Abstract
We describe three new species: Canthon mezcalis Moctezuma, Sánchez-Huerta, and Halffter sp. nov. from the Valles Centrales region in Oaxaca, Mexico; Canthon hondurensis Moctezuma and Sánchez-Huerta sp. nov. from Honduras; and Canthon woodruffi Moctezuma and Sánchez-Huerta sp. nov. from Guanacaste, Costa Rica. Through the examination of external and genital morphology, Bayesian inference, and genetic distances based on the Kimura two-parameter model of nucleotide substitution, we confidently determined that the C. indigaceus species group is a monophyletic unit, which is integrated by a set of cryptic species.
1. Introduction
The New World genus Canthon Hoffmannsegg, 1817 is one of the most important dung beetle groups. The most recent revision of Canthon considered the existence of at least 174 species and subspecies [1,2]; however, online databases suggest that it currently includes at least 164 species and 40 subspecies [3]. Recent DNA-based phylogenetic studies suggest that Canthon is a non-monophyletic genus [4,5,6], and additional work is required to clearly define the arbitrary limits stablished between the subspecific and specific ranks [2,7,8,9,10] and to deal with the high number of yet-to-be-described species included within Canthon [2,9].
The subgenus Canthon Hoffmannsegg, 1817 is distributed from northern USA to central Argentina. The diagnostic characters of this subgenus are the marginate anterior edge of the metafemur and separation of the pygidium from the propygidium by a keel [11]. Most of the species of this subgenus are coprophagous and considered as insects of the prairies, savannahs, or similar formations, although there are cases of species that inhabit the tropical and temperate forests [2,11]. The subgenus Canthon is currently organized in the following species groups: C. bispinus, C. cyanellus, C. humectus, C. indigaceus, C. juvencus, and C. pilularius [2,6,11]. The C. indigaceus species group is found from the southern USA to Central America, and all taxa included within this species group were originally considered to be full species: Canthon indigaceus LeConte, 1866; C. chevrolati Harold, 1868; and C. chiapas Robinson, 1948. However, all these taxa were downranked to subspecies of C. indigaceus for decades [2,11], until a recent work described a new species and revised the status of the previously described taxa [10]. The purpose of this study is to describe three new species of the C. indigaceus species group. In addition, we determine if the C. indigaceus species group is a monophyletic unit and if the currently recognized species deserve full species status.
2. Materials and Methods
The holotype of C. mezcalis sp. nov. will be deposited in the Texas A&M University Insect Collection, College Station, TX, USA (TAMU). The type series of both C. hondurensis sp. nov. and C. woodruffi sp. nov. are part of the ex-Gonzalo Halffter Collection, currently housed by the Colección Entomológica Dr. Miguel Angel Morón Ríos, Instituto de Ecología, A.C., Xalapa, Veracruz, Mexico (IEXA). The genital structures of taxonomic interest were extracted and clarified in a 10% KOH solution for 2 h at 70 °C. The aedeagus and endophallites were stored in a microvial with glycerine and pinned under the holotype. Figures were taken using a Leica Z16APOA stereomicroscope and the manufacturer’s software (z-stack image capture method), as well as an Olympus DP70 camera integrated into an Olympus SZX7 stereomicroscope using the manufacturer’s software. Species distribution was gathered from the literature [10,12].
DNA extraction, PCR, sequencing, and alignment techniques followed those carried out by Halffter et al. [6]. Genetic distances were calculated with the software Mega [13] based on the Kimura two-parameter model. The phylogenetic hypothesis was recovered by Bayesian inference using MrBayes v3.2.3 [14]. The best-fit nucleotide substitution model for each locus (i.e., COI, 16S rRNA, and Wingless) was selected using the Akaike information criterion [15] in jMODELTEST v2.0.2 [16]. Monte Carlo Markovian Chains (MCMCs) were implemented in two parallel analyses with one cold and three hot chains each. Fifty million generations were used for the combined dataset. A Bayesian factor test showed that the different molecular markers could be concatenated in a single matrix but were declared as independent partitions. Trees and parameters were sampled every 2000 generations. The majority-rule consensus tree was obtained, with the respective posterior probabilities, after a burn-in discarding the initial 25% of the trees [14]. The resulting tree was dated with the aid of BEAST v1.10.2 software [17]. The nucleotide substitution models were unlinked, as were the parameters used as priors for each locus. The models and parameters used were those indicated by jMODELTEST. The ‘Uncorrelated relaxed clock’ model was selected. A mutation rate of 0.0178 s/s/Myr was set for the COI marker; for the 16S, the rate set was 0.0049 s/s/Myr, whereas, for the nuclear Wingless, the selected rate was 0.0012 s/s/Myr [18]. The Yule process was used to estimate the speciation tree model. The MCMCs were run for twenty million generations, with sampling trees and parameters every 2000 generations. The analysis consisted of four independent runs to ensure that all parameters achieved statistical stationarity. The program TRACER v1.6 [19] was used to assess the stationarity of the MCMCs, effective sample sizes (ESSs > 200), and the 95% highest posterior density for interval spanning. The resulting trees and parameters from each run were combined using LogCombiner v1.8.2, applying a 25% burn-in of the initial data. The nodes evaluated for the final tree were those with a PP value > 0.9.
It is important to stress that all the previously described taxa included in the document (i.e., C. chevrolati, C. chiapas, and C. indigaceus) are defined according to Rivera-Gasperín et al. [10]. Because the aforementioned authors did not revise type material (except for the photographs of five syntypes of C. chevrolati), and since lectotype designation is missing for C. chevrolati, the original definition of these species may be different from that suggested by Rivera-Gasperín et al. [10]. Distributional data were gathered from the literature [10,12] and newly studied specimens.
3. Results
3.1. New Species Description
Canthon mezcalis Moctezuma, Sánchez-Huerta, and Halffter sp. nov.
urn:lsid:zoobank.org:act:DA12C8ED-8C28-4A55-AD18-E3041787937C
Type locality. Mexico: Oaxaca State, municipality of Tlacolula de Matamoros, locality of Tlacolula de Matamoros.
Type material. Holotype: male labeled (Figure 1): “MEXICO, Oaxaca, Tlacolula de Matamoros, 3/IX/2016, C. D. exc. vaca, 96°29′53.3″ O, 16°58′55.5″ N, matorral, 1595 m, Moctezuma & Sánchez-Huerta Col”. “México: Papalutia, Oaxaca, PT.Ch.004, PT031016JV, José Luis y Victor Col”. (TAMU).
Description: Length 12.4 mm, width of pronotum 6.5 mm. The body shape is dorsally and ventrally very convex and smooth (Figure 1). Head: Clypeus highly eroded, bidentate, clypeal teeth slightly marked, coloration blueish-black on the front and the genae, becoming black towards the clypeus. Microsculpture formed by fine separated points, becoming rugged towards the clypeus. Anterior edge of the clypeus with no margination or setae. Ventral surface of the clypeus black in color, with long, abundant setae; ventral surface and internal edge of the clypeal teeth without setae. The ventral structure of the clypeus is formed by a poorly defined fold, which gradually diminishes towards the sides. Rounded genae, blueish-black in color. Eyes narrow; ventral margin of the canthus poorly defined, with scarce, thick setae. Antennal mass blackish, covered by grayish setae. Palpiger and stipe with spiniform setae; mentum with a U-shaped carina; lateral zones of mentum covered with large spiniform setae, anterior edge of the submentum with fine spiniform setae towards the sides; submentum separated by a line of setae and a slight concavity. Thorax: Pronotum convex; microsculpture of the center of the pronotal disk is smooth, with small, superficially impressed punctures; the microsculpture becomes scabriculous towards frontal and lateral edges of the pronotum. Lateral angles of the pronotum are very acute and wide. Lateral edges of the pronotum rounded and without indentation, forming a wide obtuse angle. Posterior angles of the pronotum are slightly visible, marked by the end of the lateral edge of the pronotum. Proepisternum concave with setae. Separation of proepisternum and proepimerum with no keel indicated. Line of scarce setae, parallel to the external edge of the proepimerum. Mid-zone of the metasternum is smooth. Legs: Profemur with dense points and setae on its ventral surface. Posterior edge of the profemur with long setae. Protibia tridentate, teeth of greater size towards the apex. Between the apical and medial teeth, three small teeth are developed. Between the medial and basal teeth, three to four small teeth are present. The apical half of the protibia is widened, narrowing before the basal tooth, and continuing to narrow towards the base. The ventral surface of the mesofemur has distinctly impressed and moderately abundant punctures with setae. The posterior dorsal edge of the mesotibia is covered with setae. Metafemur anteriorly marginate; ventral surface of metafemur with glabrous microsculpture, and with sparse and distinctly impressed punctures, which sometimes bear very short and thick setae; anterior edge of metafemur with thick setae, while posterior edge is glabrous. Tarsi of the three pairs of legs are black in color. Elytra: Blueish-black in color. Elytral striae are superficially marked but clearly visible; striae IV and V are deeply impressed on the humerus (Figure 1). Stria I is deeply impressed on the apex of the elytron (Figure 6A). The striae present very fine and scattered punctures. The interstriae are flat, and interstriae III and IV have small and acute tubercles; punctation of the interstriae is very fine and dispersed, and the microsculpture has a smooth appearance. The microsculpture is scabriculous on the elytral edges. Abdomen: Microsculpture is smooth with superficially impressed and sparse punctures. Blueish-black in color. Tergite VIII: With basal and sub-oval concavity. Superficially impressed and sparse punctures, microsculpture is scabriculous. Tergite VIII and tergite VII are separated by a keel. Base arched. The border is distinctly marked (Figure 7A). Aedeagus: Apical dent of parameres sub-quadrangular in lateral view but rounded in frontal view (Figure 4A).
Etymology: The specific epithet of the new species is related to the so-called Mezcal Route tour (“Ruta del Mezcal” in Spanish), the area in which the holotype was collected. The Mezcal Route tour is recognized as such due to the important production and sale of this Mexican traditional beverage (Mezcal) in the Mexican state of Oaxaca.
Diagnosis: The holotype of the new species (Figure 1) is separated from the other species of the C. indigaceus species group by the medial and rounded concavity near the base of tergite VIII (Figure 7A); striae IV and V are deeply impressed on the humerus (Figure 1); striae I is deeply impressed on the apex of the elytron (Figure 6A); interstriae III and IV have small and acute tubercles; and circular sclerite is shown in Figure 5A.
Geographic distribution and ecology: The new species is only known from the type locality, in the Valles Centrales region of Oaxaca state, in Mexico (Figure 9). These valleys present a semidry temperate climate. During the years 2016, 2017, and 2018, direct collection was performed on cow dung pats on a dirt track road, in a semi-open area surrounded by shrubby vegetation, leading to the discovery of the only known specimen of the new species on a cow pat. Intensive and unsuccessful sampling with 30 pitfall traps, 24 h-active and baited with human feces, was performed in the same study area during the same period. The new species was collected at the same time as other Scarabaeinae, such as C. humectus sayi Robinson, 1918, Phanaeus damocles Harold, 1863, Onthophagus etlaensis Kohlmann, Escobar-Hernández and Arriaga-Jiménez, 2019, and O. gibsoni Howden and Génier, 2004.
Molecular markers accession numbers: 16s (PV563797), COI (PV564242), wingless (PV590060).

Figure 1.
Dorsal habitus of Canthon mezcalis sp. nov. (holotype).
Canthon hondurensis Moctezuma and Sánchez-Huerta sp. nov.
urn:lsid:zoobank.org:act:F2FABEE5-1906-4AEF-AF6C-4F835C084BE7
Type locality. Honduras: El Loarque.
Type material. Holotype: male labeled (Figure 2): “HOND: EL LOARQUE. VIII-30-1968. B. K. Dozier” “Canthon indigaceus chevrolati Harold, 1868. FZ Vaz-de-Mello det. 2004” “Ex-Gonzalo Halffter Salas. IEXA-INECOL”. Paratypes: 2 males, 1 female, with the same data as the holotype.
Description: Length 9.88 mm, width of pronotum 4.83 mm. The body shape is dorsally and ventrally very convex and smooth (Figure 2). Head: Clypeus bidentate, clypeal teeth distinctly marked, coloration blueish-black on the front and the genae, becoming black towards the clypeus. Microsculpture formed by fine separated points, becoming rugged towards the clypeus. Anterior edge of the clypeus with margination and setae. Ventral surface of the clypeus black in color, with long, abundant setae; ventral surface and internal edge of the clypeal teeth without setae. The ventral structure of the clypeus is formed by a poorly defined fold, which gradually diminishes towards the sides. Rounded genae, blueish-black in color. Eyes narrow; ventral margin of the canthus poorly defined, with scarce, thick setae. Antennal mass blackish, covered by setae. Palpiger and stipe with spiniform setae; lateral zones of mentum covered with large spiniform setae, anterior edge of the submentum with fine spiniform setae towards the sides; submentum separated by a line of setae and a slight concavity. Thorax: Pronotum convex; microsculpture of the center of the pronotal disk is smooth, with small, superficially impressed punctures; the microsculpture becomes scabriculous towards frontal and lateral edges of the pronotum. Lateral angles of the pronotum are very acute and wide. Lateral edges of the pronotum rounded and without indentation, forming a wide obtuse angle. Posterior angles of the pronotum are slightly visible, marked by the end of the lateral edge of the pronotum. Proepisternum concave with setae. Separation of proepisternum and proepimerum with no keel indicated. Line of scarce setae, parallel to the external edge of the proepimerum. Mid-zone of the metasternum is smooth. Legs: Profemur with dense points and setae on its ventral surface. Posterior edge of the profemur with long setae. Protibia tridentate, teeth of greater size towards the apex. Between the apical and medial teeth, three small teeth are developed. Between the medial and basal teeth, three to four small teeth are present. The apical half of the protibia is widened, narrowing before the basal tooth, and continuing to narrow towards the base. Ventral surface of mesofemur with distinctly impressed and moderately abundant punctures with setae. Posterior dorsal edge of mesotibia covered with setae. Metafemur anteriorly marginate; ventral surface of metafemur with glabrous microsculpture, and with sparse and distinctly impressed punctures, which sometimes bear very short and thick setae; anterior edge of metafemur with thick setae, while posterior edge is glabrous. Tarsi of the three pairs of legs are black in color. Elytra: Blueish-black in color. Elytral striae superficially marked, becoming deeply impressed towards the base (Figure 2); striae I, II, III, and IV are deeply impressed on the apex of the elytron (Figure 6D). Striae with very fine and scattered punctures. Interstriae flat and smooth, more roughened towards the base, interstriae II and IV with small and acute tubercles, and interstriae V and VI with minute and almost completely reduced tubercles; punctation of the interstriae very fine and dispersed, and the microsculpture has a smooth appearance. The microsculpture is scabriculous on the elytral edges (Figure 2). Abdomen: Microsculpture is smooth with superficially impressed and sparse punctures. Blueish-black in color. Tergite VIII: Convex, without apparent concavity towards the base. Superficially impressed and sparse punctures, microsculpture scabriculous. Base arched. Border is distinctly marked (Figure 7D). Aedeagus: Apical dent of parameres sub-quadrangular in lateral view but rounded in frontal view (Figure 4B).
Etymology: The specific epithet of the new species is derived from Honduras, where the type locality is located.
Diagnosis: The new species is separated from the other species of the C. indigaceus species group by the following character combination: tergite VIII convex without apparent concavities (Figure 7D); elytral striae superficially marked, becoming deeply impressed towards the base (Figure 2); striae I, II, III, and IV are deeply impressed on the apex of the elytron (Figure 6D); interstriae II and IV with small and acute tubercles; interstriae V and VI with minute and almost completely reduced tubercle, circular endophallite, as in Figure 5D.
Geographic distribution and ecology: The new species is only known from the type locality in Honduras (Figure 9). The accurate location of the type locality “El Loarque” is unknown to us; however, it may refer to the “Colonia Loarque” in the environs of Tegucigalpa. The ecology and natural history of the new species are unknown, but it might be a copro-necrophagous beetle.

Figure 2.
Dorsal habitus of Canthon hondurensis sp. nov. (holotype).
Canthon woodruffi Moctezuma and Sánchez-Huerta sp. nov.
urn:lsid:zoobank.org:act:5663366B-08D6-44CE-8000-D43881F1B489
Type locality. Costa Rica: Guanacaste, 6 km S of La Cruz.
Type material. Holotype: male labeled (Figure 3): “COSTA RICA: PROV. OF GUANACASTE, 6 KM.S. OF LA CRUZ, RT. #1” “R. E. WOODRUFF COLL. 11 VI 64” “Canthon indigaceus LeConte A. Solís Det” “Ex-Gonzalo Halffter Salas. IEXA-INECOL”. Paratypes: 2 males, 3 females. 2 males, 2 females with same data as holotype; 1 female labeled: “Finca Pacifica, Canas Guanacaste Prov. COSTA RICA” “13-VI-66 Carrion tr.” “S. Peck Collector” “R.E. Woodruff Collection” “Canthon indigaceus LeConte A. Solís Det” “Ex-Gonzalo Halffter Salas. IEXA-INECOL”.
Description: Length 9.24 mm, width of pronotum 4.63 mm. Body shape is dorsally and ventrally very convex and smooth (Figure 3). Head: Clypeus bidentate, clypeal teeth distinctly marked, coloration blueish-black on the front and the genae, becoming black towards the clypeus. Microsculpture formed by fine separated points, becoming rugged towards the clypeus. Anterior edge of the clypeus with margination and setae. Ventral surface of the clypeus black in color, with long, abundant setae; ventral surface and internal edge of the clypeal teeth without setae. The ventral structure of the clypeus is formed by a poorly defined fold, which gradually diminishes towards the sides. Rounded genae, blueish-black in color. Eyes narrow; ventral margin of the canthus poorly defined, with scarce, thick setae. Antennal mass blackish, covered by setae. Palpiger and stipe with spiniform setae; lateral zones of mentum covered with large spiniform setae, anterior edge of the submentum with fine spiniform setae towards the sides; submentum separated by a line of setae and a slight concavity. Thorax: Pronotum convex; microsculpture of the center of the pronotal disk smooth, with small, superficially impressed punctures; the microsculpture becomes scabriculous towards frontal and lateral edges of pronotum. Lateral angles of the pronotum are very acute and wide. Lateral edges of the pronotum rounded and without indentation, forming a wide obtuse angle. Posterior angles of the pronotum are slightly visible, marked by the end of the lateral edge of the pronotum. Proepisternum concave with setae. Separation of proepisternum and proepimerum with no keel indicated. Line of scarce setae, parallel to the external edge of the proepimerum. Mid-zone of the metasternum is smooth. Legs: Profemur with dense points and setae on its ventral surface. Posterior edge of the profemur with long setae. Protibia tridentate, teeth of greater size towards the apex. Between the apical and medial teeth, three small teeth are developed. Between the medial and basal teeth, three to four small teeth are present. The apical half of the protibia widened, narrowing before the basal tooth, and continuing to narrow towards the base. The ventral surface of the mesofemur has distinctly impressed and moderately abundant punctures with setae. Posterior dorsal edge of mesotibia covered with setae. Metafemur anteriorly marginate; ventral surface of metafemur with glabrous microsculpture, and with sparse and distinctly impressed punctures, which sometimes bear very short and thick setae; anterior edge of metafemur with thick setae, while the posterior edge is glabrous. Tarsi of the three pairs of legs are black in color. Elytra: Blueish-black in color. Elytral striae superficially marked, becoming deeply impressed towards the base (Figure 3); stria I is deeply impressed on the apex of the elytron (Figure 6E). Striae with very fine and scattered punctures. Interstriae flat and smooth, more roughened towards the base, striae II and IV with small and acute tubercles; punctation of the interstriae very fine and dispersed, and microsculpture with a smooth appearance. Microsculpture is scabriculous on the elytral edges. Abdomen: Microsculpture is smooth with superficially impressed and sparse punctures. Blueish-black in color. Tergite VIII: Convex, without apparent concavity towards the base. Superficially impressed and sparse punctures, microsculpture scabriculous. Base arched. The border is distinctly marked (Figure 7E). Aedeagus: The apical dent of the parameres is sub-quadrangular in lateral view but rounded in frontal view (Figure 4C).
Etymology: The specific epithet of the new species is dedicated to R. E. Woodruff, the collector of the type series.
Diagnosis: The new species is diagnosed by the following character combination: tergite VIII convex without apparent concavity (Figure 7E), elytral striae superficially marked, becoming deeply impressed towards the base (Figure 3), striae I and occasionally III deeply impressed on the apex of the elytron (Figure 6E), interstriae III and IV with small and acute tubercles, and circular sclerite as in Figure 5E.
Geographic distribution and ecology: The new species is only known from northwestern Costa Rica, in the Province of Guanacaste (Figure 9); however, its distribution probably reaches southern Nicaragua and Panama. The new species is known to be attracted to dung and carrion, and to have a wide tolerance to seasonality and human disturbance.

Figure 3.
Dorsal habitus of Canthon woodruffi sp. nov. (holotype).
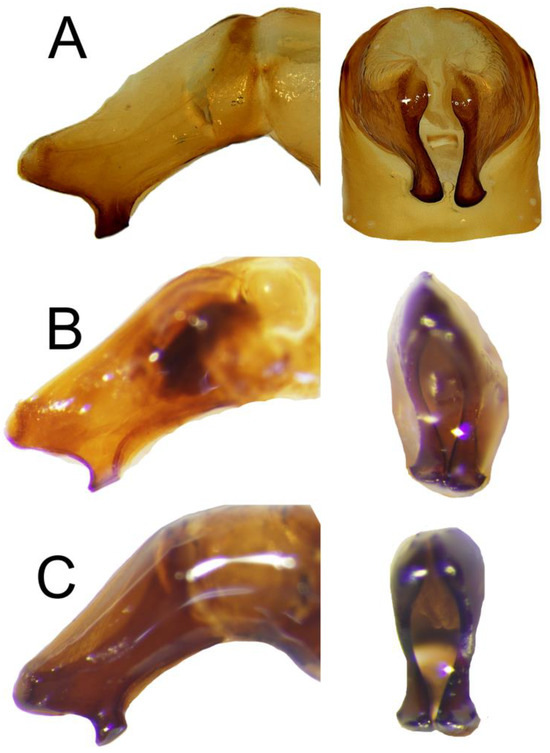
Figure 4.
Apex of parameres of (A) C. mezcalis, (B) C. hondurensis, and (C) C. woodruffi. Left: lateral view, right: frontal view.
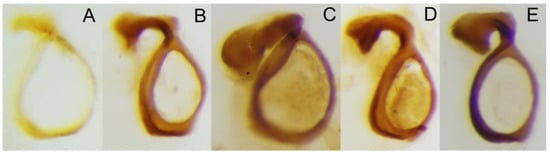
Figure 5.
Circular endophallite of (A) C. mezcalis, (B) C. cuixmala, (C) C. chiapas sensu Rivera-Gasperín et al. [10], (D) C. hondurensis, and (E) C. woodruffi.
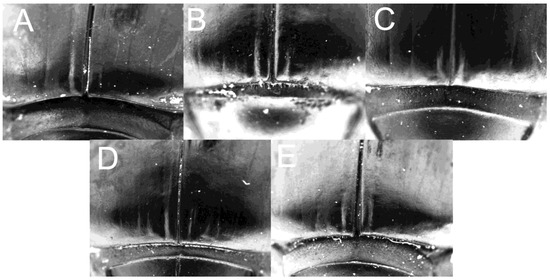
Figure 6.
Apex of elytra of (A) C. mezcalis, (B) C. cuixmala, (C) C. chiapas sensu Rivera-Gasperín et al. [10], (D) C. hondurensis, and (E) C. woodruffi.

Figure 7.
Tergite VIII of (A) C. mezcalis, (B) C. cuixmala, (C) C. chiapas sensu Rivera-Gasperín et al. [10], (D) C. hondurensis, and (E) C. woodruffi.
3.2. Phylogenetic Reconstruction
The length of the concatenated alignment for the three loci had a length of 1922 base pairs (Wingless = 375 bp, 16S rRNA = 780 bp, COI = 767 bp). The sequences used in this analysis have been used previously [6] and can be retrieved freely from GenBank. The best-fit nucleotide substitution model for the nuclear Wingless was TIM3 (nst = 6) + Gamma (0.271); for the mitochondrial 16S rRNA, it was TPM2uf (nst = 6) + Gamma (0.0210); for the mitochondrial COI, it was GTR (nst = 6) + Gamma (0.99) + I (0.537). The phylogenetic relationships of monophyletic clades represented in our study (Figure 6) are supported with PP values ≥ 0.91 (except for C. chevrolati + C. chiapas + C. chevrolati + C. indigaceus with PP = 0.83). Our results suggest that the C. indigaceus species group forms a monophyletic unit, which probably started to diversify around 0.65 Myr, during the middle Pleistocene, with a cladogenetic event that gave origin to C. cuixmala and to the ancestor of the other species of the C. indigaceus species group included in our study. A subsequent speciation event, ca. 0.45 Myr, probably gave rise to the new species C. mezcalis and to the ancestor of C. indigaceus, C. chevrolati, and C. chiapas (Figure 8).
Mean pairwise genetic distance estimated without including C. mezcalis was 8.99%, while the interspecific mean pairwise genetic distance was 0.84%, supporting the idea that C. mezcalis is a fully established species, and the C. indigaceus species group is made up by a set of cryptic species with high genetic variation (Table 1). At least three transitions were identified in the COI fragment that could likely be used as barcoding markers (Table 2).

Table 1.
Genetic distances based on the Kimura two-parameter model of nucleotide substitution.

Table 2.
Positions identified in the COI sequences for potential use in barcoding.
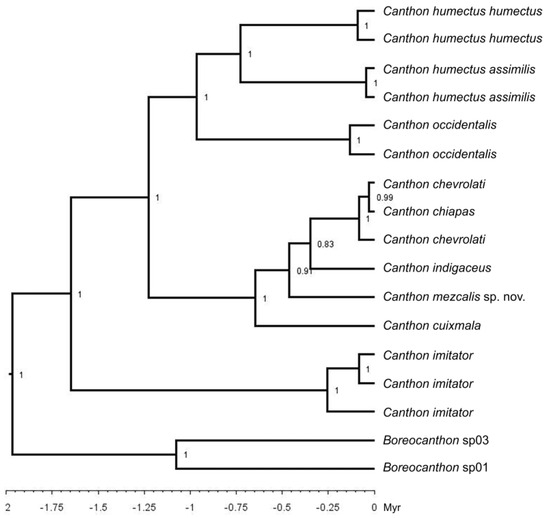
Figure 8.
Chronogram for the interrelationships of the Canthon indigaceus species group and closely related allies. Numbers indicate Bayesian posterior probabilities (PPs).
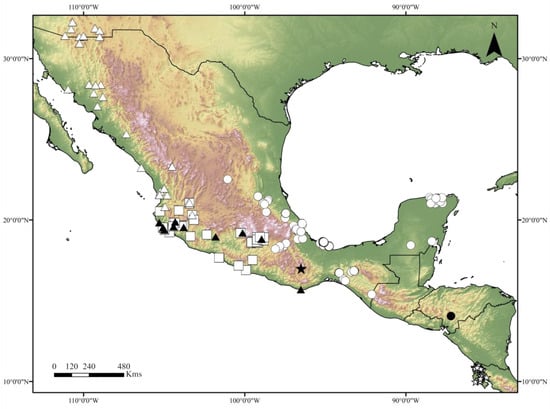
Figure 9.
Distribution of the Canthon indigaceus species group, modified from [10,11,12]. Black star: C. mezcalis sp. nov., black dot: C. hondurensis sp. nov., white star: C. woodruffi, white dot: C. chiapas sensu Rivera-Gasperín et al. [10], black triangle: C. cuixmala, white triangle: C. indigaceus sensu Rivera-Gasperín et al. [10], white square: C. chevrolati sensu Rivera-Gasperín et al. [10].
4. Discussion
Characters found in the external morphology and genitalia suggest that C. mezcalis sp. nov., C. hondurensis sp. nov., and C. woodruffi sp. nov. must be included within the C. indigaceus species group, as defined by previous authors [2,10,11]. In addition, both the Bayesian tree and mean corrected genetic distances suggest that the C. indigaceus species group is a monophyletic taxonomical unit formed by cryptic species that underwent a “recent” speciation process. Moreover, its species can be confidently diagnosed by a small set of genital traits. According to the Bayesian phylogeny and genetic distances, at least the previously described C. indigaceus and C. cuixmala deserve full species status, as previously suggested [10], while the taxonomic status of C. chiapas and C. chevrolati must be revised because C. chiapas became nested within two specimens of C. chevrolati. Two likely hypotheses are suggested to explain the relationship between both C. chiapas and C. chevrolati: First, that C. chiapas must be considered as a population within C. chevrolati, and both names should be considered as synonyms. Second, both C. chiapas and C. chevrolati are not adequately defined by previous authors [2,10,11]. In this regard, the holotypes of C. chevrolati and C. chiapas must be revised, and a lectotype designation is needed for C. chiapas in future studies to ensure a better definition of these species.
Even though only one specimen of C. mezcalis has been collected to date, we are certain that this corresponds to a new species as suggested by our results (morphological traits and COI autapomorphies). Interestingly, C. mezcalis is very similar to C. chiapas sensu Rivera-Gasperín et al. [10] in external and aedeagus morphology; however, the phylogeny suggests that it is more closely related to other lineages within the C. indigaceus species group. It would be advised to examine more specimens in the future to conduct a more detailed description of C. mezcalis and its possible intraspecific variation. We tried to increase the size of the type series of C. mezcalis by performing two of the more widely used and successful methods to collect Scarabaeinae (direct collection on cow dung pats and pitfall traps baited with human dung) over three consecutive years; however, the results were not favorable, and this study represents the only one historical record of a species allied to C. indigaceus from the Central Valleys of Oaxaca to date [2,10,11,12]. Consequently, it is possible to consider that C. mezcalis may be a non-coprophagous beetle. It is known so far that non-coprophagous Scarabaeinae used to be extremely rare in conventional sampling because these species are not usually attracted to dung [20].
According to the revision of external (Figure 1, Figure 2, Figure 3, Figure 6 and Figure 7) and genital morphology (Figure 4 and Figure 5) in our study, the circular endophallite (Figure 5) was the structure that best fits with the species recognized by the Bayesian phylogeny (Figure 8) and genetic distances analyses of the C. indigaceus species group. This endophallite (Figure 5) shows an adequate degree of interspecific variation, while its morphology remains homogeneous within populations of the same species, which makes it a keystone to diagnose taxa without available sequences for molecular studies, such as the cases of C. hondurensis (Figure 2) and C. woodruffi (Figure 3), which could not be included within our phylogenetic analysis (Figure 8). However, they were both adequately recognized as different species. Other morphological structures, such as the elytral striae and interstriae (Figure 1, Figure 2, Figure 3 and Figure 6), tergite VIII (Figure 7), and parameres (Figure 4A–C), are not completely reliable. The head, elytra (Figure 1, Figure 2, Figure 3 and Figure 6), and tergite VIII (Figure 7) can be severely affected by erosion, leading to unexpected “interspecific” variation, as previously recognized by Halffter [11]. On the other hand, the shape of the parameres (Figure 4A–C) is similar in C. chiapas sensu Rivera-Gasperín et al. [10], C. mezcalis, C. hondurensis, and C. woodruffi. Our results suggest that at least C. chiapas sensu Rivera-Gasperín et al. [10] and C. mezcalis are not sister species (Figure 8); however, their parameres are almost identical (Figure 4A), a fact that suggests that paramere morphology could be a plesiomorphic character and must be used with caution for systematics studies in Canthon. Furthermore, C. chiapas sensu Rivera-Gasperín et al. [10] is hypothesized to be the sister species of C. chevrolati sensu Rivera-Gasperín et al. [10], despite both species showing highly differentiated parameres. The taxonomical value of the circular endophallite (Figure 5) is not only recognized by us, but also by previous works that focused on other Deltochilini [9,21,22,23,24,25,26].
As previously stated, our results suggest that the C. indigaceus species group diversified during the Pleistocene (Figure 8). Consequently, this species group fits well with the typical Neotropical distributional pattern [6,9,10,12,27]. Typical Neotropical taxa are recognized by their South American origin and dispersion/diversification in the Mexican Transition Zone during the Pliocene–Pleistocene period [12,27]. Typical Neotropical taxa are particularly diverse in tropical rainforests; however, they can inhabit open lowland habitats from South America to the coastal plains and high plateaus of Mexico and the USA (Figure 9), such as the case of the C. indigaceus species group [2,11,12,27]. A similar case of a typical Neotropical Deltochilini that was proven to diversify during the Pleistocene is that of the C. cyanellus species complex [23].
Author Contributions
Conceptualization, V.M., J.L.S.-H., A.E.d.l.M., J.N.-S. and G.H.; methodology, V.M., J.L.S.-H., A.E.d.l.M., J.N.-S. and G.H.; investigation, V.M., J.L.S.-H., A.E.d.l.M., J.N.-S. and G.H.; data curation, V.M., A.E.d.l.M. and J.N.-S.; writing—original draft, V.M., J.L.S.-H., A.E.d.l.M. and G.H.; writing—review and editing V.M., J.L.S.-H. and A.E.d.l.M.; visualization V.M., J.L.S.-H. and A.E.d.l.M.; project administration V.M., A.E.d.l.M., J.N.-S. and G.H.; funding acquisition V.M., A.E.d.l.M. and G.H. All authors have read and agreed to the published version of the manuscript.
Funding
The General Directorate of the Instituto de Ecología, A. C. (grant number 20035/30916) and the Sectorial Research Fund for Education SEP-CONACYT Mexico (grant number CB-257039) funded this research.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The first author thanks SECIHTI Mexico for a grant for postgraduate studies and postdoctoral fellowship (CVU no. 486765). Keith Phillips translated this text from the original Spanish version. Unfortunately, the last author passed away before the final version of this manuscript was completed. The commentaries by two anonymous reviewers helped to improve this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halffter, G.; Martínez, A. Revisión monográfica de los Canthonina Americanos, Part IV, Clave para géneros y subgéneros. Folia Entomol. Mex. 1977, 38, 29–107. [Google Scholar]
- Halffter, G. Subtribu Canthonina. In Atlas de los Escarabajos de México. Coleoptera: Lamellicornia. Vol. II. Familias Scarabaeidae, Trogidae, Passalidae y Lucanidae; Morón, M.A., Ed.; Argania Editio: Barcelona, España, 2003; pp. 22–43. [Google Scholar]
- Schoolmeesters, P. World Scarabaeidae Database. 2025. Available online: https://www.catalogueoflife.org/data/dataset/1027 (accessed on 26 April 2025).
- Monaghan, M.T.; Inward, J.G.D.; Hunt, T.; Vogler, A.P. A molecular phylogenetic analysis of the Scarabaeinae (dung beetles). Mol. Phylogenet. Evol. 2007, 45, 674–692. [Google Scholar] [CrossRef]
- Tarasov, S.; Dimitrov, D. Multigene phylogenetic analysis redefines dung beetles relationships and classification (Coleoptera: Scarabaeidae: Scarabaeinae). BMC Evol. Biol. 2016, 16, 257. [Google Scholar] [CrossRef]
- Halffter, G.; Espinosa de los Monteros, A.; Nolasco-Soto, J.; Arriaga-Jiménez, A.; Rivera-Gasperín, S. Bajacanthon, a new subgenus for the Mexican Deltochilini (Coleoptera: Scarabaeidae: Scarabaeinae) fauna. Diversity 2022, 14, 109. [Google Scholar] [CrossRef]
- Halffter, G.; Rivera-Cervantes, L.G.; Halffter, V. Diversificación del grupo humectus del género Canthon (Coleoptera: Scarabaeidae: Scarabaeinae) en el occidente de México. Acta Zool. Mex. 2015, 31, 208–220. [Google Scholar] [CrossRef]
- Carpintero-Hensen, M.; Medina-Hernández, M.I.; da Silva, P.G.; Amore, V.; Lobo, J.M. Distribution of Canthon rutilans rutilans and Canthon rutilans cyanescens along spatio-temporal and temperature gradients. Insects 2018, 9, 124. [Google Scholar] [CrossRef]
- Nolasco-Soto, J.; González-Astorga, J.; Espinosa de los Monteros, A.; Favila, M.E. Evolutionary history and diversity in the ball roller beetle Canthon cyanellus. Front. Ecol. Evol. 2023, 10, 1066439. [Google Scholar] [CrossRef]
- Rivera-Gasperín, S.L.; Escobar-Hernández, F.; Arellano, L. A new species in the Canthon indigaceus species group (Coleoptera: Scarabaeidae: Scarabaeinae) from the Mexican Pacific Coast. Taxonomy 2025, 5, 11. [Google Scholar] [CrossRef]
- Halffter, G. Monografía de las especies norteamericanas del género Canthon hoffsg. (Coleopt., Scarab.). Ciencia 1961, 20, 225–320. [Google Scholar]
- Halffter, G.; Morrone, J.J. An analytical review of Halffter’s Mexican transition zone, and its relevance for evolutionary biogeography, ecology and biogeographical regionalization. Zootaxa 2017, 4226, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Strecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. MBE 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Huelsenbeck, J.P. Comparative performance of Bayesian and AIC-based measures of phylogenetic model uncertainty. Syst. Biol. 2006, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. JModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Anastasiou, J.; Vogler, A.P. Revisiting the insect mitochondrial molecular clock: The Mid-Aegean trench calibration. MBE 2010, 27, 1659–1672. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1.6. 2014. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 27 April 2025).
- Moctezuma, V. Spatial autocorrelation in a Mexican dung beetle ensemble: Implications for biodiversity assessment and monitoring. Ecol. Ind. 2021, 125, 107548. [Google Scholar] [CrossRef]
- Moctezuma, V.; Sánchez-Huerta, J.L.; Vaz-de-Mello, F.; Halffter, G. Revalidación y redescripción de Deltochilum (Calhyboma) burmeisteri (Coleoptera: Scarabaeidae), con una clave para las especies relacionadas. Rev. Mex. Biodiv. 2021, 92, e923712. [Google Scholar] [CrossRef]
- Silva, F.A.B.; Vaz-de-Mello, F.; Barclay, M.V.L. An updated key to the millipede-hunting subgenus Aganhyboma Kolbe, 1893 of the genus Deltochilum Eschscholtz, 1822 (Coleoptera: Scarabaeidae: Scarabaeinae), with description of a new species from Bolivia and Peru. ISE 2018, 3, 1–9. [Google Scholar] [CrossRef]
- González-Alvarado, A.; Vaz-de-Mello, F.Z. Taxonomic review of the subgenus Hybomidium Shipp 1897 (Coleoptera: Scarabaeidae: Scarabaeinae: Deltochilum). Ann. Soc. Entomol. Fr. (N. S.) 2014, 50, 431–476. [Google Scholar] [CrossRef]
- Silva, F.A.B.; Louzada, J.; Vaz-de-Mello, F. A revision of the Deltochilum subgenus Aganhyboma Kolbe, 1893 (Coleoptera: Scarabaeidae: Scarabaeinae). Zootaxa 2015, 3925, 451–504. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.C.; Vaz-de-Mello, F.Z. A new giant species of Deltochilum subgenus Deltohyboma (Coleoptera, Scarabaeidae, Scarabaeinae) from Colombia, with notes on D. spinipes Paulian, 1938. Zootaxa 2014, 3802, 276–284. [Google Scholar] [CrossRef] [PubMed]
- González, F.A.A.; Molano, F.R.; Medina, C.A.U. Los subgéneros Calhyboma, Hybomidium y Telhyboma (Coleoptera: Scarabaeidae: Scarabaeinae: Deltochilum) en Colombia. Rev. Colomb. Entomol. 2009, 35, 253–274. [Google Scholar] [CrossRef]
- Moctezuma, V.; Espinosa de los Monteros, A.; Halffter, G. Phylogenetic analyses of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae) provide new insights into the Mexican Transition Zone theory. Zootaxa 2024, 5415, 501–528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).