Morphological and Mitochondrial Evidence Supporting New Records of Leatherleaf Slugs (Gastropoda: Veronicellidae) in Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Morphological Analyses
2.2. DNA Extraction, PCR, Sequencing
3. Results
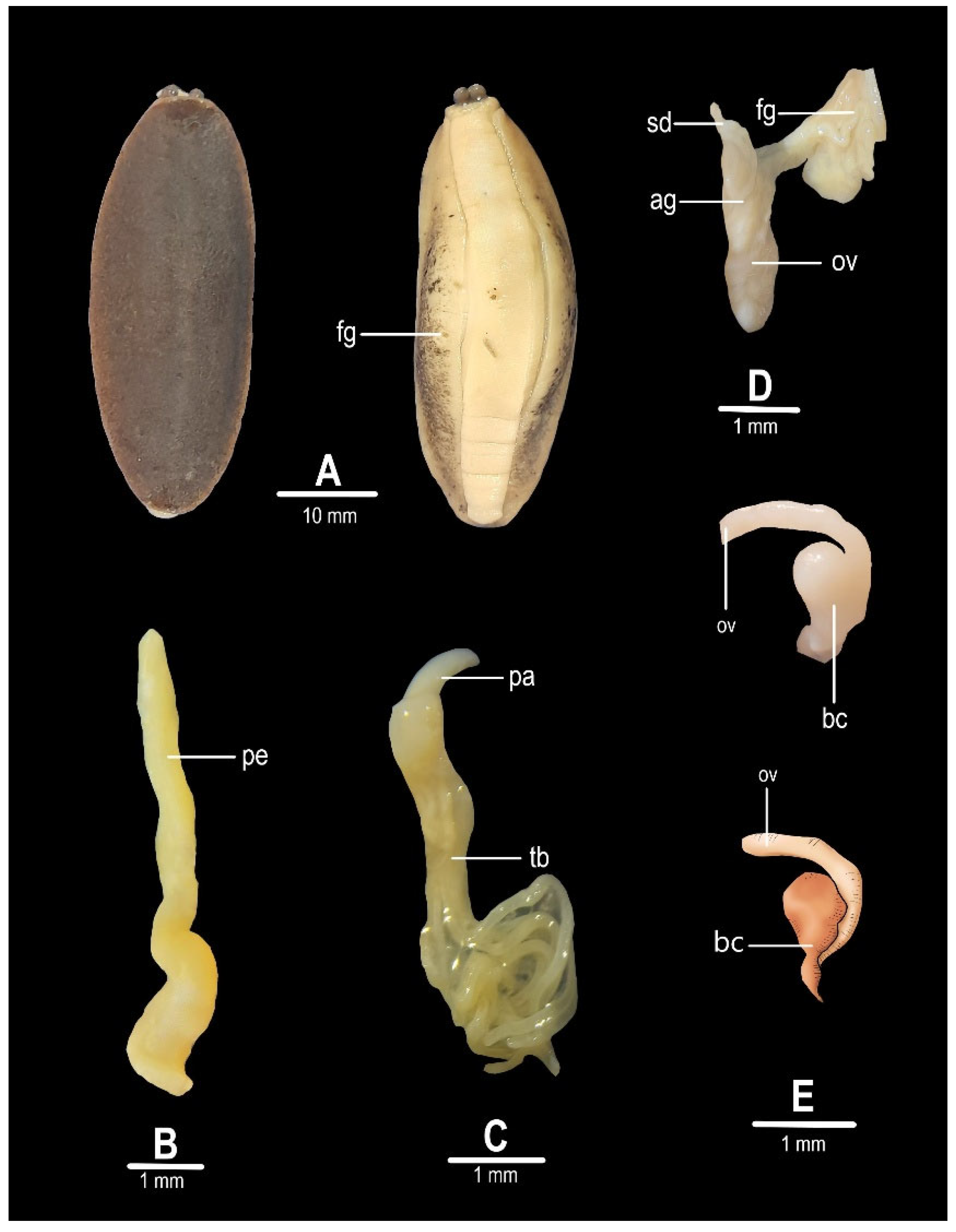
3.1. Morphological Analysis
3.1.1. Simrothula prismatica (Simroth, 1914)
3.1.2. Diplosolenodes occidentalis (Guilding, 1824)
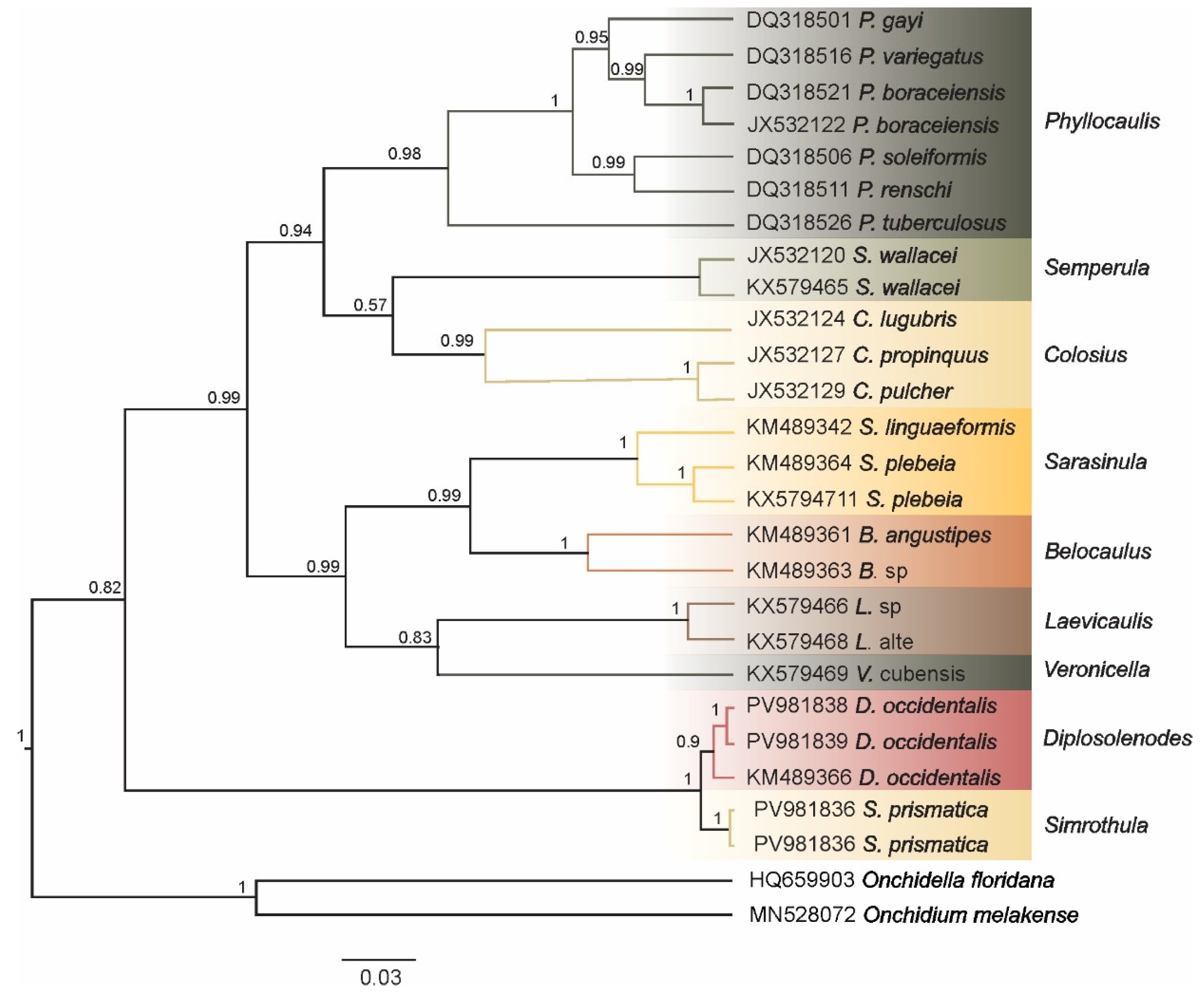
3.2. Molecular Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ag | Albumen gland |
| fg | Female genital pore |
| ov | Oviduct |
| pa | Papilla |
| pb | Penis base |
| pe | Penis |
| tb | Tubule |
| vd | Vas deferens |
References
- Kim, J.R.; Hayes, K.A.; Yeung, N.W.; Cowie, R.H. Identity and Distribution of Introduced Slugs (Veronicellidae) in the Hawaiian and Samoan Islands1. Pac. Sci. 2016, 70, 477–493. [Google Scholar] [CrossRef]
- Hirano, T.; Kagawa, O.; Fujimoto, M.; Saito, T.; Uchida, S.; Yamazaki, D.; Ito, S.; Shariar, S.M.; Sawahata, T.; Chiba, S. Species Identification of Introduced Veronicellid Slugs in Japan. PeerJ 2022, 10, e13197. [Google Scholar] [CrossRef]
- Burch, J.B.; Pearce, T.A. Terrestrial gastropoda. In Soil Biology Guide; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]
- Fisher, P.; Crosse, H. Mission Scientifique au Mexique et dans l’Amérique Centrale: Études sur les Mollusques Terrestres et Fluviatiles du Mexique et du Guatemala; Imprimerie Nationale: Paris, France, 1870; Volume 1. [Google Scholar]
- von Martens, E. Biologia Centrali Americana: Land and Freshwater Mollusca; University of Wisconsin-Madison: Madison, WI, USA, 1890. [Google Scholar]
- Baker, H.B. North American Veronicellidae. Proc. Acad. Nat. Sci. Phila. 1925, 77, 157–184. [Google Scholar]
- Naranjo-García, E.; Thomé, J.W.; Castillejo, J. A Review of the Veronicellidae from Mexico (Gastropoda: Soleolifera). Rev. Mex. Biodivers. 2007, 78, 41–50. [Google Scholar]
- Alvarez-Cerrillo, L.; Yáñez-Rivera, B.; Araiza-Gómez, V. Non-native terrestrial slugs from Sinaloa, Mexico: Deroceras laeve (O. F. Müller, 1774) and Sarasinula plebeia (P. Fischer, 1868) (Mollusca, Gastropoda). Biodivers. Data J. 2022, 10, e87666. [Google Scholar] [CrossRef] [PubMed]
- de Luna, M.; García-Barrios, R.; Hernández-González, V.A.; Gámez-Cárdenas, A.I. Nuevos registros de babosas de cuero (Gastropoda: Veronicellidae) para Nuevo León, México, con un listado actualizado de las especies registradas en Norteamérica. ACTA Zool. Mex. NS 2024, 40, 1–12. [Google Scholar] [CrossRef]
- Yeung, N.W.; Hayes, K.A.; Cowie, R.H. Effects of Washing Produce Contaminated with the Snail and Slug Hosts of Angiostrongylus cantonensis with Three Common Household Solutions. Hawaii J. Med. Public Health 2013, 72, 83–86. [Google Scholar]
- Kim, J.R.; Hayes, K.A.; Yeung, N.W.; Cowie, R.H. Diverse Gastropod Hosts of Angiostrongylus cantonensis, the Rat Lungworm, Globally and with a Focus on the Hawaiian Islands. PLoS ONE 2014, 9, e94969. [Google Scholar] [CrossRef]
- Barbosa, T.A.; Thiengo, S.C.; Fernandez, M.A.; Ramos-de-Souza, J.; Gomes, S.R. The Zoonotic Angiostrongylus cantonensis and the Veterinary Parasite Aelurostrongylus abstrusus Infecting Terrestrial Gastropods from Urban Areas of Macapá, Brazilian Amazon Region. Pathogens 2024, 13, 255. [Google Scholar] [CrossRef]
- Araujo, H.B.D. Controle de lesmas. Rev. Agronômica 1952, 16, 363–366. [Google Scholar]
- Robinson, D.G.; Fields, A. The Leatherleaf Slugs (Family Veronicellidae). In Regional Workshop: Mollusk Pests of Economic Importance; Centro Kellog, Escuela Agricola Panamericana Zamorano: San Antonio de Oriente, Honduras, 2010. [Google Scholar]
- Thomé, J.W. Estado Atual da Sistemática Dos Veronicellidae (Mollusca; Gastropoda) Americanos, com Comentários Sobre sua Importância Econômica, Ambiental e na Saúde. Biociências 1993, 1, 61–75. [Google Scholar]
- Hata, T.Y.; Hara, A.H.; Hu, B.K.-S. Molluscicides and Mechanical Barriers against Slugs, Vaginula plebeia Fischer and Veronicella cubensis (Pfeiffer) (Stylommatophora: Veronicellidae). Crop Prot. 1997, 16, 501–506. [Google Scholar] [CrossRef]
- Chiaradia, L.A.; Milanez, J.M.; Graeff-Teixeira, C.; Thomé, J.W. Lesmas: Pragas da agricultura e ameaça à saúde humana. Agropecuária Catarin. 2004, 17, 70–74. [Google Scholar] [CrossRef]
- Survey of Slug and Snail Pests on Subsistence and Garden Crops in the Islands of the American Pacific: Guam, and the Northern Mariana Islands. the Federated States of Micronesia and American Samoa, with Special Reference to Samoa. Available online: https://www.researchgate.net/publication/269097114_Survey_of_slug_and_snail_pests_on_subsistence_and_garden_crops_in_the_islands_of_the_American_Pacific_Guam_and_the_Northern_Mariana_Islands_the_Federated_States_of_Micronesia_and_American_Samoa_with_s (accessed on 26 June 2025).
- Linares, E.L.; Vera, M.L. Catálogo de los Moluscos Continentales de Colombia; Universidad Nacional de Colombia, Facultad de Ciencias, Instituto de Ciencias Naturales: Bogota, Columbia, 2011; Volume 23, pp. 1–285. [Google Scholar]
- Guilding, L. Transactions of the Linnean Society of London; Richard Taylor: London, UK, 1823; pp. 322–324. [Google Scholar]
- Thompson, F.G. An Annotated Checklist and Bibliography of the Land and Freshwater Snails of Mexico and Central America. Bull. Fla. Mus. Nat. Hist. 2011, 50, 1–299. [Google Scholar] [CrossRef]
- Breure, A.S.H.; Roosen, M.T.; Ablett, J.D. Land and Freshwater Molluscs of Mainland Ecuador: An Illustrated Checklist. Iberus 2022, 40, 1–290. [Google Scholar]
- Salvador, R.B.; Miranda, M.S.; Silva, F.S.; Oliveira, C.D.C.; Arruda, J.O.; Cavallari, D.C.; Gomes, S.R.; La Pasta, A.; Pena, M.S.; Ovando, X.M.C.; et al. Checklist of the Terrestrial Gastropods of Brazil. J. Conchol. 2024, 45, 142–185. [Google Scholar] [CrossRef]
- Branson, B.A. The Mussels (Unionaceae: Bivalvia) of Oklahoma-Part 3: Lampsilini. Proc. Okla. Aca. Sci. 1980, 64, 29–35. [Google Scholar]
- Dundee, D.S. Observations on the Veronicellid Slugs of the Southern United States. Nautilus 1977, 91, 108–114. [Google Scholar]
- Simroth, H.A. Beitrag zur Kentniss der Nacktschnecken Columbiens. Zugleich Eine Uebersicht Ueber Die Neotropische Nacktschneck-en Fauna Überhaupt. Mém. Société Neuchâtel. Sci. Nat. 1914, 5, 270–341. [Google Scholar]
- Thomé, J.W. Redescrição dos tipos de Veronicellidae (Mollusca, Gastropoda) Neotropicais: III. Espécies Depositadas no II, Zoologisches Institut und Museum da Universidade de Göttingen, Alemanha. Iheringia 1970, 38, 73–88. [Google Scholar]
- Thomé, J.W. Redescrição dos tipos de veronicellidae (Mollusca, Gastropoda) Neotropicais: VIII. Espécies Depositadas no “Institut für Spezielle Zoologie und Zoologisches Museum” de Berlim, Alemanha Oriental. Arq. Zool. 1972, 21, 235–281. [Google Scholar] [CrossRef][Green Version]
- Thomé, J.W. Os Gêneros Da Família Veronicellidae Nas Américas (Mollusca, Gastropoda). Iheringia 1975, 48, 3–56. [Google Scholar][Green Version]
- Thomé, J.W.; Gomes, S.R.; Silva, R.S. Redescription of the Genus and Species Heterovaginina limayana (Lesson, 1830) (Gastropoda, Soleolifera, Veronicellidae). Nautilus 2002, 116, 79–88. [Google Scholar][Green Version]
- Gomes, S.R.; Thomé, J.W. Anatomia Comparada de Cinco Espécies da Família Veronicellidae (Gastropoda, Soleolifera) Ocorrentes nas Regiões Australiana e Oriental. Biociências 2001, 9, 137–151. [Google Scholar][Green Version]
- Gomes, S.R.; Thome, J.W. Diversity and Distribution of the Veronicellidae (Gastropoda: Soleolifera) in the Oriental and Australian Biogeographical Regions. Mem. Qld. Mus. 2004, 49, 589–601. [Google Scholar][Green Version]
- Gomes, S.R.; Picanco, J.B.; Mendes, I.L.V.; Thome, J.W. A new species of Simrothula (Gastropoda, Soleolifera, Veronicellidae) from Northern Brazil. Zootaxa 2006, 1329, 59–68. [Google Scholar] [CrossRef]
- Palumbi, S.R. What can Molecular Genetics Contribute to Marine Biogeography? An urchin’s tale. J. Exp. Mar. Biol. Ecol. 1996, 203, 75–92. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bouckaert, R.R. An Efficient Coalescent Epoch Model for Bayesian Phylogenetic Inference. Syst. Biol. 2022, 71, 1549–1560. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.-E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- Thomé, J.W. Redescrição dos tipos de Veronicellidae (Mollusca, Gastropoda) neotropicais: X. Os tipos de Diplosolenodes occidentalis (Guilding, 1825) no British Museum (Natural History), Londres. Rev. Bras. Zool. 1984, 2, 411–417. [Google Scholar] [CrossRef]
- Thomé, J.W. Redescrição dos tipos de Veronicellidae (Mollusca, Gastropoda) Neotropicais: VII, Espécies Depositads no “Muséum National d’Histoire Naturelle”, Paris, França. Iheringia Zool. 1971, 40, 27–52. [Google Scholar]
- Daglio, E.D.; de Lucia, M.; Gomes, S.R.; Gutiérrez, D.E. First Records of the Bean-Slug Sarasinula plebeia (Gastropoda: Veronicellidae) in Argentina. Papéis Avulsos Zool. 2020, 60, e20206047. [Google Scholar] [CrossRef]
- Gomes, S.R. Filogenia Morfológica de Veronicellidae, Filogenia Molecular de Phyllocaulis Colosi e Descrição de Uma Nova Espécie para a Família (Mollusca, Gastropoda, Pulmonata). Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2007. [Google Scholar]
- Branson, B.A. The Slugs (Gastropoda: Pulmonata) of Oklahoma and Kansas with New Records. Trans. Kans. Acad. Sci. 1962, 65, 110. [Google Scholar] [CrossRef]
- Gomes, S.R. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KM489366 (accessed on 23 October 2024).
- Gomes, S.R.; Britto Da Silva, F.; Mendes, I.L.V.; Thomé, J.W.; Bonatto, S.L. Molecular Phylogeny of the South American Land Slug Phyllocaulis (Mollusca, Soleolifera, Veronicellidae). Zool. Scr. 2010, 39, 177–186. [Google Scholar] [CrossRef]
- Santin, R.A.; Miquel, S.E. Veronicellidae in Argentina: Taxonomy, Morphology and Distribution (Mollusca: Gastropoda: Systellomatophora). Arch. Für Molluskenkd. Int. J. Malacol. 2015, 144, 105–123. [Google Scholar] [CrossRef]
- Sommer, R.M.; Cowie, R.H. Invasive Traits of Veronicellid Slugs in the Hawaiian Islands and Temperature Response Suggesting Possible Range Shifts under a Changing Climate. J. Molluscan Stud. 2020, 86, 147–155. [Google Scholar] [CrossRef]
- Ali, R.F.; Robinson, D.G. Four Records of New to Egypt Gastropod Species Including the First Reported Tropical Leatherleaf Slug Laevicaulis Alte (d’A. de Férussac, 1822) (Pulmonata: Veronicellidae). Zool. Ecol. 2020, 30, 138–156. [Google Scholar] [CrossRef]
- Ali, R.F.; Robinson, D.G.; Liberto, F. Morphological Description of Laevicaulis stuhlmanni (Simroth, 1895) (Pulmonata, Veronicellidae) from Egypt. Biodivers. Data J. 2022, 10, e85495. [Google Scholar] [CrossRef]
- Capinera, J.L.; Rodrigues, C.G. Biology and Control of the Leatherleaf Slug Leidyula floridana (Mollusca: Gastropoda: Veronicellidae). Fla. Entomol. 2015, 98, 243–253. [Google Scholar] [CrossRef]
- Da Mota, D.J.G.; Rocco, S.C.; Luca, L.R.D.; Dos Santos, J.A.; Werneck, E.F.P.; Baccin, A.D.O.; Gava, R.; Pereira-Chioccola, V.L.; De Melo, L.C.V. First Record of Natural Infection by Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) in Tanychlamys indica (Godwin-Austen, 1883) in the City of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2025, 120, e240192. [Google Scholar] [CrossRef]


| Species | GenBank Accession Number |
|---|---|
| Phyllocaulis gayi | DQ318501 |
| Phyllocaulis variegatus | DQ318521 |
| Phyllocaulis boraceiensis | DQ318506 |
| Phyllocaulis boraceiensis | JX532122 |
| Phyllocaulis soleiformis | DQ318506 |
| Phyllocaulis renschi | DQ318511 |
| Phyllocaulis tuberculosus | DQ318526 |
| Semperula wallacei | JX532120 |
| Semperula wallacei | KX579465 |
| Colosius lugubris | JX532124 |
| Colosius propinquus | JX532127 |
| Colosius pulcher | JX532129 |
| Sarasinula linguaeformis | KM489342 |
| Sarasinula plebeia | KM489364 |
| Sarasinula plebeia | KX579471 |
| Laevicaulis alte | KX579468 |
| Laevicaulis sp. | KX579466 |
| Belocaulus sp. | KM489363 |
| Belocaulus angustipes | KM489361 |
| Veronicella cubensis | KX579469 |
| Diplosolenodes occidentalis | KM489366 |
| Characters | Simrothula prismatica | Diplosolenodes occidentalis | Sarasinula plebeia | Leidyula moreleti | Belocaulus angustipes |
|---|---|---|---|---|---|
| Notum color | The center has a reddish base that changes to ochre towards the sides. The middle is light colored | Light brown to yellowish with light, very short lines, regularly distributed along the entire notum and dark spots | Brownish with fine black dots distributed randomly | Light gray to brown with two well defined, parallel, darker longitudinal lines | Velvety black sometimes with an inconspicuous light stripe |
| Hyponotum color | Whitish with gray to blackish markings | Light beige with few dark spots | Light beige | Pale, sole pale ochreous | Black |
| Penial tubules | 15 arrow-shaped tubules | 16 uniform tubules long and 6 short (Thome 1985), with a limited number exhibiting bifurcation | 4–9 tubules | With 35–40 tubules, the inner ones are shorter and the outer ones longer [28] | 13–22 tubules |
| Penis glans | Arrowhead papilla, elongated and conical | The glans is an elongated conical papilla tapering to a point | Elongated with a conical papilla | Short arrowhead papilla [39] | The gland is a conical papilla |
| Canalis junctor | Typical, without constriction where it connects to the bursa copulatrix | Long, thick and sinuous | Thick and short [40] | Penetrates the bursa directly and not the duct | |
| Bursa copulatrix | Elongated with a digitiform expansion | Ovoid, the duct is distinguished by two types of tissue | Globular [40] | Ovoid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Andrade, A.D.; Araiza-Gómez, V.; Naranjo-García, E.; Ruiz, E.A. Morphological and Mitochondrial Evidence Supporting New Records of Leatherleaf Slugs (Gastropoda: Veronicellidae) in Mexico. Taxonomy 2025, 5, 58. https://doi.org/10.3390/taxonomy5040058
González-Andrade AD, Araiza-Gómez V, Naranjo-García E, Ruiz EA. Morphological and Mitochondrial Evidence Supporting New Records of Leatherleaf Slugs (Gastropoda: Veronicellidae) in Mexico. Taxonomy. 2025; 5(4):58. https://doi.org/10.3390/taxonomy5040058
Chicago/Turabian StyleGonzález-Andrade, Amalia Daniela, Victoria Araiza-Gómez, Edna Naranjo-García, and Enrico Alejandro Ruiz. 2025. "Morphological and Mitochondrial Evidence Supporting New Records of Leatherleaf Slugs (Gastropoda: Veronicellidae) in Mexico" Taxonomy 5, no. 4: 58. https://doi.org/10.3390/taxonomy5040058
APA StyleGonzález-Andrade, A. D., Araiza-Gómez, V., Naranjo-García, E., & Ruiz, E. A. (2025). Morphological and Mitochondrial Evidence Supporting New Records of Leatherleaf Slugs (Gastropoda: Veronicellidae) in Mexico. Taxonomy, 5(4), 58. https://doi.org/10.3390/taxonomy5040058






