Anopheles neivai (Diptera: Culicidae) Morphogenetic Analysis from the Pacific Coast to the Premontane Humid Forest of Colombia
Abstract
1. Introduction
2. Materials and Methods
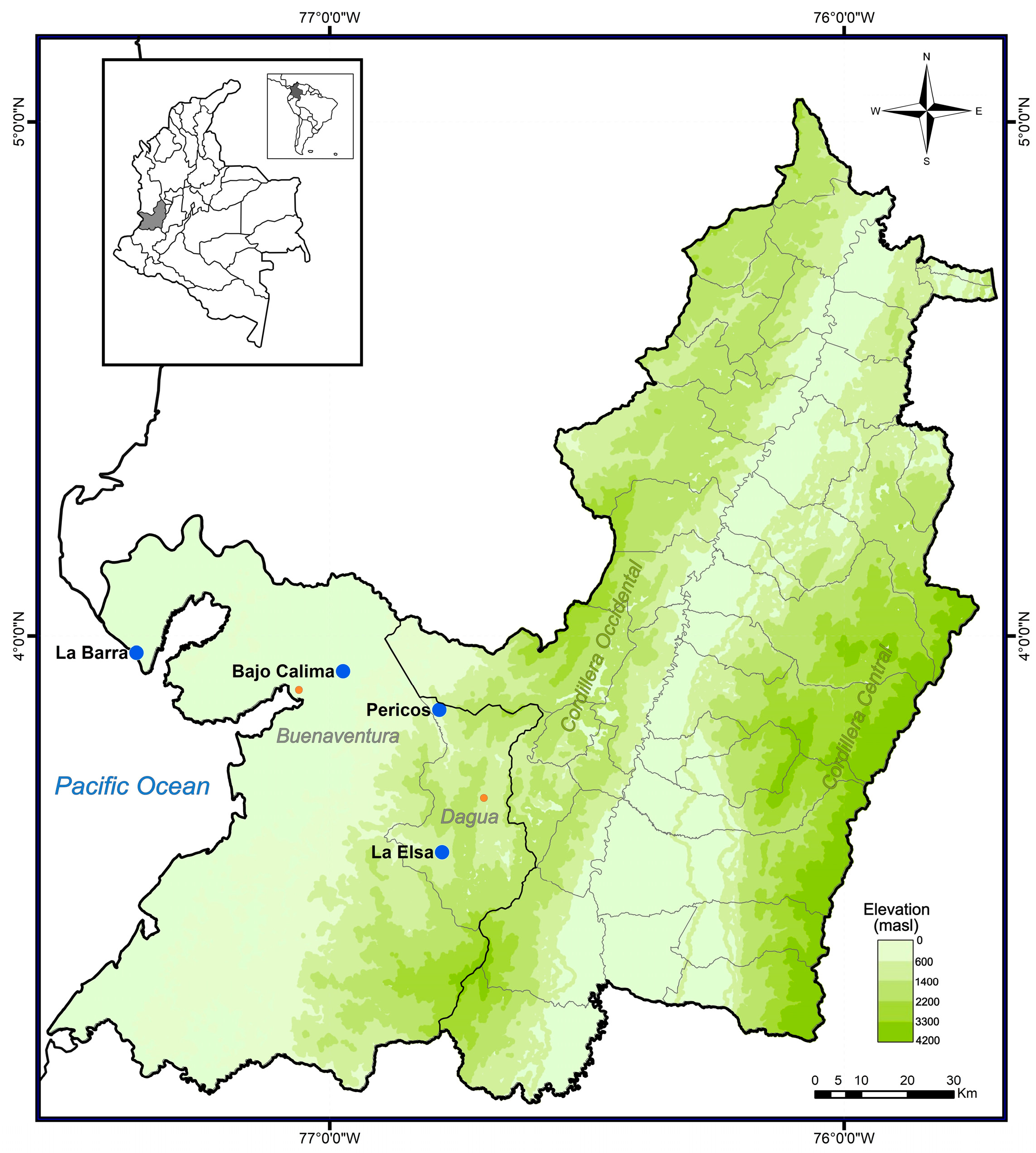
2.1. Study Sites
2.2. Specimen Collecting/Data Collection
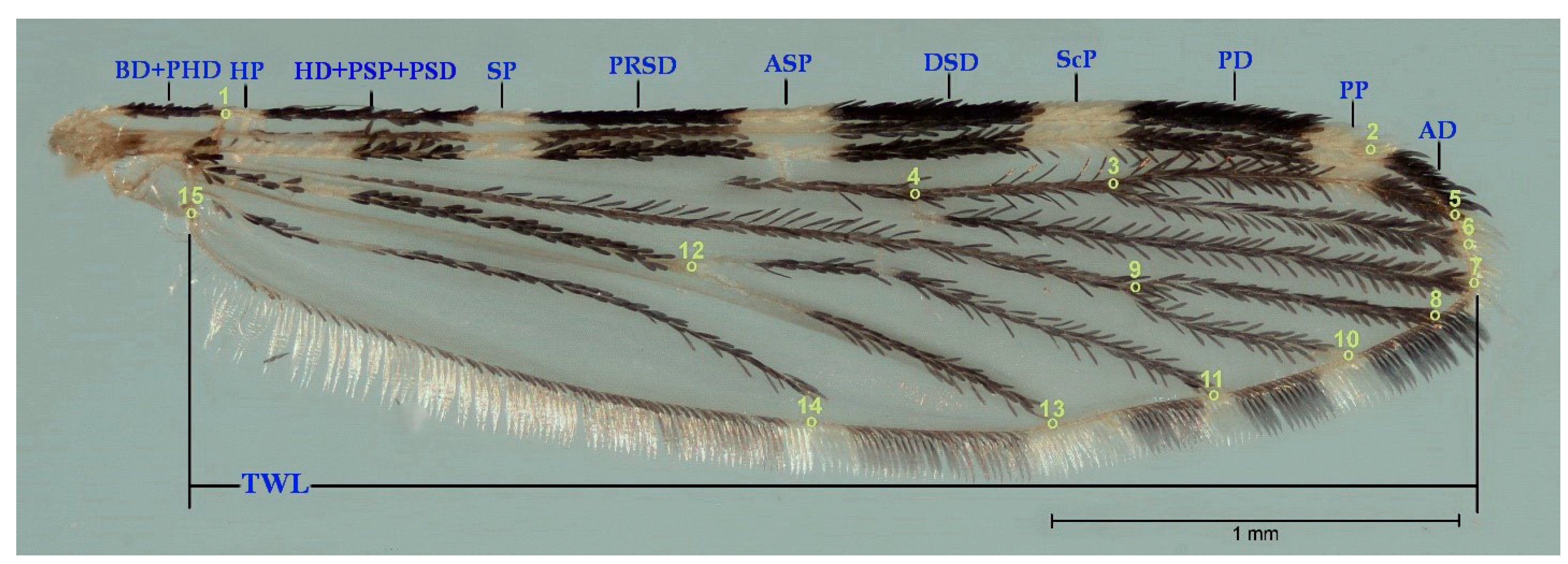
2.3. Morphological Analysis: Traditional Morphology
2.4. Morphological Analysis: Geometric Morphometrics
2.5. Extraction, Amplification, and DNA Sequencing
2.6. Alignment and Sequence Analysis
2.7. FST Y QST Population Parameter Evaluation
- : proportion of variance explained by groups.
- : Mean squares between groups.
- : Mean squares within groups.
3. Results
3.1. Morphology Analysis
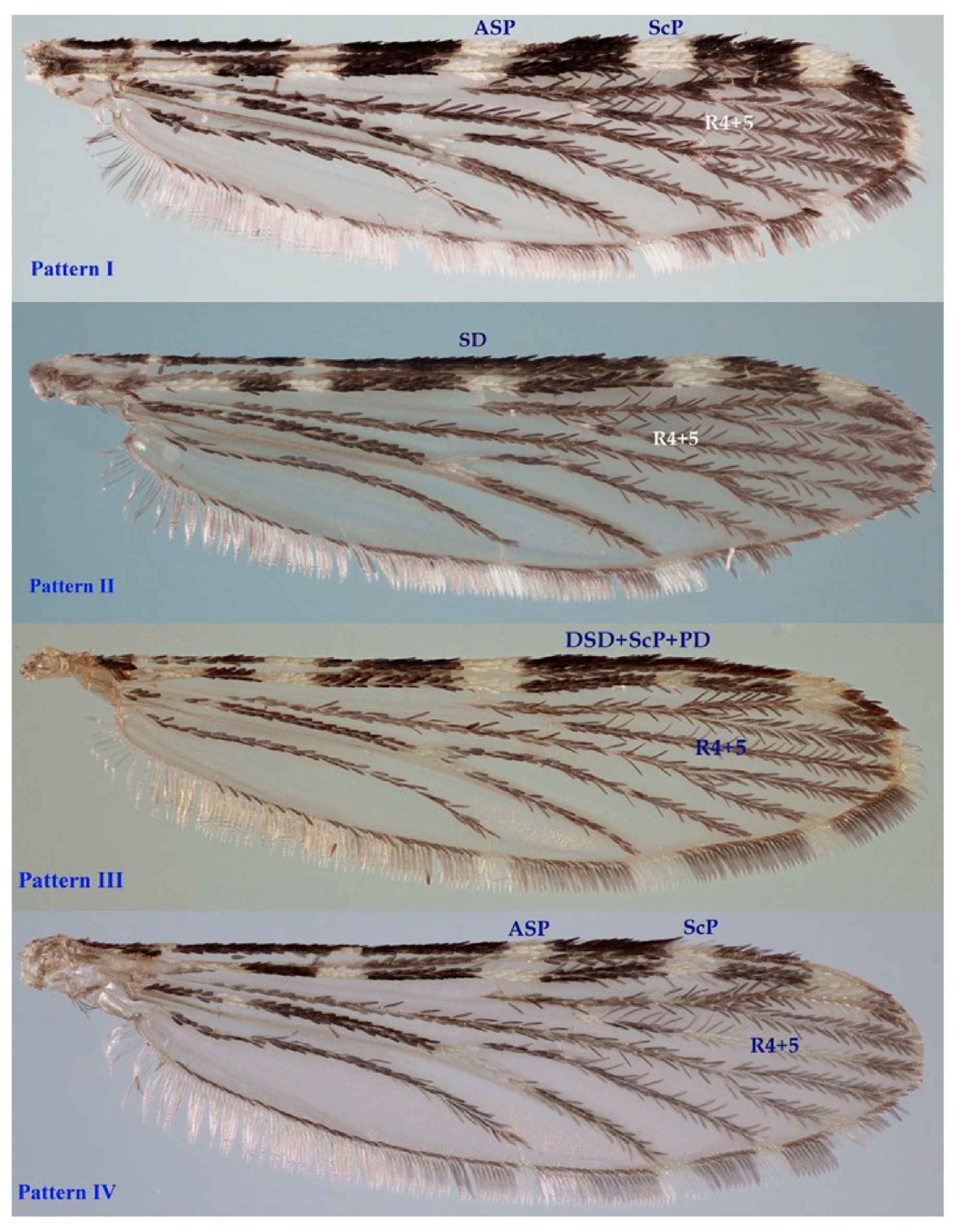
3.2. Morphology Analysis: Traditional Morphology
3.3. Morphology Analysis: Geometric Morphometrics
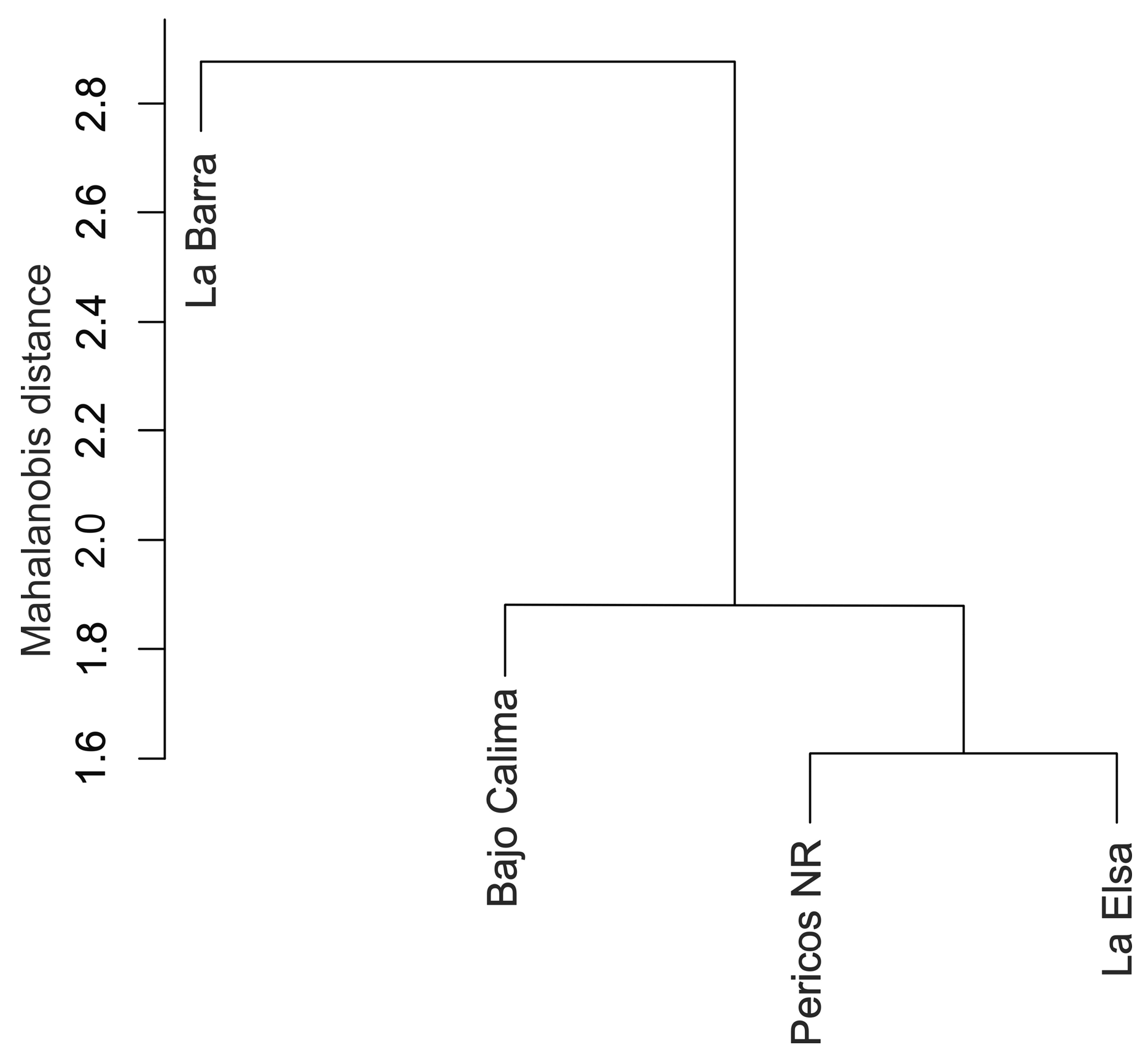
3.4. Molecular Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirci, B.; Lee, Y.; Lanzaro, G.C.; Alten, B. Altitudinal genetic and morphometric variation among populations of Culex theileri Theobald (Diptera: Culicidae) from northeastern Turkey. J. Vector Ecol. 2012, 37, 197–209. [Google Scholar] [CrossRef]
- Laporta, G.Z.; Ramos, D.G.; Ribeiro, M.C.; Sallum, M.A.M. Habitat suitability of Anopheles vector species and association with human malaria in the Atlantic Forest in south-eastern Brazil. Mem. Do Inst. Oswaldo Cruz 2011, 106, 239–245. [Google Scholar] [CrossRef]
- Parker, A.; Gardner, A.; Perez, M.; Allan, B.; Muturi, E. Container Size Alters the Outcome of Interspecific Competition Between Aedes aegypti (Diptera: Culicidae) and Aedes albopictus. J. Med. Entomol. 2019, 56, 708–715. [Google Scholar] [CrossRef]
- Prudhomme, J.; Velo, E.; Bino, S.; Kadriaj, P.; Mersini, K.; Gunay, F.; Alten, B. Altitudinal variations in wing morphology of Aedes albopictus (Diptera, Culicidae) in Albania, the region where it was first recorded in Europe. Parasite 2019, 26, 55. [Google Scholar] [CrossRef]
- Reiskind, M.; Zarrabi, A. Is bigger really bigger? Differential responses to temperature in measures of body size of the mosquito, Aedes albopictus. J. Insect Physiol. 2012, 58, 911–917. [Google Scholar] [CrossRef]
- Gómez, G.F.; Márquez, E.J.; Gutiérrez, L.A.; Conn, J.E.; Correa, M.M. Geometric morphometric analysis of Colombian Anopheles albimanus (Diptera: Culicidae) reveals significant effect of environmental factors on wing traits and presence of a metapopulation. Acta Trop. 2014, 135, 75–85. [Google Scholar] [CrossRef]
- Mayekar, H.; Solanki, P.; Arya, H.; Aradhya, R.; Suravajhala, P.; Loeschcke, P.; Rajpurohit, S. Tropical High-Altitude Insects Show Limited Capacity to Handle High Temperatures. bioRxiv 2023. [Google Scholar] [CrossRef]
- González, R.; Cárdenas, H.; Marín-Londoño, O.A. Temperature effect on the phenotypic expression of characters of costal spots Nyssorhynchus triannulatus (Diptera: Culicidae: Anophelinae). Rev. Colomb. De Entomol. 2021, 47, e8456. [Google Scholar] [CrossRef]
- Chatpiyaphat, S.; Sumruayphol, S.; Dujardin, J.P.; Samung, Y.; Phayakkaphon, A.; Cui, L.; Ruangsittichai, J.; Sungvornyothin, S.; Sattabongkot, J.; Sriwichai, P. Geometric morphometrics to distinguish the cryptic species Anopheles minimus and An. harrisoni in malaria hot spot villages, western Thailand. Med. Veter-Entomol. 2020, 35, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Cella, W.; Junior, R.; Pimenta, P.; Monteiro, W. Morphometry of the wings of Anopheles aquasalis in simulated scenarios of climate change. Rev. Da Soc. Bras. Med. Trop. 2024, 57, e00704-2024. [Google Scholar] [CrossRef]
- Leyton Ramos, L.M.; Aguirre Obando, O.A.; Duque, J.E.; García-Merchán, V.H. Effect of altitude on wing metric variation of Aedes aegypti (Diptera: Culicidae) in a region of the Colombian Central Andes. PLoS ONE 2020, 15, e0228975. [Google Scholar] [CrossRef]
- Lorenz, C.; Marques, T.C.; Sallum, M.; Suesdek, L. Altitudinal population structure and microevolution of the malaria vector Anopheles cruzii (Diptera: Culicidae). Parasites Vectors 2014, 7, 581. [Google Scholar] [CrossRef]
- Escovar, J.; González, R.; Quiñones, M. Anthropophilic biting behaviour of Anopheles (Kerteszia) neivai Howard, Dyar & Knab associated with Fishermen’s activities in a malaria-endemic area in the Colombian Pacific. Mem Inst Oswaldo Cruz. 2013, 108, 1057–1064. [Google Scholar] [PubMed] [PubMed Central]
- Bourke, B.; Wilkerson, R.; Ruiz-Lopez, F.; Justi, S.; Pecor, D.; Quiñones, M.; Navarro, J.; Alarcón-Ormaza, J.; Alarcón-Ormaza, J., Jr.; González, R.; et al. High levels of diversity in Anopheles Subgenus Kerteszia revealed by species delimitation analyses. Genes 2023, 14, 344. [Google Scholar] [CrossRef]
- González, R.; Carrejo, N. Introducción al Estudio Taxonómico de Anopheles de Colombia: Claves y Notas de Distribución, 2nd ed.; Programa Editorial Universidad del Valle: Cali, Colombia, 2009. [Google Scholar]
- Ahumada, M.; Orjuela, L.; Pareja, P.; Conde, M.; Cabarcas, D.; Cubillos, E.; López, J.; Beier, J.; Herrera, S.; Quiñones, M. Spatial distributions of Anopheles species in relation to malaria incidence at 70 localities in the highly endemic Northwest and South Pacific coast regions of Colombia. Malar. J. 2016, 15, 407. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Lerma, J.; Solarte, Y.; Giraldo-Calderón, G.; Quiñones, M.; Ruiz-López, F.; Wilkerson, R.; González, R. Malaria vector species in Colombia: A review. Mem. Inst. Oswaldo Cruz 2011, 106, 223–238. [Google Scholar] [CrossRef]
- Murillo, C.; Jaramillo, C.; Quintero, J.; Suarez, M. Biología de Anopheles (Kerteszia) neivai H., D. & K., 1913 (DIPTERA: CULICIDAE) en la costa Pacífica de Colombia. Estructura etaria y transmisión de malaria. Rev. Saude Publica 1989, 23, 363–367. Available online: https://www.scielosp.org/pdf/rsp/1989.v23n5/363-367/es (accessed on 18 September 2024).
- Murillo, C.; Astaiza, R.; Fajardo, P. Biología de Anopheles (Kerteszia) neivai H., D. & K., 1913 (Diptera:Culicidae) en la costa Pacífica de Colombia. III. Medidas de luminosidad y el comportamiento de picadura. Rev. Saude Publica 1988, 22, 109–112. [Google Scholar]
- Word Health Organization (WHO). WHO Guidelines for Malaria 2023. Available online: https://www.who.int/publications/i/item/guidelines-for-malaria (accessed on 18 September 2024).
- López-Rubio, A.; Suaza-Vasco, J.; Solari, S.; Gutiérrez-Builes, L.; Porter, C.; Uribe, S. Intraspecific phylogeny of Anopheles (Kerteszia) neivai Howard, Dyar & Knab, 1913, based on mitochondrial and nuclear ribosomal genes. Infect. Genet. Evol. 2019, 67, 183–190. [Google Scholar] [CrossRef] [PubMed]
- López-Rubio, A.; Suaza, J.; Porter, C.; Uribe, S.; Bedoya, G.; Vélez, I. Phylogenetic signal at the Cytb-SertRNA-IG1-ND1 mitochondrial region in Anopheles (Kerteszia) neivai Howard, Dyar & Knab, 1913. Biomédica 2017, 37, 143–154. [Google Scholar] [PubMed]
- López-Rubio, A.; Suaza-Vasco, J.; Marcet, P.; Ruíz-Molina, N.; Cáceres, L.; Porter, C.; Uribe, S. Use of DNA barcoding to distinguish the malaria vector Anopheles neivai in Colombia. Zootaxa 2016, 4175, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, T.; McCairns, R.; O’Hara, R.; Merilä, J. QST–FST comparisons: Evolutionary and ecological insights from genomic heterogeneity. Nat. Rev. Genet. 2013, 14, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Gentry, A.; Dodson, C. Contributions of non-trees to species richness of a tropical rainforest. Biotropica 1987, 19, 149–156. [Google Scholar] [CrossRef]
- Faber-Langendoen, D.; Gentry, A. The structure and diversity of rain forests at Bajo Calima, Chocó Region, Western Colombia. Biotropica 1991, 23, 2–11. [Google Scholar] [CrossRef]
- Mejía, M. Litoral Pacífico colombiano y cuenca del Atrato: Clima y uso de la tierra. Cuad. Geogr. 1991, 3, 63–68. [Google Scholar]
- CONIF (Corporación Nacional de Investigación y Fomento Forestal). Participación Comunitaria Para Manejo de Bosques Secundarios del Bajo Calima; CVC: Bogotá, Colombia, 1997. [Google Scholar]
- CVC (Corporación Autónoma Regional del Valle del Cauca). Memoria del Proyecto de Protección del Ecosistema de la Quebrada Pericos. 2007. Available online: https://es.scribd.com/document/22251256/Memoria-Area-en-Conservacion-Microcuenca-Quebrada-Pericos-Pacifico-Colombiano-Buenaventura (accessed on 24 August 2020).
- Rodríguez, N. Ecosistemas de los Andes Colombianos; Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt: Bogotá, Colombia, 2006. [Google Scholar]
- Vargas, F.; Bolaños, M. Presencia de reptiles en la región de Anchicayá, Pacífico Colombiano, a través de un gradiente de deforestación. Caldasia 1999, 21, 235–238. [Google Scholar]
- Harrison, B.; Ruiz-López, F.; Calderón, G.; Savage, H.; Pecor, J.; Wilkerson, R. Anopheles (Kerteszia) lepidotus (Diptera: Culicidae), not the malaria vector we thought it was: Revised male and female morphology; larva, pupa, and male genitalia characters; and molecular verification. Zootaxa 2012, 3218, 1–17. [Google Scholar] [CrossRef]
- Wilkerson, R.; Peyton, E. Standardized nomenclature for the costal wing spots of the genus Anopheles and other spotted-wing mosquitoes (Diptera: Culicidae). Med. Èntomol. 1990, 27, 207–224. [Google Scholar] [CrossRef]
- Gareth, J.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: Berlin/Heidelberg, Germany, 2013; Available online: https://www.statlearning.com/ (accessed on 18 February 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 21 March 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models. R package Version 3.1-143. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 21 March 2020).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://www.john-fox.ca/Companion/ (accessed on 21 March 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Dujardin, J. Morphometrics applied to medical entomology. Infect. Genet. Evol. 2008, 8, 875–890. [Google Scholar] [CrossRef]
- Landis, J.; Koch, G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Bender, W.; Spierer, P.; Hogness, D. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the Bithorax complex in Drosophila melanogaster. J. Mol. Biol. 1983, 168, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Birungi, J.; Munstermann, L. Genetic Structure of Aedes albopictus (Diptera: Culicidae) Populations Based on Mitochondrial ND5 Sequences: Evidence for an independent invasion into Brazil and United States. Ann. Entomol. Soc. Am. 2002, 95, 125–132. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. Available online: https://pubmed.ncbi.nlm.nih.gov/7881515/ (accessed on 19 November 2019).
- Tálaga, S.; Leroy, C.; Guidez, A.; Girod, R.; Dejean, A.; Murienne, J. DNA reference libraries of French Guianese mosquitoes for barcoding and metabarcoding. PLoS ONE 2017, 12, e0176993. [Google Scholar] [CrossRef]
- Geneious Prime 2020.1. 2020. Available online: https://www.geneious.com (accessed on 14 April 2020).
- Edgar, R. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- NCBI (National Center for Biotechnology Information). Basic Local Alignment Search Tool. 2020. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 25 June 2020).
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Bandelt, H.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.; Bryant, D. PopART: Full-Feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. Available online: https://popart.maths.otago.ac.nz/ (accessed on 27 June 2020). [CrossRef]
- Excoffier, L.; Lischer, H. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Oliveira, T.; Foster, P.; Bergo, E.; Nagaki, S.; Sanabani, S.; Marinotti, O.; Marinotti, P.; Sallum, M. Mitochondrial Genomes of Anopheles (Kerteszia) (Diptera: Culicidae) from the Atlantic Forest, Brazil. J. Med. Entomol. 2016, 53, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, V.; Kremer, A. Genetic variability at neutral markers, quantitative trait loci and traits in a subdivided population under selection. Genetics 2003, 164, 1205–1219. [Google Scholar] [CrossRef]
- Zavortink, T. A review of the subgenus Kerteszia of Anopheles. Contrib. Am. Entomol. Inst. 1973, 9, 1–37. [Google Scholar]
- Harbach, R.; Navarro, J. A new species of Anopheles subgenus Kerteszia (Diptera: Culicidae) from Venezuela. Insect Syst. Evol. 1996, 27, 205–207. [Google Scholar] [CrossRef]
- Cova-García, P.; Pulido, J.; Escalante, C. Anopheles (Kerteszia) hilli (Diptera, Culicidae), una nueva especie de Venezuela. Boletín Dir. Malariol. Saneam. Ambient. 1976, 16, 344–353. [Google Scholar]
- Cova-García, P.; Pulido, J.; Escalante, C. Anopheles (Kerteszia) gonzalezrinconesi n. sp. (Diptera, Culicidae) de Venezuela. Boletín Dir. Malariol. Saneam. Ambient. 1977, 17, 140–149. [Google Scholar]
- Montoya-Lerma, J.; Murillo, C.; Solarte, Y. Variación fenotípica de Anopheles (K) neivai (Diptera: Culicidae) en la costa Pacífica de Colombia. Colomb. Médica 1987, 8, 25–27. [Google Scholar] [CrossRef]
- Blanke, A. Analysis of modularity and integration suggests evolution of dragonfly wing venation mainly in response to functional demands. J. R. Soc. Interface 2018, 15, 20180277. [Google Scholar] [CrossRef]
- Benítez, H.A.; Püschel, T.A.; Suazo, M.J. Drosophila Wing Integration and Modularity: A Multi-Level Approach to Understand the History of Morphological Structures. Biology 2022, 11, 567. [Google Scholar] [CrossRef]
- Gómez, G.; Correa, M. Discrimination of Neotropical Anopheles species based on molecular and wing geometric morphometric traits. Infect. Genet. Evol. 2017, 54, 379–386. [Google Scholar] [CrossRef]
- Rodríguez-Zabala, J.; González, R.; Correa, M.; Gómez, G. Análisis morfométrico de dos poblaciones de Anopheles (Anopheles) calderoni (Diptera: Culicidae) del suroccidente colombiano. Rev. Mex. Biodivers. 2016, 87, 966–971. [Google Scholar] [CrossRef]
- Shelomi, M. Where are we now? Bergmann’s rule Sensu Lato in insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.; Astaiza, R.; Fajardo, O. Biología de Anopheles (Kerteszia) neivai H., D. & K., 1913 (Diptera: Culicidae) en la costa pacífica de Colombia I. Fluctuación de la población larval y características de sus criaderos. Rev. Saude Publica 1988, 22, 94–100. [Google Scholar] [PubMed]
- Whitlock, M.; McCauley, D. Indirect measures of gene flow and migration: FST≠1/(4Nm+1). Heredity 1999, 82, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Vázquez-Domínguez, E.; Castañeda-Rico, S.; Garrido-Garduño, T.; Gutiérrez-García, T. Avances metodológicos para el estudio conjunto de la información genética, genealógica y geográfica en análisis evolutivos y de distribución. Rev. Chil. Hist. Nat. 2009, 82, 277–297. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Bu, W. Exploring Large-Scale Patterns of Genetic Variation in the COI Gene among Insecta: Implications for DNA Barcoding and Threshold-Based Species Delimitation Studies. Insects 2022, 13, 425. [Google Scholar] [CrossRef]
- Miller, J.; Wood, B.; Hamilton, M. FST and QST under neutrality. Genetics 2008, 180, 1023–1037. [Google Scholar] [CrossRef]
- Carstens, B.C.; Richards, C.L. Integrating coalescent and ecological niche modeling in comparative phylogeography. Evolution 2007, 61, 1439–1454. [Google Scholar] [CrossRef]

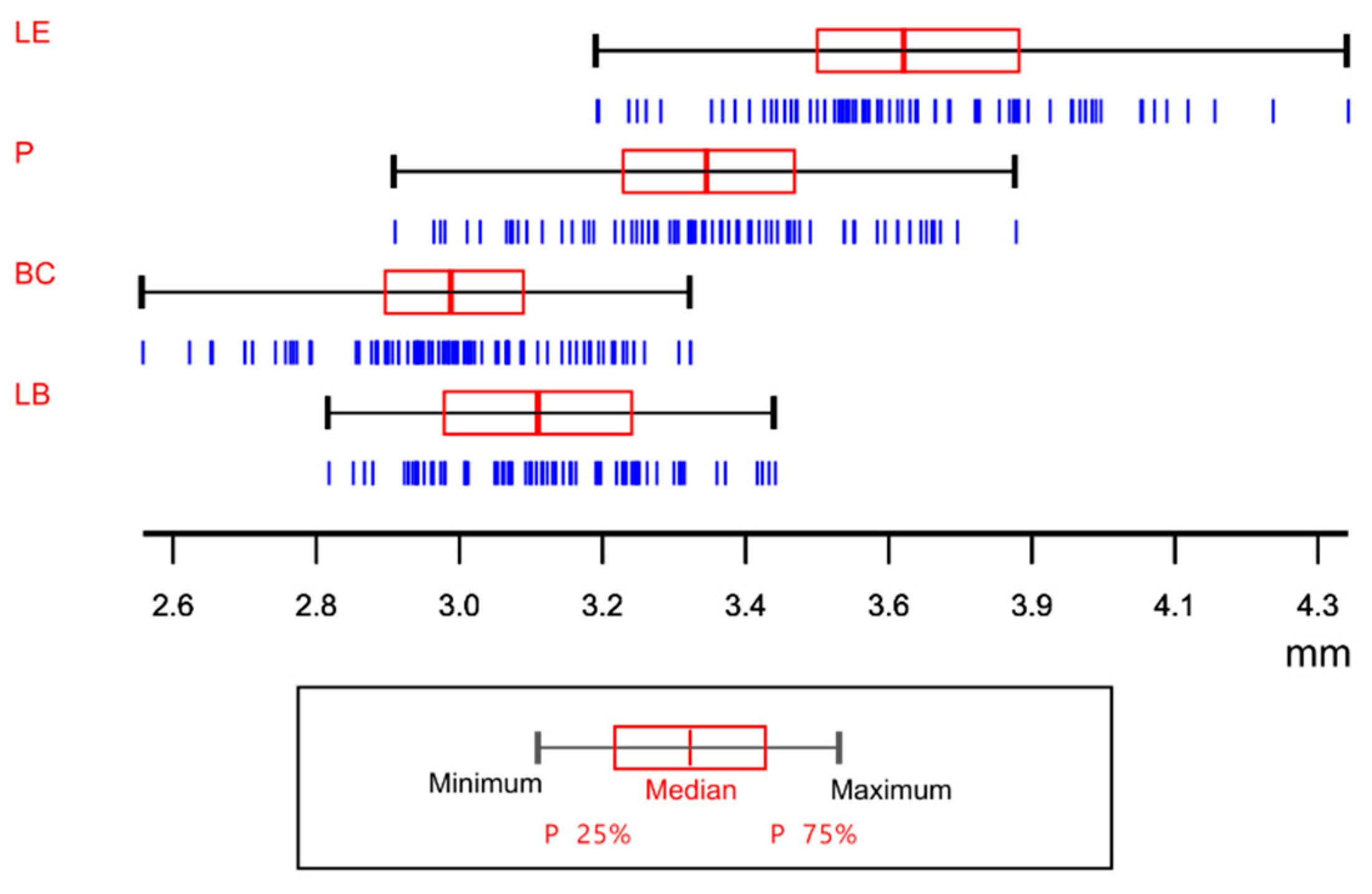

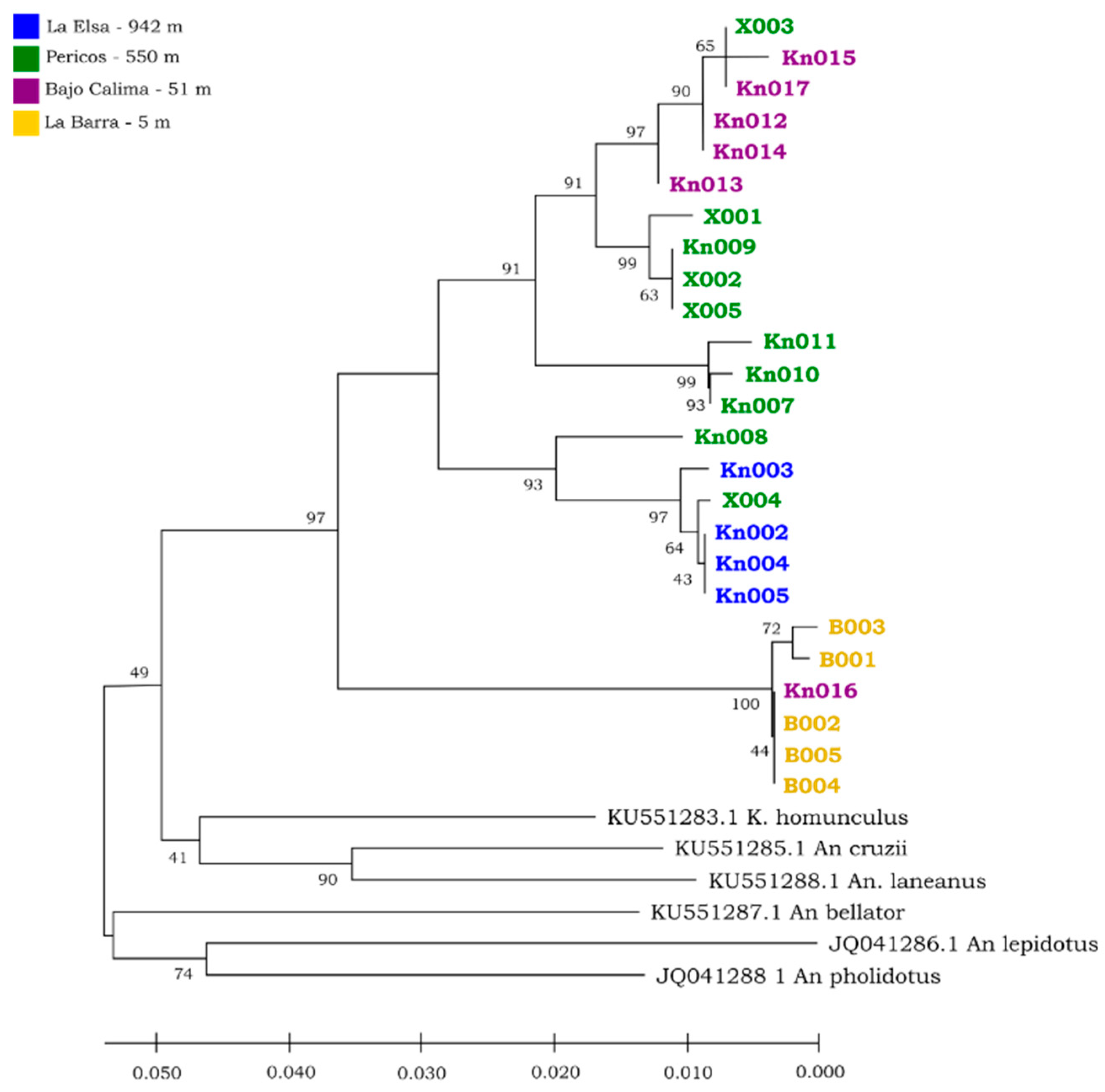

| Variable | R2 | Model | R2 Model |
|---|---|---|---|
| BD + PHD | 0.4560 | BD + PHD~HP + Population * | 0.428 |
| HP | 0.2779 | HP~BDPHD + HDPSD + Population * | 0.237 |
| HD + PSP + PSD | 0.5980 | HD + PSP + PSD ~ SP + PRSD * | 0.533 |
| SP | 0.6158 | SP~HD + PSP + PSD + PRSD * | 0.609 |
| PRSD | 0.6484 | PRSD~HD + PSP + PSD + SP + ASP * | 0.534 |
| ASP | 0.5699 | ASP~PRSD + DSD * | 0.438 |
| DSD | 0.5715 | DSD~ASP + SCP + PD * | 0.523 |
| SCP | 0.5461 | SCP~PD + DSD * | 0.433 |
| PD | 0.4679 | PD~SCP + PP + AD * | 0.375 |
| PP | 0.327 | PP~PD + AD + SCP + DSD * | 0.222 |
| AD | 0.1318 | AD~All variables * | 0.132 |
| Variable | F | d.f. | p-Value | QST | QST/FST |
|---|---|---|---|---|---|
| BD + PHD | 11.26 | 337 | <0.0001 * | 0.1086 | 0.1755 |
| HP | 4.11 | 340 | 0.0069 * | 0.0355 | 0.0574 |
| HD + PSP + PSD | 34.07 | 340 | <0.0001 * | 0.2810 | 0.4541 |
| SP | 35.36 | 340 | <0.0001 * | 0.2887 | 0.4666 |
| PRSD | 35.50 | 340 | <0.0001 * | 0.2895 | 0.4680 |
| ASP | 27.34 | 340 | <0.0001 * | 0.2374 | 0.3836 |
| DSD | 40.61 | 340 | 0.5681 | 0.3188 | 0.5152 |
| ScP | 22.32 | 340 | <0.0001 * | 0.2012 | 0.3252 |
| PD | 69.83 | 337 | <0.0001 * | 0.4508 | 0.7285 |
| PP | 18.59 | 33 | <0.0001 * | 0.1796 | 0.2903 |
| AD | 20.95 | 295 | 0.8397 | 0.2144 | 0.3465 |
| TWL | 181.81 | 341 | <0.0001 | 0.6811 | 1.1008 |
| Variable | LB-BC | LB-PNR | LB-LE | BC-PNR | BC-LE | PNR-LE |
|---|---|---|---|---|---|---|
| BD + PHD | 0.0003 * | <0.0001 * | 0.0130 * | 0.0130 * | <0.0001 * | 0.1090 |
| HP | 0.2530 | 0.1970 | 0.2000 | 0.9940 | 0.0002 * | 0.0002 * |
| HD + PSP + PSD | 0.00008 * | 0.0001 * | <0.0001 * | 0.9990 | 0.6630 | 0.7720 |
| SP | 0.0010 * | 0.00004 * | <0.0001 * | 0.6610 | 0.3600 | 0.9820 |
| PRSD | 0.00001 * | <0.0001 * | 0.00001 * | 0.2070 | 0.9990 | 0.1750 |
| ASP | >0.9999 | 0.0020 * | 0.2410 | 0.0028 * | 0.2660 | 0.1970 |
| SCP | 0.0790 | 0.0600 | 0.0480 * | 0.9950 | 0.9970 | 0.9990 |
| PD | 0.2530 | 0.1970 | 0.2000 | 0.9940 | 0.0002 * | 0.0002 * |
| PP | 0.0250 * | 0.2210 | 0.9900 | 0.8220 | 0.0033 * | 0.0740 |
| TWL | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * |
| Population | H/n | Polymorphic Sites | h | π | D | Fs |
|---|---|---|---|---|---|---|
| B. Calima | 5/6 | 25 | 0.93 | 0.025 | −1.27 | −1.16 |
| Pericos | 6/10 | 25 | 0.84 | 0.025 | 0.02 | −3.83 * |
| B/tura | 4/8 | 24 | 0.75 | 0.030 | 0.74 | −2.05 |
| Guatemala | 6/8 | 22 | 0.92 | 0.021 | −0.62 | −2.83 * |
| B. Solano | 6/9 | 7 | 0.88 | 0.005 | −1.18 | −9.16 * |
| Nuquí | 4/8 | 6 | 0.78 | 0.005 | −0.99 | −7.66 * |
| Portobelo | 3/6 | 3 | 0.73 | 0.004 | 0.86 | −5.14 * |
| La Elsa | 2/4 | 2 | 0.50 | 0.002 | −0.70 | −3.13 * |
| La Barra | 2/5 | 1 | 0.40 | 0.001 | −0.81 | −7.58 * |
| Litoral | 2/5 | 1 | 0.60 | 0.001 | 1.22 | −6.27 * |
| Tumaco | 2/8 | 1 | 0.25 | 0.0007 | −1.05 | −18.8 * |
| Acandí | 1/2 | 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| Guyana | 1/4 | 0 | 0.00 | 0.000 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-García, N.; Canas-Bermúdez, S.; González-Obando, R.; Cárdenas, H.; Rivera-Franco, N. Anopheles neivai (Diptera: Culicidae) Morphogenetic Analysis from the Pacific Coast to the Premontane Humid Forest of Colombia. Taxonomy 2025, 5, 48. https://doi.org/10.3390/taxonomy5030048
Vargas-García N, Canas-Bermúdez S, González-Obando R, Cárdenas H, Rivera-Franco N. Anopheles neivai (Diptera: Culicidae) Morphogenetic Analysis from the Pacific Coast to the Premontane Humid Forest of Colombia. Taxonomy. 2025; 5(3):48. https://doi.org/10.3390/taxonomy5030048
Chicago/Turabian StyleVargas-García, Nicole, Sebastián Canas-Bermúdez, Ranulfo González-Obando, Heiber Cárdenas, and Nelson Rivera-Franco. 2025. "Anopheles neivai (Diptera: Culicidae) Morphogenetic Analysis from the Pacific Coast to the Premontane Humid Forest of Colombia" Taxonomy 5, no. 3: 48. https://doi.org/10.3390/taxonomy5030048
APA StyleVargas-García, N., Canas-Bermúdez, S., González-Obando, R., Cárdenas, H., & Rivera-Franco, N. (2025). Anopheles neivai (Diptera: Culicidae) Morphogenetic Analysis from the Pacific Coast to the Premontane Humid Forest of Colombia. Taxonomy, 5(3), 48. https://doi.org/10.3390/taxonomy5030048






