1. Introduction
Age estimation is crucial to forensic anthropology, serving as a fundamental element in the biological profile. This profile typically includes estimations of age, sex, population affinity, and stature, all of which are useful in limiting the pool of missing persons to which unknown human remains should be compared. In addition to narrowing potential matches with missing persons, accurate and precise age estimation can provide insights into the life history of the deceased, including health, nutrition, and social status.
Traditional methods for skeletal age estimation often result in broad age ranges that can limit their utility in forensic contexts. These methods, developed primarily from reference populations that do not represent the diversity of modern cases, often fail to account for individual variability in aging processes [
1,
2]. Additionally, the lack of standardized protocols for integrating multiple age indicators leads to inconsistencies in age estimates [
3]. These challenges highlight the need for improved methodologies that can provide more precise and reliable age estimates by considering a wider range of biological and environmental factors.
Age estimation research has largely focused on developing accurate and precise predictions of chronological age based on skeletal remains. Despite advancements in age estimation techniques, these efforts are limited due to a significant gap in understanding how skeletal age indicators and chronological age are affected by health, lifestyle, and environmental exposures. Here, we present a pilot study examining how health proxies via cause of death relate to age estimation. This research is one step towards improving the accuracy and precision of forensic analyses by integrating health factors into the age estimation process.
1.1. The Science of Aging
The process of aging is a complex progression that begins with growth and development and eventually leads to various degenerative changes. Questions about why we age, how we age, and the rate at which aging occurs continue to be central to scientific inquiry. Researchers aim to better understand health, diseases, genetics, and biology through the lens of aging. For biological anthropologists, the science of aging is essential to accurately interpret the biological and skeletal changes that inform research questions related to development, mortality, and population health. The evolutionary dynamics that underpin aging and longevity result from an interplay between genetics, life-history traits, and environmental factors [
4].
1.1.1. Biological Age
In aging research, chronological age (CA) refers to the number of years, months, and days a person has been alive, serving as the baseline for other age values. Biological age (BA), on the other hand, has multiple interpretations depending on the context. BA is a physiological measure representing the rate at which an individual has physically aged [
5,
6,
7]. In contemporary research, BA often relates to cellular-level aging, quantified through telomere shortening, DNA methylation, the epigenetic clock, and other molecular biomarkers [
7,
8,
9]. An epigenetic clock is a mathematical model that uses DNA methylation levels in tissues and blood to estimate biological age. DNA methylation naturally occurs with age, but the rate of the epigenetic clock varies among individuals [
10]. Studying the epigenome allows researchers to link the genotype and phenotype to understand how the aging process changes in response to the environment [
11,
12,
13]. Analyses have demonstrated that BA better predicts late-life depression, overall mortality, intensive care unit mortality, and stroke-related mortality than does chronological age [
14,
15,
16,
17].
Studies of BA via the epigenetic clock have led to investigations into the factors influencing its acceleration and deceleration [
18,
19]. While some research has focused on population-specific analyses, examining epigenetic age differences by race (the term “race” is used because it reflects the term used in the cited article or the referenced forms), sex, or socioeconomic status [
20,
21,
22], most studies test the predictive power of cellular changes for age-related morbidity and mortality at the individual level [
23,
24]. The concept of biological age has expanded beyond the cellular level to include biomarker measures at the level of organ systems [
8,
9,
25,
26,
27]. Advancements in medical technology have improved our ability to collect biomarker data. High predictive value was found in CT-based body composition measures, including aortic calcification, muscle density, liver fat, and bone mineral density [
28,
29]. This relationship has been further illustrated in studies examining the impact of socioeconomic disadvantage on CT-based body composition measures [
30].
1.1.2. Skeletal Age
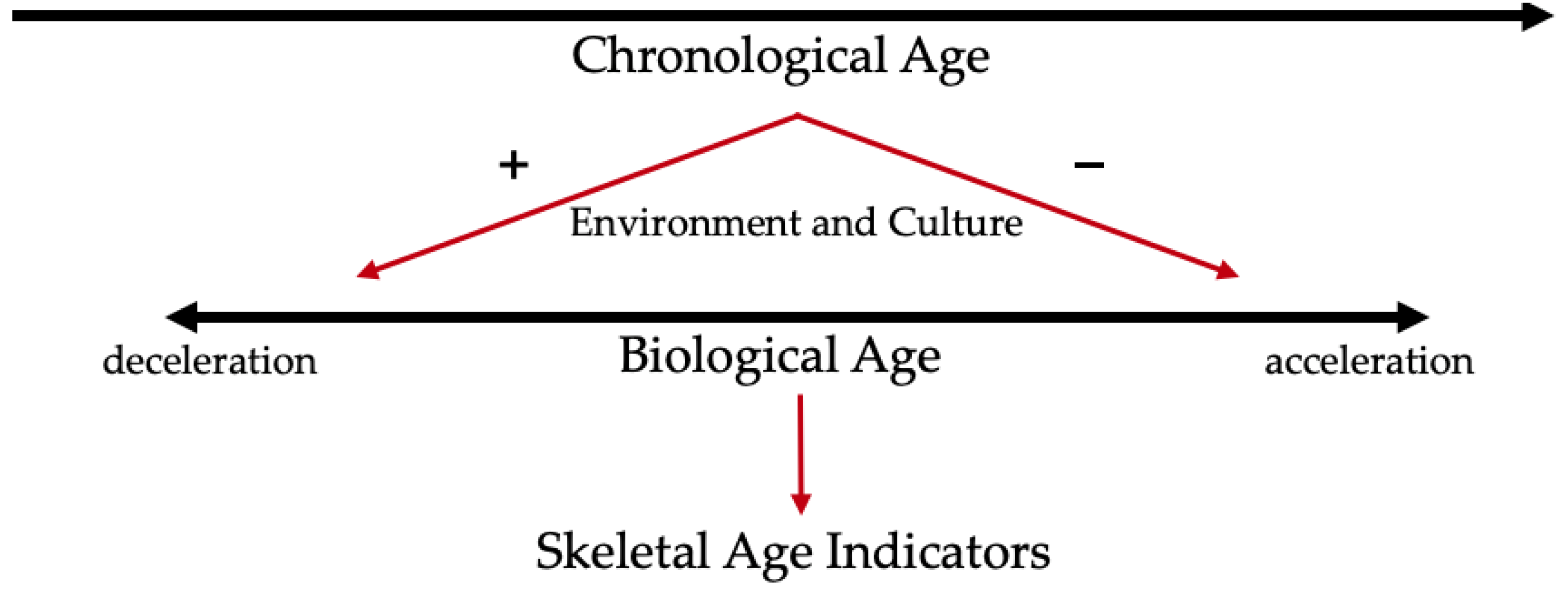
Identifying characteristics of aging in the skeleton should be approached the same way as with any other organ system in the body. Skeletal age (SA) is an estimation of CA but may more closely reflect BA. Just as BA is dependent on biological, social, and environmental factors, so too is SA (
Figure 1). These various factors are not mutually exclusive Discrepancies that exist between BA and CA should then also exist between SA and CA. Nawrocki (2010) explains that SA comes from morphological skeletal indicators that express “the skeleton’s continuing adaptation to biomechanical stress and participation in mineral metabolism, growth, remodeling, and disease” [
31]. Because the accretion of biomechanical and physiological changes is not linear or regular, errors exist when using skeletal indicators to create an SA to estimate CA [
31].
Behavior, social structures, health, stress, and the environment impact individuals throughout their lives, and the skeleton’s plastic nature means it reflects these influences. Methods developed for bioarchaeology and forensic anthropology are grounded in the understanding that degenerative changes occur over time. As people age, degenerative processes commence, and skeletal changes follow a general trajectory. However, this trajectory is not uniform, especially in older ages [
32,
33]. Age estimation methods could be improved if data reflecting these environmental and cultural variables could be incorporated. For example, population-specific correction factors based on research correlating environmental variables with skeletal degeneration can be created, or modifier variables that reflect socioeconomic status can be introduced into age estimation methods.
1.2. Age Estimation in Forensic Anthropology
The earliest methods of age estimation in forensic anthropology recognized the patterned trajectory of dental and skeletal development and degeneration. Juvenile age estimation methods generally rely on dental development, skeletal maturation and size, and epiphyseal union [
34,
35,
36,
37]. These processes occur at specific ages during adolescence and early adulthood, providing a patterned timeline for age estimation [
37,
38].
In adults, age estimation methods rely on morphological changes related to patterns of degeneration. Useful age indicators show unidirectional change with advancing age [
1]; however, the rate and degree of change vary and are dependent on cultural and environmental influences throughout life, as well as individual variation [
39,
40]. This makes adult age estimation less straightforward than the more canalized process associated with juvenile development. Although histomorphometric methods exist [
41,
42,
43], methods that draw on gross examination of skeletal elements are more common. These methods are as accurate as histomorphometric methods and are cost-effective and non-destructive.
The pubic symphysis has long been recognized as a region of interest to examine patterned degeneration [
44,
45], and continued research has worked towards refining this method, although general problems still exist, such as broad age range outputs and the results reflect the composition of the reference population [
46]. Other commonly used adult age estimation methods include examination of the auricular surface [
47,
48,
49], cranial sutures [
50,
51], and the sternal end of the fourth rib [
52,
53]. Age estimation methods continue to evolve, incorporating advancements in technology and statistical modeling. Transition Analysis integrates age-related data from various skeletal components using advanced statistical methods to generate accurate age estimates and intervals. This approach addresses the limitations of earlier methods, which relied on broad, phase-based classifications of anatomical regions [
44,
45]. Unlike these earlier methods, Transition Analysis accounts for the fact that aging and degenerative changes, even within a single skeletal location, do not progress at uniform rates [
50,
54]. Applying modern statistical methods, including Bayesian inference and machine learning algorithms, to age estimation allows for the integration of various data sources and provides probabilistic estimates, which are more robust and reliable [
50,
55,
56,
57].
1.3. Problems with Adult Age Estimation Methods
Broad age ranges produce imprecise age estimations that may hinder investigations. Additionally, a lack of standardized protocols for combining multiple age estimation methods further complicates the process. While methods like transition analysis [
50] attempt to integrate various skeletal features, there is no consensus on how to systematically synthesize data from different indicators [
3].
The lack of diversity in reference samples introduces weakness in age estimation methods. Traditional methods were primarily developed using skeletal collections from White/European American and Black/African American populations [
45,
48,
52,
53]. These reference samples do not adequately represent the genetic and environmental diversity found in modern forensic cases, leading to inaccuracies when these methods are applied to different populations [
58,
59]. Further, lifestyle, nutrition, and health status can significantly impact skeletal aging, causing deviations from the patterns observed in reference populations [
60].
Tests of age estimation methods on diverse population groups and investigations of new methods confirm that rates of aging vary among groups, so population-specific standards are necessary [
61,
62,
63,
64,
65]. However, practitioners continue to use the older methods because they have become standard and have been reported to be accurate [
3,
66]. This accuracy is misleading because precision is low. If an age range produced by any method is 40 years, there is a high probability that the individual’s chronological age will fall within the estimated range by nature of the human life span. Variations in the biological process of aging are fundamental sources of error for estimates of skeletal age [
60,
67]. Rather than continuing to test the accuracy of existing methods on various populations worldwide and finding they produce accurate results that are of limited value, research should shift to exploring whether incorporating population-specific underlying factors, such as social and physical environments, might improve precision.
Validation studies also demonstrate that factors such as population, geographic region, socioeconomic status, and body size influence the accuracy of age estimation methods. Studies show that methods developed for one population may not be directly applicable to another without significant adjustments, thereby affecting the reliability of age estimates in diverse forensic cases [
68,
69,
70,
71,
72].
1.4. Theoretical Perspectives for Considering the Social and Physical Environments
Social experiences inscribe themselves on the body, influencing aging and health outcomes. The body serves as a canvas for social experiences, with physical health and aging directly reflecting these experiences [
73,
74,
75]. Marginalized individuals may experience accelerated aging due to chronic stressors like discrimination and limited resources, and continuous exposure to adversity can lead to early health deterioration and faster biological aging, impacting biological age markers [
76,
77,
78,
79]. The systematic ways in which social structures harm or disadvantage individuals perpetuate health inequalities and create discrepancies in aging [
80,
81,
82,
83]. With an understanding of how the environment and social structures influence biological aging, anthropologists must consider how these same factors influence skeletal aging.
Secular change and regional variation can also impact biological profile methods. Secular change refers to the pattern of change in human growth and development over time due to factors such as improvements in nutrition, healthcare, and overall living conditions [
84,
85]. These changes can significantly impact the accuracy of forensic methods if they are not considered. Boldsen et al. (2002) highlighted how influences of lifestyle and environment create variation in aging [
50]. Discrepancies occur from applying methods developed on historical or archaeological samples to modern individuals, and therefore contemporary populations provide the best comparative data to draw from because it reduces biases from secular change [
86]. These same kinds of discrepancies may also exist across contemporary populations, making it increasingly important to update and refine forensic methods to reflect these changes.
Here, to explore the relationship between health and aging, we present a pilot study examining patterns among cause of death, estimated skeletal age, and chronological age to consider that factors other than age may be contributing to variations in skeletal age indicators.
2. Materials and Methods
2.1. Sample
The study utilized skeletal remains from the Maxwell Documented Skeleton Collection at the University of New Mexico. This collection comprises individuals acquired through pre-death donations, post-death donations by family, or through the Office of the Medical Investigator. The collection includes individual data on sex, age, population affinity, and cause of death for most donors, with recent donations also providing health and occupational information. The subsample for this research was selected from self- and family donations (n = 55).
The sample consisted of 14 females and 41 males, with ages ranging from 19 to 60 years and an average age of 44.2 years (
Figure 2). The sample was limited to those under 60 to reflect a forensic sample and limit the likelihood that everyone in the sample has age-related pathologies. While the sex distribution reflects that of the Maxwell collection, the age distribution for the sample used in this study is younger than the collection’s average to facilitate the examination of age estimation discrepancies within a more specific age group. All but three individuals self-identified or were next-of-kin identified as White; thus, variations in age estimation based on race were not relevant.
Cause of death information obtained from the Office of the Medical Investigator was available for all individuals. Two broad categories were defined: disease (
n = 26) and trauma (
n = 29) (
Table 1). The disease category includes cancer, cardiac arrest, or hypertension. It also included deaths related to alcoholism and drug use. These are included in the disease category because addiction is a disease, and long-term alcohol and drug use can lead to health issues [
87]. The trauma category represents deaths caused by gunshot wounds, blunt force trauma, stab wounds, and motor vehicle accidents. Although the manner of death for these cases may vary, the primary focus here is on the cause of death, and these types of fatalities all result from traumatic events. There were five individuals with a cause of death categorized as “Other”. These were incorporated into the trauma category because they included death from acute causes such as aspiration and carbon monoxide poisoning.
For this study, cause of death is used as a proxy for health status. Those who died due to disease are assumed to have been unhealthy and succumbed to poor health. Diseases take a physiological toll on the body and affect one or many bodily systems. The chronic effects of a disease process, as well as potential treatments, may influence the aging process and subsequently impact skeletal aging. In this study, we assumed that those who died from trauma were in better health as their death was unrelated to pre-existing morbidities. Based on the documented cause of death, in no instance was the trauma potentially caused by an underlying disease or co-morbidity.
2.2. Morphological Indicators of Age
Adult ages were estimated using two methods: Lovejoy et al. (1985) for the auricular surface (AS) and Brooks and Suchey (1990) for the pubic symphysis (PS) [
45,
48]. The Lovejoy method for the AS involves scoring morphological features and selecting the age range that best fits the overall presentation of traits. The age ranges were assigned scores of 1 through 8. Mean ages and 95% confidence intervals for each phase came from the Osborne et al., 2004 adjustments to the Lovejoy method [
49]. Similarly, the Suchey–Brooks method involves assessing various morphological features of the PS and assigning a phase from 1 to 6 that best corresponds to the observed traits. Each phase is associated with a 95% confidence interval and a mean age. For both methods, the mean age was used in the analysis. When possible, both the right and left PS and AS were scored.
2.3. Statistical Analysis
To understand how skeletal age relates to the true chronological age, the difference between the estimated mean age from the corresponding scores and chronological age was used (SA-CA). A negative value indicates the individual was older than the mean age and vice versa. Simply using accuracy rates for the estimated age ranges is not sufficient because the age ranges produced by the Lovejoy method are too narrow, and those from the Suchey–Brooks method are too broad.
All statistical analyses were performed in the computing software R, version 4.3.2 [
88]. We used a Kruskal–Wallis test to determine if there were any significant differences between the right and left scores for the PS and the AS. After testing for normality, we performed an ANOVA test to test the relationship between the SA-CA values and the cause of death groups. To show the relationship between CA and SA, Z-scores were calculated to standardize the SA ranges.
3. Results
3.1. Auricular Surface and Cause of Death
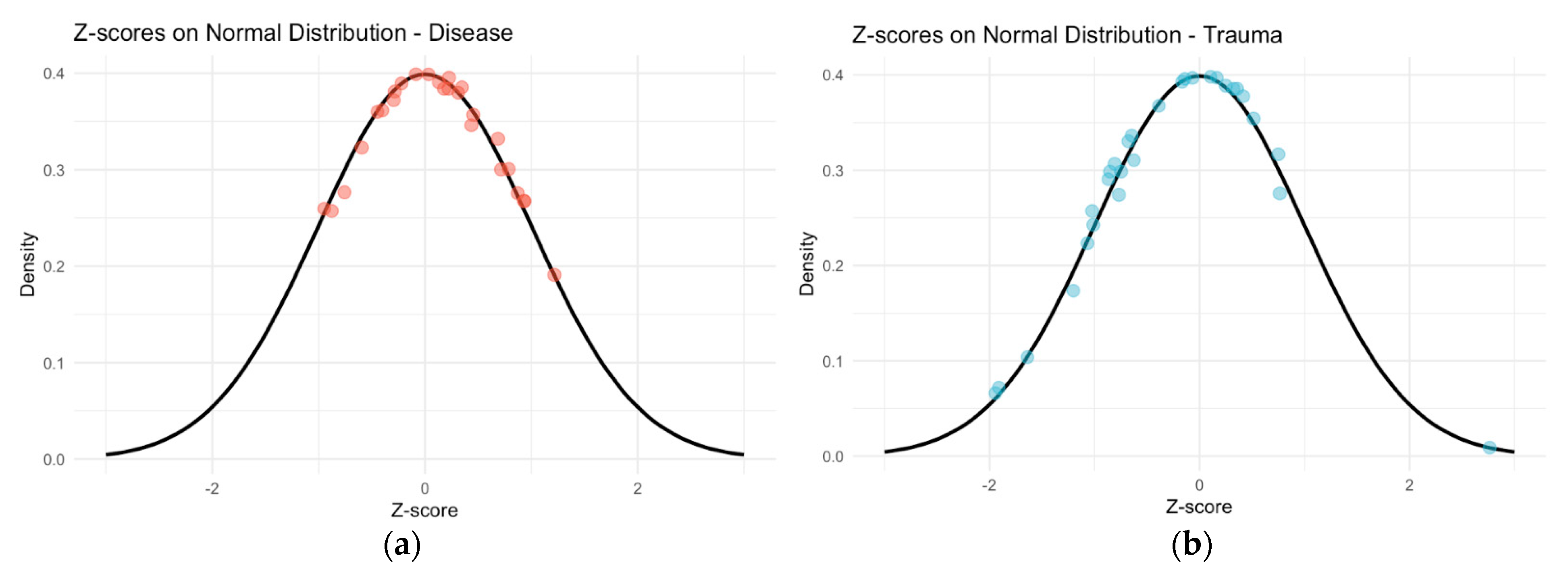
There was no significant difference between the right and left scores of the AS (p = 0.484), so the left scores were used in the analysis, and the right score was used when the left could not be scored, but the right side was available. The Shapiro–Wilks test of normality indicated that all data were normally distributed. Accuracy using AS was high, with 91% of individuals falling within the respective 95% prediction interval.
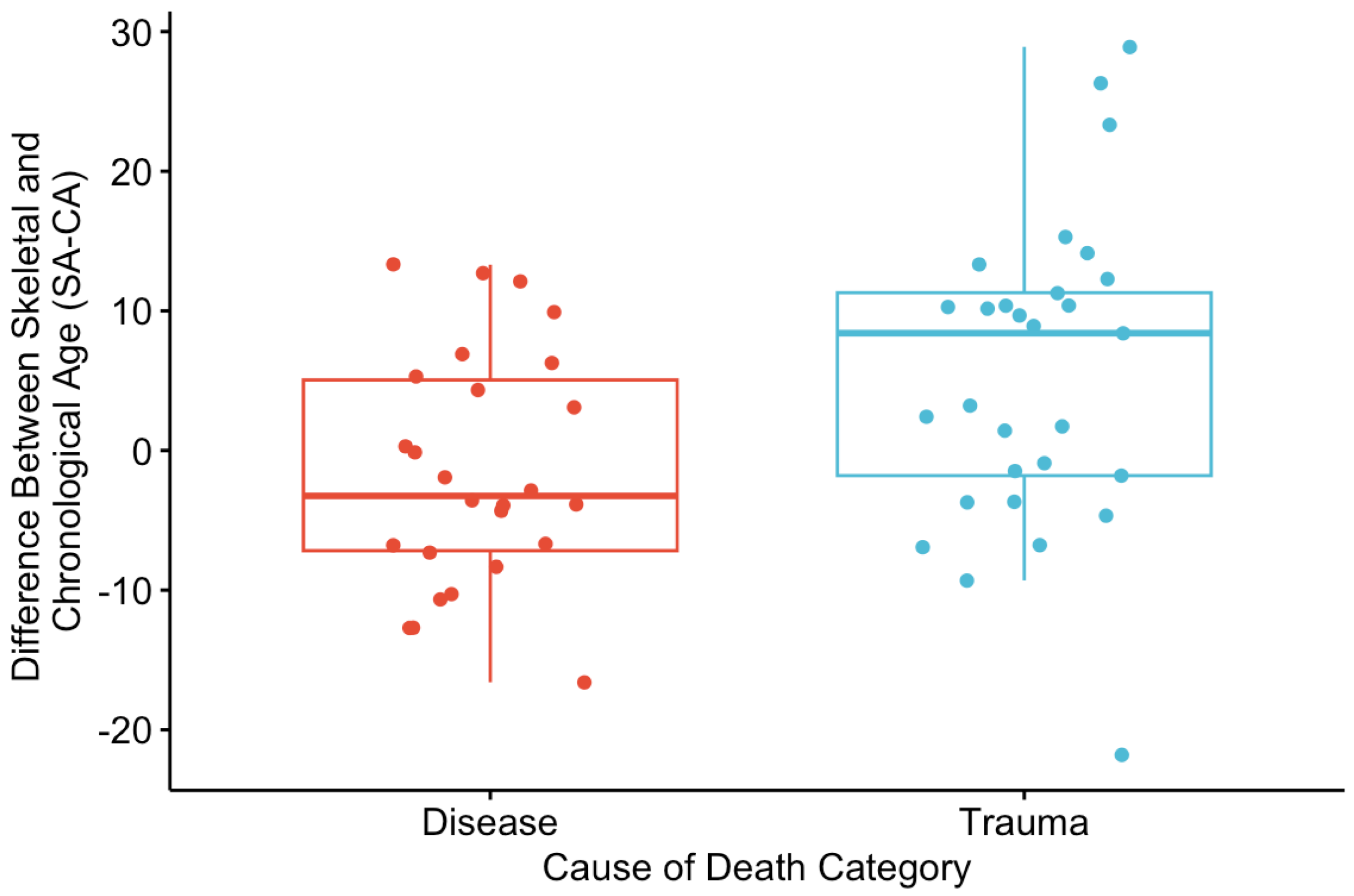
There was a significant difference in SA-CA between the disease and trauma categories of death (
p < 0.05) (
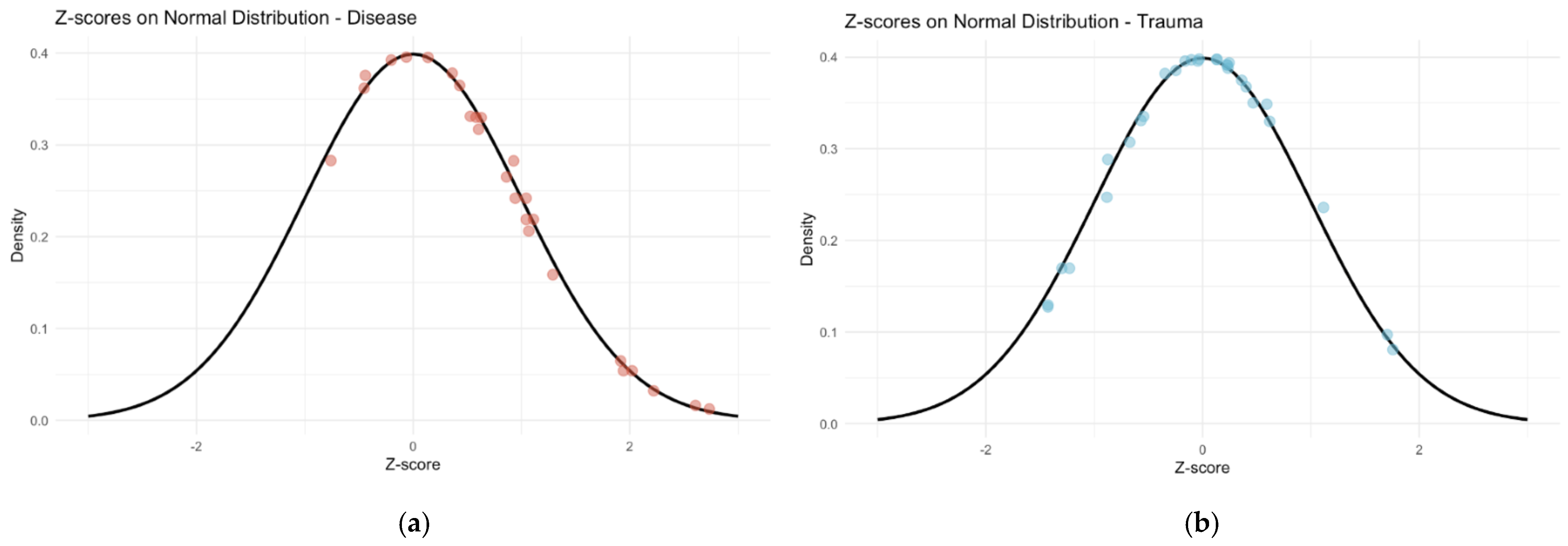
Figure 3). For the disease category, the mean SA-CA was −1.48, while for the trauma category, the mean was 5.54. The standardized AS results indicate that chronological ages are greater than the mean for disease-related deaths and less than the mean for deaths trauma-related deaths (
Figure 4).
3.2. Pubic Symphysis and Cause of Death
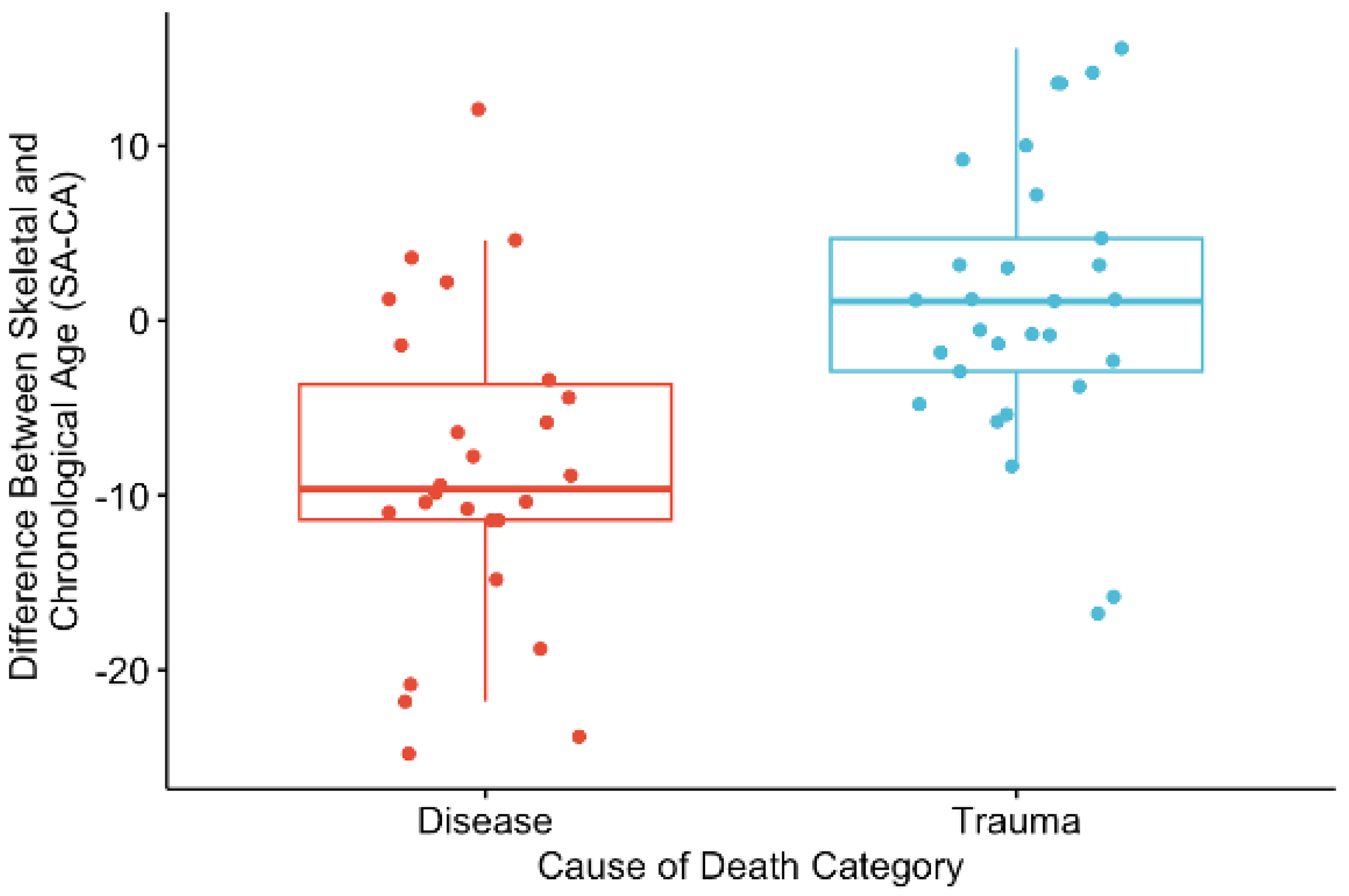
There was no significant difference between the right and left scores of the PS (p = 0.973 for PS), so left scores were used in the analysis, and the right score was used when the left could not be scored, but the right side was available. The Shapiro–Wilks test of normality indicated that data were normally distributed. Accuracy using PS was high, with 95% of individuals falling within the respective 95% prediction interval.
The results for the PS are also significant and in the same direction as what was seen for the AS. There was a significant difference in SA-CA between the disease and trauma categories of death (
p < 0.001) (
Figure 5). The mean SA-CA for those who died of disease was −8.61, while for the trauma-related deaths, the mean SA-CA was 1.07. This pattern indicates that when the cause of death was disease, the chronological age fell within the upper end of the corresponding skeletal age range (
Figure 6a). For deaths related to trauma, the chronological age was, on average, within the lower end of the corresponding skeletal age range, with the chronological age being less than the mean (
Figure 6b).
4. Discussion
4.1. Overview of Findings
This study examined the relationship between cause of death categories and adult age estimations. All individuals in the sample were described as White, so population affinity was not a source of variation in SA. Cause of death, categorized as disease- or trauma-related, was used as a proxy for health status. The results here show potential trends that can be further examined to test whether incorporating other environmental and lifestyle factors into age estimation methods proves to be fruitful. This work serves as an example of how we could consider other social and environmental factors, particularly when estimating age from skeletal remains. The significant patterned discrepancies in SA and CA correlated with the cause of death highlight how cause of death affects where people fall on the age distribution. The CA of individuals who died from disease-related causes was significantly skewed towards the upper end of the produced age range and was greater than the point estimate. The opposite pattern was found for those who died of trauma-related causes. The CA in this group was, on average, below the point estimate. These findings indicate a measurable relationship between the cause of death and the ranges produced by skeletal age estimation methods.
The results from the auricular surface and pubic symphysis support and complement each other in a way that further evidences the skewed distribution of CA within SA ranges by cause of death category. For the auricular surface, the results more strongly show that the CA for trauma-related deaths falls in the lower end of the age range, as indicated by the average SA-CA equaling 6.33. Here, disease-related deaths are less skewed but are still, on average, in the upper end of the age range. Conversely, for the pubic symphysis, the stronger result was for the disease category for cause of death. For this method, there is stronger evidence that the CA for disease-related death falls in the upper end of the age range, as indicated by the average SA-CA equaling −8.61. For trauma-related deaths, the average SA-CA falls in the lower end of the age range, but the data points are more centralized.
Based on theoretical frameworks on stress and aging, there was an expectation that those who were of poor health and died of disease would have shown signs of accelerated aging compared to those who died of trauma. However, this was not found. Rather, the results indicate that those who died of disease tend to fall above the point estimate, while those who died of trauma fall below the point estimate. One possible explanation for this is that the sample used here consisted only of White individuals. The concept of accelerated aging is discussed in the context of marginalization. Because this is a homogenous sample, variations in rates of aging by population group are not being observed. While these results suggest the potential for creating more precise age ranges, they also underscore the importance of extending analyses to include additional population groups.
4.2. Implications for Forensic Practice
The current study’s results have uncovered relationships between CA, estimated SA ranges, and the cause of death, which serves as a proxy for health. It may be that other biological profile methods are similarly impacted. Previous studies have found that certain forensic anthropology methods do not work for certain groups [
61,
62,
63,
64,
65]. The shifts in our approaches to these methods represent a recognition of how forensic anthropology is biocultural and how cultural and environmental contexts must now be better incorporated into biological profile analyses.
For age estimation, incorporating population differences should be considered in the same way they have been for other biological profile methods [
89,
90,
91,
92,
93,
94,
95,
96,
97]. Current methods have incorporated the fact that there are differences in aging between males and females, but limited analyses have examined the environmental factors that can affect skeletal aging. Various authors have noted that regional variation, lifestyle, diet, and other environmental factors influence the rate and nature of aging [
47,
65,
98,
99]. The SWGANTH standards for age estimation note that for analyzing mature remains, “factors of the environment and life history of the individual can introduce non-age-related variation in the expression of degenerative traits and thus represent a potential source of error” [
100]. However, no analyses have been performed to identify if patterns of expression exist, and no adjustments have been made to incorporate these factors into age estimation methods. The complexity and variability of the aging process after growth and development have caused adult skeletal age estimation to be difficult to refine and improve [
98].
There has been a growing call to incorporate social and environmental forces into forensic anthropology methods, as highlighted by this special issue and similar initiatives [
87]. Much of the focus has been on demonstrating that methods do not work with the same accuracy and reliability outside the US. However, our project highlights discrepancies in age estimations related to a proxy for health within a US population.
Going into the future, forensic age estimation methods must account for both population and environmental factors, recognizing that these influences are intertwined and impact skeletal aging. Systematic errors in age estimation can have significant consequences in forensic anthropology and the broader criminal justice system. Imprecise or inaccurate age estimates can hinder investigation and identification efforts both in the US and abroad [
40,
101,
102,
103,
104]. These imprecisions make it difficult to exclude large portions of the population when dealing with unknown decedents [
2,
102,
104]. Marginalized individuals are disproportionately affected by incorrect age estimations due to a lack of consideration for embodied stress [
66,
105]. While cognitive and systemic biases are documented in other areas of the criminal justice system [
106,
107,
108], biological profile estimations generally appear to be impartial [
109,
110]. Despite this, methodological improvements are needed to further narrow down the pool of missing persons and aid in identification.
4.3. Limitations and Future Directions
While our study provides valuable insights, it is not without limitations. The methods used for age estimation, the Brooks and Suchey (1990) method for the pubic symphysis and the Lovejoy et al. (1985) method for the auricular surface, have inherent limitations [
45,
48]. Both methods rely on morphological changes that can be influenced by various factors beyond age, such as physical activity, nutrition, and overall health. Additionally, the sample size, while sufficient for detecting significant trends, is relatively small, and larger studies are needed to generalize these findings to broader populations. The homogeneity of the sample is both a limitation and a strength. The lack of diversity does not accurately represent today’s population and is not a true forensic population. However, by not including race as an additional variable, an added level of complexity was removed. This allowed for testing the relationship between cause of death and skeletal age estimations alone to be tested.
Note that the cause of death is likely an imperfect proxy for health. Diseases affect the body at various rates and may or may not alter the skeleton. For trauma deaths, it is possible that these individuals did have underlying health conditions not accounted for here. Despite these limitations, the results presented here show a consistent pattern of age under- and over-estimation correlated with the cause of death category. In forensic analyses of unidentified skeletal remains, practitioners can observe visible signs of trauma or disease, which may occur simultaneously. From this, inferences can be drawn about the cause of death and the individual’s health status. Further examinations of the effects of the environment and lifestyle on skeletal aging could lead to adjustments in age estimations based on the condition of the skeleton and the presence of trauma and/or disease.
Ongoing research and the development of more refined, context-specific methods are essential. Future research should aim to refine age estimation methods by incorporating a wider range of social and environmental variables. Developing models that include health status, occupation, lifestyle, and other relevant factors could improve the accuracy of forensic age estimations. Moreover, expanding studies to include more diverse populations and larger sample sizes will help validate the findings and ensure they are broadly applicable. Additionally, efforts should focus on creating standardized protocols that integrate multiple indicators and account for individual and population variations. Improving the accuracy and reliability of age estimation methods in forensic anthropology will require a multifaceted approach that includes updating reference populations, refining existing methods, and developing new techniques tailored to diverse populations.
One potential area for future research is the impact of chronic illnesses on skeletal aging. For instance, conditions such as osteoporosis, arthritis, and other degenerative diseases can significantly alter skeletal morphology and complicate age estimations [
62,
111,
112]. Understanding how these conditions affect skeletal features could lead to more accurate age estimation methods that account for the health status of individuals.
Another area for future exploration is the influence of occupational stress on skeletal aging. Studies have shown that individuals engaged in physically demanding occupations may exhibit different patterns of skeletal aging compared to those with less physically demanding jobs [
63,
113,
114]. Incorporating occupational data into age estimation models could enhance the accuracy and reliability of forensic analyses.
5. Conclusions
This study demonstrates the potential of incorporating social and environmental factors into forensic age estimation methods, particularly based on the influence of health status as proxied by the cause of death. The findings reveal a significant relationship between cause of death and discrepancies in skeletal and chronological age estimations, highlighting that individuals who died from disease-related causes tend to be in the upper end of the estimated skeletal age ranges, while those who died from trauma-related causes tend to be in the lower end of estimated skeletal age ranges. This underscores the possibility of refining current forensic anthropology methods to better account for biocultural influences, such as health disparities, that affect aging. By addressing these discrepancies, forensic practice can become more precise and equitable, particularly for marginalized populations who may be disproportionately affected by current limitations in age estimation methods. The study emphasizes that both population-specific and environmental factors must be integrated into forensic analyses to enhance the precision and reliability of age estimations in forensic anthropology.
Author Contributions
Conceptualization, N.S.A. and H.J.H.E.; methodology, N.S.A. and H.J.H.E.; software, N.S.A.; validation, N.S.A. and H.J.H.E.; formal analysis, N.S.A.; investigation, N.S.A.; resources, N.S.A.; data curation, N.S.A.; writing—original draft preparation, N.S.A.; writing—review and editing, N.S.A. and H.J.H.E.; visualization, N.S.A.; supervision, H.J.H.E.; project administration, N.S.A. and H.J.H.E.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors would like to thank the Maxwell Museum for allowing access to the Documented Skeletal Collection to collect data for this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Milner, G.R.; Boldsen, J.L. Skeletal Age Estimation: Where We Are and Where We Should Go. In A Companion to Forensic Anthropology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 224–238. [Google Scholar] [CrossRef]
- Ubelaker, D.H.; Khosrowshahi, H. Estimation of Age in Forensic Anthropology: Historical Perspective and Recent Methodological Advances. Forensic Sci. Res. 2019, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garvin, H.M.; Passalacqua, N.V. Current Practices by Forensic Anthropologists in Adult Skeletal Age Estimation. J. Forensic Sci. 2011, 57, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Vazquez, J.M.; Sudmant, P.H. The Evolution of Aging and Lifespan. Trends Genet. 2023, 39, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Jazwinski, S.M.; Kim, S. Examination of the Dimensions of Biological Age. Front. Genet. 2019, 10, 263. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, W.; Chen, X. Common Methods of Biological Age Estimation. Clin. Interv. Aging 2017, 12, 759–772. [Google Scholar] [CrossRef]
- Ryan, C.P. “Epigenetic Clocks”: Theory and Applications in Human Biology. Am. J. Hum. Biol. 2021, 33, e23488. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; Danese, A.; Harrington, H.; Israel, S.; Levine, M.E.; Schaefer, J.D.; et al. Quantification of Biological Aging in Young Adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110. [Google Scholar] [CrossRef]
- Gruenewald, T.L.; Seeman, T.E.; Ryff, C.D.; Karlamangla, A.S.; Singer, B.H. Combinations of Biomarkers Predictive of Later Life Mortality. Proc. Natl. Acad. Sci. USA 2006, 103, 14158–14163. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA Methylation-Based Biomarkers and the Epigenetic Clock Theory of Ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Holliday, R. Epigenetics: A Historical Overview. Epigenetics 2006, 1, 76–80. [Google Scholar] [CrossRef]
- Waddington, C.H. Towards a Theoretical Biology. Nature 1968, 218, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic Regulation of Aging: Implications for Interventions of Aging and Diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Wall, M.M.; Chen, C.; Levine, M.E.; Yaffe, K.; Roose, S.P.; Rutherford, B.R. Biological Age, Not Chronological Age, Is Associated with Late-Life Depression. J. Gerontol. Ser. A 2018, 73, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Morgan, D.J.; Johnstone, M.; Edibam, C. Biological Age Is Superior to Chronological Age in Predicting Hospital Mortality of the Critically Ill. Intern. Emerg. Med. 2023, 18, 2019–2028. [Google Scholar] [CrossRef]
- Levine, M.E. Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately Than Chronological Age? J. Gerontol. Ser. A 2013, 68, 667–674. [Google Scholar] [CrossRef]
- Soriano-Tárraga, C.; Mola-Caminal, M.; Giralt-Steinhauer, E.; Ois, A.; Rodríguez-Campello, A.; Cuadrado-Godia, E.; Gómez-González, A.; Vivanco-Hidalgo, R.M.; Fernández-Cadenas, I.; Cullell, N.; et al. Biological Age Is Better than Chronological as Predictor of 3-Month Outcome in Ischemic Stroke. Neurology 2017, 89, 830–836. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A Systematic Review of Biological, Social and Environmental Factors Associated with Epigenetic Clock Acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.K.; Beach, S.R.H.; Philibert, R.A.; Cutrona, C.E.; Gibbons, F.X.; Barr, A. Economic Hardship and Biological Weathering: The Epigenetics of Aging in a U.S. Sample of Black Women. Soc. Sci. Med. 2016, 150, 192–200. [Google Scholar] [CrossRef]
- Bey, G.; Pike, J.; Palta, P.; Zannas, A.; Xiao, Q.; Love, S.-A.; Heiss, G. Biological Age Mediates the Effects of Perceived Neighborhood Problems on Heart Failure Risk Among Black Persons. J. Racial Ethn. Health Disparities 2022, 10, 3018–3030. [Google Scholar] [CrossRef]
- Graf, G.H.; Crowe, C.L.; Kothari, M.; Kwon, D.; Manly, J.J.; Turney, I.C.; Valeri, L.; Belsky, D.W. Testing Black-White Disparities in Biological Aging Among Older Adults in the United States: Analysis of DNA-Methylation and Blood-Chemistry Methods. Am. J. Epidemiol. 2022, 191, 613–625. [Google Scholar] [CrossRef]
- Robertson, T.; Batty, G.D.; Der, G.; Fenton, C.; Shiels, P.G.; Benzeval, M. Is Socioeconomic Status Associated with Biological Aging as Measured by Telomere Length? Epidemiol. Rev. 2013, 35, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic Clock: A Promising Biomarker and Practical Tool in Aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.; Fiorito, G.; Hernandez, B.; Polidoro, S.; O’Halloran, A.M.; Hever, A.; Ni Cheallaigh, C.; Lu, A.T.; Horvath, S.; Vineis, P.; et al. GrimAge Outperforms Other Epigenetic Clocks in the Prediction of Age-Related Clinical Phenotypes and All-Cause Mortality. J. Gerontol. Ser. A 2021, 76, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Geronimus, A.T.; Hicken, M.T.; Pearson, J.A.; Seashols, S.J.; Brown, K.L.; Cruz, T.D. Do US Black Women Experience Stress-Related Accelerated Biological Aging? Hum. Nat. 2010, 21, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Pacheco, N.L.; Smith, J.T.; Evans, M.K. The Accelerated Aging Phenotype: The Role of Race and Social Determinants of Health on Aging. Ageing Res. Rev. 2022, 73, 101536. [Google Scholar] [CrossRef]
- Sprott, R.L. Biomarkers of Aging and Disease: Introduction and Definitions. Exp. Gerontol. 2010, 45, 2–4. [Google Scholar] [CrossRef]
- O’Connor, S.D.; Graffy, P.M.; Zea, R.; Pickhardt, P.J. Does Nonenhanced CT-Based Quantification of Abdominal Aortic Calcification Outperform the Framingham Risk Score in Predicting Cardiovascular Events in Asymptomatic Adults? Radiology 2018, 290, 108–115. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Graffy, P.M.; Zea, R.; Lee, S.J.; Liu, J.; Sandfort, V.; Summers, R.M. Automated CT Biomarkers for Opportunistic Prediction of Future Cardiovascular Events and Mortality in an Asymptomatic Screening Population: A Retrospective Cohort Study. Lancet Digit. Health 2020, 2, e192–e200. [Google Scholar] [CrossRef]
- Lee, M.H.; Zea, R.; Garrett, J.W.; Summers, R.M.; Pickhardt, P.J. AI-Generated CT Body Composition Biomarkers Associated with Increased Mortality Risk in Socioeconomically Disadvantaged Individuals. Abdom. Radiol. 2024, 49, 1330–1340. [Google Scholar] [CrossRef]
- Nawrocki, S.P. The nature and sources of error in the estimation of age at death from a skeleton. In Age Estimation of the Human Skeleton, 1st ed.; Latham, K.E., Finnegan, J.M., Eds.; Charles C Thomas Publisher: Springfield, IL, USA, 2010; pp. 79–101. [Google Scholar]
- Agarwal, S.C. Bone Morphologies and Histories: Life Course Approaches in Bioarchaeology. Am. J. Phys. Anthropol. 2016, 159 (Suppl. S61), 130–149. [Google Scholar] [CrossRef]
- Gibson, R.C. Minority Aging Research: Opportunity and Challenge. J. Gerontol. 1989, 44, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief Communication: The London Atlas of Human Tooth Development and Eruption. Am. J. Phys. Anthr. 2010, 142, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.L.; Thompson, G.W.; Popovich, F. Age of Attainment of Mineralization Stages of the Permanent Dentition. J. Forensic Sci. 1976, 21, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Moorrees, C.F.; Fanning, E.A.; Hunt, E.E. Age variation of formation stages for ten permanent teeth. J. Dent. Res. 1963, 42, 1490–1502. [Google Scholar] [CrossRef]
- Scheuer, L.; Black, S. Developmental Juvenile Osteology; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar] [CrossRef]
- Schaefer, M.; Black, S.; Scheuer, L. Juvenile Osteology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Kemkes-Grottenthaler, A. Aging through the ages: Historical perspectives on age indicator methods. In Paleodemography: Age Distributions from Skeletal Samples, 1st ed.; Hoppa, R.D., Vaupel, J.W., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 48–72. [Google Scholar]
- Schmitt, A. Age-at-Death Assessment Using the Os Pubis and the Auricular Surface of the Ilium: A Test on an Identified Asian Sample. Int. J. Osteoarchaeol. 2004, 14, 1–6. [Google Scholar] [CrossRef]
- Dudar, J.; Pfeiffer, S.; Saunders, S. Evaluation of Morphological and Histological Adult Skeletal Age-at-Death Estimation Techniques Using Ribs. J. Forensic Sci. 1993, 38, 677–685. [Google Scholar] [CrossRef]
- Kerley, E.R. The Microscopic Determination of Age in Human Bone. Am. J. Phys. Anthropol. 1965, 23, 149–163. [Google Scholar] [CrossRef]
- Stout, S.D.; Paine, R.R. Brief Communication: Histological Age Estimation Using Rib and Clavicle. Am. J. Phys. Anthr. 1992, 87, 111–115. [Google Scholar] [CrossRef]
- Todd, T.W. Age Changes in the Pubic Bone. I. The Male White Pubis. Am. J. Phys. Anthropol. 1920, 3, 285–334. [Google Scholar] [CrossRef]
- Brooks, S.; Suchey, J.M. Skeletal Age Determination Based on the Os Pubis: A Comparison of the Acsádi-Nemeskéri and Suchey-Brooks Methods. Hum. Evol. 1990, 5, 227–238. [Google Scholar] [CrossRef]
- Hartnett, K.M. Analysis of Age-at-Death Estimation Using Data from a New, Modern Autopsy Sample-Part I: Pubic Bone. J. Forensic Sci. 2010, 55, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Buckberry, J.L.; Chamberlain, A.T. Age estimation from the auricular surface of the ilium: A revised method. Am. J. Phys. Anthropol. 2002, 119, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, C.O.; Meindl, R.S.; Pryzbeck, T.R.; Mensforth, R.P. Chronological Metamorphosis of the Auricular Surface of the Ilium: A New Method for the Determination of Adult Skeletal Age at Death. Am. J. Phys. Anthropol. 1985, 68, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.L.; Simmons, T.L.; Nawrocki, S.P. Reconsidering the Auricular Surface as an Indicator of Age at Death. J. Forensic Sci. 2004, 49, JFS2003348-7. [Google Scholar] [CrossRef]
- Boldsen, J.L.; Milner, G.R.; Konigsberg, L.W.; Wood, J.W. Transition Analysis: A New Method for Estimating Age from Skeletons. In Paleodemography; Hoppa, R.D., Vaupel, J.W., Hoppa, R.D., Vaupel, J.W., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 73–106. [Google Scholar] [CrossRef]
- Meindl, R.S.; Lovejoy, C.O. Ectocranial Suture Closure: A Revised Method for the Determination of Skeletal Age at Death Based on the Lateral-Anterior Sutures. Am. J. Phys. Anthropol. 1985, 68, 57–66. [Google Scholar] [CrossRef]
- Işcan, M.Y.; Loth, S.R.; Wright, R.K. Metamorphosis at the Sternal Rib End: A New Method to Estimate Age at Death in White Males. Am. J. Phys. Anthr. 1984, 65, 147–156. [Google Scholar] [CrossRef]
- Işcan, M.Y.; Loth, S.R.; Wright, R.K. Age Estimation from the Rib by Phase Analysis: White Females. J. Forensic Sci. 1985, 30, 853–863. [Google Scholar] [CrossRef]
- Milner, G.R.; Boldsen, J.L. Transition Analysis: A Validation Study with Known-age Modern American Skeletons. Am. J. Phys. Anthr. 2012, 148, 98–110. [Google Scholar] [CrossRef]
- DiGangi, E.A.; Bethard, J.D.; Kimmerle, E.H.; Konigsberg, L.W. A New Method for Estimating Age-at-Death from the First Rib. Am. J. Phys. Anthr. 2009, 138, 164–176. [Google Scholar] [CrossRef]
- Getz, S.M. The Use of Transition Analysis in Skeletal Age Estimation. WIREs Forensic Sci. 2020, 2, e1378. [Google Scholar] [CrossRef]
- Langley-Shirley, N.; Jantz, R.L. A Bayesian Approach to Age Estimation in Modern Americans from the Clavicle. J. Forensic Sci. 2010, 55, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Iscan, M.Y.; Loth, S.R. Osteological manifestations of age in the adult. In Reconstruction of Life from the Skeleton, 1st ed.; Iscan, M.Y., Kennedy, K.A.R., Eds.; Alan R. Liss, Incorporated: New York, NY, USA, 1989; pp. 23–40. [Google Scholar]
- Katz, D.; Suchey, J.M. Race Differences in Pubic Symphyseal Aging Patterns in the Male. Am. J. Phys. Anthropol. 1989, 80, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hoppa, R.D. Population Variation in Osteological Aging Criteria: An Example from the Pubic Symphysis. Am. J. Phys. Anthropol. 2000, 111, 185–191. [Google Scholar] [CrossRef]
- Corsini, M.-M.; Schmitt, A.; Bruzek, J. Aging Process Variability on the Human Skeleton: Artificial Network as an Appropriate Tool for Age at Death Assessment. Forensic Sci. Int. 2005, 148, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D. Forensic Age Estimation in Human Skeletal Remains: Current Concepts and Future Directions. Leg. Med. 2010, 12, 1–7. [Google Scholar] [CrossRef]
- Mays, S. The Effect of Factors Other than Age upon Skeletal Age Indicators in the Adult. Ann. Hum. Biol. 2015, 42, 332–341. [Google Scholar] [CrossRef]
- Rissech, C.; Wilson, J.; Winburn, A.P.; Turbón, D.; Steadman, D. A Comparison of Three Established Age Estimation Methods on an Adult Spanish Sample. Int. J. Leg. Med. 2012, 126, 145–155. [Google Scholar] [CrossRef]
- Schmitt, A.; Murail, P.; Cunha, E.; Rougé, D. Variability of the Pattern of Aging on the Human Skeleton: Evidence from Bone Indicators and Implications on Age at Death Estimation. J. Forensic Sci. 2002, 47, 1203–1209. [Google Scholar] [CrossRef]
- Hughes, C.E.; Juarez, C.; Yim, A.-D. Forensic Anthropology Casework Performance: Assessing Accuracy and Trends for Biological Profile Estimates on a Comprehensive Sample of Identified Decedent Cases. J. Forensic Sci. 2021, 66, 1602–1616. [Google Scholar] [CrossRef]
- Bocquet-Appel, J.-P.; Masset, C. Farewell to Paleodemography. J. Hum. Evol. 1982, 11, 321–333. [Google Scholar] [CrossRef]
- Calce, S.E.; Rogers, T.L. Evaluation of Age Estimation Technique: Testing Traits of the Acetabulum to Estimate Age at Death in Adult Males*. J. Forensic Sci. 2011, 56, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Merritt, C.E. Inaccuracy and Bias in Adult Skeletal Age Estimation: Assessing the Reliability of Eight Methods on Individuals of Varying Body Sizes. Forensic Sci. Int. 2017, 275, 315.e1–315.e11. [Google Scholar] [CrossRef] [PubMed]
- Michopoulou, E.; Negre, P.; Nikita, E.; Kranioti, E.F. The Auricular Surface as Age Indicator in a Modern Greek Sample: A Test of Two Qualitative Methods. Forensic Sci. Int. 2017, 280, e1–e246. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Maestro, N.; Benito, M.; Sánchez, J.A.; Márquez-Grant, N.; Trejo, D.; Ríos, L. Sex and Age at Death Estimation from the Sternal End of the Fourth Rib. Does Íşcan’s Method Really Work? Leg. Med. 2018, 31, 24–29. [Google Scholar] [CrossRef]
- Spake, L.; Cardoso, H.F.V. Are We Using the Appropriate Reference Samples to Develop Juvenile Age Estimation Methods Based on Bone Size? An Exploration of Growth Differences between Average Children and Those Who Become Victims of Homicide. Forensic Sci. Int. 2018, 282, 1–12. [Google Scholar] [CrossRef]
- Csordas, T.J. Embodiment as a Paradigm for Anthropology. Ethos 1990, 18, 5–47. [Google Scholar] [CrossRef]
- Gravlee, C.C. How Race Becomes Biology: Embodiment of Social Inequality. Am. J. Phys. Anthropol. 2009, 139, 47–57. [Google Scholar] [CrossRef]
- Keppel, K.G.; Pearcy, J.N.; Wagener, D.K. Trends in Racial and Ethnic-Specific Rates for the Health Status Indicators: United States, 1990–98. Healthy People 2000 Stat. Notes 2002, 23, 1–16. [Google Scholar]
- Geronimus, A.T. The Weathering Hypothesis and the Health of African-American Women and Infants: Evidence and Speculations. Ethn. Dis. 1992, 2, 207–221. [Google Scholar]
- Geronimus, A.T. Weathering: The Extraordinary Stress of Ordinary Life in an Unjust Society, 1st ed.; Little Brown Spark: New York, NY, USA, 2023. [Google Scholar]
- Das, A. How Does Race Get “Under the Skin”?: Inflammation, Weathering, and Metabolic Problems in Late Life. Soc. Sci. Med. 2013, 77, 75–83. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.-K.; Klopack, E.; Beach, S.R.H.; Gibbons, F.X.; Philibert, R.A. The Effects of Social Adversity, Discrimination, and Health Risk Behaviors on the Accelerated Aging of African Americans: Further Support for the Weathering Hypothesis. Soc. Sci. Med. 2021, 282, 113169. [Google Scholar] [CrossRef] [PubMed]
- Galtung, J. Violence, Peace, and Peace Research. J. Peace Res. 1969, 6, 167–191. [Google Scholar] [CrossRef]
- Klaus, H.D. The Bioarchaeology of Structural Violence: A Theoretical Model and a Case Study. In The Bioarchaeology of Violence; Martin, D.L., Harrod, R.P., Eds.; University Press of Florida: Gainesville, FL, USA, 2012. [Google Scholar] [CrossRef]
- Leatherman, T.; Goodman, A.H. Critical Biocultural Approaches in Medical Anthropology. In A Companion to Medical Anthropology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 29–48. [Google Scholar] [CrossRef]
- Martin, D.L.; Harrod, R.P. Bioarchaeological Contributions to the Study of Violence. Am. J. Phys. Anthropol. 2015, 156 (Suppl. S59), 116–145. [Google Scholar] [CrossRef] [PubMed]
- Albanese, J. A Critical Review of the Methodology for the Study of Secular Change Using Skeletal Data. Ont. Archaeol. 2008, 85–88, 139–155. [Google Scholar]
- Hoppa, R.D.; Vaupel, J.W. Paleodemography: Age Distributions from Skeletal Samples; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Dirkmaat, D.C.; Cabo, L.L.; Ousley, S.D.; Symes, S.A. New Perspectives in Forensic Anthropology. Am. J. Phys. Anthropol. 2008, 137 (Suppl. S47), 33–52. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health. 2014. Available online: https://www.who.int/publications/i/item/global-status-report-on-alcohol-and-health-2014 (accessed on 25 July 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. Available online: https://www.R-project.org/ (accessed on 14 June 2024).
- Scientific Working Group for Forensic Anthropology (SWGANTH). Sex Assessment. 2010. Available online: https://www.nist.gov/system/files/documents/2018/03/13/swganth_sex_assessment.pdf (accessed on 25 July 2024).
- Adams, D.M.; Blatt, S.H.; Flaherty, T.M.; Haug, J.D.; Isa, M.I.; Michael, A.R.; Smith, A.C. Shifting the Forensic Anthropological Paradigm to Incorporate the Transgender and Gender Diverse Community. Humans 2023, 3, 142–165. [Google Scholar] [CrossRef]
- Tallman, S.; Kincer, C.; Plemons, E. Centering Transgender Individuals in Forensic Anthropology and Expanding Binary Sex Estimation in Casework and Research. Forensic Anthropol. 2022, 5, 161–180. [Google Scholar] [CrossRef]
- Spradley, M.K.; Jantz, R.L. Sex Estimation in Forensic Anthropology: Skull versus Postcranial Elements. J. Forensic Sci. 2011, 56, 289–296. [Google Scholar] [CrossRef]
- Ubelaker, D.H.; DeGaglia, C.M. Chapter 17—Factors of Population Variation in Sex Estimation Methodology. In Sex Estimation of the Human Skeleton; Klales, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 281–293. [Google Scholar] [CrossRef]
- Chandler, P.J.; Bock, R.D. Age Changes in Adult Stature: Trend Estimation from Mixed Longitudinal Data. Ann. Hum. Biol. 1991, 18, 433–440. [Google Scholar] [CrossRef]
- Cline, M.G.; Meredith, K.E.; Boyer, J.T.; Burrows, B. Decline of Height with Age in Adults in a General Population Sample: Estimating Maximum Height and Distinguishing Birth Cohort Effects from Actual Loss of Stature with Aging. Hum. Biol. 1989, 61, 415–425. [Google Scholar]
- Niskanen, M.; Maijanen, H.; McCarthy, D.; Junno, J.-A. Application of the Anatomical Method to Estimate the Maximum Adult Stature and the Age-at-Death Stature. Am. J. Phys. Anthropol. 2013, 152, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Kamnikar, K.; Appel, N.; Rangel, E.; Adolphi, N.; Ousley, S.; Edgar, H.; Abeyta-Brown, A. Stature Estimation Equations for Modern American Indians in the American Southwest. Forensic Sci. Int. 2024, 361, 112151. [Google Scholar] [CrossRef] [PubMed]
- Algee-Hewitt, B.F.B. Age Estimation in Modern Forensic Anthropology. In Forensic Anthropology: An Introduction; Langley, N.R., Tersigni-Tarrant, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Stinson, S.; Bogin, B.; O’Rourke, D.H. Human Biology: An Evolutionary and Biocultural Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Scientific Working Group for Forensic Anthropology (SWGANTH). Age Estimation. 2013. Available online: https://www.nist.gov/system/files/documents/2018/03/13/swganth_age_estimation.pdf (accessed on 25 July 2024).
- Martrille, L.; Ubelaker, D.H.; Cattaneo, C.; Seguret, F.; Tremblay, M.; Baccino, E. Comparison of Four Skeletal Methods for the Estimation of Age at Death on White and Black Adults*. J. Forensic Sci. 2007, 52, 302–307. [Google Scholar] [CrossRef]
- Reineke, R.; Halstead, C. Identifying dead migrants: Examples from the United States–Mexico border. Fatal. Journeys 2017, 3, 77–98. [Google Scholar]
- Rissech, C.; Estabrook, G.F.; Cunha, E.; Malgosa, A. Estimation of Age-at-Death for Adult Males Using the Acetabulum, Applied to Four Western European Populations*. J. Forensic Sci. 2007, 52, 774–778. [Google Scholar] [CrossRef]
- Christensen, A.M.; Passalacqua, N.V.; Bartelink, E.J. Chapter 10—Age Estimation. In Forensic Anthropology, 2nd ed.; Christensen, A.M., Passalacqua, N.V., Bartelink, E.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 307–349. [Google Scholar] [CrossRef]
- Byrnes, J.F.; Sandoval-Cervantes, I. The Marginalized in Death: A Forensic Anthropology of Intersectional Identity in the Modern Era; Rowman & Littlefield: Lanham, MD, USA, 2022. [Google Scholar]
- Appel, N.S.; Edgar, H.J.H.; Berry, S.D.; Hunley, K. Error and Bias in Race and Ethnicity Descriptions in Medical Examiner Records in New Mexico: Consequences for Understanding Mortality among Hispanic/Latinos. Forensic Sci. Int. Synerg. 2023, 7, 100338. [Google Scholar] [CrossRef]
- Nakhaeizadeh, S.; Dror, I.E.; Morgan, R.M. Cognitive Bias in Forensic Anthropology: Visual Assessment of Skeletal Remains Is Susceptible to Confirmation Bias. Sci. Justice 2014, 54, 208–214. [Google Scholar] [CrossRef]
- Dror, I.E. Biases in Forensic Experts. Science 2018, 360, 243. [Google Scholar] [CrossRef]
- Appel, N.S.; Lynch, P.; Hughes, C.E. Identification trends in forensic anthropology cases at the New Mexico Office of the Medical Investigator. In Proceedings of the American Academy of Forensic Science, Denver, CO, USA, 19–24 February 2024. [Google Scholar]
- Hughes, C.; Yim, A.-D.; Juarez, C.; Servello, J.; Thomas, R.; Passalacqua, N.; Soler, A. Investigating Identification Disparities in Forensic Anthropology Casework. PLoS ONE 2023, 18, e0290302. [Google Scholar] [CrossRef]
- Boldsen, J.L.; Milner, G.R.; Ousley, S.D. Paleodemography: From Archaeology and Skeletal Age Estimation to Life in the Past. Am. J. Biol. Anthropol. 2022, 178 (Suppl. S74), 115–150. [Google Scholar] [CrossRef]
- Brickley, M.; Ives, R. Age-Related Bone Loss and Osteoporosis. In The Bioarchaeology of Metabolic Bone Disease; Academic Press: San Diego, CA, USA, 2008; pp. 151–183. [Google Scholar] [CrossRef]
- Agarwal, S.C. Understanding Bone Aging, Loss, and Osteoporosis in the Past. In Biological Anthropology of the Human Skeleton; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 385–414. [Google Scholar] [CrossRef]
- Merritt, C.E. The Influence of Body Size on Adult Skeletal Age Estimation Methods. Am. J. Phys. Anthropol. 2015, 156, 35–57. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).