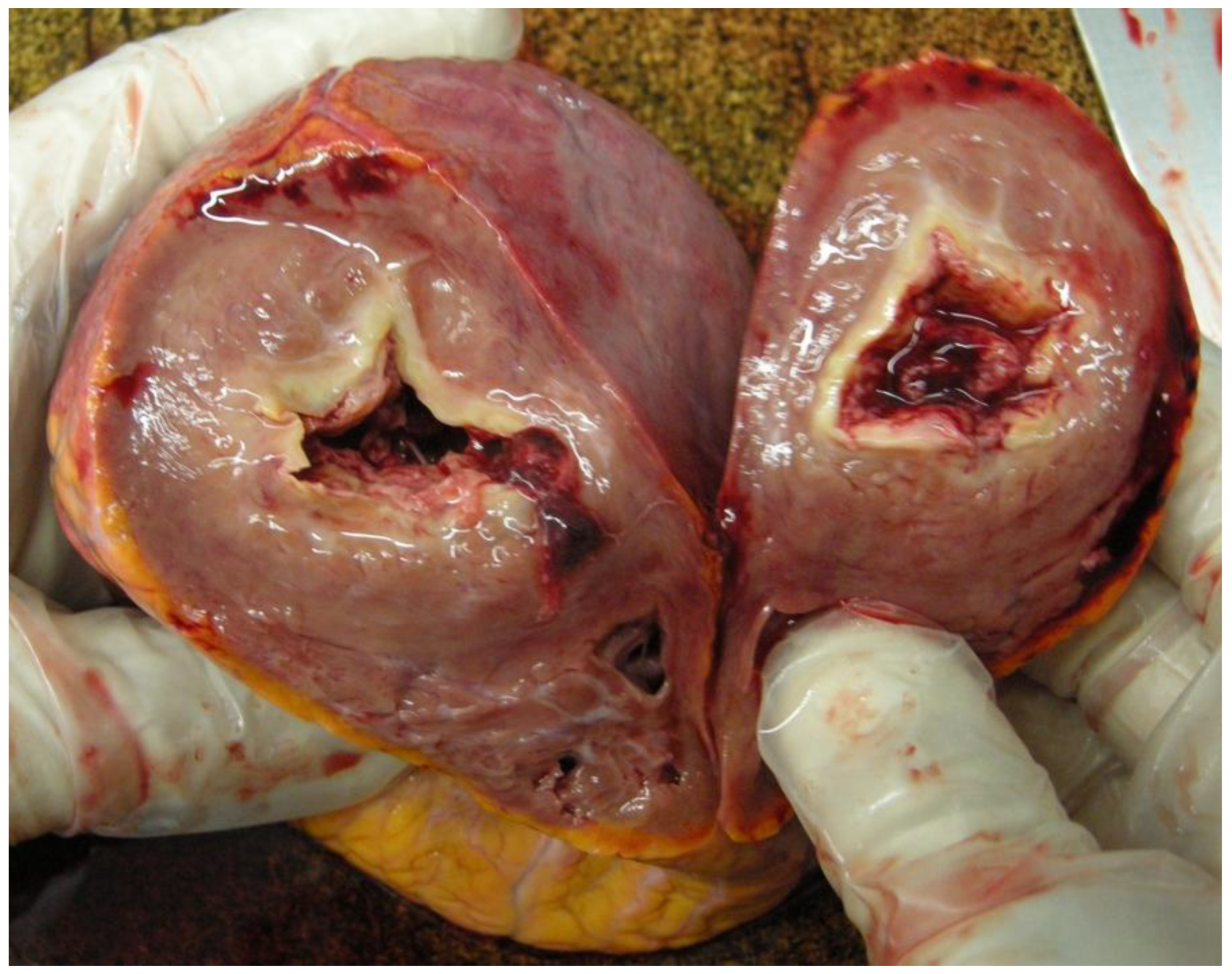
- The myocardial thickness had increased. The posterior wall measured 1.2 cm, the lateral wall measured 1.8 cm, the anterior wall measured 1.5 cm, the ventricular septal measured 1.6 cm, and the right ventricular wall measured 0.4 cm [Figure 2].
- -
- Examination of the coronary artery tree showed a subcritical stenosis in the proximal tract of the left anterior descending artery, complicated by an occlusive thrombus. Instead, the right coronary artery remained patent.
- -
- The right ventricular wall was partially replaced by fibrotic tissue.
- -
- -
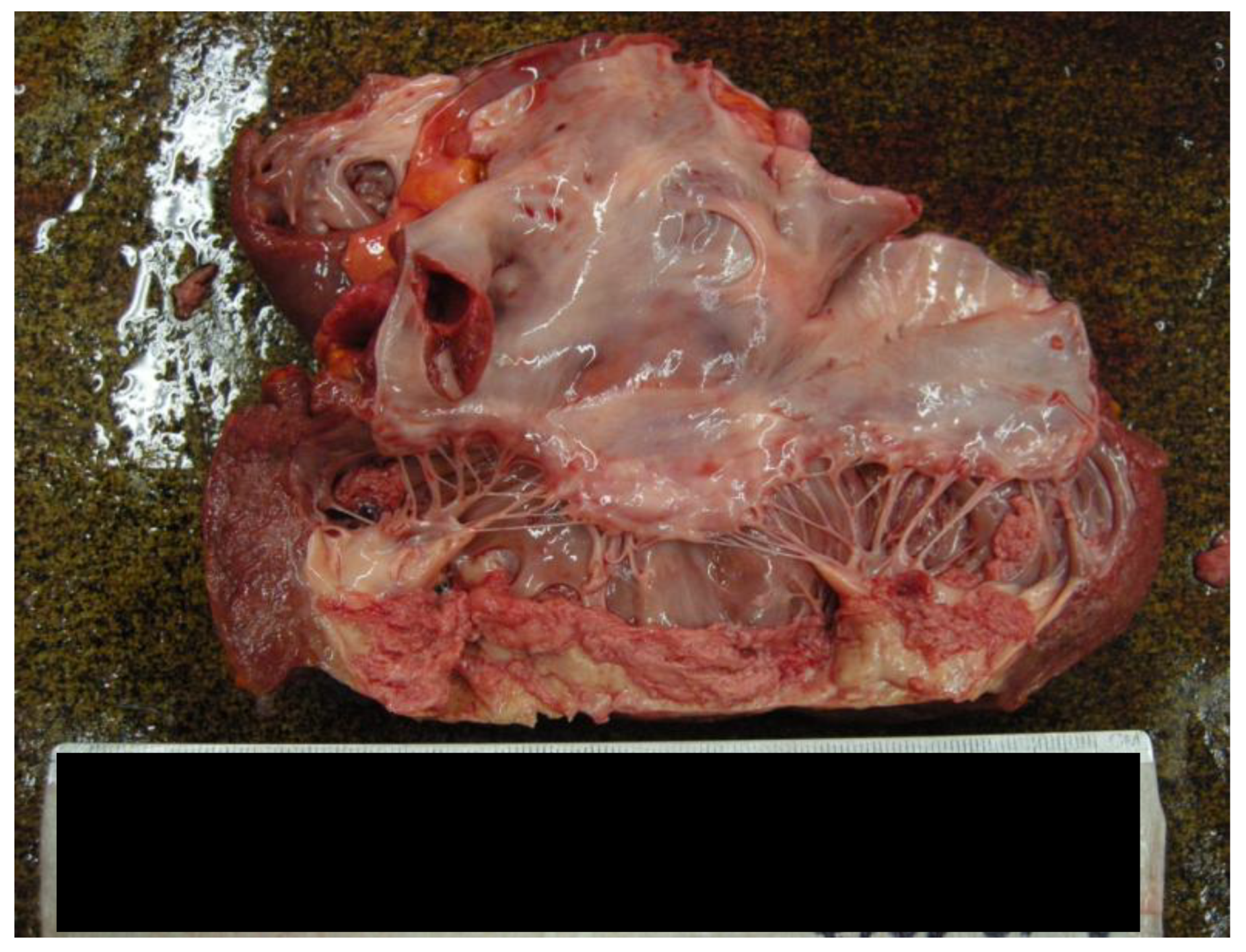
- The cusps of the tricuspid and mitral valves appeared slightly retracted, measuring 11 cm and 10.5 cm in length, respectively. The pulmonary valve measured 8 cm, and the aortic valve was 7.5 cm.
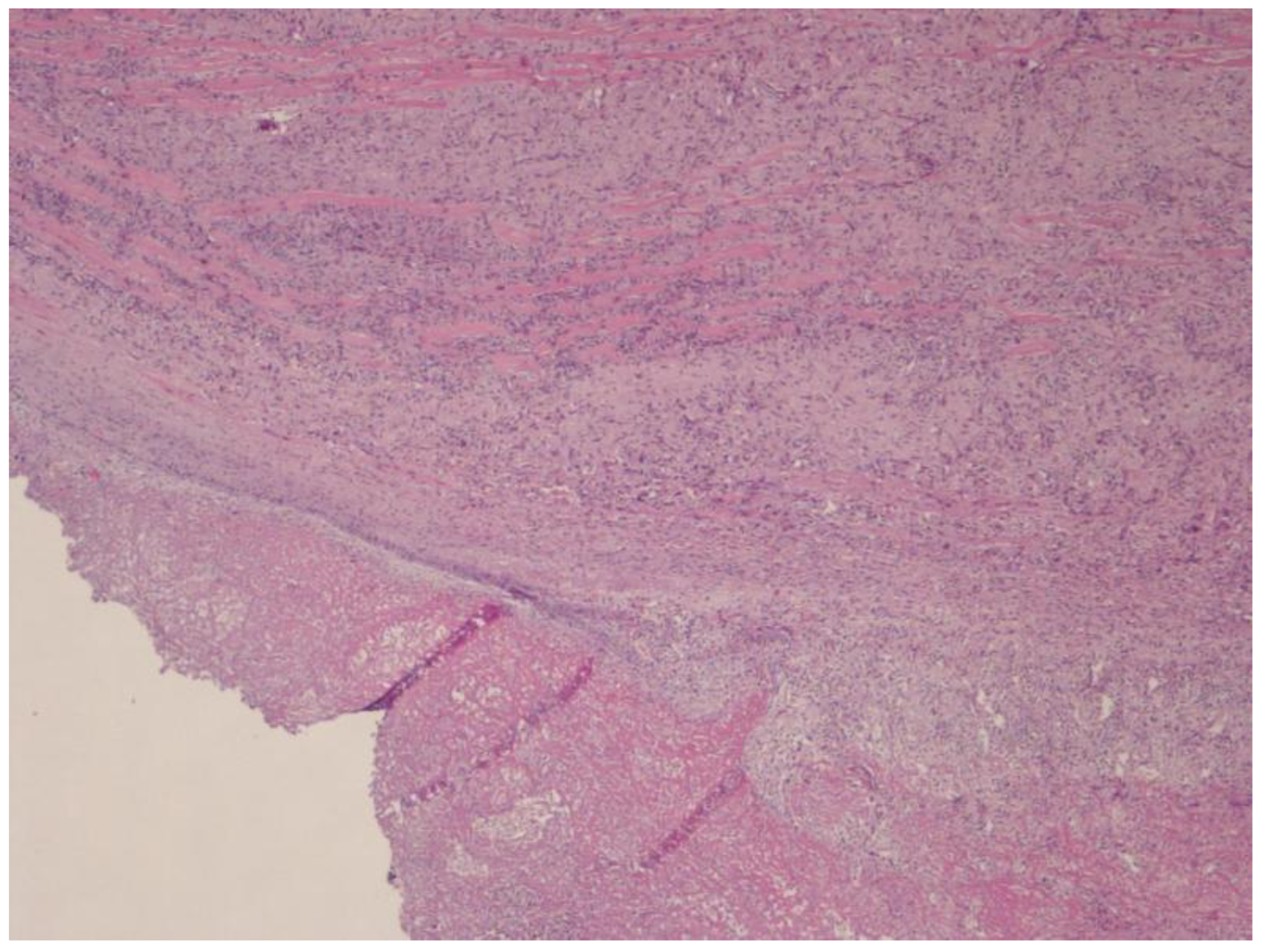
2.4. Histological Finding
2.5. Cause of Death
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutierrez, P.S.; Campos, F.P.F.D. Endomyocardial fibrosis. Autops Case Rep. 2017, 7, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Mocumbi, A.O.; Freers, J.; Lachaud, M.; Mirabel, M.; Ferreira, B.; Narayanan, K.; Celermajer, D.S.; Sidi, D.; Jouven, X.; et al. Tropical Endomyocardial Fibrosis: Natural History, Challenges, and Perspectives. Circulation 2016, 133, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Mocumbi, A.O.; Ferreira, M.B.; Sidi, D.; Yacoub, M.H. A Population Study of Endomyocardial Fibrosis in a Rural Area of Mozambique. N. Engl. J. Med. 2008, 359, 43–49. [Google Scholar] [CrossRef] [PubMed]
- De Gaspari, M.; Toscano, G.; Bagozzi, L.; Metra, M.; Lombardi, C.; Rizzo, S.; Angelini, A.; Perazzolo Marra, M.; Gerosa, G.; Basso, C. Endomyocardial fibrosis and myocardial infarction leading to diastolic and systolic dysfunction requiring transplantation. Cardiovasc. Pathol. 2019, 38, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Mocumbi, A.O.; Stothard, J.R.; Correia-de-Sá, P.; Yacoub, M. Endomyocardial Fibrosis: An Update After 70 Years. Curr. Cardiol. Rep. 2019, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, Y.; Zhao, P. Application of Routine Echocardiography Combined with Contrast-Enhanced Echocardiography in the Diagnosis of Endomyocardial Fibrosis. Discov. Med. 2023, 35, 1052. [Google Scholar] [CrossRef] [PubMed]
- Tigani, S.E.; Khalil, S.; Saad, H.A.; Khalil, S.I. Endomyocardial fibrosis in Sudan: Clinical and echocardiographic features Cardiovasc. J. Afr. 2017, 28, 208–214. Available online: https://journals.co.za/doi/10.5830/CVJA-2016-079 (accessed on 22 January 2025).
- Vásquez-Rodríguez, J.F.; Medina-Mur, R.I.; Giraldo, L.E.; Juan-Guardela, M.L.; Gelves, J.; Jaimes, C.P. Endomyocardial fibrosis: A restrictive cardiomyopathy in developing countries. Arch. De Cardiol. De México 2021, 91, 196–201. [Google Scholar] [CrossRef]
- Grimaldi, A.; Alfieri, O.; Camici, P.G.; Zoppei, G.; Olivotto, I. “Mal d’Africa” e cuore: I misteri della fibrosi endomiocardica. G. Ital. Cardiol. 2011, 12, 484–491. [Google Scholar]
- Théry, G.; Faroux, L.; Deleuze, P.; Metz, D. Idiopathic endomyocardial fibrosis in a Western European: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Reddy, S.; Ôunpuu, S.; Anand, S. Global Burden of Cardiovascular Diseases. Circulation 2001, 104, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Endomyocardial Fibrosis in a European Woman and Its Successful Surgical Treatment-Abstract-Europe PMC. Available online: https://europepmc.org/article/med/916720 (accessed on 18 December 2024).
- López Ramón, M.; García de la Calzada, M.D.; Domínguez Cunchillos, M.; Salazar Mena, J. Tropical endomyocardial fibrosis in Spain. An. Pediatr. 2013, 78, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Cağli, K.; Uygur, B.; Ozlü, F.; Gölbaşi, Z. Endomyocardial disease: A case report. Turk. Kardiyol. Dern. Ars. 2009, 37, 136–140. [Google Scholar] [PubMed]
- Gyr, K.; Fernex, M.; Wick, R.; Burckhardt, D. African endomyocardial fibrosis. Report of a case seen in Basel. Dtsch. Med. Wochenschr. 1970, 95, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Haumer, M.; Poelzl, G.; Wiedemann, D.; Kliegel, A.; Ullrich, R.; Gartlehner, G.; Zuckermann, A.; Müller, L.; Mayr, H.; et al. A case report of a 40-year-old woman with endomyocardial fibrosis in a non-tropical area: From initial presentation to high urgent heart transplantation. BMC Cardiovasc. Disord. 2019, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Pavia, M.; Cugini, A.; Buffa, J. Restrictive cardiomyopathy caused by endomyocardial fibrosis. Description of a case observed in Piedmont. Minerva Cardioangiol. 1985, 33, 357–361. [Google Scholar] [PubMed]
- Mocumbi, A.O.; Yacoub, S.; Yacoub, M.H. Neglected tropical cardiomyopathies: II. Endomyocardial fibrosis. Heart 2008, 94, 384–390. [Google Scholar] [PubMed]
- Mandoli, G.E.; D’Ascenzi, F.; Vinco, G.; Benfari, G.; Ricci, F.; Focardi, M.; Cavigli, L.; Concetta Pastore, M.; Sisti, N.; De Vivo, O.; et al. Novel Approaches in Cardiac Imaging for Non-invasive Assessment of Left Heart Myocardial Fibrosis. Front. Cardiovasc. Med. 2021, 8, 614235. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; La Sala, L. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. Int. J. Mol. Sci. 2022, 23, 10944. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Avishay, D.M.; Jones, C.R.; Shaikh, J.D.; Kaur, R.; Aljadah, M.; Kichloo, A.; Shiwalkar, N.; Keshavamurthy, S. Sudden cardiac death: Epidemiology, pathogenesis and management. Rev. Cardiovasc. Med. 2021, 22, 147–158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosi, L.; Nicolì, S.; Ferorelli, D.; Straface, A.; Benevento, M.; Solarino, B. Migratory Flows and Endomyocardial Fibrosis: A Mysterious Disease in Western Countries. Forensic Sci. 2025, 5, 11. https://doi.org/10.3390/forensicsci5010011
Ambrosi L, Nicolì S, Ferorelli D, Straface A, Benevento M, Solarino B. Migratory Flows and Endomyocardial Fibrosis: A Mysterious Disease in Western Countries. Forensic Sciences. 2025; 5(1):11. https://doi.org/10.3390/forensicsci5010011
Chicago/Turabian StyleAmbrosi, Laura, Simona Nicolì, Davide Ferorelli, Antonio Straface, Marcello Benevento, and Biagio Solarino. 2025. "Migratory Flows and Endomyocardial Fibrosis: A Mysterious Disease in Western Countries" Forensic Sciences 5, no. 1: 11. https://doi.org/10.3390/forensicsci5010011
APA StyleAmbrosi, L., Nicolì, S., Ferorelli, D., Straface, A., Benevento, M., & Solarino, B. (2025). Migratory Flows and Endomyocardial Fibrosis: A Mysterious Disease in Western Countries. Forensic Sciences, 5(1), 11. https://doi.org/10.3390/forensicsci5010011







