Abstract
The genera: Lerniana Delicado et Hauffe, 2022, Trichonia Schütt, 1980, and two clades (“Radomaniola” elongata Radoman, 1973, and an unnamed clade referred to as “Radomaniola” sp. 1, sp. 2) whose assignment to any genus remains unknown, form sister group with the genus Radomaniola Szarowska, 2007 (Hydrobiidae W. Stimpson, 1865, subfamily Horatiinae D. W. Taylor, 1966). The paper deals with all these clades sister to Radomaniola. Cytochrome c oxidase subunit I (COI) sequences have been used to infer phylogenetic relationships between the snails collected at 15 localities in southern Greece and one in Montenegro. Thirty-two haplotypes represent eight Molecular Taxonomical Units (mOTUs) of the species level, four of them within the genus Lerniana: L. seminula (Frauenfeld, 1863), L. tritonum (Bourguignat, 1852), and two other of these four species are both described as new. First of them has been found at seven localities in Peloponnese, Attica and southern Thessaly, the second only at one locality, sympatrically with the former species. “Radomaniola” elongata does not belong to Radomaniola, and its relationships remain unknown, similarly as in other unnamed clade, whose genus-level assignment cannot currently be resolved. The shells, protoconchs, radulae, female reproductive organs and penes are presented, also for Trichonia trichonica Radoman, 1973, for which the genus assignment remains undecided based on our molecular results. The study clearly illustrates how fragmentary is our knowledge is on the real biodiversity of the minute truncatelloid gastropods, whose morphology—simple and variable—makes species distinction hardly possible. Informed decisions on species and habitat protection should consider the above.
Keywords:
nomenclature; phylogeny; cytochrome oxidase; shell; reproductive organs; radula; Radomaniola 1. Introduction
The truncatelloid fauna of the Balkans was studied for more than hundred years [1,2,3,4,5,6,7,8]. Especially the monographs of Radoman [9] and Szarowska [10] reviewed these gastropods, Delicado et al. [11] included also the Balkan species. Despite this long history of studies, the Balkan truncatelloid fauna, one of the richest worldwide, remains still rather poorly known. Falniowski et al. [1] in their study on the genus Radomaniola Szarowska, 2007 detected a group of species forming a distinct clade, provisionally named Radomaniola s. lato. Later, Delicado & Hauffe [2] confirmed the distinctness of this clade and describe it as a new genus Lerniana Delicado et Hauffe, 2022.
Bourguignat [3] described Hydrobia tritonum, from: “Graeciam, in aquis paludosis lacus Lernae, habitat.” [“Ce Mollusque habite sous les feuilles des plantes aquatiques des eaux fangeuses du marais de Lerne, en Grèce.” (Bourguignat [4]; as Bithina tritonum)], which means: lives under the leaves of aquatic plants in the muddy waters of the marsh in Lérna, Greece. Roth [5], Frauenfeld [6], Westerlund & Blanc [7] and Martens [8] transferred H. tritonum to the genus Amnicola A. Gould & Haldeman, 1840, in Handelman [12] (for review of the nomenclatoric chaos connected with Amnicola [13]).
Later, Schütt [14] considered H. tritonum a representative of the family Cochliopidae Tryon, 1866, and more precisely of the genus Semisalsa Radoman, 1974. Falniowski et al. [1] examined the female reproductive organs and penes of topotypes of H. tritonum, and demonstrated that, despite the penis habitus somewhat resembling typical Semisalsa, the anatomy is clearly typical of Radomaniola [10] (replacement name for Orientalina Radoman, 1978). This assignment was confirmed molecularly, although in molecular tree Radomaniola tritonum belonged to a distinct clade, sister with the one grouping most of the other Radomaniola (thus Radomaniola s. stricto clade). This clade was provisionally defined as Radomaniola s. lato [1].
In the clade Radomaniola s. lato [1] there were five examined populations whose assignment to any known species was impossible, as well as four populations, and eleven haplotypes, identified as Amnicola seminula Frauenfeld, 1863. Amnicola seminula was described from Arcadia (Peloponnisos, Greece) without a more precisely defined locality. Schütt [14] classified it as belonging to the genus Belgrandiella A. J. Wagner, 1928. In fact, Belgrandiella does not occur in Greece, and Schütt’s “Belgrandiella” contains the representatives of three genera and two families [15]. Schütt [14] erroneously synonymized Amnicola filiola Westerlund, 1881, described from “Graecia, Patras [Patrai], Fonte Salevale” [16] with A. seminula.
Delicado and Hauffe [2] confirmed molecular distinctness of the part of the clade Radomaniola s. lato of Falniowski et al. [1], consisting of three species: R. tritonum, R. seminula and R. feheri (Georgiev, 2013), and described this clade as a new genus Lerniana Delicado et Hauffe, 2022, with the type species Hydrobia tritonum Bourguignat, 1852 (even though their identification of H. tritonum was different from the one of Falniowski et al.; molecularly their H. tritonum belonged to A. seminula in Falniowski et al. [1] see Supplementary File).
Apart from Lerniana, there were found some other taxa [1], phylogenetically closer to Lerniana than to Radomaniola. One of them was Orientalia elongata Radoman, 1973, described from Montenegro, spring near monastery Vranjina, near Virpazar Town. Radoman [16] introduced the genus Trichonia Radoman,1973, (however, creating a nomen nudum), with the type species T. kephalovrissonia Radoman, 1973, from Kephalovrisson spring by the main road Messolongi-Agrinion in Greece. However, the locality was later destroyed [17]. T. kephalovrissonia was reported also from Thérmon, NE of Trichonida Lake, and the sequences of the snails from this place were published [1]. Schütt [14] published a diagnosis of Trichonia, Schütt, 1980, with T. trichonica Radoman, 1973 from Lake Trichonida (Trichonis) as the type species. The shells of T. trichonica were photographed and described [9,14,18,19]. There are no molecular data on this species. Our materials, collected in 1985, were fixed in formalin, thus no molecular data are available.
Recently we have detected some more haplotypes which either belong or are closely related to the genus Lerniana, certainly not Radomaniola. Moreover, not all available data from the GenBank have been previously analyzed, so detailed phylogenetic studies are necessary. The aim of our study is to provide more data on the genus Lerniana, from more localities, but also about “Radomaniola”elongata, and Trichonia, the clades whose phylogenetic position remains unclear.
2. Material and Methods
The snails were collected at 16 localities (Figure 1, Figure 2 and Figure 3, Table 1), 15 of them in Greece, one in Montenegro, in the period 2001–2008, and 2012–2022. Fourteen of them were the same as in the study of Falniowski et al. [1]. For the molecular study snails were washed twice in 80% analytically pure ethanol and left to stand in this solution for ca. 12 h, after which the solution was changed twice in 24 h. Finally, after a few days, the 80% solution was exchanged for a 96% solution of ethanol and the material was stored at –20 °C. Snails for the morphological study were fixed in 10% buffered formalin.
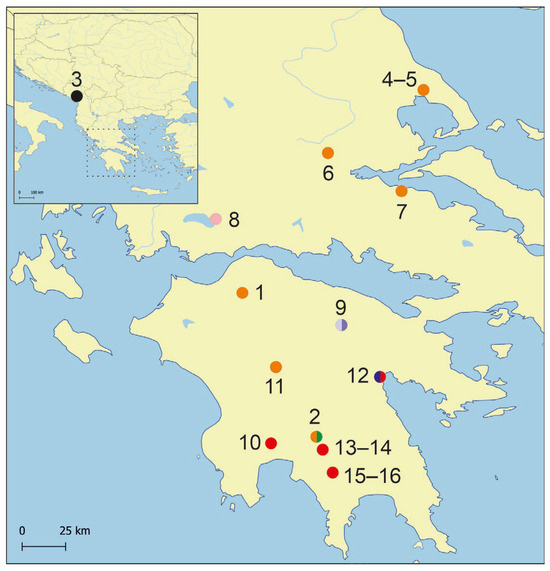
Figure 1.
Map showing distribution of the studied localities. Colours for the distinguished mOTUs as in molecular tree below.

Figure 2.
Studied localities: (A). spring of Achilles, ESE of Kalamakion, NW of Lamia (6), type locality of Lerniana dianadelicadoae. (B). spring in city centre in Thérmon, NE of Trichonida Lake (8). (C). spring Piges Pamisou, Peloponnese (10). Photographs Andrzej Falniowski.

Figure 3.
Studied localities: (A). spring between Palaiochori and Karkalou, WNW of Tripolis, Peloponnese (11). (B). spring S of Koumousta, Taigetos Mts., Peloponnese (15). (C,D). spring at Mili (Lérni), Peloponnese (12). (E). spring S of Koumousta, Taigetos Mts., Peloponnese (16). Photographs Andrzej Falniowski.

Table 1.
Sampled localities. M and G—localities from Falniowski et al. [1], D—localities from Delicado & Hauffe [2].
The shells were photographed with a Canon EOS 50D digital camera, under a Nikon SMZ18 microscope. The dissections were done under a Nikon SMZ18 microscope with dark field, equipped with Nikon DS-5 digital camera, whose captured images were used to draw female reproductive organs with a graphic tablet. Seven morphometric parameters of the shell were measured, following the scheme already proved to reflect differences in the truncatelloid shell biometry [10,15], by one person using ImageJ v. 1.53g image analysis software [20]. The linear measurements were logarithmically transformed, to reduce the influence of outliers and make distribution closer to the normality (although the latter being simply unrealistic in such case). Principal component analysis (PCA) based on the correlation matrix was computed, applying a descriptive, non-stochastic approach—the only approach adequate to such low number of objects and no normality or at least ellipticity of the distribution. The original observations were projected into PC space to show morphological similarity relationships between the specimens without any a priori classification. For PCA, the Clust Vis 2.0 web tool [21] was used. Protoconchs were cleaned using an ultrasonic cleaner, mounted and examined, the radulae were extracted with Clorox, applying the techniques described by Falniowski [22]. Protoconchs and radulae were photographed using a HITACHI S-4700 scanning electron microscope. Penes were photographed under Motic B3 Professional microscope with dark field. Morphological terminology after Hershler & Ponder [23].
DNA was extracted from whole fixed specimens; tissues were hydrated in tris–EDTA (TE) buffer (3 × 10 min); then total genomic DNA was extracted with the Sherlock extraction kit (A&A Biotechnology), and the final product was dissolved in 20 μL of TE buffer. The extracted DNA was stored at −80 °C at the Department of Malacology, Institute of Zoology and Biomedical Research, Jagiellonian University in Kraków (Poland).
Mitochondrial cytochrome c oxidase subunit I (COI) locus was sequenced. Details of PCR conditions, primers used and sequencing technique were given in Szarowska et al. [24]. Sequences were initially aligned in the MUSCLE [25] program in MEGA 7 [26] and then checked in Bioedit 7.1.3.0 [27]. Uncorrected p-distances were calculated in MEGA 7. The estimation of the proportion of invariant sites and the saturation test for entire data sets [28,29] were performed using DAMBE [30]. In the phylogenetic analysis, additional sequences from GenBank were used as reference [1,2,11] (Table 1). The sequences were selected based on an alignment containing all Radomaniola sequences from GenBank and on searching of the BOLDSystems database. The COI dataset were analysed using approaches based on Bayesian inference (BI) and maximum likelihood (ML). For RAxML analysis, the jModelTest2 via the CIPRES Science Gateway [31] was used to find the best-fitting model of nucleotide substitution. The model HKY+I was used. The ML analysis was conducted in RAxML-NG v. 0.8.0 [32] via web service available at https://raxml-ng.vital-it.ch/ (accessed on 25 August 2024), with 10 random and 10 parsimony starting trees. In the BI analysis, the HKY+I model was selected using MrModelTest 2.4 [33]. The analyses were run using MRBAYES v. 3.2.7a [34] with defaults of most priors. Two simultaneous analyses were performed, each with 10,000,000 generations, with one cold chain and three heated chains, starting from random trees and sampling the trees every 1000 generations. The first 25% of the trees were discarded as burn-in. The analyses were summarised as a 50% majority-rule tree. Convergence was checked in TRACER v.1.7.1 [35], in all cases Effective Sample Size exceeded 200. FigTree v. 1.4.4 [36] was used to visualize the trees. Molecular Taxonomical Units (mOTUs)—the terminal clades inferred in phylogenetic analysis, which means taxons whose systematic status remains undetermined, were tested with species delimitation techniques. Three species delimitation methods were performed: Poisson Tree Processes (PTP) [37], Automatic Barcode Gap Discovery (ABGD) [38] and Assemble Species by Automatic Partitioning (ASAP) [39]. The bPTP approach was run using the web server https://species.h-its.org/ptp// (accessed on 28 August 2024), with 100,000 MCMC generations, 100 thinning and 0.1 burn-in. We used the RAXML output phylogenetic tree. The ABGD and ASAP approaches used the web servers (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html/ (accessed on 30 August 2024) and (https://bioinfo.mnhn.fr/abi/public/asap/ (accessed on 30 August 2024) respectively, with the default parameters. In all delimitation methods we used only Radomaniola sequences. The Fastachar application [40] and DeSignate [41] were used to distinguish species from another species, based on COI sequences, by determining the Molecular Diagnostic Characters (MDCs). Two types of characters were accepted: binary positions (the character state in the query group is different from the uniform character state in the reference group) and asymmetric positions (the character state in the query group is different from the non-uniform character state in the reference group).
Acronyms in the systematic part
MNHW—Museum of Natural History of the University of Wrocław, Poland
ZMUJ—Zoological Museum of Jagiellonian University, Kraków, Poland
3. Results
3.1. Molecular Part
We obtained five new COI sequences of Lerniana (457 bp, GenBank accession numbers PX096765-PX096769). The test for the substitution saturation analysis showed an ISS (0.73) significantly smaller than the critical ISS value (0.93), indicating that sequences were not saturated and thus useful in phylogenetic reconstruction. The topologies of the resulting phylograms were identical in both the ML and BI phylogram analyses, thus we present the phylogram computed with RAxML. In our tree (Figure 4) a well-supported (bootstrap 74%) clade, distinct from the one grouping Radomaniola, consisted of Lerniana (bootstrap support 82%, “Radomaniola” elongata (Radoman, 1973) (bootstrap support only 60%), unclassified clade consisted of probably two undescribed species (bootstrap support 78%), and Trichonia (bootstrap support 96%). The p-distances between these four clades varied from 0.037 to 0.089. The species delimitation indicated eight distinct mOTUs (A–H), probably at the species level, with p-distances between 0.026–0.106 (Table 2). These mOTUs differed from the representatives of the genera Radomaniola and Anagastina by 6.4 to 13.9%, always over 10% for Anagastina.
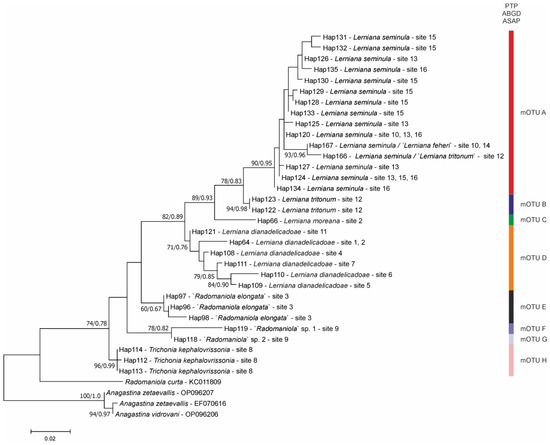
Figure 4.
Maximum likelihood tree based on cytochrome c oxidase subunit I (COI). Bootstrap supports (>60%) and Bayesian probabilities are given. Haplotypes and localities are marked. Color bars indicated particular mOTUs, according to three delimitation methods used. Colors correspond to Figure 1.

Table 2.
Uncorrected p-distances between the distinguished mOTUs, calculated for COI sequences (A—Lerniana seminula, B—L. tritonum, C—L. moreana, D—L. dianadelicadoe, E—“Radomaniola” elongata, F—“Radomaniola” sp. 1, G—“Radomaniola” sp. 2, H—Trichona kephalovrissonia).
Within the Lerniana there were four species: L. seminula (mOTU A), found at our four localities and represented by 13 haplotypes. Within L. seminula clustered also the sequences of Delicado & Hauffe [2], assigned by them to L. seminula, ‘L. tritonum’ and ‘L. feheri’ and representing two more haplotypes. It should be noted that ‘L. feheri’ was identical with L. seminula. Two haplotypes found in two specimens represented L. tritonum (mOTU B) (bootstrap support 94%). The other haplotype (mOTU C) belongs to the new for science species Lerniana moreana Grego & Jaszczyńska, which is described below, found at Aqueduct source in Virtaliotiko Farangi, in Kastorio, Laconia, Peloponnese, in a spring 33 km N of village—our locality 2 (Table 1, Figure 1). At the same locality, sympatrically, there was found another species of (mOTU D) unknown so far: Lerniana dianadelicadoae Falniowski & Jaszczyńska, which is also described below. The latter species (bootstrap support 71%) was also found at five other localities, one of them also at Peloponnese and the other four in SE continental Greece (Attica, Thessaly), and was represented by six haplotypes.
3.2. Systematic Part
Truncatelloidea Gray, 1840
Hydrobiidae W. Stimpson, 1865
Horatiinae D. W. Taylor, 1966
Lerniana Delicado et Hauffe, 2022; type species: Amnicola seminula Frauenfeld, 1863
(see the Supplementary File)
Lerniana seminula (Frauenfeld, 1863)
Lerniana feheri (Georgiev, 2013)
Shell: (Figure 5A–F) up to 1.86 mm high, ovate-conical, broad, with relatively low spire, with about 4.5 whorls, spire low, its height 14–21% of the shell height, body whorl broad. Teleoconch whorls moderately convex, evenly rounded, growing regularly in diameter. Aperture narrow ovoid, outer lip simple, parietal lip broad and complete, umbilicus absent or slit-like. Teleoconch moderately thick-walled, its wall translucent, with delicate growth lines, periostracum whitish or yellowish. Shell measurements: Table 3, Figure 6. Operculum paucispiral, smooth on its both surfaces.
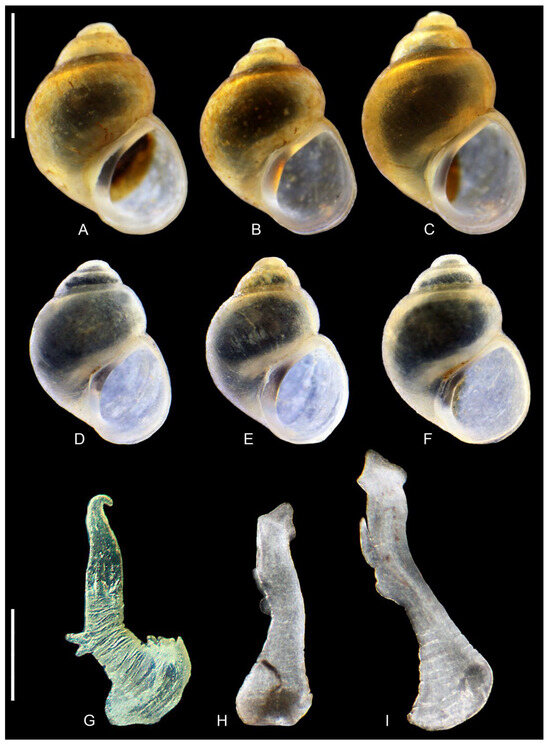
Figure 5.
Lerniana seminula: (A–F). shells. (A–C). locality 10. (D–F). locality 15. (G–I). penes, locality 15. Bar equals 1 mm for shells and 200 µm for penes.

Table 3.
Shell biometry of the sequenced specimens, symbols as in Figure 4. Measured variables: a—shell height; b—body whorl breadth; c—aperture height; d—spire height; e—aperture breadth; α—apex angle measured between the lines tangential to the spire; β—angle between the body whorl suture and the line perpendicular to the columella.
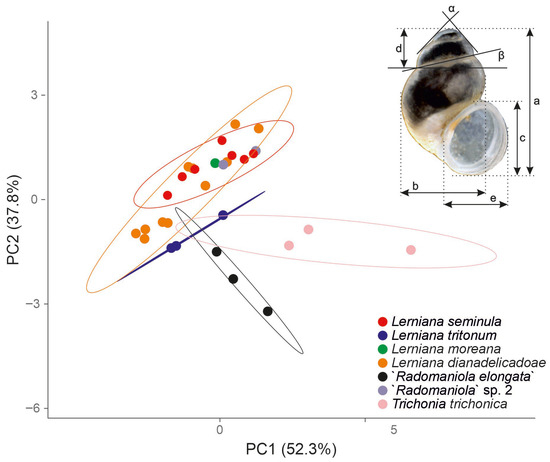
Figure 6.
Principal component analysis (PCA). Projection into PC1 (52.3% of the total variation) and PC2 (37.8%); cumulatively they explain 90.2% of the total variation. The shell measurements are also shown. Abbreviations: a—shell height; b—body whorl breadth; c—aperture height; d—spire height; e—aperture breadth; α—apex angle measured between the lines tangential to the spire; β—angle between the body whorl suture and the line perpendicular to the columella. Measurements’ values given in Table 3.
Soft parts morphology and anatomy: Head and mantle intensely pigmented black. Female reproductive organs (Figure 7A) with moderately big and spherical bursa copulatrix, moderately broad loop of oviduct and long and narrow, straight receptaculum seminis. In comparison with the figure S7J of Delicado & Hauffe [2] the bursa is spherical, not sac-shaped as in their study, and the receptaculum somewhat smaller. Penis (Figure 5G–I) long and narrow, its tip broad (with delicate outgrowths on its left and right side: Figure 5H,I) or sharp and narrow (Figure 5G); perhaps the terminal outgrowths have been caused by contraction. On the left side of the medial part of the penis there is double outgrowth, consisted of two narrow extensions, vas deferens often visible inside the penis.
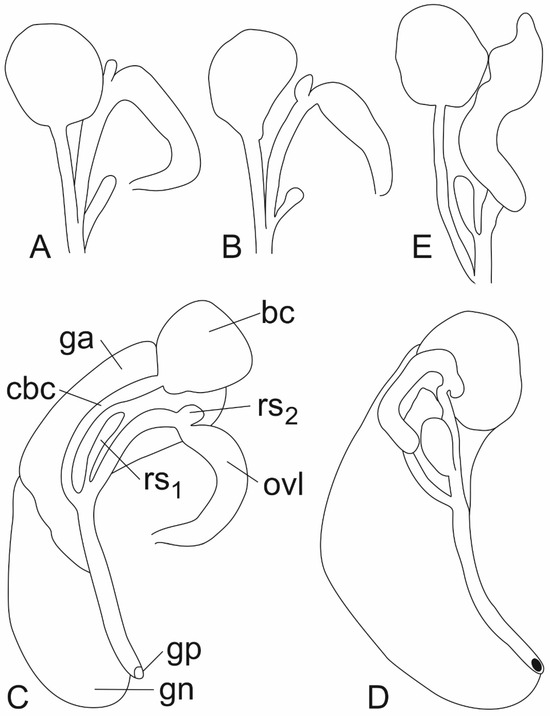
Figure 7.
Female reproductive organs: (A). Lerniana seminula, (bursa copulatrix, receptaculum seminis and loop of oviduct), locality 16. (B). Lerniana tritonum, female reproductive organs (bursa copulatrix, receptaculum seminis and loop of oviduct), locality 12. (C). “Radomaniola” elongata. (D). “Radomaniola” sp., locality 9. (E). Trichonia trichonica (bc—bursa copulatrix, cbc—duct of bursa copulatrix, ga—albuminoid gland, gn—nidamental gland, gp—gonoporus, ovl—loop of (renal) oviduct, rs1—distal receptaculum seminis, rs2—proximal receptaculum seminis).
Distribution and habitat: Found at six localities (Figure 1), all of them at Peloponnese. Found in springs, like localities 10 (Figure 2C) and 16 (Figure 3E), but also in marshes like locality 15 (Figure 3B), often among macrophytes. Also sympatrically with L. tritonum.
Remark: Considering the molecular tree as well as the variable morphology, L. feheri should be accepted as junior synonym of L. seminula.
Lerniana tritonum (Bourguignat, 1852)
Shell: (Figure 8A–C) up to 1.90 mm high, ovate-conical, slender, with spire relatively higher than in L. seminula, with about 4.5 whorls, the spire’s height about 26–28% of the shell height, body whorl less broad than in L. seminula. Teleoconch whorls moderately convex, evenly rounded, growing regularly in diameter. Aperture narrow ovoid, outer lip simple, parietal lip complete but much less broad than in L. seminula, umbilicus more visible that in L. seminula. Teleoconch moderately thick-walled, its wall translucent, with delicate growth lines, periostracum whitish or yellowish. Shell measurements: Table 3, Figure 6. Operculum smooth on both surfaces.

Figure 8.
Lerniana tritonum, locality 12. (A–C). shell. (D–G). penis. Bar equals 1 mm for shells and 200 µm for penes.
Soft parts morphology and anatomy: Head and mantle intensely pigmented black. Female reproductive organs (Figure 7B) with moderately big and broad bursa copulatrix, somewhat irregular in shape, loop of oviduct somewhat broader than in L. seminula, straight receptacula seminis, the distal one (rs1) smaller and shorter than in that species. Penis (Figure 8D–G) flat and broader than in L. seminula, its tip narrow. On the left side of the medial part of the penis there is double outgrowth, its extensions much broader than in L. seminula, vas deferens not visible inside the penis.
Distribution and habitat: Found only at its type locality at Mili (Lérni), ancient Lerna, at a canal draining the marshland, among macrophytes and on gravel. After Delicado & Hauffe [2] may be sympatric with L. seminula.
Lerniana dianadelicadoae Falniowski & Jaszczyńska, sp. nov.
Radomaniola sp. from the locality G14 [1, figs 3 and 8]
- LSID urn:lsid:zoobank.org:act:FEF8E391-3B88-44D3-8C58-CB872E3EA076
- GenBank sequence numbers: PX096765 - PX096768, KC011734, KC011747, KC011762, KC011766, KC011772
- mOTU D
Holotype: Ethanol-fixed specimen (Figure 9I) collected on 15th of September 2007 by Magdalena Szarowska and Andrzej Falniowski at Spring of Achilles, ESE of Kalamakion, NW of Lamia (locality 6: Figure 1), 38°59′13″ N, 22°22′43″ E, 737 m a.s.l. S Thessaly, Greece (Figure 2A), Museum of Natural History of the University of Wrocław, Poland, voucher number MNHW-1524.
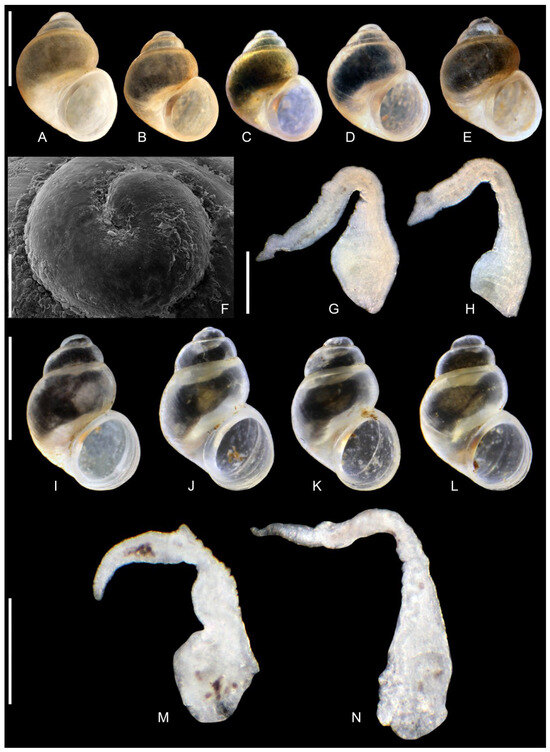
Figure 9.
Lerniana dianadelicadoae sp. nov. (A–H). locality 1. (I–N). locality 6, (A–E,I–L). shells ((I). holotype). (F). protoconch. (G,H,M,N). penes. Bar equals 1 mm for shells, 100 µm for protoconch and 200 µm for penes.
Paratypes: 30 ethanol-fixed specimens, collected at the type locality (Spring of Achilles, ESE of Kalamakion, NW of Lamia (locality 6), 38°59′13″ N, 22°22′43″ E, 737 m a.s.l. S Thessaly, Greece), voucher number ZMUJ2732–62.
Description: Shell (Figure 9A–E,I–L) up to 1.65 mm high, ovate-conical, broad, with spire relatively low or moderately low, with about 4 whorls, spire height 14–17% of the shell height at the type locality (and up 20% in some other populations). Teleoconch whorls slightly convex, evenly rounded, growing regularly in diameter. Aperture narrow ovoid, outer lip simple, parietal lip broad and complete, umbilicus broad or slit-like. Teleoconch moderately thick-walled, its wall translucent, with delicate growth lines, periostracum whitish or yellowish. Shell measurements: Table 3, Figure 6. Protoconch (Figure 9F) growing abruptly, no sharp border between proto- and teleoconch, its microsculpture with regular net of depressions Operculum smooth on its both surfaces.
Radula: (Figure 10A) with central tooth with blunt and massive median cusp and short (less than half of the central cusp length) moderately sharp cusps following formula:
9 – 1 – 9 or 10 – 1 – 10
1–1 1–1
1–1 1–1
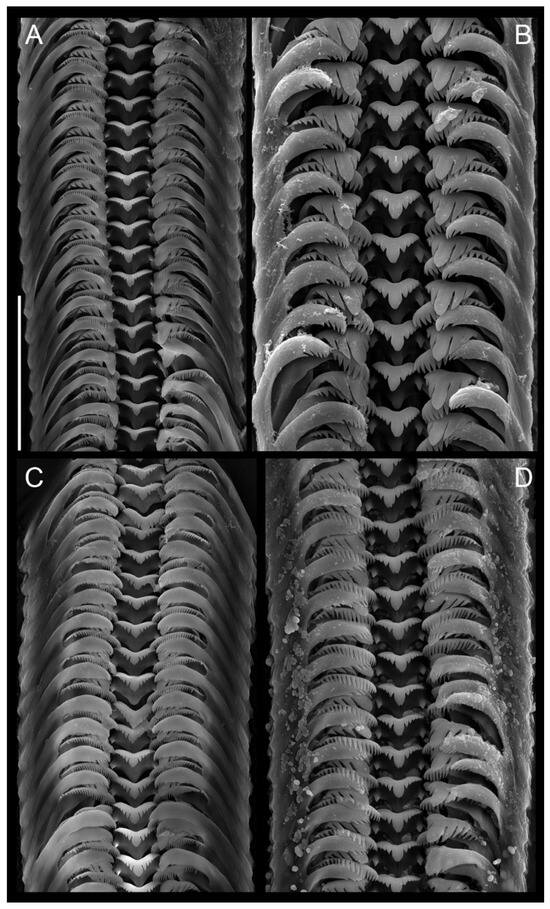
Figure 10.
Radulae. (A). Lerniana dianadelicadoae sp. nov. (B). “Radomaniola” elongata. (C). “Radomaniola” sp., locality 9. (D). Trichonia trichonica. Bar equals 50 µm.
Lateral tooth with 3 – 1 – 4 blunt cusps, the biggest one broad and rounded. Inner marginal tooth with 32 long and sharp cusps, outer marginal tooth with 12–16 relatively big cusps on the terminal edge.
Soft parts morphology and anatomy: Head and mantle pigmented intensely black. Female reproductive organs (Figure 11) with moderately big spherical bursa copulatrix, broad and prominent loop of oviduct, proximal receptaculum seminis (rs2) [42] relatively broad, distal one (rs1) [42] small and spherical. Penis (Figure 9G,H,M,N) long and narrow, with no outgrowth on its left side, vas deferens visible inside.
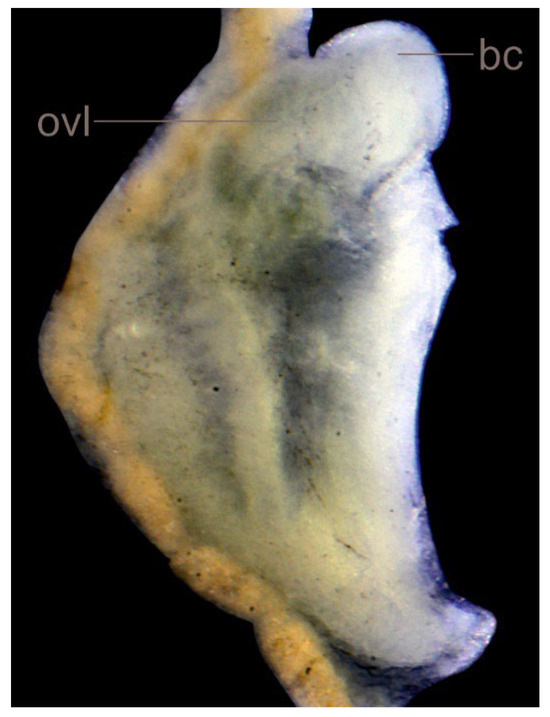
Figure 11.
Lerniana dianadelicadoae sp. nov., female reproductive organs.
Differential diagnosis: As seen in Figure 6, as well as in Table 3, shell size and proportions wider than in Lerniana seminula, the latter within the range for L. dianadelicadoae, S. tritonum distinct in proportions, but not in size. The parietal lip similar as in L. seminula, but broader than in L. tritonum. Loop of oviduct in L. dianadelicadoae more broad and massive, proximal receptaculum bigger distal one spherical, all the three character states distinguish L. dianadelicadoae from both L. seminula and L. tritonum. The penis, with neither single nor double outgrowth on its left side differs L. dianadelicadoae from the two other species of Lerniana. The Molecular Diagnostic Characters in comparison with other Lerniana species: binary: 147 (C), 195 (C), 342 (G); asymmetric: 213 (A).
Derivation of name: Species name to honour Dr. Diana Delicado, a splendid malacologist deeply devoted to the study of the Truncatelloidea.
Distribution and habitat: Apart from the type locality, this new species has been found at six other localities (Figure 1), in Peloponnese (localities 1, 2 and 11), S Thessaly (localities 4 and 5), and Attica (locality 7). Inhabits springs and fountains, also small springs (like locality 11: Figure 3A). In comparison with the other species of Lerniana, the geographic range of L. dianadelicadoe spans over about 210 km, from Laconia to Thessaly. It is the only representative of this genus outside the Peloponnese recorded so far. This may explain the high intraspecific genetic diversity (p = 0.021). In the molecularly based phylogram it still from the single mOTU D. In L, seminula (mOTU A), whose range spans only about 70 km, the intraspecific diversity is lower (p = 0.013). The shells of the specimens from the type locality in Thessaly (locality 6: Figure 9I–L) are more slender and have higher spire than the shells from the Peloponnese (locality 1: Figure 9A–E and locality 2: Figure 12A). Slenderer shells with higher spire were also found at the localities 4 and 5, the ones less slender and with lower spire—at the other Peloponnese locality 11, but also at the locality 7 in Attica. In general, however, there was a continuum in the shell habitus, and this N-S variability seems of the clinal character. One can speculate that there is some congruence between these proportions and genetic divergence visible in the phylogram (Figure 4), but the biometrical data are too limited for any more justified conclusion.
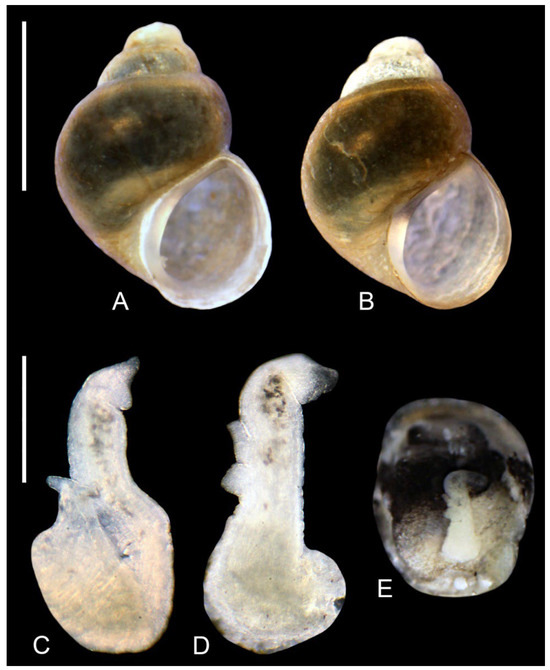
Figure 12.
Lerniana, locality 2: (A,B). shell. (A). L. dianadelicadoae sp. nov. (B). L. moreana sp. nov. (C–E). penis, L. moreana sp. nov. Bar equals 1 mm; for shells and 200 µm for penes.
Lerniana moreana Grego & Jaszczyńska, sp. nov.
- LSID urn:lsid:zoobank.org:act:9C5EE953-14E9-4A50-B97A-CB35F72CA3E4
- GenBank sequence number: PX096769
- mOTU C
Holotype: Ethanol-fixed specimen (Figure 12B) collected on 25th of June 2018 by Jozef Grego, at spring 3.3 km N of Kastorio village at Aqueduct source in Virtaliotiko Farangi, Laconia, Peloponnese, Greece (our locality 2: Figure 1), 37°10′01.2″ N 22°18′25.9″ E, voucher number ZMUJ2763.
Paratypes: 20 ethanol-fixed specimens, collected at the type locality, voucher number ZMUJ2764–ZMUJ2783.
Description: Shell (Figure 12B) up to 1.53 mm high, ovate-conical, broad, with spire low or moderately low, with about 4 whorls, spire height 15% of the shell height. Teleoconch whorls nearly flat, growing regularly in diameter. Aperture narrow ovoid, apex flat, outer lip simple, parietal lip broad and complete, umbilicus slit-like. Teleoconch moderately thick-walled, its wall translucent, with delicate growth lines, periostracum yellowish. Shell measurements: Table 3, Figure 6. Operculum smooth on its both sides.
Soft parts morphology and anatomy: Head and mantle pigmented intensely black. Female reproductive organs unknown. Penis (Figure 12C–E) bent, proximally broad, distally narrow with sharp tip, with small outgrowth close to the tip of the penis, vas deferens running in zigzag inside.
Differential diagnosis: The shell undistinguishable from the one of L. dianadelicadoae, which is clearly visible in Figure 6. The penis differs in its terminal outgrowth and proportions. Molecularly these species occurring at the locality 2 in sympatry differ clearly. The Molecular Diagnostic Characters in comparison with other Lerniana species: binary: 29 (A).
Derivation of name: Species name reflects geographic location of its type locality (Figure 1), the specific epithet moreana refers to Morea, the medieval name of Peloponnese Peninsula.
Distribution and habitat: Known only from the type locality, where it occurs in sympatry with L. dianadelicadoae. The habitat is represented by a large, scattered spring zone in humid broadleaf forest with a few springs captured into local aqueduct and as tap water source for Kastorio village. The aqueduct was used to run a small, now abandoned powerplant at mouth of the valley, and as supply of the local irrigation channels. Live specimens were found all over the spring zone on fallen leaves and stones.
Incertae sedis: unknown genus assignment
“Radomaniola” elongata (Radoman, 1973)
Shell: (Figure 13A–C) up to 3.05 mm high, turriform (Figure 13A) or ovate-conical (Figure 13B,C), slender, with high spire, with 4½–5½ whorls, spire height up to 46% of the shell height, body whorl narrow. Teleoconch whorls moderately convex, evenly rounded, growing regularly in diameter, in turriform specimens suture deeper. Aperture narrow ovoid or nearly circular, outer lip simple, parietal lip moderately broad and complete, umbilicus slit-like. Teleoconch moderately thick-walled, its wall translucent, with delicate growth lines, periostracum whitish or yellowish. Shell measurements: Table 3, Figure 6. Protoconch (Figure 13D) broad, its surface with delicate depressions. Operculum smooth on both surfaces.
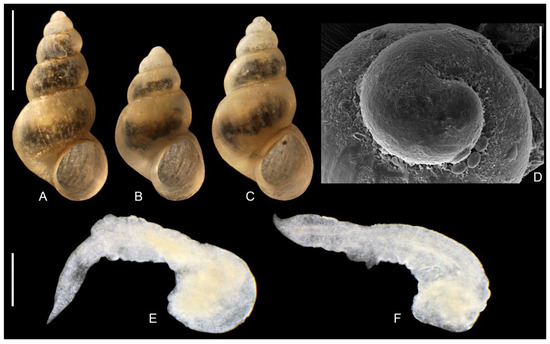
Figure 13.
“Radomaniola” elongata. (A–C). shells. (D). protoconch. (E,F). penes. Bar equals 1 mm for shells, 100 µm for protoconch and 200 µm for penes.
Radula: (Figure 10B) with central tooth with blunt and massive median cusp and cutting edge triangular in shape, following formula:
5 – 1 – 5 or (6)5 – 1 – 5(6)
2 – 2 2 – 2
2 – 2 2 – 2
Lateral tooth with (3)2 – 1 – 2(3) rather sharp and massive, the biggest one broad and rounded. Inner marginal tooth with 13–14 long and sharp cusps, outer marginal tooth with 7–11 cusps on the terminal edge.
Soft parts morphology and anatomy: Head and mantle intensely pigmented black. Female reproductive organs (Figure 7C) with moderately big bursa copulatrix irregular in shape, moderately broad loop of oviduct, proximal receptaculum seminis (rs2) small and spherical in shape, distal one (rs1) very long and narrow. It has to be noted that the female reproductive organs presented in Falniowski et al. [1] for the specimen from the type locality of “Radomaniola” elongata (Figure 11 in their study) were erroneously identified as Radomaniola curta curta (the shell from this locality M17: Figure 5 in their study, was typical of “Radomaniola” elongata). Penis (Figure 13E,F) flat and narrow, with sharp tip and with no outgrowths (or only vestigial ones), vas deferens often visible inside the penis.
Distribution and habitat: Found at spring on island Vranjina, Skadar Lake area Montenegro (Figure 1), at its type locality.
“Radomaniola” sp. 1 and 2
At the locality 9, spring near Stimfalia Lake, between Bouzion and Kalanoi, Peloponnesus, Greece, there were found two haplotypes, evidently representing two distinct species, clearly distinct molecularly but undistinguishable morphologically, at least with the material available for examination (see Discussion).
Shell: (Figure 14A,B) up to 1.82 mm high, ovate-conical, broad, with spire relatively low or moderately low, with about 4 whorls, spire height 16–20% of the shell height, Teleoconch whorls slightly convex, evenly rounded, growing regularly in diameter. Aperture ovoid, outer lip simple, parietal lip moderately broad and complete, umbilicus broad or slit-like. Teleoconch moderately thick-walled, its wall translucent, with delicate growth lines, periostracum whitish or yellowish. Shell measurements: Table 3, Figure 6. Protoconch (Figure 14C) broad, no sharp border between proto- and teleoconch, its microsculpture with regular net of depressions, relatively broad and shallow. Operculum smooth on its both surfaces.

Figure 14.
“Radomaniola” sp., locality 9. (A,B). shells, (C). protoconch. (D–G). penes. Bar equals 1 mm for shells, 100 µm for protoconch and 200 µm for penes.
Radula: (Figure 10C) with central tooth with median cusp broad but sharp, and cutting edge in the form of flat triangle, following formula:
8 – 1 – 8
1 – 1
1 – 1
Lateral tooth with (4)3– 1 – 5(6) rather sharp and massive, the biggest one broad. Inner marginal tooth with 27–29 long and sharp cusps, outer marginal tooth with 8–10 cusps on the terminal edge.
Soft parts morphology and anatomy: Head and mantle intensely pigmented black. Female reproductive organs (Figure 7D) with big and spherical bursa copulatrix, narrow loop of oviduct, proximal receptaculum seminis (rs2) small and spherical in shape, distal one (rs1) big and bulky. Penis (Figure 14D–G) bent and flat, proximally broad, distally narrow, with no outgrowths on its left side, characteristic of Radomaniola, with two small outgrowths on its right side. Vas deferens not visible inside the penis.
Distribution and habitat: Found only in a spring at the locality 9.
Trichonia Schütt, 1980; type species: Trichonia trichonica Radoman, 1973
Trichonia trichonica Radoman, 1973
Our materials were collected in 1985 and fixed in formalin. Thus, it has not been possible to obtain sequences. The radula and protoconch were described and illustrated by Szarowska [10], the female reproductive organs were illustrated by Radea et al. [19], the penis was neither described nor illustrated.
Shell: (Figure 15A–C) up to 2.60 mm high, turriform, slender, with high spire, with 4½–5½ whorls, spire height 35–39% of the shell height, body whorl narrow. Teleoconch whorls moderately convex, evenly rounded, growing regularly in diameter. Aperture narrow ovoid, outer lip simple, parietal lip complete, moderately broad or narrow. umbilicus slit-like or covered. Teleoconch moderately thick-walled, its wall translucent, growth lines well visible, periostracum whitish or yellowish. Shell measurements: Table 3, Figure 6. Protoconch (Figure 15D,E) broad, its surface with a net of delicate depressions. Operculum smooth on its both surfaces, similar as shown by Szarowska [10].
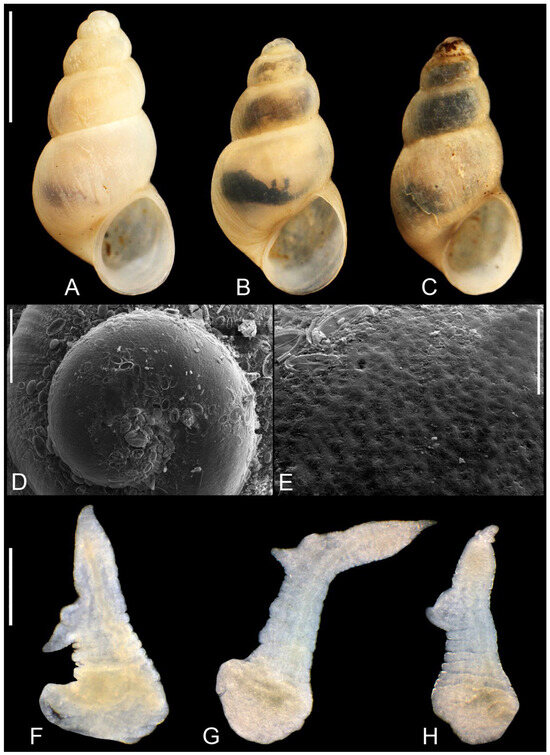
Figure 15.
Trichonia trichonica. (A–C). shell. (D,E). protoconch. (F–H). penes. Bar equals 1 mm for shells, 100 µm for protoconch and 200 µm for penes.
Radula: (Figure 10D) with central tooth with blunt and massive median cusp and cutting edge triangular in shape, following formula:
5 – 1 – 5 or (6)5 – 1 – 5(6) or 6 – 1 – 6
1 – 1 1 – 1 1 – 1
1 – 1 1 – 1 1 – 1
Lateral tooth with 3 – 1 – 3 rather sharp and massive cusps, the biggest one broad but sharp. Inner marginal tooth with 17–18 long and sharp cusps, outer marginal tooth with 18 cusps on the terminal edge. The radula identical with the one described and illustrated by Szarowska [10].
Soft parts morphology and anatomy: Head and mantle intensely pigmented black. Female reproductive organs (Figure 7E) with moderately big bursa copulatrix spherical in shape, broad and bulky loop of oviduct, proximal receptaculum seminis (rs2) small and spherical in shape, broader and shorter than presented by Radea et al. [19], and the distal one (rs1) elongately pyriform in shape, bigger than the one presented by Radea et al. [19]. Penis (Figure 15F–H) flat and narrow, with sharp tip and one bilobed outgrowth, vas deferens visible inside the penis.
Distribution and habitat: Described from Trichonida (Trichonis) Lake in SW continental Greece. Collected not from sublittoral, but from puddle-like springs around the lake. Later this oligotrophic lake was polluted, eutrophicated, and the water level decreased a couple of meters (due to the water intake from the drills for the agriculture), and majority of the fauna inhabiting this unique ancient lake disappeared (personal observations in 2003). However, extensive field work of Radea et al. [18,19] resulted in the collection of three living specimens at the depth 2–4 m (thus hardly a sublittoral), in the North part of the Trichonida Lake, and also in the spring in Neromanna, 3 km NE of Triconida Lake. Frogley & Preece [43] reported this species from the Krya’s Spring at the northern bank of Pamvoris Lake, near Ioannina. However, without molecular data the identification was doubtful, the spring was completely dry in 2003 (AF personal observation), and Radea et al. [18] did not found any shell at that locality.
Trichonia kephalovrissonia Radoman, 1973
Collected at the locality 8 (Figure 2B), spring in the city centre of Thérmon, NE of Trichonida Lake. Its’ shell and penis were figured in Falniowski et al. [1]. In the present paper only its’ sequences have been used.
4. Discussion
- (1)
- “Radomaniola” elongata clearly belongs neither to the genus Radomaniola, nor to Lerniana, it is distinct both morphologically and molecularly. However, with only COI sequence, it is not possible to infer its phylogenetic relationships. Probably it represents a distinct genus, but some fresh material is badly needed, to sequence more loci. It is close to Lerniana phylogenetically, but not geographically, since Montenegro is far from southern Greece. Similarly. the phylogenetic relationships of another clade of “Radomaniola”, consisted of two rather molecularly distinct species, remain unclear.
Delicado & Hauffe [2] stated: “Most of the molecular species delimitation methods merged R. feheri and R. seminula into one group. Our anatomical examinations also revealed close similarities between the populations attributed to these two species. This points to synonymizing of the two species. However, the priority [sic] of the species name is not clear. Our study includes topotypes of the species R. feheri but not of R. seminula, which was described earlier [6,44]. Populations of the latter species have been attributed to R. seminula [1] because their shape and size resemble those of the lectotype selected by Schütt [14]. However, the populations studied by Schütt [14] and Falniowski et al. [1] could have been misidentified, because they are not located directly within the type locality region of R. seminula (i.e., the Archadia regional unit” [6]). Obviously, the case has nothing in common with the priority rule: the priority of seminula is undisputable. Arcadien of Frauenfeld [6] is Arcadia, while “Archadia” is used in some fantasy stories.
Glöer & Hirschfelder [45] described a new genus, Tridentalia Glöer et Hirschfelder, 2023, with its type species T. lykaia Glöer et Hirschfelder, 2023, from Ano Karies, Arcadia, Peloponnese, thus from the terra typica of Lerniana seminula. Their locality is situated between our localities 10 (G4, D) and 11 (G6). Tridentalia lykaia, has shell and penis habitus (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7, p. 128) identical to those of L. seminula (Figure 5A–F,H,I) in our study. Despite the fact that Glöer & Hirschfelder [45] presented neither female reproductive organs, nor molecular data, Tridentalia lykaia is most probably a junior synonym of Lerniana seminula.
- (2)
- Lerniana tritonum (mOTU B) is evidently distinct, both morphologically and molecularly from L. seminula (mOTU A). On the other hand, molecular identity and slight morphological differences between L. feheri and L. seminula legitimate, despite the doubts presented by Delicado & Hauffe [2], synonymizing of L. feheri as a younger synonym of L. seminula.
Delicado & Hauffe [2] published two COI sequences of ‘Lerniana tritonum’ collected somewhere in Myli, different from the one published by Falniowski et al. [1], clearly belonged to L. seminula. We should also note that in the time of the collection of the materials of Falniowski et al. [1], in 2003, the type locality of L. tritonum was already nearly destroyed. It should also be noted, that in our samples we found two species in sympatry at 15 localities, and even three at one locality. Thus, even sampling at the same locality might have resulted in the collection of another species [46].
- (3)
- It is noteworthy that L. tritonum has been found only at its type locality so far, while L. seminula at six localities, all of them at Peloponnese. Newly described L. dianadelicadoae expands the geographic range of Lerniana—it can be found also in Attica (locality 7) and south Thessaly (Figure 1: locality 4–6). Another newly described species L. moreana has been found at only one locality (Figure 1: locality 2) at Peloponnese, in sympatry with L. dianadelicadoae.
- (4)
- “Radomaniola” elongata in our tree computed for all the 168 COI sequences of Radomaniola and allied taxa (also including the sequences of Delicado & Hauffe [2]), the tree not presented in this study, clustered outside of the clade grouping Radomaniola. As stated above, without freshly collected material, enabling to study other loci, its phylogenetic relationships remain enigmatic. Apart from its characteristically high-spired shell, all the other character states can be found also in Radomaniola.
- (5)
- Trichonia kephalovrissonia in the COI/18S tree [1] belonged to the clade grouping all the species of Radomaniola, also “R. elongata”, although the clade was formed by unresolved polytomy. This suggested the assignment of Trichonia kephalovrissonia to the genus Radomaniola, and this suggestion was followed by Radea et al. [18]. The assignment of T. kephanovrissonnia to the genus Radomaniola was later confirmed by the molecular data of Delicado & Hauffe [2], thus followed in WoRMS (Editorial Board) 2024 [47]. However, our COI tree places T. kephalovrissonia within this clade (bootstrap support 74%), not with Radomaniola. richonia kephalovrissonia falls close to the three “Radomaniola” species (“Radomaniola” elongata, “Radomaniola” sp. 1, “Radomaniola” sp. 2), and the phylogenetic position of all the four taxa remains unclear. It has to be noted that there are still no molecular data for Trichonia trichonica.
- (6)
- In the principal component analysis (Figure 6) PC1 and PC2 explained cumulatively 90.1% of the total variation, the other PCs explained less than expected with a broken-stick model [48,49]. PC1 reflects mostly size differences, morphological polymorphism, and sexual dimorphism, PC2 differences in shape [50]. As could be seen in Figure 6, PC1 reflected small shells of Trichonia trichonica different from all the other taxa. The latter overlapped in the PC1 space, only the ranges of variation were different. Also, in PC2 space the most variable Lerniana dianadelicadoae overlapped with less variable L. seminula, L. moreana and “Radomaniola”sp. 1. Lerniana tritonum and especially “Radomaniola” elongata differed in PC2. Obviously, with such unnumerous specimens measured, and not representative samples, these conclusions should be interpreted with caution, as rather preliminary, but still PCA seems useful as a summary of the morphometric data.
Our study clearly illustrates how fragmentary is our knowledge on the real biodiversity of the minute truncatelloid gastropods, whose morphology—simple and variable—makes hardly possible species distinction. This makes no more than tentative all decisions concerning species and habitat protection. The freshwater habitats in Greece, especially springs, are either endangered or already destroyed [17]. European Red List of Non-marine Molluscs [51] presents dramatically bad state of most of the freshwater species. It has to be noted, however, that many of the species listed there should be synonymized, as younger synonyms. On the other hand, numerous studies, like the present one, uncover numerous cryptic or hard to discern species. Fragmentary data result also in supposed extinction of still existing species [52]. All these strongly supports the urgent need of more extensive studies on these snails.
Supplementary Materials
Nomenclature The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/taxonomy5030045/s1. Ref. [53] are cited in Supplementary Materials.
Author Contributions
Conceptualization, A.J., S.H. and A.F.; Methodology, A.J., S.H. and A.O.; Formal analysis, S.H. and A.O.; Investigation, A.J., A.O. and S.H.; Resources, J.G. and A.F.; Writing—original draft, A.J., S.H. and A.F.; Writing—review & editing, A.J., S.H. and A.F.; Visualization, A.J.; Funding acquisition, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the grant N18/DBS/000012, from the Institute of Zoology and Biomedical Research, Jagiellonian University.
Data Availability Statement
All the sequences are deposited in GenBank.
Acknowledgments
We are grateful for the assistance of Anna Łatkiewicz (Laboratory of FE Scanning Microscopy and Microanalysis, Institute of Geological Sciences, Jagiellonian University, Krakow, Poland) for her help with the SEM. We would like also to express our gratitude to two anonymous Reviewers, whose valuable and detailed comments and corrections have resulted in much better manuscript.
Conflicts of Interest
The author declare no conflict of interest.
References
- Falniowski, A.; Szarowska, M.; Glöer, P.; Pešić, V. Molecules vs morphology in the taxonomy of the Radomaniola/Grossuana group of Balkan Rissooidea (Mollusca: Caenogastropoda). J. Conchol. 2012, 41, 19–36. [Google Scholar]
- Delicado, D.; Hauffe, T. Shell features and anatomy of the springsnail genus Radomaniola (Caenogastropoda: Hydrobiidae) show a different pace and mode of evolution over five million years. Zool. J. Linn. Soc. 2022, 196, 393–441. [Google Scholar] [CrossRef]
- Bourguignat, J.-R. Testacea Novissima Quæ Cl. de Saulcy in Itinere per Orientem Annis 1850 et 1851, Collegit; Baillière: Paris, France, 1852; pp. 1, 5–31. Available online: https://gallica.bnf.fr/ark:/12148/bpt6k99037b/f1.image (accessed on 10 August 2025).
- Bourguignat, J.-R. Catalogue raisonné des mollusques terrestres et fluviatiles recueillis par M. F. de Saulcy pendant son voyage en Orient. In Voyage Autour de la Mer Morte et Dans les Terres Bibliques Exécuté de Décembre 1850 à Avril 1851, Livr. 15; de Saulcy, F., Ed.; Gide & J. Baudry: Paris, France, 1853; XXVI+96p, pp. 1–4, 64, Available online: http://gallica.bnf.fr/ark:/12148/bpt6k99038p (accessed on 24 August 2025).
- Roth, J.R. Spicilegium molluscorum orientalium annis 1852 et 1853 collectorum. Malakol. Blätter 1854, 2, 17–58. [Google Scholar]
- Von Frauenfeld, G. Vorläufige Aufzählung der Arten der Gattungen Hydrobia Htm. und Amnicola Gld. Hldm. in der kaiserlichen und in Cuming’s Sammlung. Verhandlungen Kais.-Königlichen Zool.-Bot. Ges. Wien Abh. 1863, 13, 17–1032. Available online: https://www.biodiversitylibrary.org/page/54758154 (accessed on 24 August 2025).
- Westerlund, C.A.; Blanc, H. Apercų sur la Faune Malacologique de la Grèce Inclus l’Epire et la Thessalie. Coquilles Extramarines; Imprimerie de Ferré & Tornese: Naples, Italy, 1879; Volume 19, pp. 1–61. Available online: https://www.molluscabase.org/aphia.php?p=sourcedetails&id=298691 (accessed on 10 August 2025).
- Martens, E. Griechische Mollusken. Gesammelt von Eberh. Von Örtzen. Arch. Für Naturgeschichten 1889, 1, 169–240. [Google Scholar]
- Radoman, P. New classification of fresh and brakish [sic!] water Prosobranchia from the Balkans and Asia Minor. Poseb. Izd. Prir. Muz. Beogr. 1973, 32, 1–30. [Google Scholar]
- Szarowska, M. Molecular phylogeny, systematics and morphological character evolution in the Balkan Rissooidea (Caenogastropoda). Folia Malacol. 2006, 14, 99–168. [Google Scholar] [CrossRef]
- Delicado, D.; Hauffe, T.; Wilke, T. Fifth mass extinction event triggered the diversification of the largest family of freshwater gastropods (Caenogastropoda: Truncatelloidea: Hydrobiidae). Cladistics 2024, 40, 82–96. [Google Scholar] [CrossRef]
- Haldeman, S.S. A Monograph of the Limniades and Other Fresh Water Univalve Shells of North America; J. Dobson: Philadelphia, PA, USA, 1840; Volume 1, pp. 1–16. [Google Scholar] [CrossRef]
- Kabat, A.R.; Hershler, R. The prosobranch snail family [Hydrobiidae (Gastropoda: Rissooidea): Review of classification and supraspecific taxa. Smithson. Contrib. Zool. 1993, 547, 1–94. [Google Scholar] [CrossRef]
- Schütt, H. Zur Kenntnis griechischer Hydrobiiden. Arch. Molluskenkd. 1980, 110, 115–149. [Google Scholar]
- Falniowski, A.; Jaszczyńska, A.; Osikowski, A.; Hofman, S. Litthabitellidae: A new family of the Truncatelloidea (Caenogastropoda). J. Nat. Hist. 2023, 57, 299–329. [Google Scholar] [CrossRef]
- Westerlund, C.A. Malakologiska bidrag. II. För Vetenskapen nya Land- och Sötvatten Mollusker. Öfversigt Af Kongliga Vetensk.-Akad. Förhandlingar 1881, 4, 50–70. [Google Scholar]
- Szarowska, M.; Falniowski, A. Destroyed and threatened localities of Rissooid snails (Gastropoda: Rissooidea) in Greece. Folia Malacol. 2011, 19, 35–39. [Google Scholar] [CrossRef]
- Radea, C.; Parmakelis, A.; Papadogiannis, V.; Charou, D.; Triantis, K.A. The hydrobioid freshwater gastropods (Caenogastropoda, Truncatelloidea) of Greece: New records, taxonomic re-assessments using DNA sequence data and update of the IUCN Red List Categories. ZooKeys 2013, 350, 1–20. [Google Scholar] [CrossRef]
- Radea, C.; Louvrou, I.; Bakolitsas, K.; Economou-Amilli, A. Local endemic and threatened freshwater hydrobiids of Western Greece: Elucidation of anatomy and new records. Folia Malacol. 2017, 25, 3–13. [Google Scholar] [CrossRef]
- Rueden, D.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, e529. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef]
- Falniowski, A. Anatomical characters and SEM structure of radula and shell in the species-level taxonomy of freshwater prosobranchs (Mollusca: Gastropoda: Prosobranchia): A comparative usefulness study. Folia Malacol. 1990, 4, 53–142. [Google Scholar] [CrossRef]
- Hershler, R.; Ponder, W.F. A review of morphological characters of hydrobioid snails. Smithson. Contrib. Zool. 1998, 600, 1–55. [Google Scholar] [CrossRef]
- Szarowska, M.; Osikowski, A.; Hofman, S.; Falniowski, A. Pseudamnicola Paulucci, 1878 (Caenogastropoda: Truncatelloidea) from the Aegean Islands: A long or short story? Org. Divers. Evol. 2016, 16, 121–139. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Xia, X. Data Analysis in Molecular Biology and Evolution; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2000; 296p. [Google Scholar]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenetics Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Xia, X. DAMBE: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; 8p. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v.2; Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hohn, A.S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.3.1; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, Scotland, 2010. [Google Scholar]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2011, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Merckelbach, L.; Borges, L. Make every species count: FastaChar software for rapid determination of molecular diagnostic characters to describe species. Mol. Ecol. Resour. 2020, 20, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Hütter, T.; Ganser, M.H.; Kocher, M.; Halkic, M.; Agatha, S.; Augsten, N. DeSignate: Detecting signature characters in gene sequence alignments for taxon diagnoses. BMC Bioinform. 2020, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Radoman, P. Hydrobioidea a superfamily of Prosobranchia (Gastropoda). I. Systematics. Monogr. Serbian Acad. Sci. Arts DXLVII Dep. Sci. 1983, 57, 1–256. [Google Scholar]
- Frogley, M.; Preece, R. A review of the aquatic Mollusca from Lake pamvotis, Ioannina, an ancient lake in NW Greece. J. Conchol. 2007, 39, 271–296. [Google Scholar]
- Georgiev, D. A new species of Radomaniola Szarowska, 2006 (Gastropoda: Hydrobiidae) from Peloponnese, Greece. Acta Zool. Bulg. 2013, 65, 293–295. [Google Scholar]
- Glöer, P.; Hirschfelder, H.-J. A new hydrobiid genus and species from Peloponnese (Greece), with a note on Achaiohydrobia (Gastropoda: Hydrobiidae). Basteria 2023, 87, 127–129. [Google Scholar]
- Falniowski, A. Species Distinction and Speciation in Hydrobioid gastropods (Mollusca: Caenogastropoda: Truncatelloidea). Arch. Zool. Stud. 2018, 1, 3. [Google Scholar] [CrossRef]
- WoRMS (Editorial Board). World Register of Marine Species. 2024. Available online: https://www.marinespecies.org (accessed on 29 July 2025).
- Rohlf, F.J. NTSYSpc, Numerical Taxonomy and Multivariate Analysis System; Version 2.0. [Computer Software and Manual]; Exeter Software: Setauket, NY, USA, 1998. [Google Scholar]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. How many principal components? Stopping rules for determining the number of non-trivial axes revisited. Comput. Stat. Data Anal. 2005, 49, 974–997. [Google Scholar] [CrossRef]
- Falniowski, A. Metody Numeryczne w Taksonomii [Numerical Techniques in Taxonomy]; Wydawnictwo Uniwersytetu Jagiellońskiego: Kraków, Poland, 2003. [Google Scholar]
- Cuttelod, A.; Seddon, M.; Neubert, E. European Red List of Non-marine Molluscs; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Szarowska, M.; Hofman, S.; Falniowski, A. Vinodolia fiumana Radoman, 1973 (Caenogastropoda: Rissooidea): Rediscovery and relationships of a species presumed extinct. Folia Malacol. 2013, 21, 135–142. [Google Scholar] [CrossRef]
- Boeters, H.D.; Glöer, P.; Slavevska-Stamenković, V. The Radomaniola/Grossuana group from the Balkan Peninsula, with a description of Grossuana maceradica n. sp. and the designation of a neotype of Paludina hohenackeri Küster, 1853 (Caenogastropoda: Truncatelloidea: Hydrobiidae). Arch. Molluskenkd. 2017, 146, 187–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).