Ovarian Weight and Uterine Volume Index Are Useful for Age Estimation in Adult Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
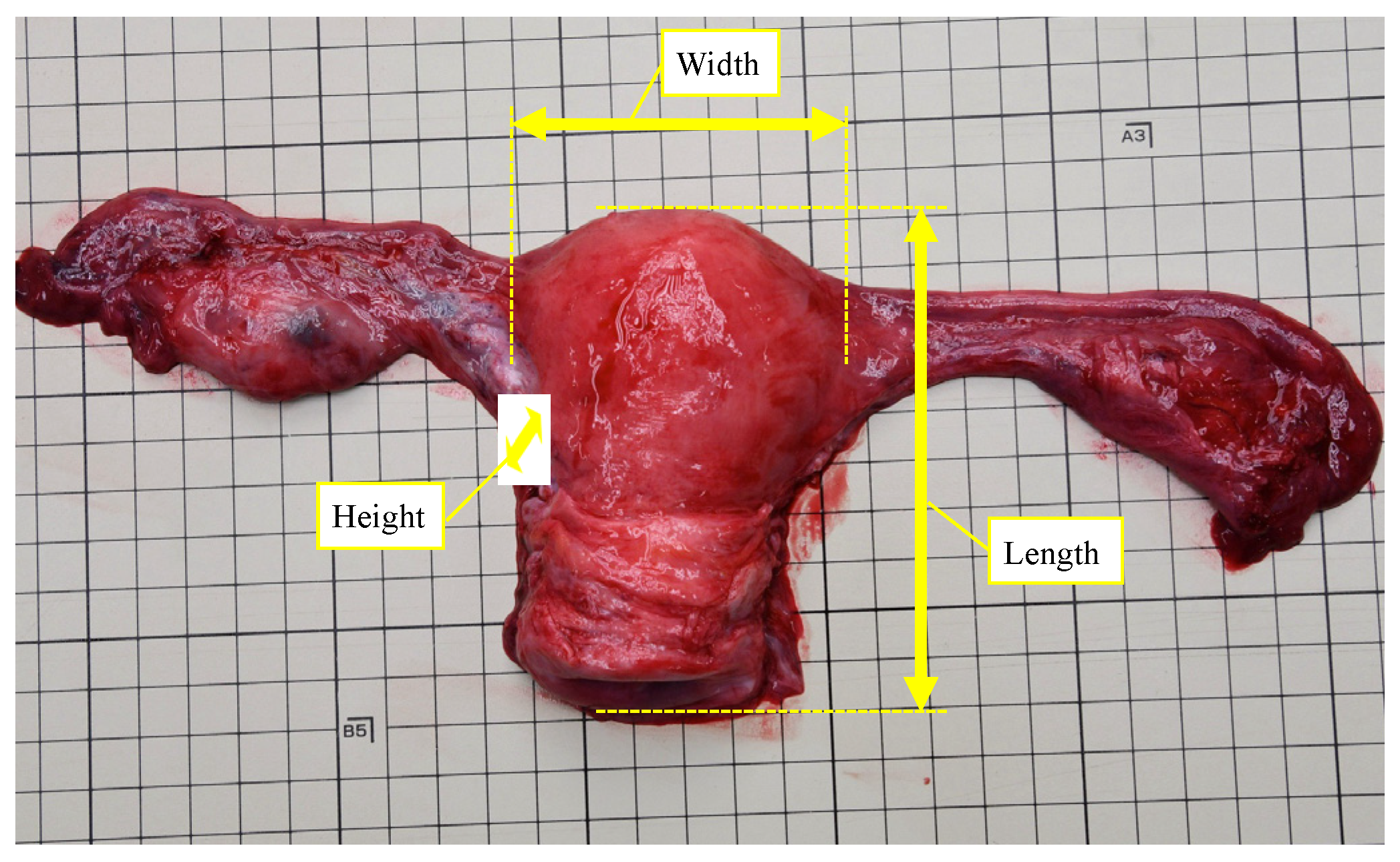
2.2. Measurement of Uterus and Ovaries
2.3. Statistical Analysis
3. Results
3.1. General Aspects
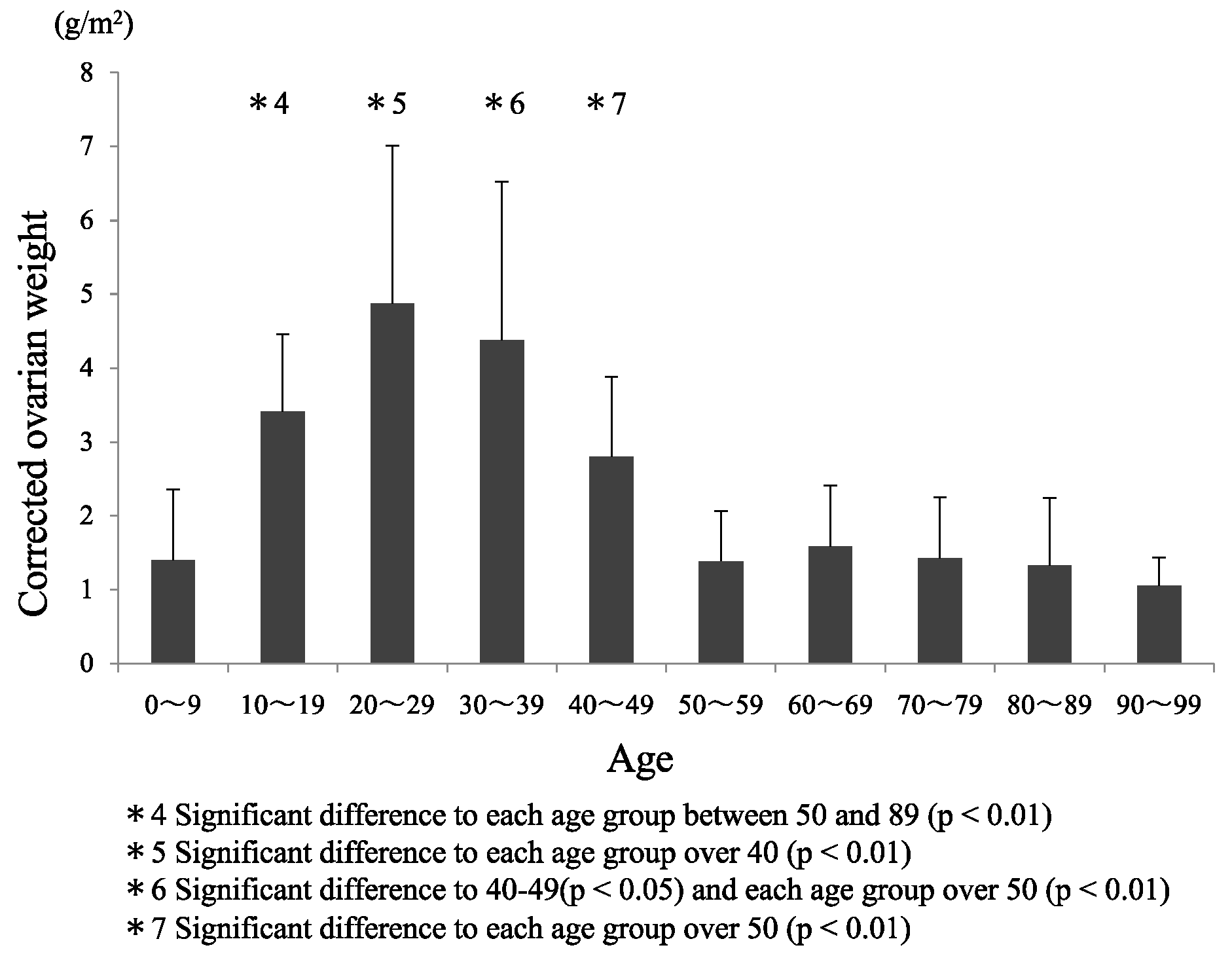
3.2. Comparisons between Age Groups
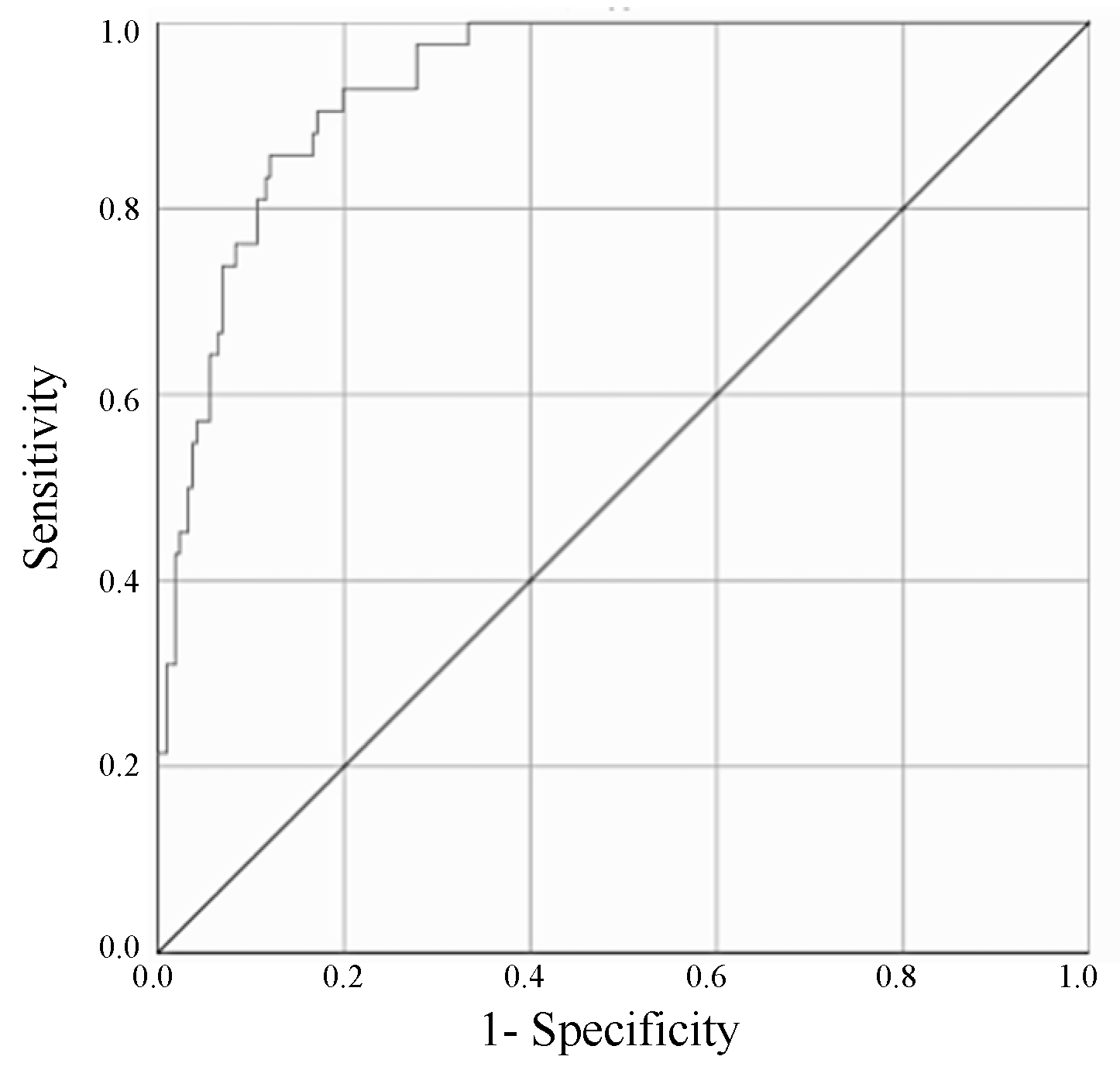
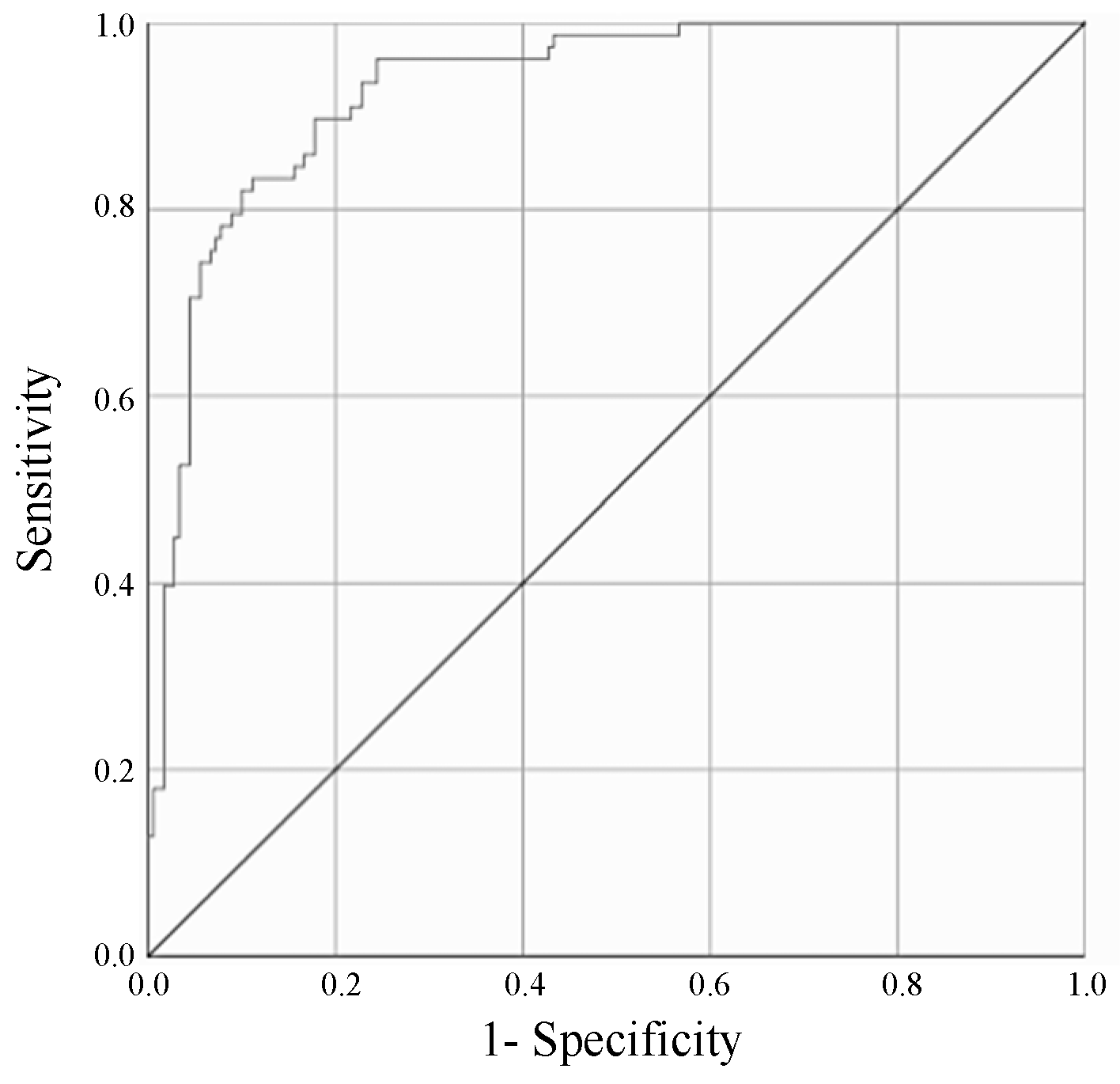
3.3. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francis, E.C.; Ann, E.R.; Bernard, G.B.L.; Frederic, C.T. Gradwohl’s Legal Medicine, 3rd ed.; The Stonebridge Press, John Wright & Sons Limited: Bristol, UK, 1976; ISBN 0-7236-0310-3. [Google Scholar]
- Bernard, K. Forensic Pathology; Edward Arnold, a division of Hodder and Stoughton Limited: London, UK, 1991; ISBN 0-7131-4550-1. [Google Scholar]
- Dorandeu, A.; Coulibaly, B.; Piercecchi-Marti, M.D.; Bartoli, C.; Gaudart, J.; Baccino, E.; Leonetti, G. Age-at-death estimation based on the study of frontosphenoidal sutures. Forensic Sci. Int. 2008, 177, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Meindl, R.S.; Lovejoy, C.O. Ectocranial suture closure: A revised method for the determination of skeletal age at death based on the lateral-anterior sutures. Am. J. Phys. Anthropol. 1985, 68, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.; Vas, Z.; Sótonyi, P.; Magyar, L.G. Skeletal age estimation in Hungarian population of known age and sex. Forensic Sci. Int. 2012, 223, 374.e1–374.e8. [Google Scholar] [CrossRef] [PubMed]
- Radoinova, D.; Tenekedjiev, K.; Yordanov, Y. Stature estimation from long bone lengths in Bulgarians. Homo 2002, 52, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S. Estimation of age from the teeth of unidentified corpses using the amino acid racemization method with reference to actual cases. Am. J. Forensic Med. Pathol. 1995, 16, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, B.; Kamalandua, A.; Zapico, S.C.; Van de Voorde, W.; Decorte, R. Improved age determination of blood and teeth samples using a selected set of DNA methylation markers. Epigenetics 2015, 10, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Ambroa-Conde, A.; Giron-Santamaria, L.; Mosquera-Miguel, A.; Phillips, C.; Casares de Cal, M.A.; Gomez-Tato, A.; Alvarez-Dios, J.; de la Puente, M.; Ruiz-Ramirez, J.; Lareu, M.V.; et al. Epigenetic age estimation in saliva and in buccal cells. Forensic Sci. Int. Genet. 2022, 61, 102770. [Google Scholar] [CrossRef] [PubMed]
- Marcante, B.; Delicati, A.; Onofri, M.; Tozzo, P.; Caenazzo, L. Estimation of Human Chronological Age from Buccal Swab Samples through a DNA Methylation Analysis Approach of a Five-Locus Multiple Regression Model. Int. J. Mol. Sci. 2024, 25, 935. [Google Scholar] [CrossRef]
- Shiga, M.; Asari, M.; Takahashi, Y.; Isozaki, S.; Hoshina, C.; Mori, K.; Namba, R.; Okuda, K.; Shimizu, K. DNA methylation-based age estimation and quantification of the degradation levels of bisulfite-converted DNA. Leg. Med. 2024, 67, 102336. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Leo, C.D.; Ramsthaler, F.; Birngruber, C.G.; Verhoff, M.A. Assessment of renal glomerulosclerosis and thickness of the carotid intima-media complex as a means of age estimation in Western European bodies. Int. J. Legal Med. 2022, 136, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci. Rep. 2019, 9, 3304. [Google Scholar] [CrossRef] [PubMed]
- Horny, L.; Adamek, T.; Chlup, H.; Zitny, R. Age estimation based on a combined arteriosclerotic index. Int. J. Legal Med. 2012, 126, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Horny, L.; Adamek, T.; Vesely, J.; Chlup, H.; Zitny, R.; Konvickova, S. Age-related distribution of longitudinal pre-strain in abdominal aorta with emphasis on forensic application. Forensic Sci. Int. 2012, 214, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, H.; Miyamori, D.; Ishikawa, N.; Ichioka, H.; Ikegaya, H. Relationship between serum prostate-specific antigen and age in cadavers. SAGE Open Med. 2020, 8, 2050312120958212. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hitosugi, M.; Takaso, M.; Nakamura, M.; Takeda, A. Affecting Factors of Prostate Volume in Forensic Autopsied Decedents. Heaithcare 2023, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Sokalska, A.; Valentin, L. Changes in ultrasound morphology of the uterus and ovaries during the menopausal transition and early postmenopause: A 4-year longitudinal study. Ultrasound Obstet. Gynecol. 2008, 31, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar] [PubMed]
- Flaws, J.A.; Rhodes, J.C.; Langenberg, P.; Hirshfield, A.N.; Kjerulff, K.; Sharara, F.I. Ovarian volume and menopausal status. Menopause 2000, 7, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, K.; Fuchs, S.C.; Spritzer, P.M. Ovarian volume in pre- and perimenopausal women: A population-based study. Menopause 2003, 10, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J.; DePriest, P.D.; Gallion, H.H.; Ueland, F.R.; Reedy, M.B.; Kryscio, R.J.; van Nagell, J.R., Jr. Ovarian volume related to age. Gynecol. Oncol. 2000, 77, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Merz, E.; Miric-Tesanic, D.; Bahlmann, F.; Weber, G.; Wellek, S. Sonographic size of uterus and ovaries in pre- and postmenopausal women. Ultrasound Obstet. Gynecol. 1996, 7, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Suhonen, S.; Sipinen, S.; Lähteenmäki, P.; Laine, H.; Rainio, J.; Arko, H. Postmenopausal oestrogen replacement therapy with subcutaneous oestradiol implants. Maturitas 1993, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rannevik, G.; Jeppsson, S.; Johnell, O.; Bjerre, B.; Laurell-Borulf, Y.; Svanberg, L. A longitudinal study of the perimenopausal transition: Altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 1995, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Faddy, M.J.; Scheffer, G.; te Velde, E.R. Antral follicle counts are related to age at natural fertility loss and age at menopause. Menopause 2004, 11, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Toner, J.P.; Seifer, D.B. Why we may abandon basal follicle-stimulating hormone testing: A sea change in determining ovarian reserve using antimüllerian hormone. Fertil. Steril. 2013, 99, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Segawa, T.; Omi, K.; Watanabe, Y.; Sone, Y.; Handa, M.; Kuroda, M.; Miyauchi, O.; Osada, H.; Teramoto, S. Age-specific values of Access anti-Mullerian hormone immunoassay carried out on Japanese patients with infertility: A retrospective large-scale study. BMC Womens Health 2019, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Seifer, D.B.; Baker, V.L.; Leader, B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil. Steril. 2011, 95, 747–750. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Sighinolfi, G.; Giulini, S.; Traglia, M.; Argento, C.; Sala, C.; Masciullo, C.; Volpe, A.; Toniolo, D. Normal serum concentrations of anti-Müllerian hormone in women with regular menstrual cycles. Reprod. Biomed. Online 2010, 21, 463–469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelson, S.M.; Messow, M.C.; Wallace, A.M.; Fleming, R.; McConnachie, A. Nomogram for the decline in serum antimüllerian hormone: A population study of 9,601 infertility patients. Fertil. Steril. 2011, 95, 736–741. [Google Scholar] [CrossRef]
- Dandolu, V.; Singh, R.; Lidicker, J.; Harmanli, O. BMI and uterine size: Is there any relationship? Int. J. Gynecol. Pathol. 2010, 29, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Bumbuliene, Z.; Klimasenko, J.; Sragyte, D.; Zakareviciene, J.; Drasutiene, G. Uterine size and ovarian size in adolescents with functional hypothalamic amenorrhoea. Arch. Dis. Child. 2015, 100, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, D.E.; Li, Y.; Tang, J.; Hu, S.; Wu, X.; Tian, Z.; Tan, H. Uterine dimensions in gravida 0 phase according to age, body mass index, and height in Chinese infertile women. Medicine 2018, 97, e12068. [Google Scholar] [CrossRef] [PubMed]
- Elsedfy, H.H.; Hamza, R.T.; Farghaly, M.H.; Ghazy, M.S. Uterine development in patients with Turner syndrome: Relation to hormone replacement therapy and karyotype. J. Pediatr. Endocrinol. Metab. 2012, 25, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A. Uterine growth in the endometrium active phase. Wien. Klin. Wochenschr. 1989, 101, 352–359. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murai, T.; Tomioka, T.; Takaso, M.; Takeda, A.; Nakamura, M.; Koshinuma, S.; Tateoka, Y.; Hitosugi, M. Ovarian Weight and Uterine Volume Index Are Useful for Age Estimation in Adult Women. Forensic Sci. 2024, 4, 211-220. https://doi.org/10.3390/forensicsci4020014
Murai T, Tomioka T, Takaso M, Takeda A, Nakamura M, Koshinuma S, Tateoka Y, Hitosugi M. Ovarian Weight and Uterine Volume Index Are Useful for Age Estimation in Adult Women. Forensic Sciences. 2024; 4(2):211-220. https://doi.org/10.3390/forensicsci4020014
Chicago/Turabian StyleMurai, Takato, Takahiro Tomioka, Marin Takaso, Arisa Takeda, Mami Nakamura, Shinya Koshinuma, Yumiko Tateoka, and Masahito Hitosugi. 2024. "Ovarian Weight and Uterine Volume Index Are Useful for Age Estimation in Adult Women" Forensic Sciences 4, no. 2: 211-220. https://doi.org/10.3390/forensicsci4020014
APA StyleMurai, T., Tomioka, T., Takaso, M., Takeda, A., Nakamura, M., Koshinuma, S., Tateoka, Y., & Hitosugi, M. (2024). Ovarian Weight and Uterine Volume Index Are Useful for Age Estimation in Adult Women. Forensic Sciences, 4(2), 211-220. https://doi.org/10.3390/forensicsci4020014





