An Overlooked New Endemic Species of Renonus DeLong, 1959 (Hemiptera: Cicadellidae: Deltocephalinae: Athysanini) from the Seasonally Dry Forest of Western Mexico †
Abstract
1. Introduction
2. Materials and Methods
2.1. Leafhopper Sampling
2.2. Morphological Terminology and Criteria
2.3. Procedures of Specimen’s Preparation
2.4. Imaging, Edits, and Vectorization
2.5. Entomological Repositories
| CNIN | Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City, Mexico. |
| USNM | National Museum of Natural History, Washington DC, USA. |
| AMNH | American Museum of Natural History, New York, USA. |
| FSCA | Florida State Collection of Arthropods, Gainesville, Florida, USA. |
| CAS | California Academy of Sciences, San Francisco, California, USA. |
| NHM | National History Museum, London, UK. |
| OSUC | Ohio State C.A. Triplehorn Insect Collection, Ohio, USA. |
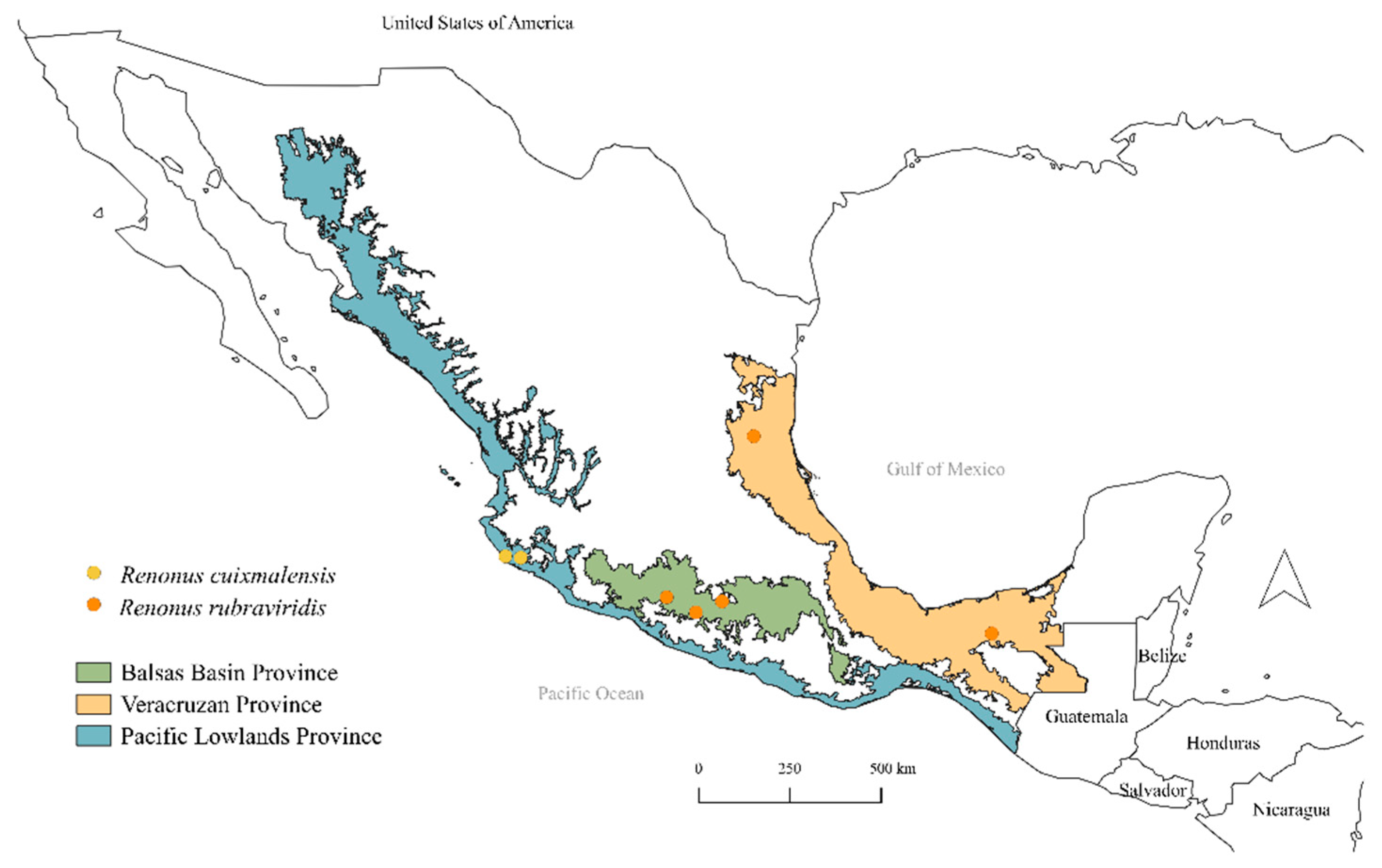
3. Results

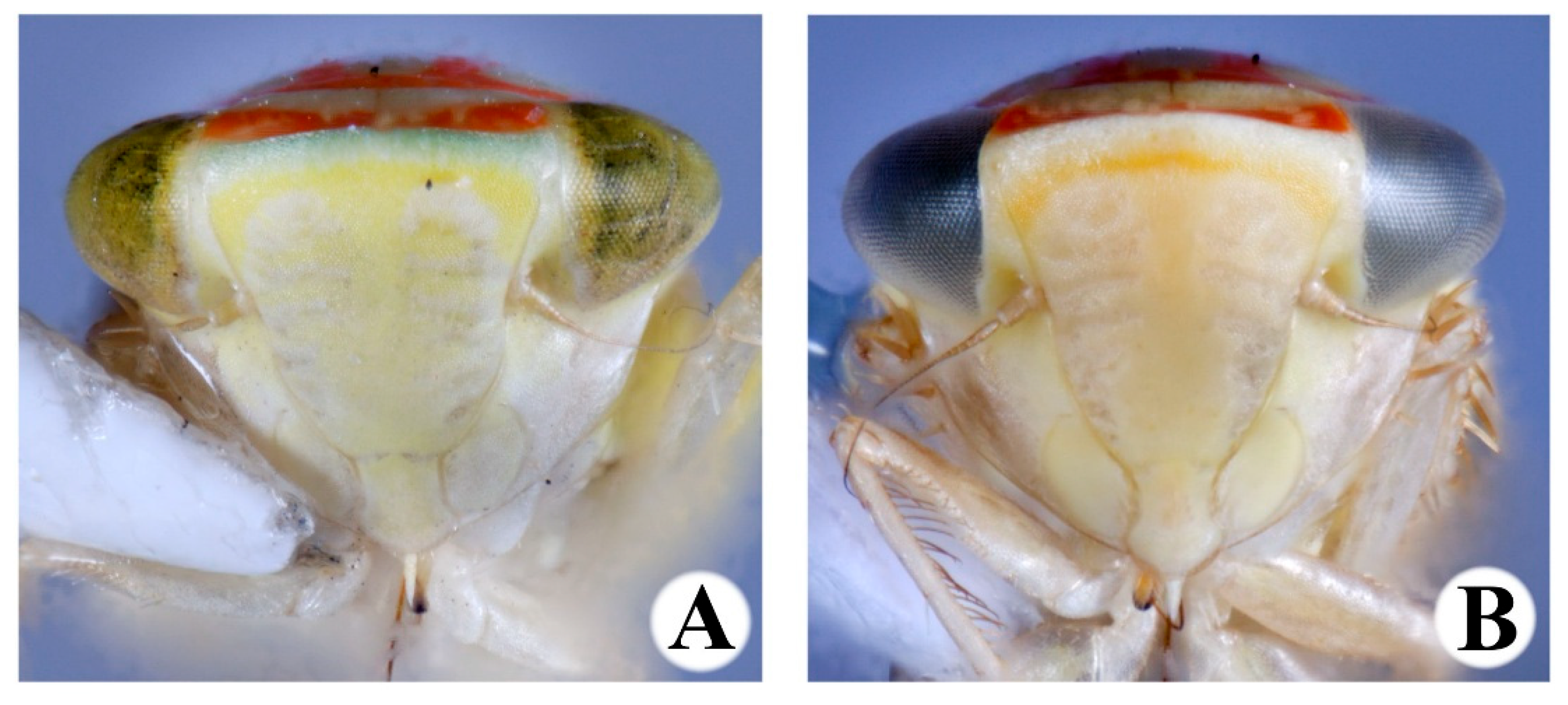
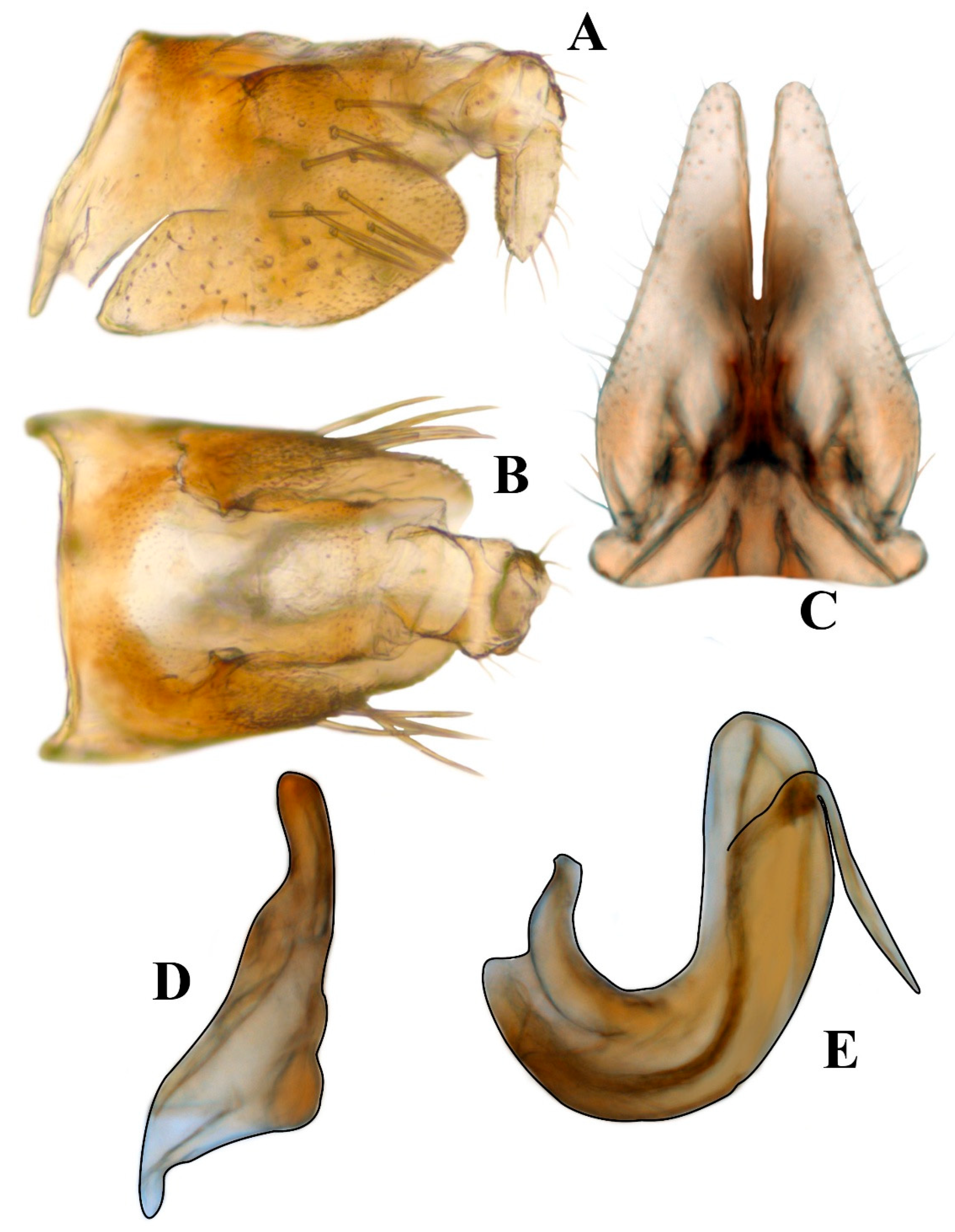


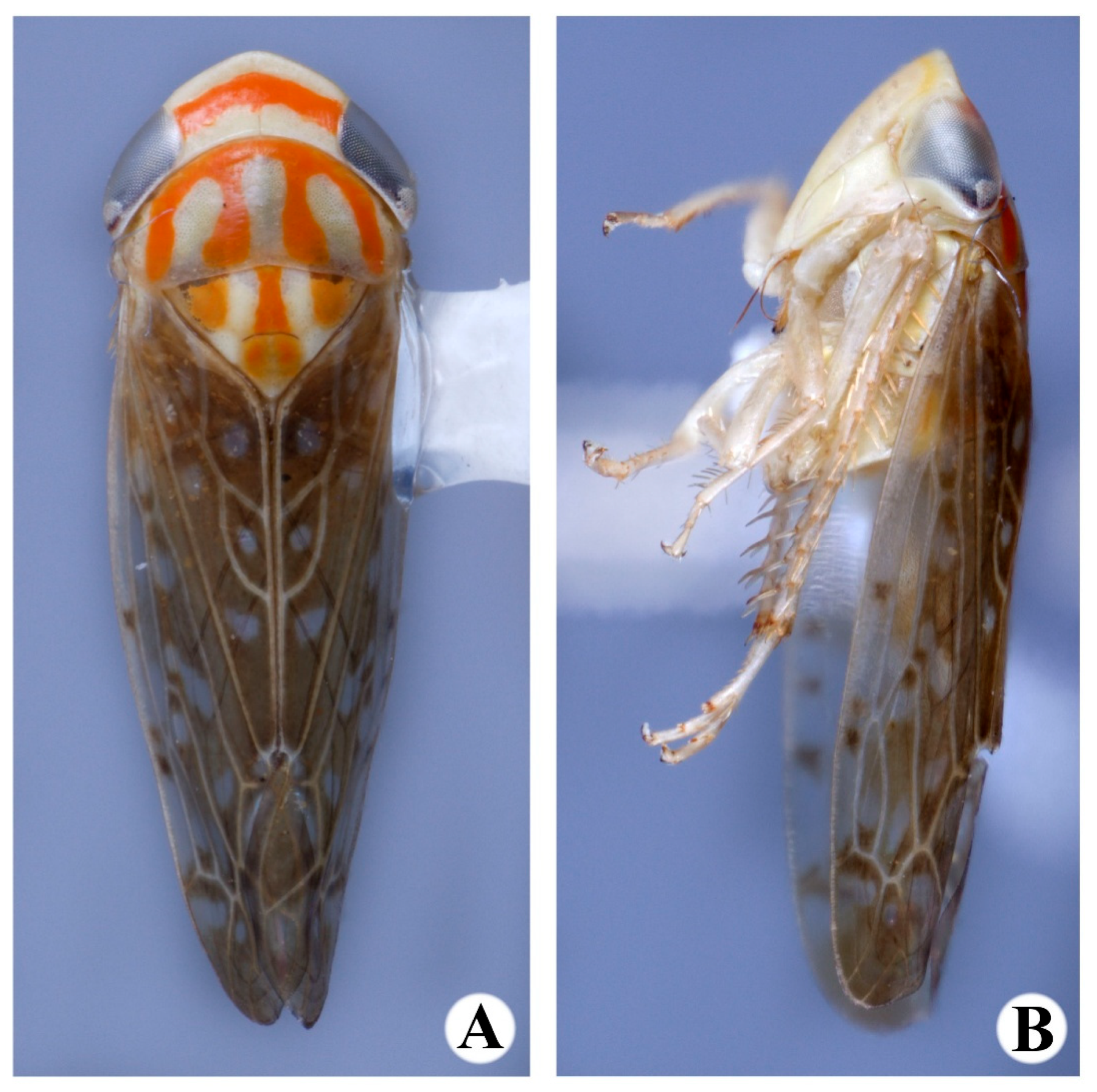
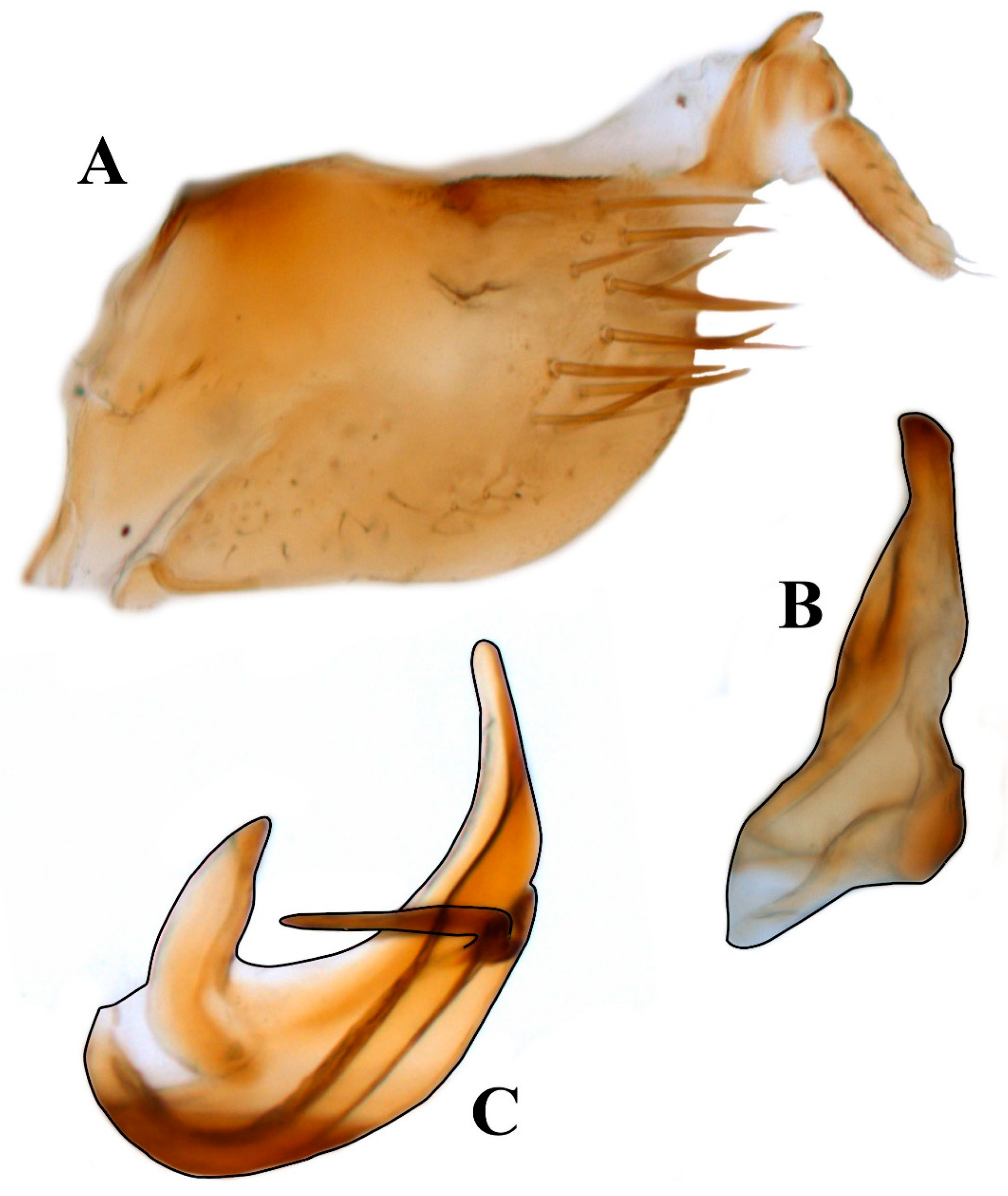
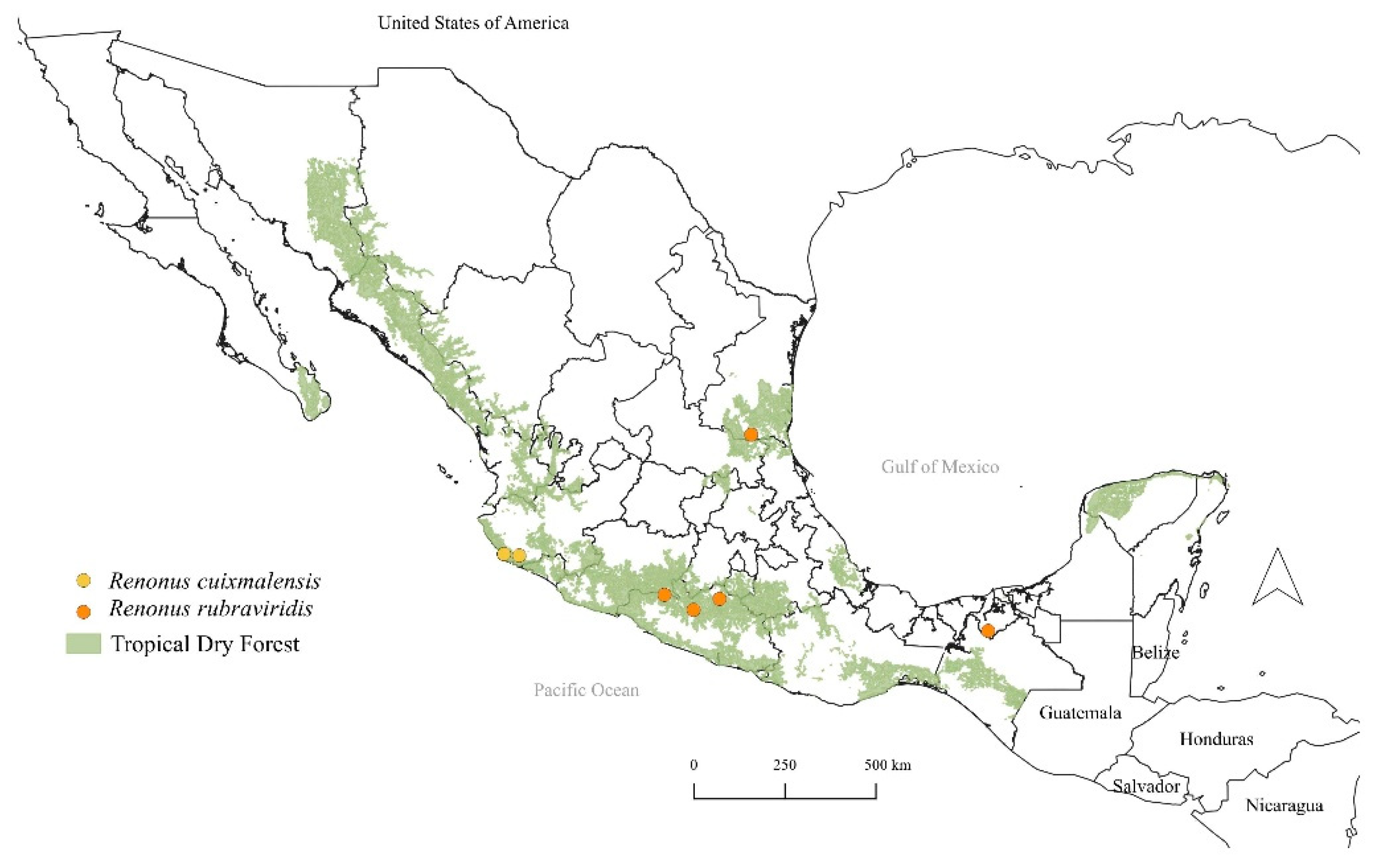
- 1.
- Posterior margin of pygofer pointed; style apex digitate; apex of aedeagus rounded; shaft with medial processes on posterior margin strongly directed anterad; ………………………………………………………………… Renonus rubraviridis DeLong, 1959
- -
- Posterior margin of pygofer truncate; style apex slightly pointed; apex of aedeagus acute; shaft with preapical processes on posterad margin directed caudad………………………………………………………………………… Renonus cuixmalensis sp. nov.
4. Discussion

Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dmitriev, D.A.; Angelova, R.; Anufriev, G.A.; Bartlett, C.R.; Blanco-Rodríguez, E.; Borodin, O.I.; Cao, Y.-H.; Cara, C.; Deitz, L.L.; Dietrich, C.H.; et al. World Auchenorrhyncha Database. TaxonPages. 2022-Onward. Available online: https://hoppers.speciesfile.org/ (accessed on 15 July 2025).
- Pinedo-Escatel, J.A.; Moya-Raygoza, G.; Dietrich, C.H.; Zahniser, J.N.; Portillo, L. Threatened Neotropical seasonally dry tropical forest: Evidence of biodiversity loss in sap-sucking herbivores over 75 years. R. Soc. Open Sci. 2021, 8, 201370. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Escatel, J.A.; Aragón-Parada, J.; Dietrich, C.H.; Moya-Raygoza, G.; Zahniser, J.N.; Portillo, L. Biogeographical evaluation and conservation assessment of arboreal leafhoppers in the Mexican Transition Zone biodiversity hotspot. Divers. Distrib. 2021, 27, 1051–1065. [Google Scholar] [CrossRef]
- Pinedo-Escatel, J.A.; Dietrich, C.H.; Zahniser, J.N.; Moya-Raygoza, G.; Portillo, L. A dichotomous key and checklist for Mexican Athysanini leafhopper genera (Hemiptera: Cicadellidae) with a new species from the Oaxacan dry tropical forest. Eur. J. Entomol. 2021, 118, 255–278. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C.H. Phylogeny of the leafhopper subfamily Deltocephalinae (Insecta: Auchenorrhyncha: Cicadellidae) and related subfamilies based on morphology. Syst. Biodivers. 2008, 6, 1–24. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C. A review of the tribes of Deltocephalinae (Hemiptera: Auchenorrhyncha: Cicadellidae). Eur. J. Taxon. 2013. [Google Scholar] [CrossRef]
- Ebeling, A.; Hines, J.; Hertzog, L.R.; Lange, M.; Meyer, S.T.; Simons, N.K.; Weisser, W.W. Plant diversity effects on arthropods and arthropod dependent ecosystem functions in a biodiversity experiment. Basic Appl. Ecol. 2018, 26, 50–63. [Google Scholar] [CrossRef]
- Van Schalkwyk, J.; Pryke, J.S.; Samways, M.J.; Gaigher, R. Congruence between arthropod and plant diversity in a biodiversity hotspot largely driven by underlying abiotic factors. Ecol. Appl. 2019, 29, e01883. [Google Scholar] [CrossRef]
- Ferraz, G.; Russell, G.J.; Stouffer, P.C.; Bierregaard, R.O.; Pimm, S.L.; Lovejoy, T.E. Rates of species loss from Amazonian forest fragments. Proc. Natl. Acad. Sci. USA 2003, 100, 14069–14073. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Aguilar-Pérez, J.G.; Pinedo-Escatel, J.A.; Valdez-Quezada, B.C. Three new Mexican species of the endemic Athysanini leafhopper genus Devolana DeLong (Hemiptera: Cicadellidae) from the tropical dry forest. J. Nat. Hist. 2019, 53, 2039–2056. [Google Scholar] [CrossRef]
- Pinedo-Escatel, J.A.; Dietrich, C.H. Nomenclatural changes and two new species in the leafhopper genus Usanus DeLong (Hemiptera: Cicadellidae) with notes on conservation status. Zootaxa 2020, 4822, 567–576. [Google Scholar] [CrossRef]
- DeLong, D.M. A new genus, Renonus, and two new species of Mexican leafhoppers (Homoptera: Cicadellidae). Ohio J. Sci. 1959, 59, 325–326. [Google Scholar]
- Dietrich, C.H. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Fla. Entomol. 2005, 88, 502–517. [Google Scholar] [CrossRef]
- Anufriev, G.A.; Emeljanov, A.F. Suborder Cicadinea (Auchenorrhyncha). In Keys to the Insects of the Far East of the USSR; Homoptera and Hemiptera; Nauka Publishing House: Leningrad, Russia, 1988; p. 496. [Google Scholar]
- Rakitov, R.A. On differentiation of cicadellid leg chaetotaxy (Homoptera: Auchenorrhyncha: Membracoidea). Russ. Entomol. J. 1998, 6, 7–27. [Google Scholar]
- Oman, P.W.; Knight, W.J.; Nielson, M.W. Leafhoppers (Cicadellidae): A bibliography, Generic Check-List and Index to the World Literature 1956–1985; CABI Databases: Wallingford, UK, 1990; p. 368. [Google Scholar]
- Zanol, K.M.R. Catalogue of the Neotropical Deltocephalinae (Hemiptera: Cicadellidae). Part III–Tribe Athysanini. Acta Biol. Paran. 2008, 37, 1–104. [Google Scholar]
- Linnavuori, R. Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Ann. Zool. Soc. Zool.-Bot. Fenn. Vanamo 1959, 20, 1–370. [Google Scholar]
- DeLong, D.M. A new genus Acunasus and eight new species of Mexican leafhoppers (Homoptera: Cicadellidae). Ann. Entomol. Soc. Am. 1945, 38, 199–206. [Google Scholar] [CrossRef]
- DeLong, D.M. Studies of the Mexican Deltocephalinae: A new genus, Conversana and three new species. Proc. Entomol. Soc. Wash. 1967, 69, 266–269. [Google Scholar]
- DeLong, D.M. A new genus (Costamia) and species of Mexican leafhopper (Homoptera: Cicadellidae). Ann. Entomol. Soc. Am. 1946, 30, 82–83. [Google Scholar] [CrossRef]
- DeLong, D.M.; Harlan, H.J. Studies of the Mexican Deltocephalinae: New species of Eutettix and two allied new genera (Homoptera: Cicadellidae). Ohio J. Sci. 1968, 68, 139–152. [Google Scholar]
- DeLong, D.M.; Hershberger, R.V. A new genus (Crassana), new subgenus (Macrasana) and new species of North American leafhoppers (Homoptera-Cicadellidae). Pan-Pac. Entomol. 1947, 23, 76–78. [Google Scholar]
- DeLong, D.M.; Hershberger, R.V. A new genus, Dampfiana, and new species of leafhopper related to Stoneana (Homoptera, Cicadellidae). Proc. Entomol. Soc. Wash. 1948, 50, 229–230. [Google Scholar]
- DeLong, D.M. Spinulana, new genus of Mexican Deltocephalinae and two new species of Spinulana (Homoptera, Cicadellidae). Ohio J. Sci. 1967, 67, 20–22. [Google Scholar]
- Oman, P.W. The Nearctic Leafhoppers (Homoptera: Cicadellidae). A Generic Classification and Check List (No. 3); Entomological Society of Washington: Washington, DC, USA, 1949. [Google Scholar]
- Cwikla, P.S.; Blocker, H.D. Neotropical genera of Deltocephalinae not included in Linnavuori’s 1959 key. Bull. Entomol. Soc. Am. 1981, 27, 170–178. [Google Scholar] [CrossRef]
- DeLong, D.M. New genera and species of Mexican and South American deltocephaline leafhoppers (Homoptera, Cicadellidae, Deltocephalinae). Rev. Peru. Entomol. 1980, 23, 63–71. [Google Scholar]
- DeLong, D.M. A new genus–Stoneana–and three new species of Mexican leafhoppers (Homoptera: Cicadellidae). Ann. Entomol. Soc. Am. 1943, 36, 448–450. [Google Scholar] [CrossRef]
- Ball, E.D. Some new genera and species of leafhoppers related to Mesamia Ball. Bull. Brooklyn Entomol. Soc. 1931, 29, 91–95. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinedo-Escatel, J.A. An Overlooked New Endemic Species of Renonus DeLong, 1959 (Hemiptera: Cicadellidae: Deltocephalinae: Athysanini) from the Seasonally Dry Forest of Western Mexico. Taxonomy 2025, 5, 37. https://doi.org/10.3390/taxonomy5030037
Pinedo-Escatel JA. An Overlooked New Endemic Species of Renonus DeLong, 1959 (Hemiptera: Cicadellidae: Deltocephalinae: Athysanini) from the Seasonally Dry Forest of Western Mexico. Taxonomy. 2025; 5(3):37. https://doi.org/10.3390/taxonomy5030037
Chicago/Turabian StylePinedo-Escatel, J. Adilson. 2025. "An Overlooked New Endemic Species of Renonus DeLong, 1959 (Hemiptera: Cicadellidae: Deltocephalinae: Athysanini) from the Seasonally Dry Forest of Western Mexico" Taxonomy 5, no. 3: 37. https://doi.org/10.3390/taxonomy5030037
APA StylePinedo-Escatel, J. A. (2025). An Overlooked New Endemic Species of Renonus DeLong, 1959 (Hemiptera: Cicadellidae: Deltocephalinae: Athysanini) from the Seasonally Dry Forest of Western Mexico. Taxonomy, 5(3), 37. https://doi.org/10.3390/taxonomy5030037




