On Brazilian Finger-Net Caddisfly Chimarra Stephens, 1829 (Trichoptera: Philopotamidae), I: Two New Species of Chimarra (Curgia) Walker, 1860 from the Caatinga and Cerrado Biomes, Northeastern Brazil †
Abstract
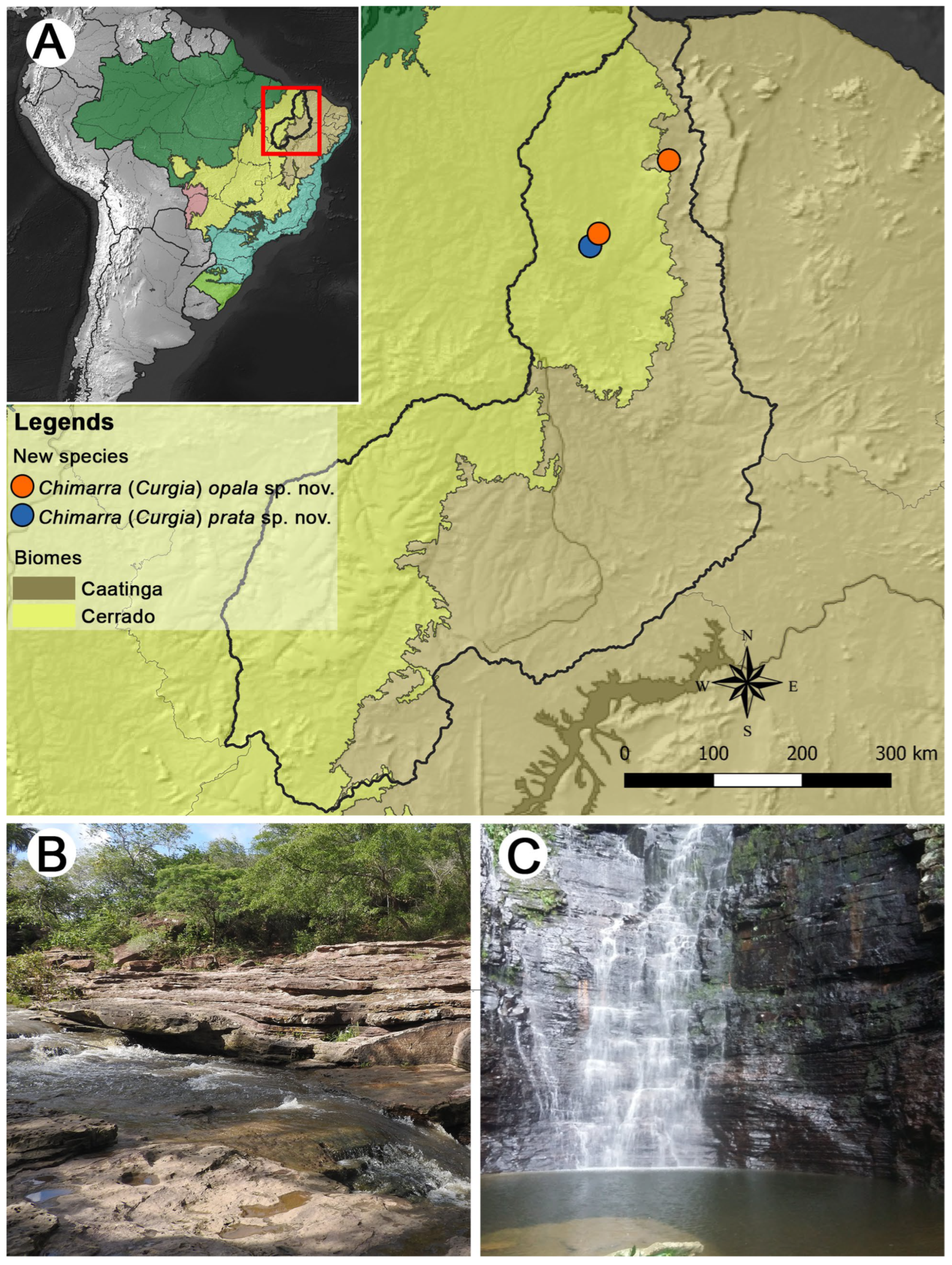
1. Introduction
2. Materials and Methods
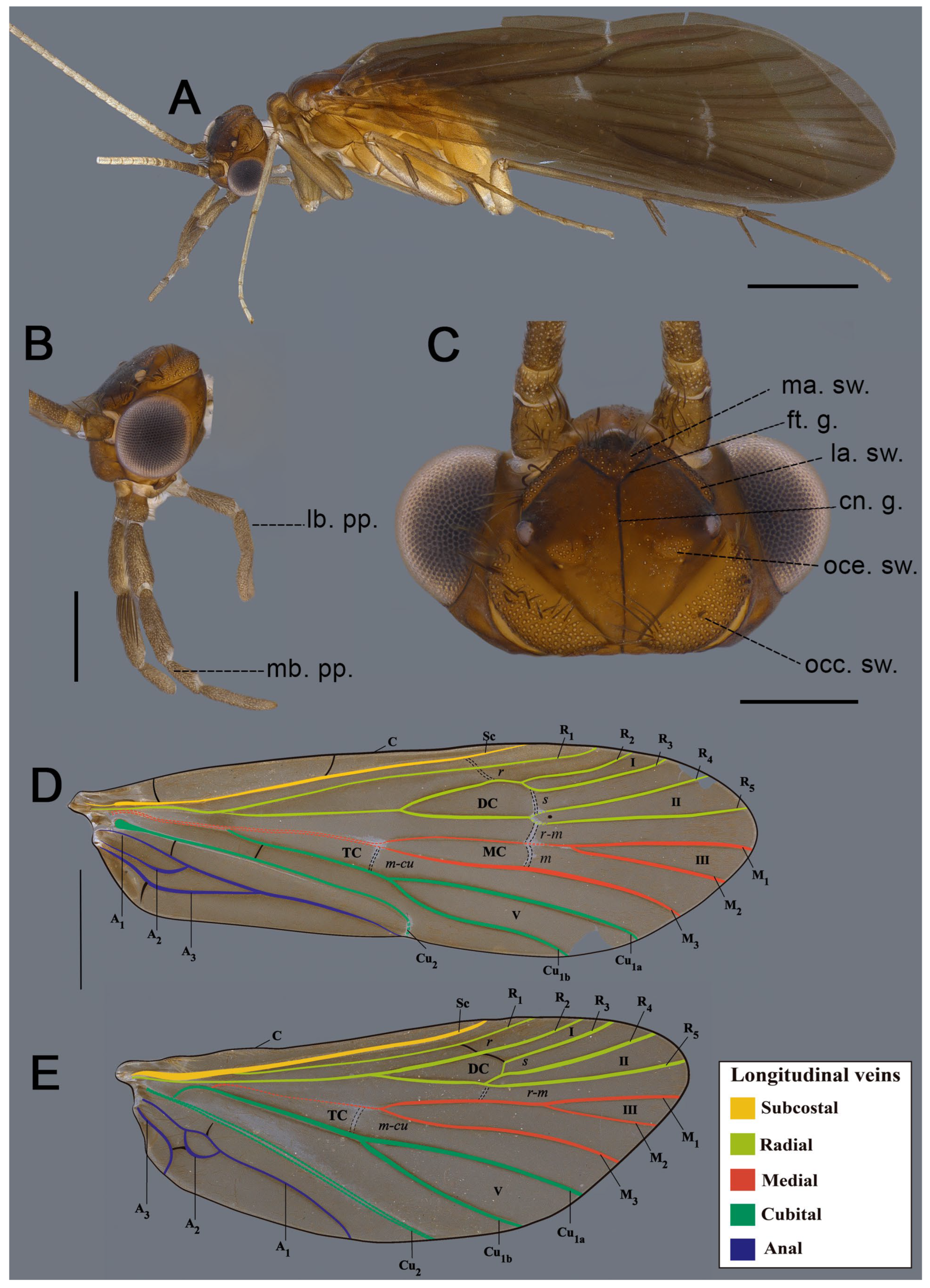
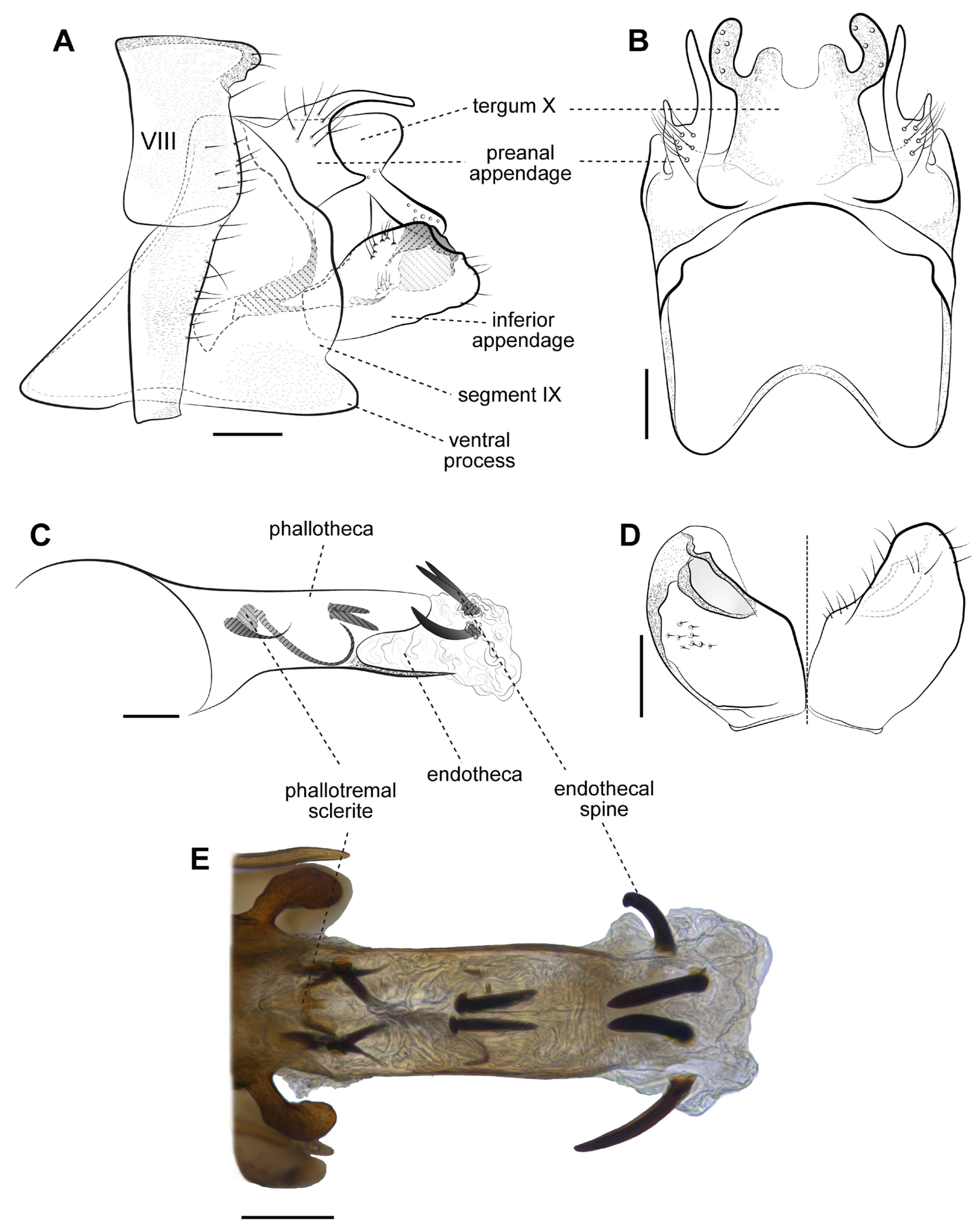
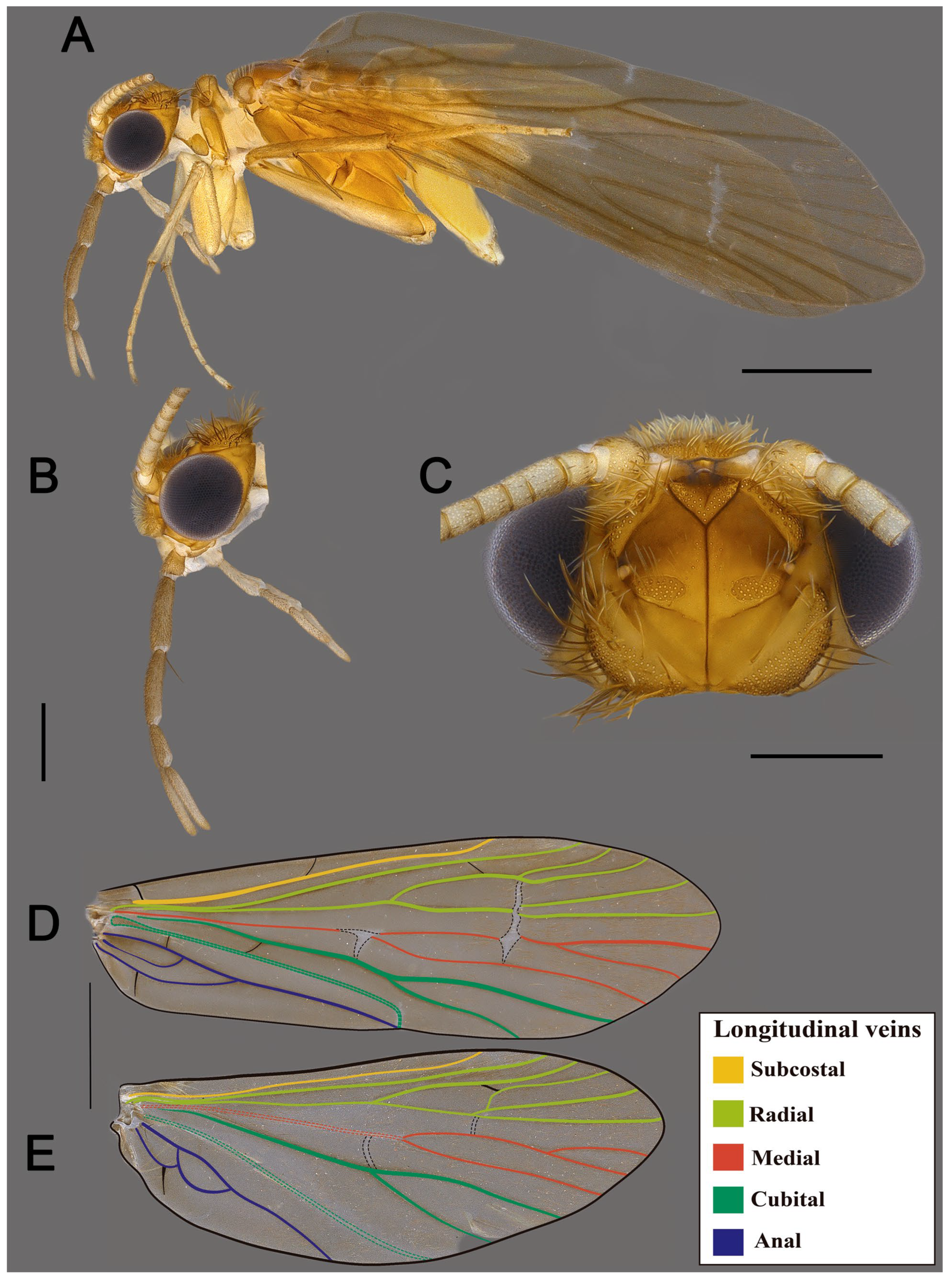
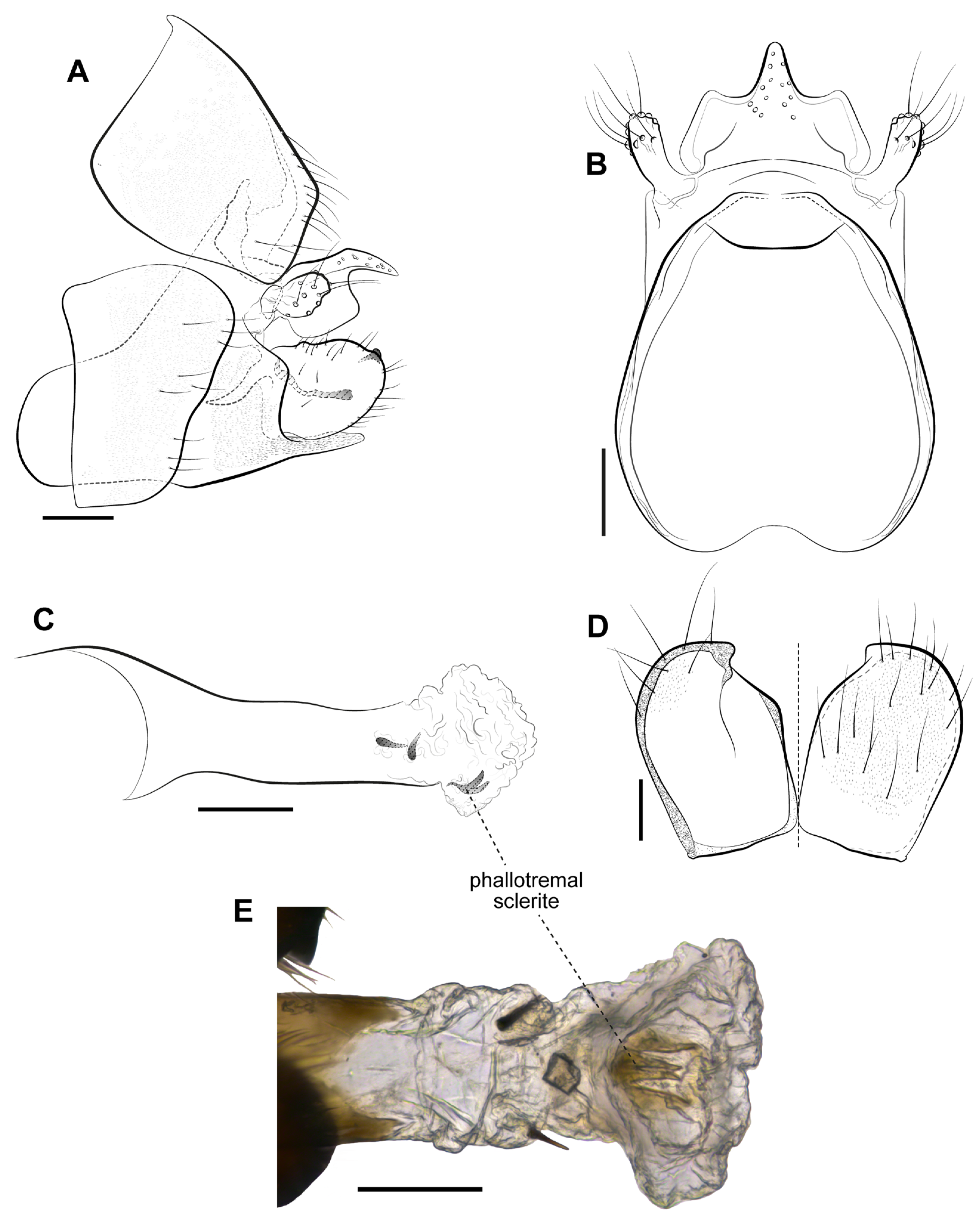
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cn. g. | coronal groove |
| ft. g. | frontal groove |
| la. sw. | lateroantennal wart |
| lb. pp. | labial palp |
| ma. sw. | medioantennal wart |
| mb. pp. | maxillary palp |
| occ. sw. | occipital wart |
| oce. sw. | ocellar wart |
| CE | Ceará |
| DF | Distrito Federal |
| GO | Goiás |
| MA | Maranhão |
| MG | Minas Gerais |
| PE | Pernambuco |
| PI | Piauí |
References
- Thomas, J.A.; Frandsen, P.B.; Prendini, E.; Zhou, X.; Holzenthal, R.W. A multigene phylogeny and timeline for Trichoptera (Insecta). Syst. Entomol. 2020, 45, 670–686. [Google Scholar] [CrossRef]
- Frandsen, P.B.; Holzenthal, R.W.; Espeland, M.; Breinholt, J.; Thomas Thorpe, J.A.; Simon, S.; Kawahara, A.Y.; Plotkin, D.; Hotaling, S.; Li, Y.; et al. Phylogenomics recovers multiple origins of portable case making in caddisflies (Insecta: Trichoptera), nature’s underwater architects. Proc. R. Soc. B Biol. Sci. 2024, 291, 20240514. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.C.; Frandsen, P.B.; Graf, W.; Thomas, J.A. Diversity and ecosystem services of Trichoptera. Insects 2019, 10, 125. [Google Scholar] [CrossRef]
- Holzenthal, R.W.; Blahnik, R.J.; Ríos-Touma, B. A new genus and new species of Ecuadorian Philopotamidae (Trichoptera). ZooKeys 2022, 1117, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Holzenthal, R.W.; Blahnik, R.J.; Prather, A.L.; Kjer, K.M. Order Trichoptera Kirby, 1813 (Insecta), caddisflies. Zootaxa 2007, 1668, 639–698. [Google Scholar] [CrossRef]
- Holzenthal, R.W.; Blahnik, R.J.; Ríos-Touma, B. New species and a new genus of Philopotamidae from the Andes of Bolivia and Ecuador (Insecta, Trichoptera). ZooKeys 2018, 780, 89–108. [Google Scholar] [CrossRef]
- Blahnik, R.J.; Andersen, T. New species of the genus Chimarra Stephens from Africa (Trichoptera, Philopotamidae) and characterization of the African groups and subgroups of the genus. ZooKeys 2022, 1111, 43–198. [Google Scholar] [CrossRef]
- Blahnik, R.J.; Holzenthal, R.W. New Neotropical species of Chimarra (Trichoptera, Philopotamidae). ZooKeys 2012, 184, 1–33. [Google Scholar] [CrossRef]
- Kjer, K.M.; Zhou, X.; Frandsen, P.B.; Thomas, J.A.; Blahnik, R.J. Moving toward species-level phylogeny using ribosomal DNA and COI barcodes: An example from the diverse caddisfly genus Chimarra (Trichoptera: Philopotamidae). Arthropod Syst. Phylogeny 2014, 72, 345–354. [Google Scholar] [CrossRef]
- Wahlberg, E.; Johanson, K.A. The age, ancestral distribution and radiation of Chimarra (Trichoptera: Philopotamidae) using molecular methods. Mol. Phylogenet. Evol. 2014, 79, 433–442. [Google Scholar] [CrossRef]
- Flint, O.S., Jr. Studies of Neotropical caddisflies, LIII: A taxonomic revision of the subgenus Curgia of the genus Chimarra (Trichoptera: Philopotamidae). In Smithsonian Contributions to Zoology; Number 594; Smithsonian Institution Press: Washington, DC, USA, 1998; Volume i–v, pp. 1–131. [Google Scholar] [CrossRef]
- Blahnik, R.J. Systematics of Chimarrita, a new subgenus of Chimarra (Trichoptera: Philopotamidae). Syst. Entomol. 1997, 22, 199–243. [Google Scholar] [CrossRef]
- Blahnik, R.J. A revision of the Neotropical species of the genus Chimarra, subgenus Chimarra (Trichoptera: Philopotamidae). In Memoirs of the American Entomological Institute; American Entomological Institute: Logan, UT, USA, 1998; pp. 1–318. [Google Scholar]
- Blahnik, R.J. Systematics of Otarrha, a new Neotropical subgenus of Chimarra (Trichoptera: Philopotamidae). Syst. Entomol. 2002, 27, 65–130. [Google Scholar] [CrossRef]
- Milne, L.J. Studies in North American Trichoptera. In Studies of North American Trichoptera; [1934–36]; Private Printing: Cambridge, MA, USA, 1936; Volume 3, pp. 56–128. [Google Scholar]
- Santos, A.P.M.; Nessimian, J.L. New species and records of Chimarra Stephens (Trichoptera, Philopotamidae) from Central Amazonia, Brazil. Rev. Bras. Entomol. 2009, 53, 23–25. [Google Scholar] [CrossRef]
- Dumas, L.L. Philopotamidae in Catálogo Taxonômico da Fauna do Brasil. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/17484 (accessed on 28 May 2025).
- Santos, A.P.M.; Dumas, L.L.; Henriques-Oliveira, A.L.; Souza, W.R.M.; Camargos, M.C.; Calor, A.R.; Pes, A.M.O. Taxonomic catalog of the Brazilian Fauna: Order Trichoptera (Insecta), diversity and distribution. Zoologia 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística—IBGE. Biomas. Available online: https://www.ibge.gov.br/geociencias/informacoes-ambientais/ (accessed on 22 June 2024).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Pennington, T.; Prado, D.E.; Pendry, C.A. Neotropical seasonally dry forests and Quaternary vegetation changes. J. Biogeogr. 2000, 27, 261–273. [Google Scholar] [CrossRef]
- Queiroz, L.P.; Cardoso, D.; Fernandes, M.F.; Moro, M.F. Diversity and Evolution of Flowering Plants of the Caatinga Domain. In Caatinga; Silva, J.M.C., Leal, I.R., Tabarelli, M., Eds.; Springer Nature: Berlin, Germany, 2017; pp. 23–63. [Google Scholar] [CrossRef]
- Brown, J.H.; Lomolino, M.V. Biogeography, 2nd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 1998; pp. 1–691. [Google Scholar]
- Lomolino, M.V. Conservation biogeography. In Frontiers of Biogeography: New Directions in the Geography of Nature; Lomolino, M.V., Heaney, L.R., Eds.; Sinauer: Sunderland, MA, USA, 2004; pp. 293–296. [Google Scholar]
- Takiya, D.M.; Santos, A.P.M.; Pinto, Â.P.; Henriques-Oliveira, A.L.; Carvalho, A.L.; Sampaio, B.H.L.; Clarkson, B.; Moreira, F.F.F.; Avelino Capistrano, F.; Gonçalves, I.C.; et al. Aquatic Insects from the Caatinga: Checklists and diversity assessments of Ubajara (Ceará State) and Sete Cidades (Piauí State) National Parks, Northeastern Brazil. Biodivers. Data J. 2016, 4, e8354. [Google Scholar] [CrossRef] [PubMed]
- Desidério, G.R.; Barcelos-Silva, P.; Souza, W.R.M.; Pes, A.M.; Azevêdo, C.A.S. Caddisflies (Insecta: Trichoptera) from Maranhão State, Northeast Region, Brazil: A new species, checklist, and new geographical records. Zootaxa 2017, 4221, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.R.M.; Lima, L.R.C.; Pes, A.M.O.; Pinheiro, U. Trichoptera (Insecta) from Pernambuco State, northeastern Brazil. J. Nat. Hist. 2013, 47, 1–10. [Google Scholar] [CrossRef]
- Moreno, L.A.S.; Desidério, G.R.; Souza, W.R.M.; Lima, L.R.C. Updated checklist of caddisflies (Insecta: Trichoptera) from the state of Piauí, Northeast Brazil, including a new species and new geographical records. Zootaxa 2020, 4838, 257–272. [Google Scholar] [CrossRef]
- Nessimian, J.L.; Santos, A.P.M.; Sampaio, B.H.L.; Dumas, L.L.; Pes, A.M.; Ferreira, N., Jr. The collapsible light tarp: A portable Pennsylvania light trap for collecting aquatic insects. An. Acad. Bras. Ciênc. 2024, 96, 1–12. [Google Scholar] [CrossRef]
- Blahnik, R.J.; Holzenthal, R.W. Collection and curation of Trichoptera, with an emphasis on pinned material. Nectopsyche Neotrop. Trichoptera Newsl. 2004, 1, 8–20. [Google Scholar]
- Kawada, R.; Buffington, M.L. A scalable and modular dome illumination system for scientific microphotography on a budget. PLoS ONE 2016, 11, e0153426. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: https://qgis.osgeo.org/ (accessed on 12 July 2021).
- Natural Earth. Available online: https://www.naturalearthdata.com/downloads/ (accessed on 29 May 2025).
- Dallwitz, M.L.; Paine, T.A.; Zucher, E.J. User’s Guide to the DELTA Editor. 2020. Available online: https://www.delta-intkey.com/www/delta-ed.htm (accessed on 13 November 2020).
- Oláh, J.; Johanson, K.A. Trinominal terminology for cephalic setose warts in Trichoptera (Insecta). Braueria 2007, 34, 43–50. [Google Scholar]
- Nielsen, A. A comparative study of the genital segments and their appendages in male Trichoptera. K. Dansk. Vidensk. Selsk. Skr. 1957, 23, 1–200. [Google Scholar]
- Mosely, M.E.; Kimmins, D.E. The Trichoptera (Caddisflies) of Australia and New Zealand; British Museum: London, UK, 1953; pp. 1–550. [Google Scholar]
- da Silva, J.M.C.; Lacher, T.E. Caatinga—South America. In Encyclopedia of the World’s Biomes; Goldstein, M.I., DellaSala, D.A., Eds.; Elsevier: Oxford, UK, 2000; pp. 554–561. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. A conservação do Cerrado brasileiro. Megadiversidade 2005, 1, 147–155. [Google Scholar]
- Colli, G.R.; Vieira, C.R.; Dianese, J.C. Biodiversity and conservation of the Cerrado: Recent advances and old challenges. Biodivers. Conserv. 2020, 29, 1465–1475. [Google Scholar] [CrossRef]
| Species | Biomes (States) | References |
|---|---|---|
| C. (Curgia) camposae Flint, 1998 | Cerrado (MG) | [11] |
| C. (Curgia) conica Flint, 1983 | Caatinga (CE); Cerrado (GO, MG) | [11] |
| C. (Curgia) cultellata Flint, 1983 | Caatinga (CE); Cerrado (DF, MG) | [11,25] |
| C. (Curgia) cipoensis Flint, 1998 | Cerrado (MG) | [11] |
| C. (Curgia) jugescens Flint, 1998 | Caatinga (PI); Cerrado (MA) | [25,26] |
| C. (Curgia) opala sp. nov. | Caatinga (PI); Cerrado (PI) | This study |
| C. (Curgia) prata sp. nov. | Cerrado (PI) | This study |
| C. (Curgia) hyoiedes Flint, 1983 | Caatinga (PE) | [27] |
| C. (Curgia) parana Flint, 1972 | Caatinga (PE, PI); Cerrado (DF, GO, MG) | [11,27,28] |
| C. (Curgia) scopuloides Flint, 1974 | Cerrado (GO) | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, L.; Desidério, G.R.; Souza, W.R.M.; Santana, V.; Bispo, P.C.; Lima, L.R.C. On Brazilian Finger-Net Caddisfly Chimarra Stephens, 1829 (Trichoptera: Philopotamidae), I: Two New Species of Chimarra (Curgia) Walker, 1860 from the Caatinga and Cerrado Biomes, Northeastern Brazil. Taxonomy 2025, 5, 44. https://doi.org/10.3390/taxonomy5030044
Moreno L, Desidério GR, Souza WRM, Santana V, Bispo PC, Lima LRC. On Brazilian Finger-Net Caddisfly Chimarra Stephens, 1829 (Trichoptera: Philopotamidae), I: Two New Species of Chimarra (Curgia) Walker, 1860 from the Caatinga and Cerrado Biomes, Northeastern Brazil. Taxonomy. 2025; 5(3):44. https://doi.org/10.3390/taxonomy5030044
Chicago/Turabian StyleMoreno, Lucas, Gleison R. Desidério, Wagner R. M. Souza, Vitória Santana, Pitágoras C. Bispo, and Lucas R. C. Lima. 2025. "On Brazilian Finger-Net Caddisfly Chimarra Stephens, 1829 (Trichoptera: Philopotamidae), I: Two New Species of Chimarra (Curgia) Walker, 1860 from the Caatinga and Cerrado Biomes, Northeastern Brazil" Taxonomy 5, no. 3: 44. https://doi.org/10.3390/taxonomy5030044
APA StyleMoreno, L., Desidério, G. R., Souza, W. R. M., Santana, V., Bispo, P. C., & Lima, L. R. C. (2025). On Brazilian Finger-Net Caddisfly Chimarra Stephens, 1829 (Trichoptera: Philopotamidae), I: Two New Species of Chimarra (Curgia) Walker, 1860 from the Caatinga and Cerrado Biomes, Northeastern Brazil. Taxonomy, 5(3), 44. https://doi.org/10.3390/taxonomy5030044









