Abstract
Athysanini is one of the largest tribes within Deltocephalinae, which is a vast cosmopolitan subfamily of leafhoppers (Hemiptera: Cicadellidae) with many genera known to occur in the Neotropical realm. Peruvian forests house up to 16 genera of Athysanini and, so far, are strongly restricted to this region. In this contribution, a new species of the leafhopper genus Andanus Linnavuori, 1959, Andanus acanthophallus sp. nov., is described based on pinned museum specimens. Illustrations and a diagnosis for all species to segregate them in the genus are provided. A dichotomous key to all known species and distributional notes are also given. The new species can be easily separated from others by (1) overall color light orange to stramineous, (2) the pronotum lacking marks or transverse medial bands, (3) a row of very long fine setae on the outer lateral margin of the subgenital plate, (4) apophysis of style straight without lateral projection but minute tooth on inner side, and (5) an aedeagal apex bifid with a pair of processes directed anterad and posterad. The valid status and current position of Andanus based on similarities to other Neotropical genera is discussed.
1. Introduction
Cicadellidae is one of the largest herbivorous insect families worldwide (Hemiptera: Auchenorrhyncha: Cicadomorpha: Membracoidea), currently comprising more than 23,000 valid species, and the New World harbors a diverse leafhopper fauna placed in 22 subfamilies [1,2]. Deltocephalinae harbors up to 7239 species and is the richest subfamily in terms of species number, divided into 40 tribes and 991 genera [1,3,4].
The tribe Athysanini in the New World comprises 126 genera with high diversity in both, the Neotropical and Nearctic realms, and in some cases could highly be specialized on host plants or habitats with distributional ranges limited and rarely are extended beyond particular habitats [5,6]. Besides previous research on Athysanini, the tribe is still hard to differentiate from other similar tribes, and placement of some genera (e.g., Platymetopius Burmeister, 1838; Brazosa Oman, 1938; or Pseudalaca Linnavuori, 1959) are questionable [4,7].
The genus Andanus Linnavuori, 1959, comprises four species restricted to the South Brazilian dominion [8], a region in the Americas with a large number of endemic arboreal leafhoppers according to Pinedo-Escatel et al. [7] and Pinedo-Escatel and Dietrich [9]. However, it remains one of the most unexplored areas in South America for leafhoppers, presumably inhabiting canopies of dense rainforests.
The genus Andanus has faced a major restructuring due to the inclusion of several species previously placed in monotypic genera such as Adlaca Lozada, 1998; Paralaca Lozada, 1998; and Perundanus Zanol, 1993. These changes now recognize those generic identities as invalid under Andanus based on comparative morphology [8]. Relationships among these and other South American leafhoppers with similar geographical and environmental preferences have been poorly explored. Despite this, the overall morphology shown in Andanus is, in part, consistently found in the Rondonia province, where other related arboreal taxa are distributed, e.g., Napo Linnavuori and DeLong, 1976, and Pseudonapo Pinedo-Escatel and Dietrich, 2020; however, internal male genitalia ostentatiously vary, aiding and validating their identity.
Herein, through meticulous revision of miscellaneous materials housed in the museums below stated, four unidentified specimens belonging to the genus Andanus were located, and after careful study, it was not possible to allocate them to any described species; thus, with a detailed examination of male terminalia and morphological discrimination, an undescribed species was found, which is here therefore extensively outlined and compared to all known species of the genus.
2. Materials and Methods
Specimens of the new taxon were submitted to an external and internal morphological study, then compared to all species of the genus, including types from museums listed below, to build a functional dichotomous key. Abdomens were removed from the pinned specimens, then cleared by standard leafhopper protocol with the following modifications: the abdomens were submerged overnight in a cold 45% KOH solution, rinsed four times with distilled water, and soaked with clove essence to remove any remaining KOH [10]. Male terminalia was retained in microvials with glycerin and pinned beneath the specimen. Labels are quoted exactly as written, and specimen labels are quoted verbatim in lines. Body length was obtained by using an ocular micrometer mounted to the stereomicroscope, corroborated by an electronic vernier. Body color is described based on dry pinned specimens.
Overall terminology herein follows Dietrich [2], wing venation follows the system proposed by Anufriev and Emeljanov [11], and leg chaetotaxy follows Rakitov [12]. Nomenclatural changes and valid names followed Oman et al. [13], Zanol [14], Linnavuori [5], and Oman [6].
Each checklist entry provides information in order as follows: taxon name, author and year of description, synonyms and original combination, citation, and distribution.
The studied materials are deposited at the Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de México, CDMX, Mexico (CNIN), and Illinois Natural History Survey, University of Illinois at Urbana-Champaign, Champaign, USA (INHS). Revised types are deposited at the National Museum of Natural History, Washington DC, USA (USNM); Illinois Natural History Survey, University of Illinois at Urbana-Champaign, Champaign, USA (INHS); Universidad Nacional Mayor de San Marcos, Peru (USML); Instituto Nacional de Pesquisas da Amazônia, Coleção Sistemática da Entomologia, Amazona, Brazil (INPA); and Coleção Entomológica Prof. José Alfredo Pinheiro Dutra, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (DZRJ).
The shapefile of the map was extracted from Google Earth [15] and vectorized in GIMP [16].
3. Results
Systematic Entomology
Family: Cicadellidae Latreille, 1825.
Subfamily: Deltocephalinae Dallas, 1870.
Tribe: Athysanini Van Duzee, 1892.
Genus: Andanus Linnavuori, 1959.
Andanus Linnavuori, 1959: 237.
Adlaca Lozada, 1998: 113.
Perundanus Zanol, 1993: 616.
Paralaca Lozada, 1998: 113.
Type species: Andanus bimaculatus Linnavuori, 1959.
Note: Pinedo-Escatel et al. [8] provided a review of generic description, a species list, and annotations on the genus.
Diagnosis: Characteristics to discriminate from other Athysanine genera are as follows: Medium-sized and moderately robust leafhoppers with yellowish overall body coloration with brown or orange marks, head rounded with crown relatively short, anterior and posterior margins parallel, surface longitudinally striate, smooth, wide between the eyes, and black marks on the anterior margin with a pair of large-round black spots adjacent to eyes. Pronotum convex; male terminalia with particular long sclerotized 10th segment, narrower than head, slightly declivous with weak lateral carina. Forewing macropterous, without extra crossveins; anal veins without crossvein. Pygofer with spines or serrations, pointed distally, without processes; subgenital plates divergent in ventral view, few macrosetae on the subgenital plate; connective with anterior arms partially fused anteriorly, often curved mesad; aedeagus very long, curved, cylindrical with complex sub and apical processes, and gonoduct sclerotized beyond atrium.
| Species list of Andanus | ||
| Andanus bimaculatus Linnavuori, 1959. | ||
| Distribution: Peru (Madre de Dios) and Brazil (Ipixuna). | ||
| Repository: National Museum of Natural History (USMN) and Illinois Natural History Survey (INHS). | ||
| Andanus acanthophallus sp. nov. | ||
| Distribution: Peru (Madre de Dios). | ||
| Repository: Colección Nacional de Insectos, Instituto de Biología, Universidad Autónoma Nacional de Mexico (CNIN). | ||
| Andanus raunoi (Zanol, 1993). | ||
| Distribution: Peru (Madre de Dios). | ||
| Repository: National Museum of Natural History (USMN). | ||
| Andanus tambopixunensis Pinedo-Escatel, Dietrich and Zahniser, 2022. | ||
| Distribution: Peru (Tambopata) and Brazil (Ipixuna). | ||
| Repository: Universidad Nacional Mayor de San Marcos (USML), Illinois Natural History Survey (INHS), Instituto Nacional de Pesquisas da Amazônia, Coleção Sistemática da Entomologia (INPA), and Coleção Entomológica Prof. José Alfredo Pinheiro Dutra, Universidade Federal do Rio de Janeiro (DZRJ). | ||
| Andanus sordidus (Lozada, 1998). | ||
| Distribution: Peru (Madre de Dios). | ||
| Repository: National Museum of Natural History (USMN). | ||
| Key to males of all known species of Andanus |
| 1. Pygofer on ventral margin with spines or serrated ………………………………‥ 2. |
| - Pygofer simple, without processes or spines …………………………………‥…. 3. |
| 2. Pygofer posterior margin strongly serrated; aedeagus with pair of sublateroapical flanges, one subdorsoapical spine, and single ventromedial spine …………………………………………………………‥……. A. sordidus (Lozada, 1998). |
| - Pygofer posterior margin with two spines; aedeagus with two bifid pair of apical processes directed anterad …………………………………… A. acanthophallus sp. nov. |
| 3. Surface of pygofer with abundant macrosetae on dorsoapical half; aedeagus with two pairs of apical spines directed caudad, without a single spine on ventral surface or long spines along shaft ……………………….……. A. bimaculatus Linnavuori, 1959. |
| - Surface of pygofer with limited macrosetae not extended to apex; aedeagus with one pair of long spines along shaft, with or without preapical spines directed anterad and a single spine on ventral surface …………….……………………………………. 4. |
| 4. Lateral lobe of style near base rounded; aedeagus without short distal spines ………………………………………………………………….…. A. raunoi (Zanol, 1993). |
| - Lateral lobe of style near base acute; aedeagus with pair of short dorsoapical and single ventroapical spine …………………………. A. tambopixunensis Pinedo-Escatel, Dietrich, Zahniser, 2022. |
Andanus acanthophallus sp. nov.
Diagnosis: This species exhibits an overall color of light orange to stramineous, and the pronotum lacks extra marks or transverse medial bands (present in A. raunoi). Anterior margin of crown has distinctly bigger black dots (0.3× smaller in A. sordidus, A. bimaculatus, and A. tambopixunensis). The species displays a row of very long fine setae on the outer lateral margin of subgenital plate, which in A. tambopixunensis and A. bimaculatus are fine or inconspicuous and absent in A. sordidus. While the apophysis of style is largely straight without lateral projection with a minute tooth is present on the inner side. The aedeagus apex is bifid with a pair of processes directed anterad and posterad with a simple and trifid apex, respectively. The style apophysis and aedeagus morphology are distinctive within the genus.
Description: Body coloration fades from light orange to stramineous and brownish. Head in lateral view rounded. Crown fades from light orange to yellowish, with pair of large hexagonal black spots next to eyes slightly on margin line (Figure 1A). Anteclypeus and lora are light orange to yellow with lateral and central ochraceous markings. Gena with outer half ochraceous and inner light orange yellow (Figure 1B). Pronotum mostly brownish with anterior margin yellowish. Scutellum light orange. Forewing semitranslucent and membrane hyaline, distant veins brown and anal and Pcu veins bold orange. Legs ochraceous with yellow specs near base of femur. Dorsum of abdomen mostly brownish with orange medially, venter ochraceous (Figure 1C).
Male genitalia: Pygofer elongate and posterior margin sharply pointed with two processes, one apical (Figure 2Aa) and another subapical (Figure 2Ab); 5–6 macrosetae on dorsum near to second third of pygofer separated from posterior margin and minute stout setae near base (Figure 2B). Anal tube as long as pygofer length, wide at base, strongly sclerotized laterally and connected through dorsum, minute stout setae present (Figure 2A–C).
Valve with anterior margin convex and posterior rounded and strongly sclerotized. Subgenital plate somewhat triangular, mesal margin straight, apex pointed, three rows with 15–17 of small stout setae running medially, another row of 11–13 macrosetae near to outer margin, and inner margin with a row of 30–35 fine elongated setae as long as the subgenital plate length (Figure 3A). Style at base bilobed, medial lobe poorly developed, apophysis long, and apex digitiform with small tooth on inner side (Figure 3B). Connective stem 2.2 times shorter than arms, length reaching midheight of style (Figure 3C). Aedeagus wide, cylindrical, as long as pygofer length, somewhat straight directed caudad; aedeagal apex bifid disposing two processes of which one is single directed caudad with tip curved caudad while second is directed anterad with trifurcated apex (Figure 3D–E). Gonoduct wide and beyond atrium slightly sclerotized. Gonopore circular on apical margin.

Figure 1.
Holotype of Andanus acanthophallus sp. nov. (A) Habitus, dorsal view. (B) Habitus, anterior view. (C) Habitus, lateral view.
Female genitalia: Sternite VII deeply excavated medially with a small notch, Figure 4.
Distribution: Restricted to Peru (Figure 5).
Etymology: The species epithet is formed by combining the Greek words “acanthus” (spiny) and “phallus” (penis) referring to the complex aedeagus ornamentation. The gender is masculine.
Measurements (mm): Male body length: 3.95–4.00; width: 1.58–1.60. Head width: 1.20–1.22; mid-length: 0.17–0.18; eye width: 0.40; eye length: 0.12–0.13; crown mid-width before eyes: 0.67–0.69; crown posterior width between eyes: 0.63–0.64; distance between ocelli: 0.45; frontoclypeus width: 0.59–0.60; frontoclypeus length: 0.78–0.80; anteclypeus width: 0.20–0.22; anteclypeus length: 0.25–0.27; lorum width: 0.12–0.14; lorum length: 0.29–0.30; gena width: 0.53–0.54; gena length: 0.32–0.33. Pronotum width: 1.24–1.26; length: 0.61–0.65. Scutellum width: 0.94–0.97; length: 0.64–0.66. Forewing length: 3.47–3.50. Male capsule pygofer height: 0.49–0.55; pygofer length: 1.51–1.55; valve width: 0.58–0.61; valve length: 0.39–0.50; subgenital plate apex width: 0.08–0.12; mid-width: 0.25–0.30; base width: 0.38–0.41; plate length: 0.80–0.81; style length: 0.50–0.53; aedeagus length: 0.81–0.84.
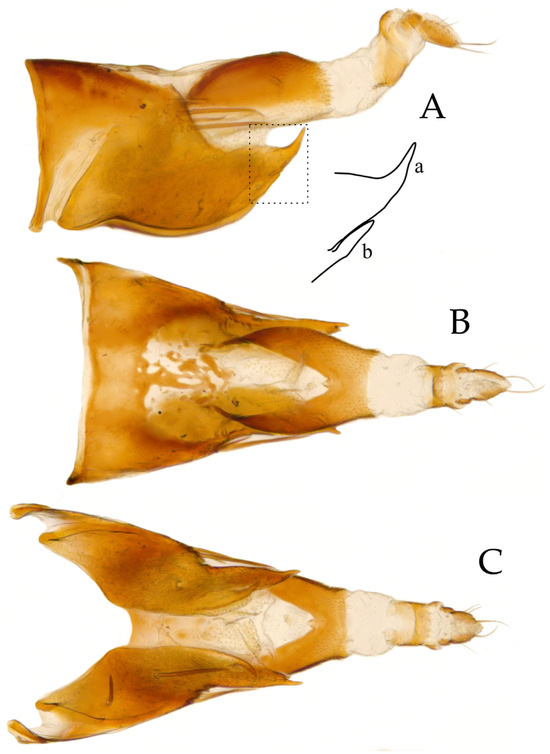
Figure 2.
Holotype of Andanus acanthophallus sp. nov. (A) Pygofer and anal tube, lateral view with line drawing of posterior margin with apical (a) and subapical (b) spines. (B) Pygofer and anal tube, dorsal view. (C) Pygofer and anal tube, ventral view.
Material examined: Holotype: male—PERU: Madre de Dios, Tambopata Research Center, on Rio Tambopata, 662ft., S13°08.305 W69°36.502, C.R. Bartlett Coll. (CNIN). Paratypes: one male and one female—PERU: Madre de Dios, Tambopata Research Center, on Rio Tambopata, 662ft., S13º08.305 W69º36.502, C.R. Bartlett Coll. (CNIN); two females—PERU: Madre de Dios, Tambopata Research Center, on Rio Tambopata, 662ft., S13º08.305 W69º36.502, C.R. Bartlett Coll. (INHS).
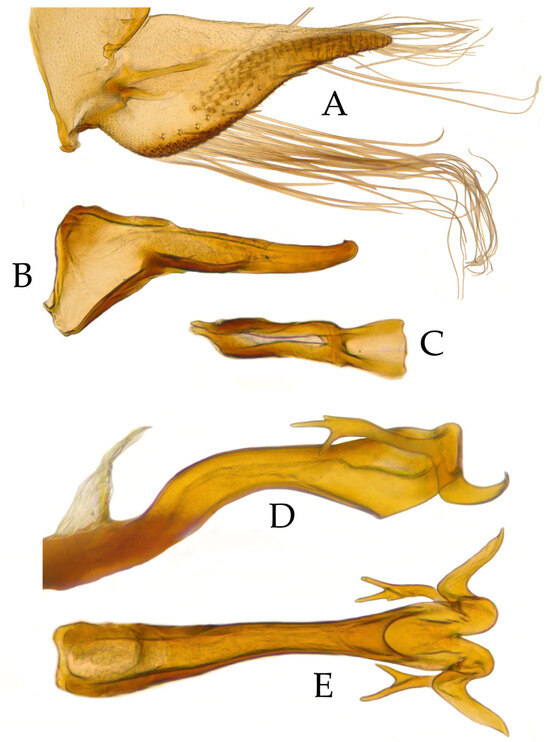
Figure 3.
Holotype of Andanus acanthophallus sp. nov. (A) Subgenital plate and valve, ventral view. (B) Style, ventral view. (C) Connective, ventral view. (D) Aedeagus, lateral view. (E) Aedeagus, ventral view.
Remarks: The new species is similar in external appearance to A. sordidus (Lozada, 1998) and A. tambopixunensis Pinedo-Escatel, Dietrich and Zahniser, 2022, but the width of crown is larger than in preceding species and has a pair of black hexagonal spots conspicuously bigger and closer to eyes than previously known species. A major difference is observed in the pygofer and aedeagus with a unique complex arrangement of apical processes in the genus.
Andanus raunoi (Zanol, 1993)
Perundanus raunoi Zanol, 1993: 616.
Diagnosis: The color is stramineous with a brown transverse medial band on the pronotum, subgenital plate parallel wide towards apex, style with anterolateral lobe strongly produced, apophysis very narrow and elongated, and aedeagus with pair of long processes directed ventrad.
Material examined: One female (USNM) PERU: Cusco, Estacion Biologica Villa Carmen, trail 8 mark 8–1924, 721 m, 12°54′08″ S, 71°24′38″ W, malaise trap, 1–7 January 2013, T. Förster.
Andanus sordidus (Lozada, 1998)
Paralaca sordida Lozada, 1998: 114.
Diagnosis: General color stramineous without bands transversally on pronotum, subgenital plate very narrow with expanded tips distally, style shorter than connective steam and anterolateral lobe strongly projecting with acute apex, apophysis of style short with blunt tip, aedeagus with single short spine at base with a pair of lateral flanges and a dorsal single spine.
Material examined: One male (USNM) PERU: Madre de Dios, Manu, Pakitza, 11°56′ S, 71°18′ W, 12–18.IX.1989, kitchen stream, malaise trap, night, N. Adams et al. Colls.
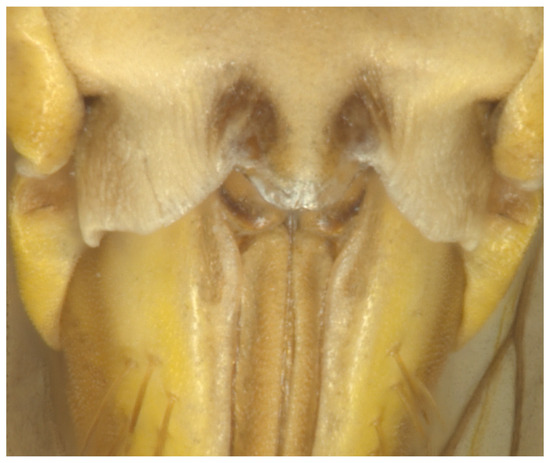
Figure 4.
Paratype of Andanus acanthophallus sp. nov. Female VII sternite, ventral view.
Andanus tambopixunensis Pinedo-Escatel, Dietrich and Zahniser, 2022
Andanus tambopixunensis Pinedo-Escatel, Dietrich and Zahniser, 2022: 3.
Diagnosis: This species can be distinguished from other species by its lack of a transverse medial band on the pronotum, subgenital plate medially sinuate and excavated toward apex, style with anterolateral lobe strongly rounded, apophysis of style without lateral projection, and aedeagus with pair of long processes near apex.
Material examined: One male (USML) PERU: Madre de Dios, Tambopata Research Center, Rio Tambopata, 662ft., 13°08.305′ S, 69°36.502′ W, C.R. Bartlett Coll.; two females (USML) PERU: Madre de Dios, Tambopata Research Center, Rio Tambopata, 662ft., 13°08.305′ S, 69°36.502′ W, C.R. Bartlett Coll.; one male (INPA) BRAZIL: Amazonas, Ipixuna, Rio Liberdade, Comunidade São Vicente no Estirão da Preta, 07°21′47″ S, 071°52′07″ W, 175 m, 11–14.v.2011, light trap, Cavichioli, Gonçalves and Takiya Colls.; one male (INHS) PERU: Madre de Dios, Tambopata Research Center, 13°08.305′ S, 69°36.502′ W, 190 m, 3–7 May 2004, C.R. Bartlett Coll.; one male (DZRJ) BRAZIL: Amazonas, Ipixuna, Rio Liberdade, Com. São Vicente no Estirão da Preta, 07°21′47″ S, 071°52′07″ W, 175 m, 11–14.v.2011, light trap, Cavichioli, Gonçalves and Takiya Colls.
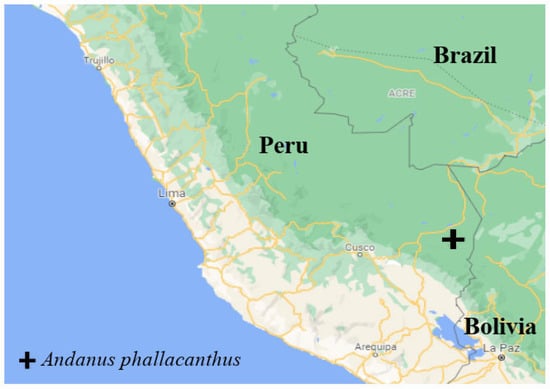
Figure 5.
Map of the distribution of the new species. Shapefile taken from Google Earth.
Andanus bimaculatus Linnavuori, 1959
Andanus bimaculatus Linnavuori, 1959: 238.
Adlaca dubiosa Lozada, 1998: 114.
Diagnosis: The crown is orange and the body stramineous without a transverse medial band on the pronotum, subgenital plate wide with narrow apex, style slender with anterolateral lobe strongly rounded, apophysis of elongated with small lateral projection, and aedeagus with pair of two apical processes.
Material examined: Two males (USNM) PERU: Madre de Dios, Manu, Pakitza, 11°56′ S, 71°18′ W, 12–18.IX.1989, malaise trap, night, N. Adams et al.; one male (INHS) PERU: Madre de Dios, Rio Tambopata, Posada Amazonas, 662ft., S12°48′08.4″ W, 69°17′59.4″, September 2004, J.R. Cryan, J.M. Urban; one male (USMN) PERU: Madre de Dios, Manu, Pakitza, 12°07′ S, 70°58′ W, 250 m, 14–23 September 1988, O. Flint, N. Adams, Trail 2, 1st stream, malaise trap, day and night.
4. Discussion
The range of distribution for Andanus is strongly biased to the eastern Peruvian forests and the extreme west rainforest of Brazil, both placed within the Brazilian dominion and suggesting a strong specialization to this exclusive environment. Unfortunately, no tree species are associated so far, but most species were taken from nearby localities and were caught using light traps that might bring evidence of a tendency to move among interconnected canopies. However, most findings were occasional and not systematic as expected, resulting in a low number of species known for the genus. Other morphologically similar genera, e.g., Napo and Pseudonapo, might also explain such biological preferences, explaining the small number of known specimens.
The Peruvian forests house up to 16 genera of the tribe Athysanini, remarkably denoting particular morphological characteristics not shared among genera that presumably occur in similar habitats, such as: Andanus; Atanus Oman, 1938; Brasilanus Linnavuori, 1959; Chimaerotettix Dietrich and Rakitov, 2002; Pseudonapo Pinedo-Escatel, 2020; Sincholata DeLong, 1982; Spaltumtettix Pinedo-Escatel and Dietrich, 2020; Huancabamba Linnavuori, 1959; Brazosa Oman, 1938; Caranavia Linnavuori, 1959; Bolotheta Kramer, 1963; Paratanus Young, 1957; Tingolix Linnavuori and DeLong, 1978; Yungasia Linnavuori, 1959; Napo Linnavuori and DeLong, 1976; Pachytettix Linnavuori, 1959, as stated in Pinedo-Escatel and Dietrich [9], based on careful study of material type by Zanol [17] and Lozada [18].
For most members of the tribe Athysanini vary significantly in distribution, and particular habitat preferences are largely unknown [8]. Even less is known about plant associations, which for most remain unclear, especially those collected with light trapping in dense forests [19]. Previous studies mentioned that those genera here considered as valid under Andanus were described based mostly on a single species with few available specimens, then the status was evaluated and validated by Pinedo-Escatel et al. [8] using all members plus other available specimens, concluding that although these species differ substantially in details of the male genital structures, it might be better to recognize them in a single genus.
Particularly, Napo and Pseudonapo share external structures and coloration to Andanus, like the short crown of the head, surface smooth, and transocular distance between the eyes, with rounded transition from crown to face are strongly shared. But notably, overall, internal male structures vary significantly, giving self-identity to even accommodate in other independent lineages (e.g., Cao et al. [20]), providing as a background that such a strategy of appearance might be explained by habitat preferences hopefully covered in further research with detailed taxonomic studies using phylogenetic inferences to elucidate the limits of these genera to evidence relationships in Athysanini.
Funding
This research was supported in full by UNAM-DGAPA-PAPIIT grant IA203825.
Data Availability Statement
Voucher of specimens reviewed are in museums cited in materials and methods.
Acknowledgments
We thank Edith Blanco-Rodriguez, who kindly imaged specimens studied during her doctorate research visit at INHS (University of Illinois, USA), Harry Brailovsky (IBUNAM) for given comments on the first draft of the manuscript, Victoria Chavez, who imaged sternite VII of the female paratype, and Cristina Mayorga (CNIN, Instituto de Biología UNAM) for providing entomological supplies and help processing specimens to accomplish this work.
Conflicts of Interest
The author declares no conflict of interest.
References
- Dmitriev, D.A.; Angelova, R.; Anufriev, G.A.; Bartlett, C.R.; Blanco-Rodríguez, E.; Borodin, O.I.; Cao, Y.-H.; Cara, C.; Deitz, L.L.; Dietrich, C.H.; et al. World Auchenorrhyncha Database. TaxonPages. 2022-onward. Retrieved on 18 January 2025. Available online: https://hoppers.speciesfile.org/otus/615181/overview (accessed on 29 April 2025).
- Dietrich, C.H. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Fla. Entomol. 2005, 88, 502–517. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C.H. Phylogeny of the leafhopper subfamily Deltocephalinae (Insecta: Auchenorrhyncha: Cicadellidae) and related subfamilies based on morphology. Syst. Biodivers 2008, 6, 1–24. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C. A review of the tribes of Deltocephalinae (Hemiptera: Auchenorrhyncha: Cicadellidae). Eur. J. Taxon. 2013, 45, 1–211. [Google Scholar] [CrossRef]
- Linnavuori, R. Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Ann. Zool. Soc. Zool. Bot. Fenn. Vanamo. 1959, 20, 1–370. [Google Scholar]
- Oman, P.W. The Nearctic leafhoppers (Homoptera: Cicadellidae). A generic classification and check list. Mem. Entomol. Soc. Wash. 1949, 3, 1–253. [Google Scholar]
- Pinedo-Escatel, J.A.; Dietrich, C.H. Review of the enigmatic Neotropical leafhopper genus Brazosa Oman and other potentially related Athysanini genera (Hemiptera: Auchenorrhyncha: Cicadellidae: Deltocephalinae), with descriptions of South American new genera and species. Zootaxa 2020, 4830, 401–454. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Cienc. 2022, 94, e20211167. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Escatel, J.A.; Dietrich, C.H.; Zahniser, J.N. Andanus tambopixunensis sp. nov., a new species of the remarkable leafhopper genus Andanus (Hemiptera: Cicadellidae: Deltocephalinae), generic redescription and two new synonyms with new placements. Zoologia 2022, 39, e21037. [Google Scholar] [CrossRef]
- Pinedo-Escatel, J.A. Notes on the techniques for collection, preservation, and mounting of Auchenorrhyncha (Insecta: Hemiptera). Bol. AMXSA 2017, 1, 3–5. [Google Scholar]
- Anufriev, G.A.; Emeljanov, A.F. Suborder Cicadinea (Auchenorrhyncha). In Keys to the Insects of the Far East of the USSR. Homoptera and Hemiptera; Nauka Publishing House: Leningrad, Russia, 1988; pp. 1–496. [Google Scholar]
- Rakitov, R.A. On differentiation of cicadellid leg chaetotaxy (Homoptera: Auchenorrhyncha: Membracoidea). Russ. Entomol. J. 1998, 6, 7–27. [Google Scholar]
- Oman, P.W.; Knight, W.J.; Nielson, M.W. Leafhoppers (Cicadellidae): A Bibliography, Generic Check-List and Index to the World Literature 1956–1985; CAB International: Wallingford, UK, 1990; p. 368. [Google Scholar]
- Zanol, K.M.R. Catalogue of the Neotropical Deltocephalinae (Hemiptera: Cicadellidae). Part III–Tribe Athysanini. Acta Biol. Paran. 2008, 37, 1–104. [Google Scholar]
- Google Earth. Version 7.3.6.10201. Available online: https://www.google.es/intl/es/earth/index.html (accessed on 28 April 2025).
- GIMP. GNU Image Manipulation Program, Community, Free Software (license GPLv3) Version 3.0.2. Available online: https://gimp.org (accessed on 28 April 2025).
- Zanol, K.M.R. Sobre o material-tipo de Andanus bimaculatus Linnavuori e descrição de um novo gênero e nova espécie (Homoptera: Cicadellidae: Deltocephalinae). Rev. Brasil Zool. 1993, 10, 613–618. [Google Scholar] [CrossRef]
- Lozada, P. Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Rev. Peru Biol. 1998, 5, 113–117. [Google Scholar] [CrossRef]
- Aguilar-Pérez, J.G.; Pinedo-Escatel, J.A.; Valdez-Quezada, B.C. Three new Mexican species of the endemic Athysanini leafhopper genus Devolana DeLong (Hemiptera: Cicadellidae) from the tropical dry forest. J. Nat. Hist. 2019, 53, 2039–2056. [Google Scholar] [CrossRef]
- Cao, Y.; Dietrich, C.H.; Zahniser, J.N.; Dmitriev, D.A. Dense sampling of taxa and characters improves phylogenetic resolution among deltocephaline leafhoppers (Hemiptera: Cicadellidae: Deltocephalinae). Syst. Entomol. 2022, 47, 430–444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
