Tetrigidae of Ethiopia: First Species Delimitation via DNA Barcoding and Description of Three New Species †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. PCR Amplification
2.4. Phylogenetic Analysis
- Species delimitation
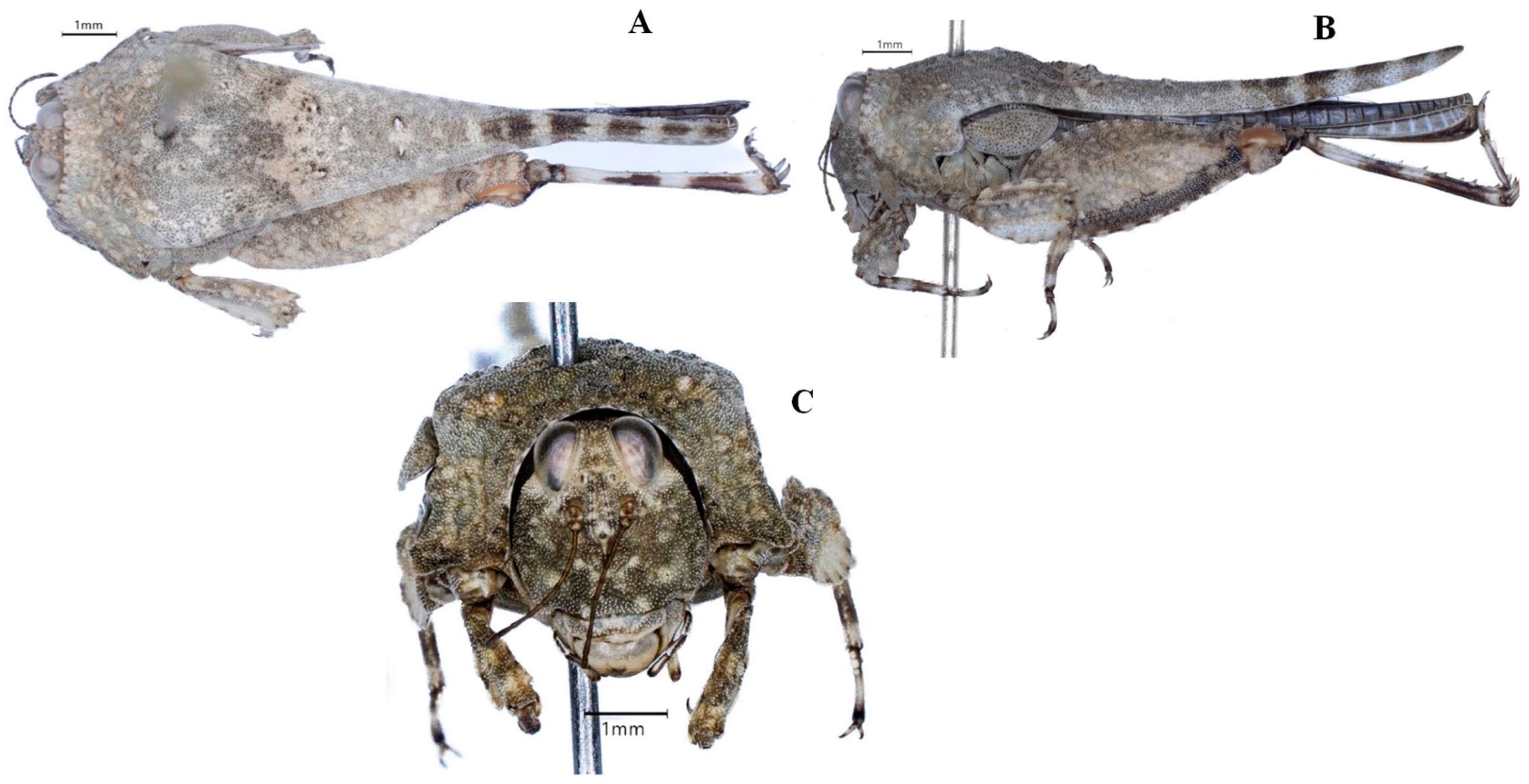
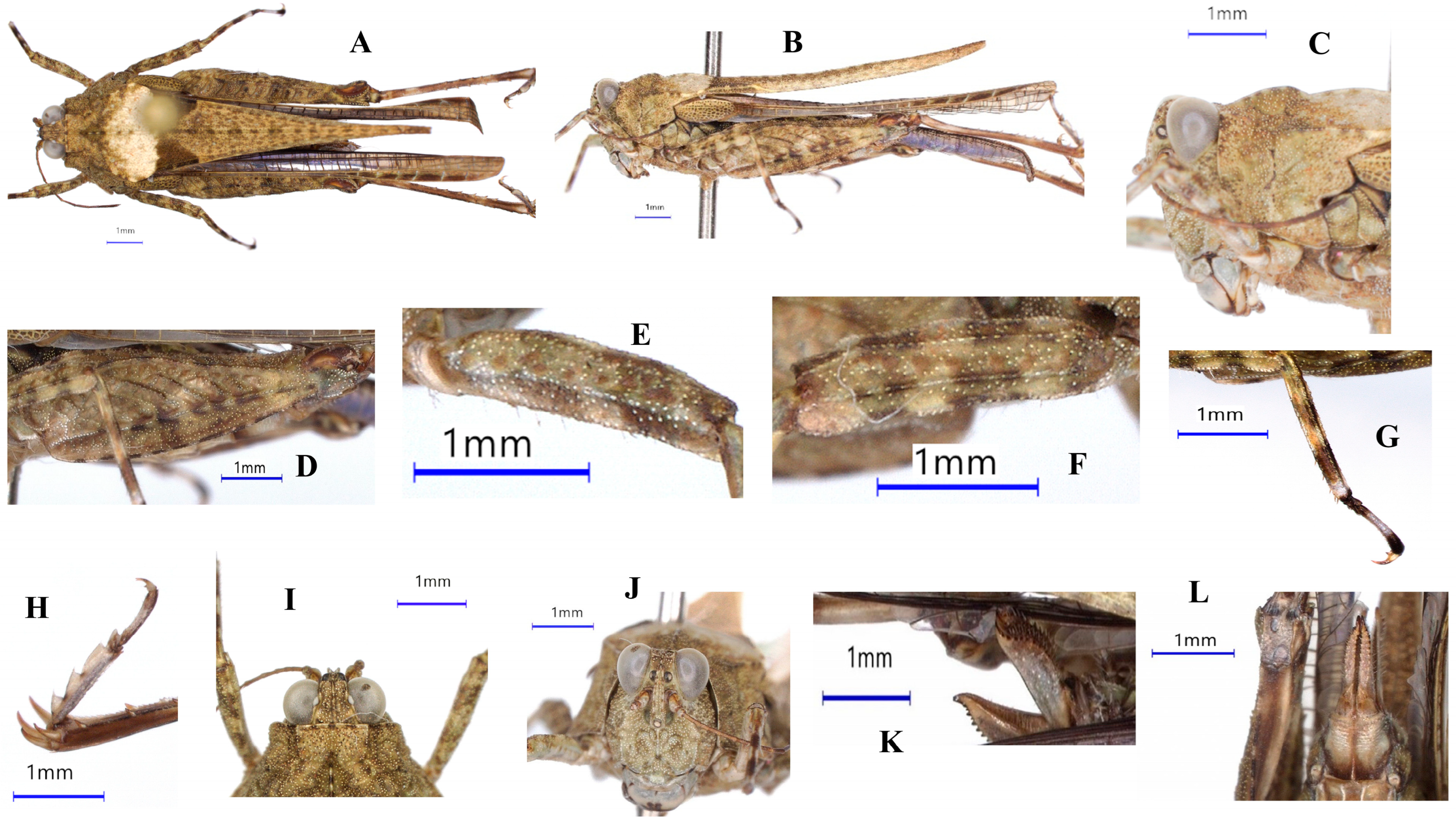
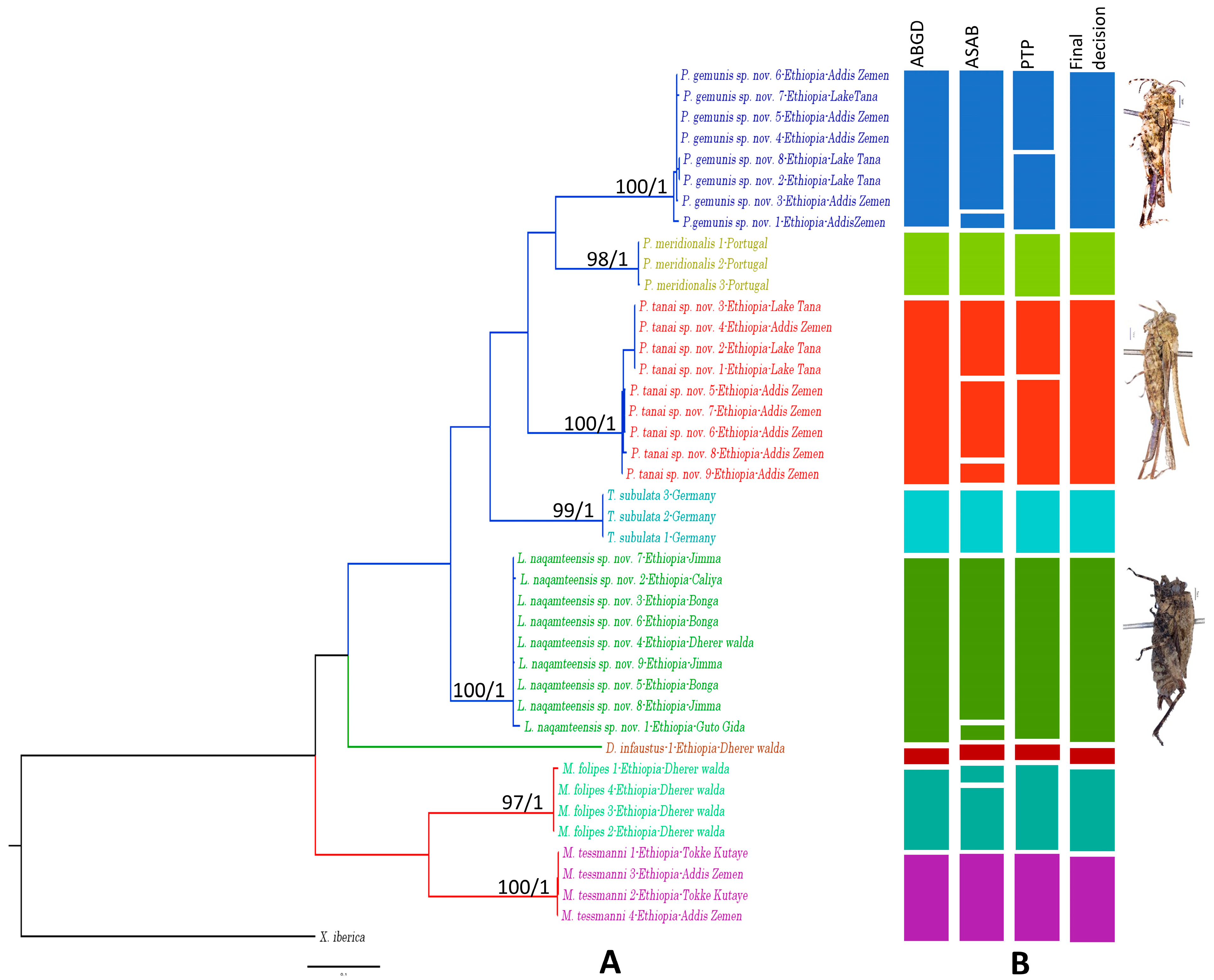
3. Results
Systematic Entomology
|
|

|
|
|
|
|
|
- Species delimitation results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paranjape, S.Y.; Bhalerao, A.M.; Naidu, N.M. On Etho-Ecological Characteristics and Phylogeny of Tetrigidae. In Evolutionary Biology of Orthopteroid Insects; Bacetti, B.M., Ed.; Ellis Horwood: New York, NY, USA; pp. 386–395.
- Yao, Y.P.; Zheng, Z.M.; Wang, N.X.; Jiang, G.F. Revision of the four species of Tetrigidae (Orthoptera: Tetrigoidea) from China based on morphological characteristics and partial sequences of three genes. Acta Entomol. Sin. 2008, 51, 855–860. [Google Scholar]
- Tumbrinck, J. Taxonomic Revision of the Cladonotinae (Orthoptera: Tetrigidae) from the Islands of South–East Asia and from Australia, with General Remarks to the Classification and Morphology of the Tetrigidae and Descriptions of New Genera and Species from New Guinea and New Caledonia. In Biodiversity, Biogeography and Nature Conservation in Wallacea and New Guinea; Telnov, D., Ed.; Entomological Society of Latvia: Riga, Latvia, 2014; pp. 350–396. [Google Scholar]
- Cadena-Castañeda, O.J.; Silva, D.S.M.; Mendes, D.M.D.M.; Pereira, M.R.; De Domenico, F.C.; Sperber, C.F. Review of the tribe Amorphopini (Orthoptera: Tetrigidae: Metrodorinae): Pygmy moss-lichen tetrigids from the Amazon rainforest. J. Orthoptera Res. 2020, 29, 45–62. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. 2024. Available online: http://Orthoptera.SpeciesFile.org (accessed on 10 October 2024).
- Kuřavová, K.; Šipoš, J.; Wahab, R.A.; Kahar, R.S.; Kočárek, P. Feeding patterns in tropical groundhoppers (Tetrigidae): A case of phylogenetic dietary conservatism in a basal group of Caelifera. Zool. J. Linn. Soc. 2017, 179, 291–302. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.-Q.; Mao, B.-Y.; Li, M. Two new species of Xistra Bolívar, 1887 (Orthoptera: Tetrigidae: Metrodorinae) from China, with a key to species of the genus. Orient. Insects 2022, 56, 408–427. [Google Scholar] [CrossRef]
- Kasalo, N.; Buzzetti, F.M.; Stancher, G.; Cambra, R.A.; Skejo, J. Contribution to the knowledge of Batrachideini (Orthoptera: Tetrigidae): Description of two new flightless genera, Naskreckiana and Procellator, and revision of the status of Eotetrix. Acta Èntomol. Musei Natl. Pragae 2023, 63, 279–292. [Google Scholar] [CrossRef]
- Devriese, H.; Nguyen, E.; Husemann, M. An identification key to the genera and species of Afrotropical Tetrigini (genera Paratettix, Leptacrydium, Hedotettix, Rectitettix nov. gen., and Alienitettix nov.gen.) with general remarks on the taxonomy of Tetrigini (Orthoptera, Tetrigidae). Zootaxa 2023, 5285, 511–556. [Google Scholar] [CrossRef] [PubMed]
- Neo, I.; Tan, M.K.; Cho, T.J.; Yeo, D.C. A faunistic study and taxonomic account of species of pygmy grasshoppers (Orthoptera: Tetrigidae) from Singapore’s last freshwater swamp forest. J. Asia-Pacific Biodivers. 2023, 17, 87–116. [Google Scholar] [CrossRef]
- Kasalo, N.; Skejo, J. The smallest Australian Tetrigidae (Orthoptera): Taxonomic revision of Peraxelpa Sjöstedt, 1932 with the descriptions of three new genera and eleven new species. Ann. Soc. Èntomol. Fr. 2024, 60, 515–546. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Williams, H.; Ormerod, S.; Bruford, M. Molecular systematics and phylogeography of the cryptic species complex Baetis rhodani (Ephemeroptera, Baetidae). Mol. Phylogenetics Evol. 2006, 40, 370–382. [Google Scholar] [CrossRef]
- Moczek, A.P. Phenotypic plasticity and diversity in insects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 593–603. [Google Scholar] [CrossRef]
- Li, R.; Ying, X.; Deng, W.; Rong, W.; Li, X. Mitochondrial genomes of eight Scelimeninae species (Orthoptera) and their phylogenetic implications within Tetrigoidea. PeerJ 2021, 9, e10523. [Google Scholar] [CrossRef]
- Wei, S.-Z.; Deng, W.-A. Two new species of the genus Macromotettixoides Zheng, Wei & Jiang (Orthoptera: Tetrigidae: Metrodorinae) from China. Orient. Insects 2022, 57, 626–642. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, R.; Deng, W. First mitogenomic characterization of Macromotettixoides (Orthoptera, Tetrigidae), with the descriptions of two new species. ZooKeys 2024, 1195, 95–120. [Google Scholar] [CrossRef]
- Li, X.-D.; Zhang, W.; Xin, L.; Ye, R.; Deng, W.-A.; Li, R. Sequence and phylogenetic analysis of the mitochondrial genome for the groundhopper Mazarredia convexa (Orthoptera: Tetrigidae). Mitochondrial DNA Part B 2020, 5, 2276–2277. [Google Scholar] [CrossRef]
- Adžić, K.; Deranja, M.; Franjević, D.; Skejo, J. Are Scelimeninae (Orthoptera: Tetrigidae) monophyletic and why it remains a question? Èntomol. News 2020, 129, 128–146. [Google Scholar] [CrossRef]
- Kasalo, N.; Skejo, J.; Husemann, M. DNA Barcoding of Pygmy Hoppers—The First Comprehensive Overview of the BOLD Systems’ Data Shows Promise for Species Identification. Diversity 2023, 15, 696. [Google Scholar] [CrossRef]
- Pina, S.; Pauperio, J.; Barros, F.; Chaves, C.; Martins, F.M.; Pinto, J.; Ferreira, S. The InBIO barcoding initiative database: DNA barcodes of Orthoptera from Portugal. Biodivers. Data J. 2024, 12, e118010. [Google Scholar] [CrossRef]
- Hawlitschek, O.; Morinière, J.; Lehmann, G.U.C.; Lehmann, A.W.; Kropf, M.; Dunz, A.; Glaw, F.; Detcharoen, M.; Schmidt, S.; Hausmann, A.; et al. DNA barcoding of crickets, katydids and grasshoppers (Orthoptera) from Central Europe with focus on Austria, Germany and Switzerland. Mol. Ecol. Resour. 2017, 17, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Kock, L.-S.; Körs, E.; Husemann, M.; Davaa, L.; Dey, L.-S. Barcoding fails to delimit species in Mongolian Oedipodinae (Orthoptera, Acrididae). Insects 2024, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Moulton, M.J.; Whiting, M.F.; Arthofer, W. Rampant nuclear insertion of mtDNA across diverse lineages within Orthoptera (Insecta). PLoS ONE 2014, 9, e110508. [Google Scholar] [CrossRef] [PubMed]
- Vuataz, L.; Sanchez, A.; Wyler, S.; Blanc, M.; Chittaro, Y. Diversity and relationships of Ampedini Gistel, 1848 (Coleoptera: Elateridae) in Switzerland and Europe. Invertebr. Syst. 2019, 33, 544–555. [Google Scholar] [CrossRef]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005, 85, 407–415. [Google Scholar] [CrossRef]
- Tang, C.Q.; Humphreys, A.M.; Fontaneto, D.; Barraclough, T.G.; Paradis, E. Effects of phylogenetic reconstruction method on the robustness of species delimitation using single-locus data. Methods Ecol. Evol. 2014, 5, 1086–1094. [Google Scholar] [CrossRef]
- Blair, C.; Bryson, R.W. Cryptic diversity and discordance in single-locus species delimitation methods within horned lizards (Phrynosomatidae: Phrynosoma). Mol. Ecol. Resour. 2017, 17, 1168–1182. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Abro, T.W.; Desta, A.B.; Debie, E.; Alemu, D.M. Endemic plant species and threats to their sustainability in Ethiopia: A systematic review. Trees For. People 2024, 17, 100634. [Google Scholar] [CrossRef]
- Teku, D.; Abebe, A.; Fetene, M. Ethiopian orthodox tewahedo church sacred forests as sanctuaries for endangered species: Key roles, challenges and prospects. Sustain. Environ. 2024, 10, 2391614. [Google Scholar] [CrossRef]
- CEPF. Eastern Afromontane Biodiversity Hotspot- Ecosystem Profile. 2012. Available online: http://www.cepf.net/Documents/Eastern_Afromontane_Ecosystem_Profile_FINAL.pdf (accessed on 2 February 2023).
- Chernet, T. Land Resources and Socioeconomic Report of Bonga, Boginda, Mankira and the Surrounding Areas in Kaffa Zone, SNNPRS, Ethiopia; Report for PPP Project on the Establishment of a Coffee Biosphere Reserve in Bonga Region; PPP Project: Addis Ababa, Ethiopia, 2008. [Google Scholar]
- NABU. The Nature and Biodiversity Conservation Union’s, Forest Status of Kafa Biosphere Reserve in the Frame of “Forest and Community Analysis”; NABU: Berlin, Germany, 2017. [Google Scholar]
- Devriese, H. Revision des Xerophyllini d’Afrique (Orthoptera, Tetrigidae). Belg. J. Entomol. 1999, 1, 21–99. [Google Scholar]
- Rohland, N.; Reich, D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2012, 22, 939–946. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Edgar, R.C. Muscle v5 enables improved estimates of phylogenetic tree confidence by ensemble bootstrapping. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S.; Battistuzzi, F.U. MEGA11: Molecular Evolutionary Genetics Analysis Version. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Guindon, S.; Jean-François, D.; Vincent, L.; Maria, A.; Wim, H.; Olivier, G. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K.; Battistuzzi, F.U. MEGA12: Molecular evolutionary genetic analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Günther, K. Acrydiinen (Orthoptera, Acrididae) von Neu Guinea hauptsächlich aus den Ausbeuten von Professor Dr. Bürgers (Deutsche Kaiserin Augusta Fluss-Expedition 1912/1913), Dr. E. Mayr (1928), G. Stein (1931) und Miss L. E. Cheesman (1933/34); Nova Guinea (NS): Leiden, The Netherlands, 1938; pp. 1–46. [Google Scholar]
- Günther, K. Revision der Acrydiinae (Orthoptera), III Sectio Amor-phopi (Metrodarae Bol. 1887, auct.). In Abhandlungen und Berichte des Staatlichen Museums für Tierkunde Dresden; State Museum of Animal Science: Dresden, Germany, 1939; pp. 16–335. [Google Scholar]
- Günther, K. Die Tetrigoidea von Afrika südlich der Sahara (Orthoptera: Caelifera). Beiträge Zur Entomolo-Gie/Contrib. Entomol. 1979, 29, 7–183. [Google Scholar]
- Zhang, R.-J.; Zhao, C.-L.; Wu, F.-P.; Deng, W.-A. Molecular data provide new insights into the phylogeny of Cladonotinae (Orthoptera: Tetrigoidea) from China with the description of a new genus and species. Zootaxa 2020, 4809, 547–559. [Google Scholar] [CrossRef]
- Madan, S.; Niko, K.; Josip, S. Definition of the pygmy grasshopper subfamily Criotettiginae (Orthoptera: Tetrigidae) with a preliminary catalogue of genera. Zool. Anz. 2025, 318, 133–151. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. 2025. Available online: http://Orthoptera.SpeciesFile.org (accessed on 7 March 2025).
- Song, C.; Lin, X.; Wang, Q.; Wang, X. DNA barcodes successfully delimit morphospecies in a superdiverse insect genus. Zool. Scr. 2018, 47, 311–324. [Google Scholar] [CrossRef]
- Stasiukynas, L.; Havelka, J.; da Silva, F.L.; Jimenez, M.F.T.; Podėnas, S.; Lekoveckaitė, A. COI Insights into Diversity and Species Delimitation of Immature Stages of Non-Biting Midges (Diptera: Chironomidae). Insects 2025, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Ling, C.; Ho, S.Y.W.; Zhu, C.-D.; Mueller, R. Comparison of methods for molecular species delimitation across a range of speciation scenarios. Syst. Biol. 2018, 67, 830–846. [Google Scholar] [CrossRef]
- Liang, J.; Wang, S.; Zhang, J.; Chen, J.; Fu, S.; Ye, Z.; Xue, H.-J.; Li, Y.; Bu, W. Species delimitation methods facilitate the identification of cryptic species within the broadly distributed species in Homoeocerus (Tliponius) (Insecta: Hemiptera: Coreidae). Insects 2025, 16, 797. [Google Scholar] [CrossRef]
- Huang, W.; Xie, X.; Huo, L.; Liang, X.; Wang, X.; Chen, X. An integrative DNA barcoding framework of ladybird beetles (Coleoptera: Coccinellidae). Sci. Rep. 2020, 10, 10063. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
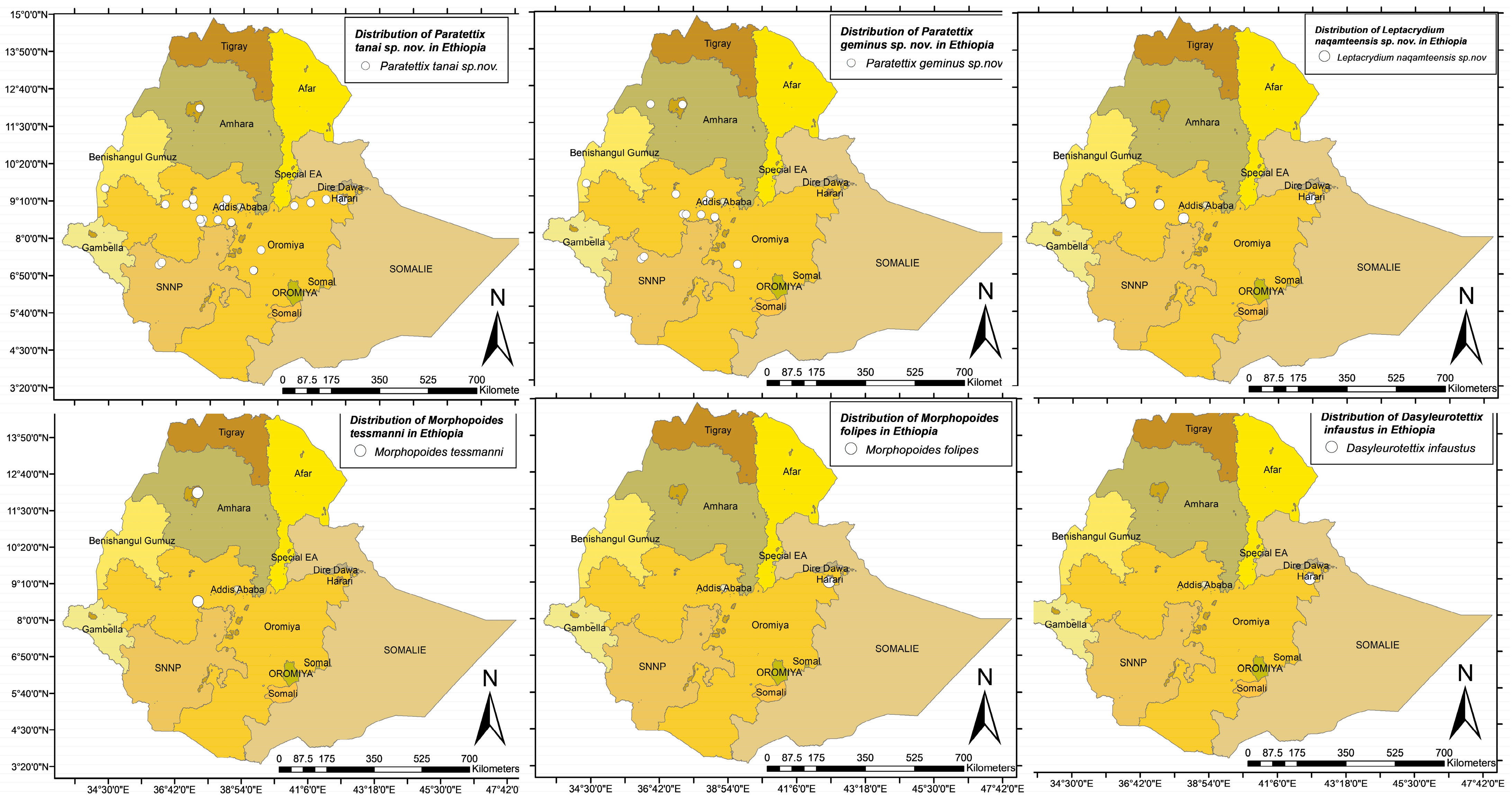
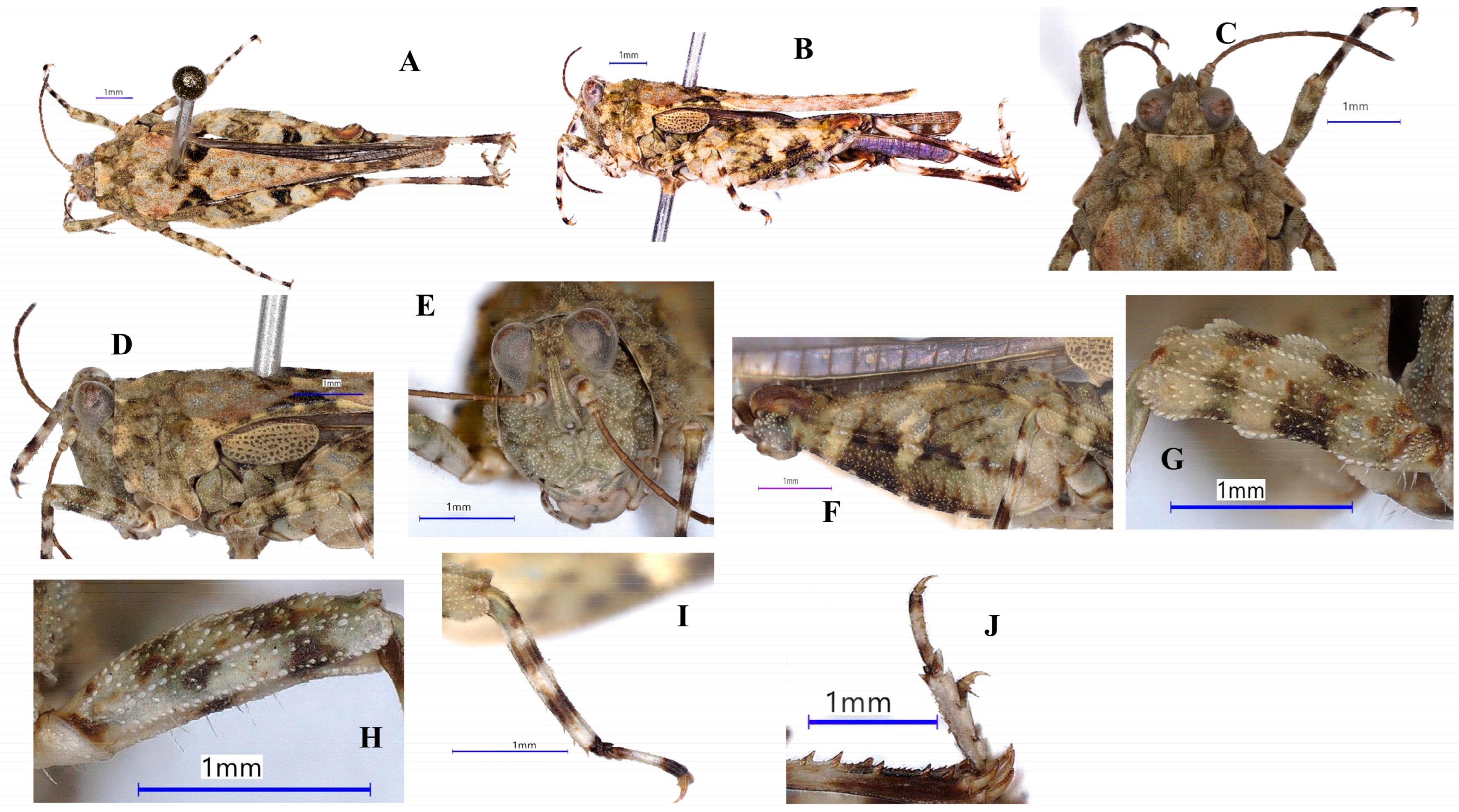
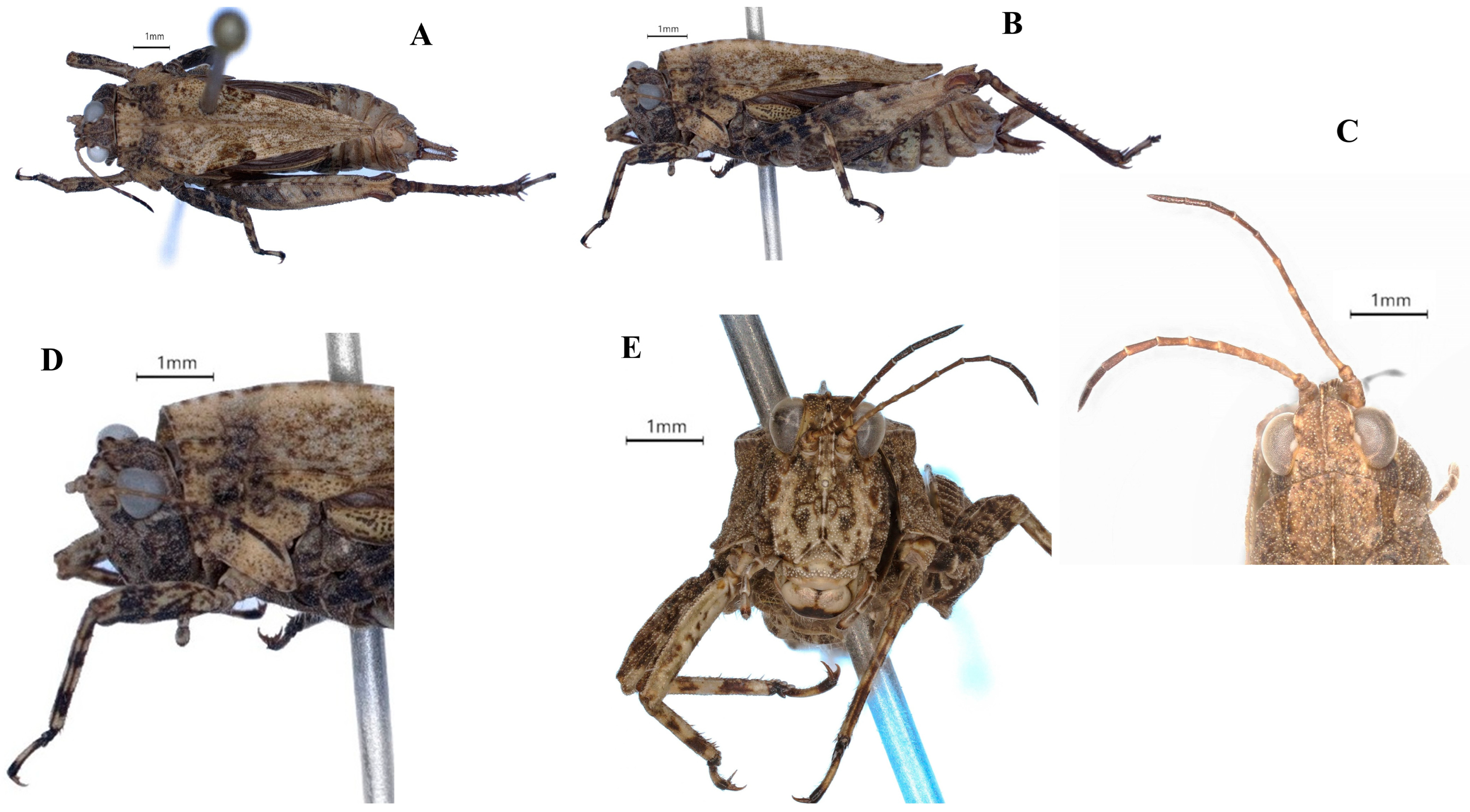
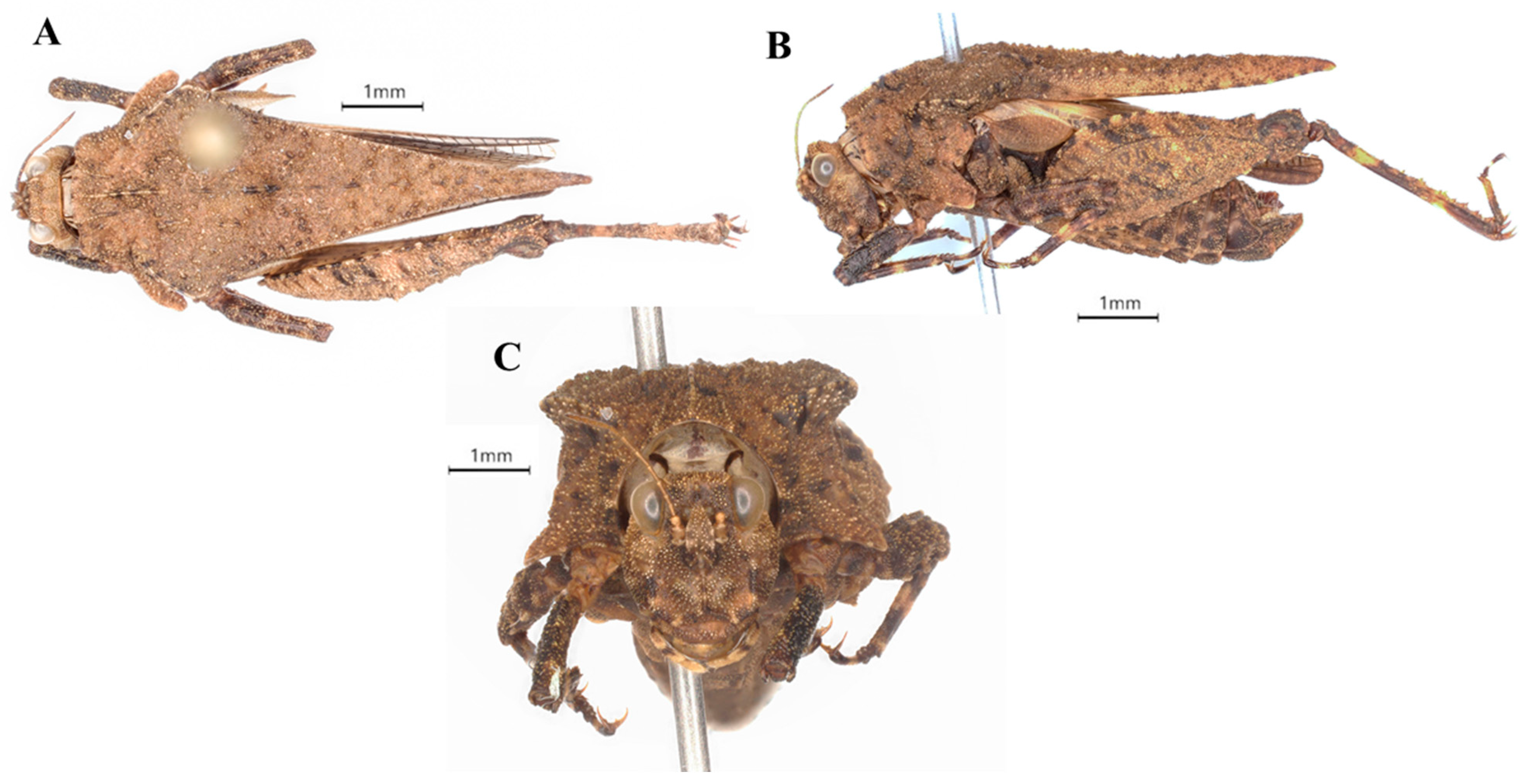

| Region | Zones | District/Town | Specific Places | Habitat Type | Species | Number of Samples | Collector | Collection Date | Altitude (m) | Longitude | Latitude |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oromiya | West Showa | Tokkee Kutaye | Boji River | River bench and shore | Morphopoides tessmanni Günther, 1939 | 2 | Tarekegn F. and Baritu T. | 10 March 2023 | 1945 | 37.4753 | 8.5918 |
| Amhara | South Gonder | Addis Zemen | River crossing the town | River bench and shore | M. tessmanni | 2 | Tarekegn F. | 25 February 2025 | 1988 | 37.4639 | 12.0719 |
| Amhara | South Gonder | Addis Zemen | River crossing the town | River bench and shore | Paratettix geminus sp. nov. | 1 | Tarekegn F. | 25 February 2023 | 1988 | 37.4639 | 12.0719 |
| Amhara | South Gonder | Addis Zemen | River crossing the town | River bench and shore | P. geminus sp. nov. | 9 | M. Husemann | 1 March 2023 | 1988 | 37.7736 | 12.1136 |
| Amhara | South Gonder | Addis Zemen | Tara Gedam | Church forest, near pond | P. tanai sp. nov. | 1 | M. Husemann | 28 March 2023 | 2291 | 37.7473 | 12.1259 |
| Amhara | Bahir Dar | Lake Tana | Zege Peninsula | Wetland | P. geminus sp. nov. | 6 | M. Husemann | 1 March 2023 | 1988 | 37.7736 | 12.1136 |
| Oromiya | E/Wallaga | Guto Gida | Calalaqi | Wetland | Leptacrydium naqamteensis sp. nov. | 1 | Tarekegn F. | 7 August 2023 | 2097 | 36.3455 | 9.6051 |
| Oromiya | W/Showa | Caliya | Gedo | River bench and shore | L. naqamteensis sp. nov. | 1 | Tarekegn F. | 8 August 2023 | 2502 | 37.2738 | 9.0470 |
| Harari | Harari | Dherer Walda | Dherer River | River bench and shore | M. folipes Hancock, 1908 | 4 | Tarekegn F. | 7 October 2023 | 1394 | 42.1424 | 9.1711 |
| Harari | Harari | Dherer Walda | Dherer River | River bench and shore | Dasyleurotettix infaustus Walker, 1871 | 1 | Tarekegn F. | 7 October 2023 | 1394 | 42.1424 | 9.1711 |
| South Ethiopia | Bonga | Bonga | Sheka River | Wetland | L. naqamteensis sp. nov. | 3 | Tarekegn F. | 14 October 2023 | 1581 | 36.1410 | 7.1713 |
| South Ethiopia | Bonga | Bonga | Sheka River | Wetland | P. tanai sp. nov. | 1 | Tarekegn F. | 14 October 2023 | 1581 | 36.1410 | 7.1713 |
| Oromiya | Jimma | Amiyo | Gojeb River | Wetland near river | L. naqamteensis sp. nov. | 3 | Tarekegn F. | 15 October 2023 | 1298 | 36.2322 | 7.2439 |
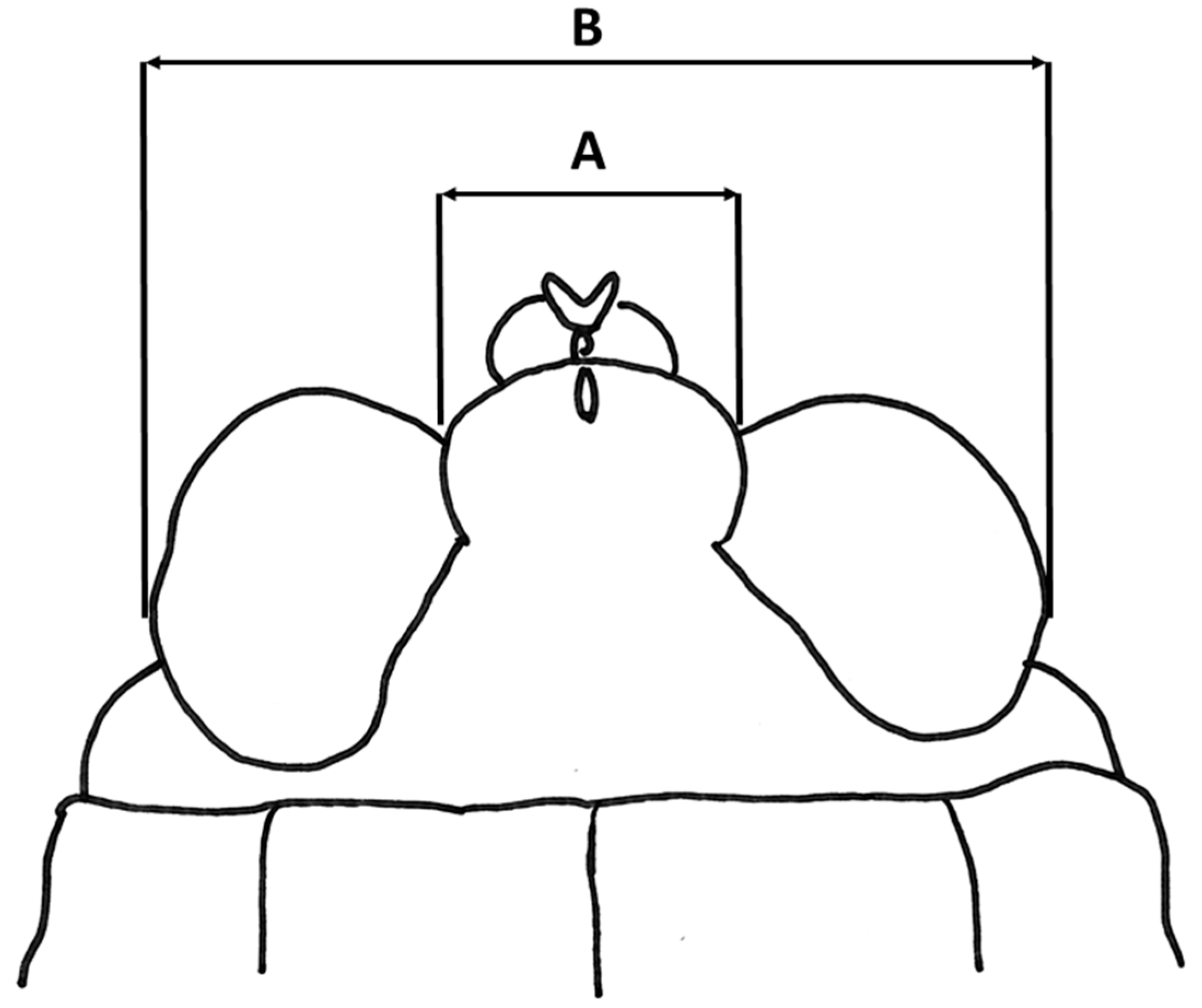
| Key Characters | Sex | P. subpustulatus | P. macrostenus | P. tanai sp. nov. | P. geminus sp. nov. | P. meridionalis |
|---|---|---|---|---|---|---|
| Frontal costa | not incurved | not incurved | not incurved | incurved | incurved | |
| Eyes D/H | <1 | <1 | 1 | >1 | >1 | |
| Fastigium/total width of eyes + fastigium | 26–30% | 24–29% | 24–28% | 29–33% | ||
| Carinae of the lateral lobes of the pronotum | not pronounced | not pronounced | not pronounced | very pronounced and curved | very pronounced and curved | |
| Middle carina of the pronotum | nearly straight | nearly straight | with one or two conspicuous elevations | with two conspicuous elevations | with one or two conspicuous elevations | |
| Pronotal disk L/B | >1 | >1 | <1 | <1 | <1 | |
| Pronotal length | female | 10–14 mm | 10–12 mm | 8.5–11.5 mm | 7.5–11 mm | 8–10 mm |
| male | 8.5–12.5 mm | 8.5–12.5 mm | 8–11 mm | 7–9 mm | 7.5–9 mm | |
| Pronotal breadth | female | 2.5–3.2 mm | 2.2–2.6 mm | 2.5–3.2 mm | 2.3–3.2 mm | 2.7–3.0 mm |
| male | 2.0–2.5 mm | 1.8–2.5 mm | 2.1–2.4 mm | 1.9–2.9 mm | 2.0–2.5 mm | |
| Borders of the midfemur | nearly straight | nearly straight | undulating | strongly undulating | strongly undulating | |
| posttibia | brown | brown | dark gray with a basal ring, sometimes with an unclear ring at the middle | dark gray with 2 clear rings | dark brown with 2 clear rings | |
| Coloration | mostly dark brown, sometimes with white patches | dark brown | spottled with darker and white patches | spottled with darker and white patches | spottled with dark and white patches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fite, T.; Devriese, H.; Kulanek, D.; Skejo, J.; Kasalo, N.; Misganaw, M.; Tefera, T.; Villinger, J.; Husemann, M. Tetrigidae of Ethiopia: First Species Delimitation via DNA Barcoding and Description of Three New Species. Taxonomy 2025, 5, 49. https://doi.org/10.3390/taxonomy5030049
Fite T, Devriese H, Kulanek D, Skejo J, Kasalo N, Misganaw M, Tefera T, Villinger J, Husemann M. Tetrigidae of Ethiopia: First Species Delimitation via DNA Barcoding and Description of Three New Species. Taxonomy. 2025; 5(3):49. https://doi.org/10.3390/taxonomy5030049
Chicago/Turabian StyleFite, Tarekegn, Hendrik Devriese, Dustin Kulanek, Josip Skejo, Niko Kasalo, Manaye Misganaw, Tadele Tefera, Jandouwe Villinger, and Martin Husemann. 2025. "Tetrigidae of Ethiopia: First Species Delimitation via DNA Barcoding and Description of Three New Species" Taxonomy 5, no. 3: 49. https://doi.org/10.3390/taxonomy5030049
APA StyleFite, T., Devriese, H., Kulanek, D., Skejo, J., Kasalo, N., Misganaw, M., Tefera, T., Villinger, J., & Husemann, M. (2025). Tetrigidae of Ethiopia: First Species Delimitation via DNA Barcoding and Description of Three New Species. Taxonomy, 5(3), 49. https://doi.org/10.3390/taxonomy5030049








