Use of the Investigator ESSplex SE QS Kit (QIAGEN) at Half PCR Reaction Volumes for the Analysis of Forensic Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Male Control DNA 9948 Analysis
2.2. Sample Analysis
2.2.1. Control Samples
2.2.2. Forensic Samples
Forensic Sample Pre-Processing
2.2.3. Informed Consent and Ethical Aspects
2.3. DNA Extraction
2.4. DNA Quantification
2.5. DNA Amplification and Typing
2.6. Data Analysis
3. Results and Discussion
3.1. Male Control DNA 9948 Analysis
3.2. Control Samples
3.3. Forensic Casework Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, P.; Kimpton, C.P.; Urquhart, A.; Oldroyd, N.; Millican, E.S.; Watson, S.K.; Downes, T.J. Automated short tandem repeats (STR) analysis in forensic casework—A strategy for the future. Electrophoresis 1995, 16, 1543–1552. [Google Scholar] [CrossRef]
- Kimpton, C.; Fisher, D.; Watson, S.; Adams, M.; Urquhart, A.; Lygo, J.; Gill, P. Evaluation of an automated DNA profiling system employing multiplex amplification of four tetrameric STR loci. Int. J. Leg. Med. 1994, 106, 302–311. [Google Scholar] [CrossRef]
- Butler, J.M.; Hill, C.R. Biology and genetics of new autosomal STR loci useful for forensic DNA analysis. Forensic Sci. Rev. 2012, 24, 15. [Google Scholar] [PubMed]
- Hares, D.R. Expanding the CODIS Core Loci in the United States. Forensic Sci. Int. Genet. 2012, 6, e52–e54. [Google Scholar] [CrossRef] [PubMed]
- Schumm, J.W.; Gutierrez-Mateo, C.; Tan, E.; Selden, R. A 27-locus STR assay to meet all United States and European law enforcement agency standards. J. Forensic Sci. 2013, 58, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, A. STR Typing and Available Multiplex Kits Including Validation Methods. In Forensic DNA Typing: Principles, Applications and Advancements; Shrivastava, P., Dash, H.R., Lorente, J.A., Imam, J., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Gaines, M.L.; Wojtkiewicz, P.W.; Valentine, J.A.; Brown, C.L. Reduced Volume PCR Amplification Reactions Using the AmpFlSTR Profiler Plus Kit. J. Forensic Sci. 2002, 47, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Bessekri, M.W.; Aggoune, A.; Lazreg, S.; Bucht, R.; Fuller, V. Comparative study on the effects of reduced PCR reaction volumes and increased cycle number, on the sensitivity and the stochastic threshold of the AmpFlSTR Identifiler® Plus kit. Forensic Sci. Int. Genet. Suppl. Ser. 2013, 4, e306–e307. [Google Scholar] [CrossRef]
- Almheiri, R.; Alghafri, R. Reduced volume for direct PCR amplification of blood reference samples using Identifiler® Direct and Globalfiler™ Express assays. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e340–e341. [Google Scholar] [CrossRef]
- Mahmood, H.K.; Salman, N.F.; Hasan, D.H.; Salih, K.M.; Sadiq, M.A.; Mohammad, B.T.; Mohammed, M.K.; Nahi, S.M.; Baqir, S.S. Validation of Half-Reaction Volumes of the Promega PowerPlex® Forensic Amplification Kits (PowerPlex® 18D Systems, PowerPlex ® 21System, PowerPlex® Fusion System and PowerPlex® Y23 System) in STR Analysis. Arab. J. Forensic Sci. Forensic Med. 2020, 2, 152–158. [Google Scholar] [CrossRef]
- Perry, J.; Munshi, T.; Haizel, T.; Iyavoo, S. Validation of reduced volume VeriFiler™ Express PCR Amplification Kit for buccal swab samples extracted using Prep-n-Go™ Buffer. J. Forensic Sci. 2022, 67, 1971–1978. [Google Scholar] [CrossRef]
- Barbaro, A.; Cormaci, P. Validation of PowerPlex® Y23 System (Promega) using reduced reaction volume. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e373–e374. [Google Scholar] [CrossRef]
- Barbaro, A.; Falcone, G. Validation of SureID® 21G Human STR Identification Kit Using Alternative Reaction Protocols. Biom. Biostat. J. 2021, 3, 114. [Google Scholar]
- Coble, M.D.; Butler, J.M. Characterization of new miniSTR loci to aid analysis of degraded DNA. J. Forensic Sci. 2009, 50, 43–53. [Google Scholar] [CrossRef]
- Mogensen, H.S.; Frank-Hansen, R.; Morling, N. Test of Investigator ESSPLEX SE QS with quality sensors. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e490–e491. [Google Scholar] [CrossRef][Green Version]
- Barbaro, A.; Samar, S.; Falcone, G.; La Marca, A. Highly efficient and automated extraction of DNA from human remains using a modified EZ1 protocol. Forensic Sci. Res. 2021, 6, 59–66. [Google Scholar] [CrossRef] [PubMed]
- EZ1 DNA Investigator Handbook—QIAGEN—Rev. 7. 2014. Available online: https://www.qiagen.com/us/resources/download.aspx?id=46064856-1b88-4b27-a825d3f616e06c08&lang=en (accessed on 28 December 2023).
- Investigator Quantiplex Pro RGQ Kit Handbook—QIAGEN—Rev. 1. 2018. Available online: https://www.qiagen.com/us/resources/download.aspx?id=57497d59-7a43-4eaf-8c94086e88742e86&lang=en (accessed on 28 December 2023).
- Vraneš, M.; Scherer, M.; Elliott, K. Development and validation of the Investigator® Quantiplex Pro Kit for qPCR-based examination of the quantity and quality of human DNA in forensic samples. FSI Genet. Suppl. Ser. 2017, 6, e518–e519. [Google Scholar] [CrossRef]
- Investigator® ESSplex SE QS Handbook, June 2021—QIAGEN—Rev. 2. 2021. Available online: https://www.qiagen.com/us/resources/resourcedetail?id=1fce64fd-9cf1-4750-a4d0-e7aa64113d7b&lang=en (accessed on 30 December 2023).
- Butler, J.M. Advanced Topics in Forensic DNA Typing: Methodology; Elsevier Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Lőrincz, É.; Norbert, M.; Katalin, A.R.; Tamás, C.; Nóra, M.M.; Attila, H. Comparison of Reduced PCR Volume PowerPlex Fusion 6C Kit Validations on Manual and Automated Systems. DNA 2024, 1, 52–63. [Google Scholar] [CrossRef]
- Developmental Validation of the Investigator® ESSplex SE QS Kit, QIAGEN—October 2015. Available online: https://www.qiagen.com/th/resources/download.aspx?id=9761730e-def1-4011-bd8a-852b4849dd6d&lang=en (accessed on 30 December 2023).
- Coyle, H. Quality Control and Duplication for Concordance in Forensic DNA Samples: Implications for Interpretation in Mixtures. IRJCS Int. Res. J. Comput. Sci. 2015, 2, 16–18. [Google Scholar]
- Verdon, T.J.; Mitchell, R.J.; van Oorschot, R.A. The influence of substrate on DNA transfer and extraction efficiency. Forensic Sci. Int. Genet. 2013, 7, 167–175. [Google Scholar] [CrossRef]
- Lee, S.B.; Shewale, J.G. DNA Extraction Methods in Forensic Analysis, Encyclopedia of Analytical Chemistry; Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Martins, C.; Lima, G.; Carvalho, M.R.; Cainé, L.; Porto, M.J. DNA quantification by real-time PCR in different forensic samples. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e545–e546. [Google Scholar] [CrossRef]
- Mulero, J.J.; Hennessy, L.K. Next-Generation STR Genotyping Kits for Forensic Applications. Forensic Sci. Rev. 2012, 24, 163. [Google Scholar]
- Davis, C.P.; King, J.L.; Budowle, B.; Eisenberg, A.J.; Turnbough, M.A. Extraction platform evaluations: A comparison of Automate Express™, EZ1® Advanced XL, and Maxwell® 16 Bench-top DNA extraction systems. Leg. Med. 2012, 14, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Frances, P.A.C.; Penidoa, C.A.F.O.; Costa, G.S.M.B.; de Almeida, R.M.; Pena, E.E.S.; Funabashi, K.; Resque, L.R. Comparison between automated DNA extraction employing the EZ1platform and manual methods using real forensic samples. Rev. Bras. Crimin. 2021, 10, 44–56. [Google Scholar] [CrossRef]

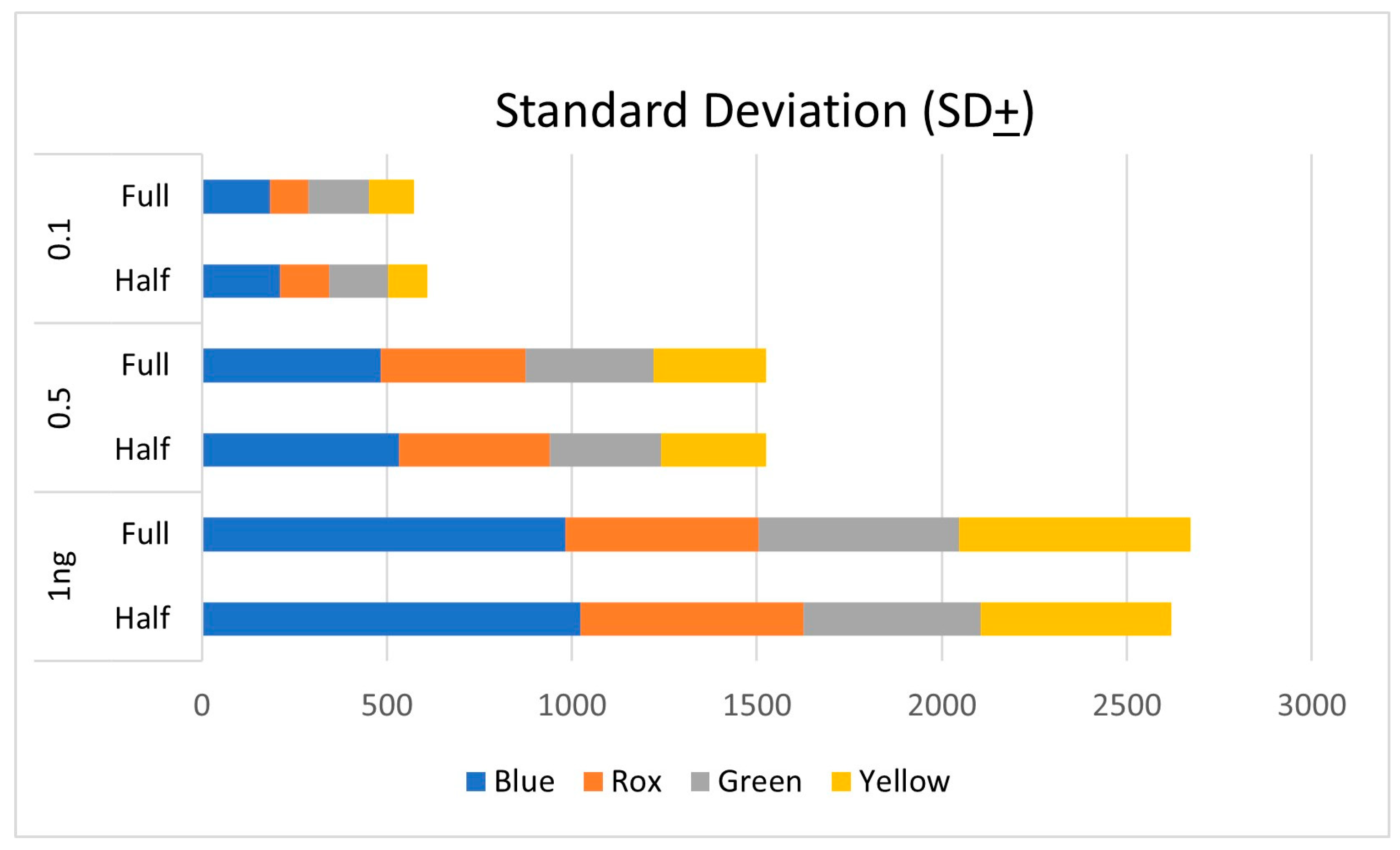

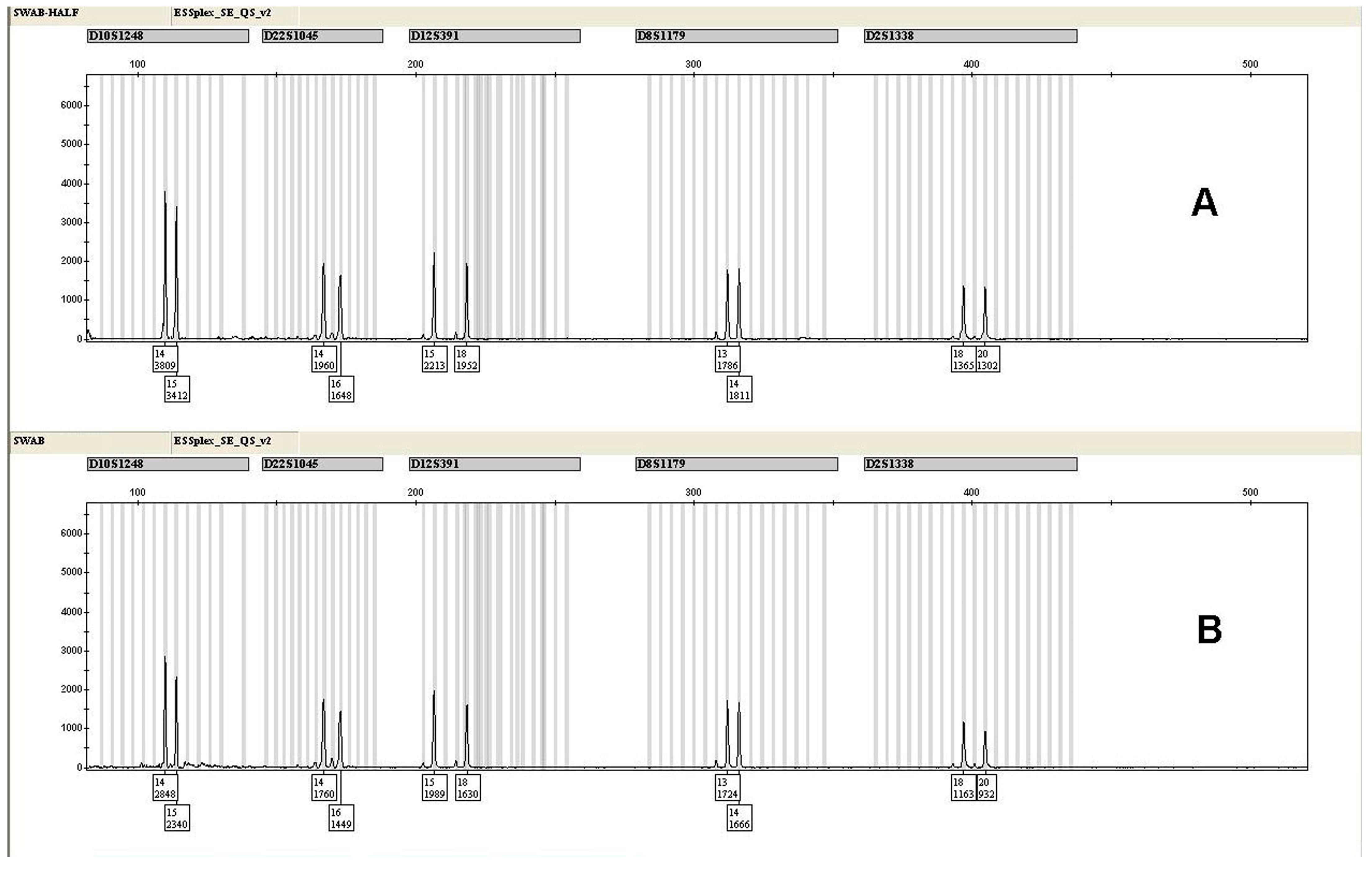
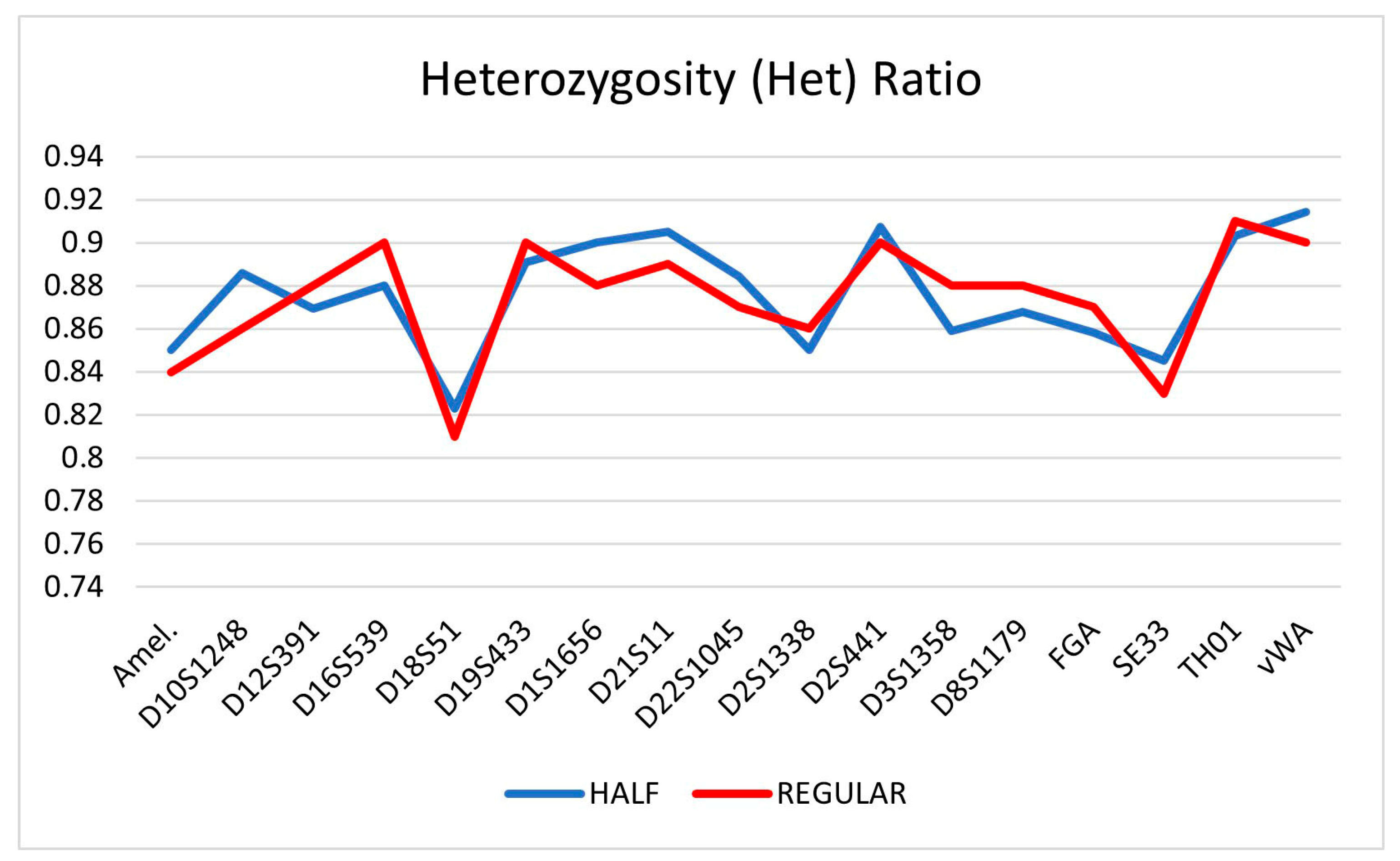

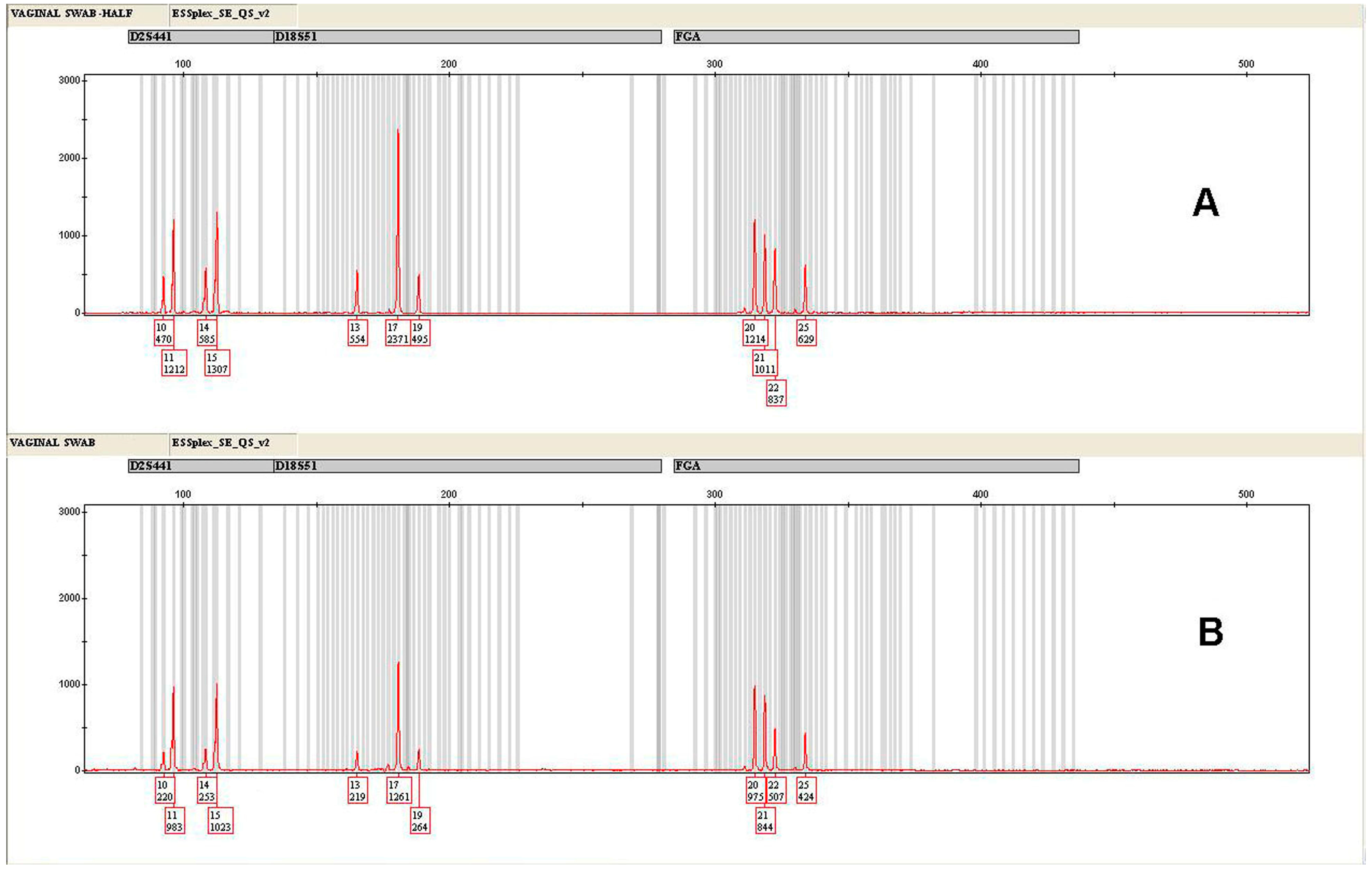
| Locus | HALF | SD (±) | REGULAR | SD (±) |
|---|---|---|---|---|
| Amel. | 0.85 | 0.10 | 0.84 | 0.11 |
| D10S1248 | 0.89 | 0.07 | 0.86 | 0.07 |
| D12S391 | 0.87 | 0.09 | 0.88 | 0.08 |
| D16S539 | 0.88 | 0.10 | 0.9 | 0.11 |
| D18S51 | 0.82 | 0.12 | 0.81 | 0.11 |
| D19S433 | 0.89 | 0.07 | 0.9 | 0.07 |
| D1S1656 | 0.90 | 0.07 | 0.88 | 0.06 |
| D21S11 | 0.91 | 0.11 | 0.89 | 0.12 |
| D22S1045 | 0.88 | 0.08 | 0.87 | 0.07 |
| D2S1338 | 0.85 | 0.12 | 0.86 | 0.11 |
| D2S441 | 0.91 | 0.08 | 0.9 | 0.09 |
| D3S1358 | 0.86 | 0.09 | 0.88 | 0.10 |
| D8S1179 | 0.87 | 0.09 | 0.88 | 0.09 |
| FGA | 0.86 | 0.11 | 0.87 | 0.10 |
| SE33 | 0.85 | 0.09 | 0.83 | 0.08 |
| TH01 | 0.90 | 0.05 | 0.91 | 0.06 |
| vWA | 0.91 | 0.09 | 0.9 | 0.10 |
| Locus | HALF | SD (±) | REGULAR | SD (±) |
|---|---|---|---|---|
| D10S1248 | 9.7 | 0.01 | 9.5 | 0.01 |
| D12S391 | 8.5 | 0.02 | 8.6 | 0.02 |
| D16S539 | 9 | 0.02 | 8.9 | 0.03 |
| D18S51 | 9.4 | 0.03 | 9.2 | 0.02 |
| D19S433 | 7 | 0.01 | 7.1 | 0.01 |
| D1S1656 | 9.3 | 0.02 | 8.9 | 0.02 |
| D21S11 | 8.8 | 0.01 | 8.2 | 0.01 |
| D22S1045 | 9.5 | 0.01 | 9.6 | 0.02 |
| D2S1338 | 9.3 | 0.02 | 9.2 | 0.02 |
| D2S441 | 4.7 | 0.01 | 4.9 | 0.01 |
| D3S1358 | 9.6 | 0.02 | 9.8 | 0.01 |
| D8S1179 | 8.5 | 0.02 | 8.6 | 0.02 |
| FGA | 9.7 | 0.01 | 9.6 | 0.01 |
| SE33 | 9.2 | 0.04 | 9 | 0.03 |
| TH01 | 5.9 | 0.008 | 6 | 0.009 |
| vWA | 8.6 | 0.02 | 8.8 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbaro, A.; Falcone, G.; La Marca, A.; Barbaro, A. Use of the Investigator ESSplex SE QS Kit (QIAGEN) at Half PCR Reaction Volumes for the Analysis of Forensic Samples. Forensic Sci. 2024, 4, 152-163. https://doi.org/10.3390/forensicsci4010009
Barbaro A, Falcone G, La Marca A, Barbaro A. Use of the Investigator ESSplex SE QS Kit (QIAGEN) at Half PCR Reaction Volumes for the Analysis of Forensic Samples. Forensic Sciences. 2024; 4(1):152-163. https://doi.org/10.3390/forensicsci4010009
Chicago/Turabian StyleBarbaro, Anna, Giacomo Falcone, Angelo La Marca, and Aldo Barbaro. 2024. "Use of the Investigator ESSplex SE QS Kit (QIAGEN) at Half PCR Reaction Volumes for the Analysis of Forensic Samples" Forensic Sciences 4, no. 1: 152-163. https://doi.org/10.3390/forensicsci4010009
APA StyleBarbaro, A., Falcone, G., La Marca, A., & Barbaro, A. (2024). Use of the Investigator ESSplex SE QS Kit (QIAGEN) at Half PCR Reaction Volumes for the Analysis of Forensic Samples. Forensic Sciences, 4(1), 152-163. https://doi.org/10.3390/forensicsci4010009







