Abstract
The freshwater cyanobacterium Raphidiopsis raciborskii is an invasive species that was originally described as tropical and is now widely distributed in temperate regions. The current taxonomic position was established based on a multilevel approach with the morphological description of natural populations as well as their ultrastructural, physiological and molecular characterization. The practical problem in identifying this species is that the morphology of the trichome in the natural environment can vary considerably during population growth. The aim of this study was to investigate the morphological variability of R. raciborskii during its blooming in a temperate floodplain lake on the Middle Danube (Europe). In both cases, only straight trichomes were found. Young trichomes with one or two acuminate ends and without heterocytes, referred to as primary filaments, can be exclusively monodominant at the beginning of bloom formation and remain dominant throughout the year, leading to taxonomic confusion. In mature populations, the different morphological forms of secondary filaments may differ in the size of the filaments and in the number and size of akinetes and heterocytes formed in the trichomes. The correct taxonomic identification and early detection of R. raciborskii in natural freshwaters is extremely important for the successful control of the spread of this potentially toxic species.
1. Introduction
Raphidiopsis raciborskii (Wołoszyńska) Aguilera, Berrendero Gómez, Kaštovský, Echenique & Salerno, is a solitary filamentous freshwater cyanobacteria species belonging to the order Nostocales [1]. It originates from the subtropics/tropics and is distributed in temperate waters [2]. Hundreds of reports published in the last decades have shown that R. raciborskii forms blooms in lakes, reservoirs and rivers around the world, and today it can be considered to be a globally invasive species [3,4]. Nevertheless, R. raciborskii has the ability to produce toxic compounds, which makes it one of the most ecologically remarkable cyanobacteria [5].
The taxonomy of prokaryotic cyanobacteria was traditionally based on cytomorphological and ecological features [6]. Accordingly, the morphology of the trichomes is an important feature for distinguishing R. raciborskii species. The filaments can be straight, sigmoidal or coiled and, in certain aquatic biotopes, all morphotypes or only certain types of filaments can occur [7]. For example, straight or bent filaments usually occurred in the dead arm of the Morava River [8], coiled filaments dominated in Lake Catemaco in Mexico [9], while straight and coiled trichomes occurred together in freshwaters in Australia [10]. Another particularly important morphological feature is that R. raciborskii can form heterocytes, specialized cells for nitrogen fixation, which are located at the end of the trichome [11]. In temperate freshwaters, it also forms akinetes, which are used for reproduction and are located in the trichomes at some distance from the heterocytes [7]. The combination of akinetes and heterocytes in the trichomes gives the R. raciborskii filaments a typical subsymmetrical appearance [12], which is the first impression during identification. Accordingly, the dimensions of the vegetative cells, trichomes, heterocytes and akinetes are the primary taxonomic criteria at the species level of this genus [11].
The taxonomy of R. raciborskii has often been reviewed on the basis of heterocyte origin and position. In the summary in [13], it was first described as Anabaena raciborskii Wołoszyńska because it has two terminal heterocytes, then as Anabaenopsis raciborskii (Wołoszyńska) Elenkin due to the terminal position of the heterocytes and, finally, as Cylindrospermopsis raciborskii due to the peculiarities of the heterocyte differentiation process. The species of the genus Cylindrospermopsis are very similar to the species of the genus Raphidiopsis Fritsch & Rich [7] and can be found under similar ecological conditions, even in the same water body [14]. The possibility of heterocyte formation in the genus Cylindrospermopsis was the main taxonomic feature that distinguished it from the genus Raphidiopsis, which does not form heterocytes [11]. More recently, Aguilera et al. [15], using a polyphasic approach that included the morphological description of the natural populations and their ultrastructural, physiological and molecular characterization, proposed to unite the two genera into a single genus, Raphidiopsis, particularly due to the genetic similarity between the genera Cylindrospermopsis and Raphidiopsis (secondary structure of the intergenic spacer region of the 16S-23S ribosomal RNA). Although there are still some uncertainties about the process of heterocyte differentiation [16], the new species name Raphidiopsis raciborskii [15] has been accepted [17] and is currently in use.
In addition to the fact that the determination of genetic relationships using the method of molecular sequencing should form the basis of the modern classification of cyanobacteria, Komárek [6] pointed out the importance of comparing the results of selected cultured strains with the common (and often precisely described) natural populations. The regular genus of cyanobacteria represents a unique type based on a combination of definable molecular, morphological and ecological criteria [18]. As an invasive species in different aquatic environments, R. raciborskii shows exceptional phenotypic plasticity [19], with a large variability in the size and number of cells per trichome [20,21]. Moreover, variability may occur due to specific ecological conditions and additional changes in trichome morphology during population growth in the annual cycle [7,22]. This can lead to practical problems in the taxonomic identification of the species [2]. The global invasion success of R. raciborskii was mainly attributed to the optimization of its growth in different environments with respect to temperature, light intensity, nutrient fluctuations and grazing pressure [23]. The morphological variation is very large, even within lakes of the same region, suggesting that local environmental factors may be more decisive for morphology than global ones [24].
Further analyses of changes in morphological traits under different environmental conditions have been carried out, mainly in conjunction with genetic studies and under experimental laboratory conditions (for a review, see [4]). For example, Saker et al. [10] found that temperature can hinder the population development of both straight and coiled trichomes and cause morphological changes in the trichomes, such as constrictions in the cell wall followed by fragmentation into single cells. Shafik et al. [25] showed that R. raciborskii exhibited tremendous morphological variability under controlled conditions in batch cultures under phosphorus starvation and in continuous cultures at two growth rates with different nitrogen forms. In the case studied by Galvanese et al. [26], the morphological characteristics (i.e., length, width, number of heterocytes and number of cells per trichome) differed between two strains of R. raciborskii under the same experimental conditions (phosphorus limitation and temperature variation), although they were grown under the same laboratory conditions for at least one year. The authors of [26] suggested that the differences could be due to the conditions in the lakes from which they originated and pointed out that the relationship between the morphological characteristics of the strains and the original environments needs to be further explored using strains from numerous lakes with different trophic status and mixing regimes.
Morphological plasticity often leads to difficulties in identification, especially in relation to morphologically and ecologically related species [27]. For example, Raphidiopsis species may morphologically resemble some species of the planktonic Nostocaceae family, such as Anabaena aphanizomenoides Forti and Aphanizomenon issatschenkoi (Ussachew) Proschkina-Larvernko [28]. Moreover, there are few in situ studies on the morphological variability of R. raciborskii and the classical taxonomic literature does not provide a detailed description of the possible variability in trichome morphology that may occur in specific water types [29].
Distinct morphological features and biological specificity (type of division, type of heterocyte or akinete formation, etc.), are a part of modern criteria for taxonomic classification [18]. The aim of this study was to investigate the morphological characteristics of the species Raphidiopsis raciborskii during its bloom period in 2007 and 2011 in a temperate floodplain lake of the Middle Danube. Traditional morphological criteria, such as the shape and size of trichomes, as well as the number and position of akinetes and heterocytes in the filaments were observed as important features of the species, which might be influenced by the specific ecological conditions of a dynamic freshwater system such as the study lake.
2. Materials and Methods
The study was conducted during the bloom formation of R. raciborskii in the summer of 2007 (June–August) and the summer of 2011 (August–September) on a monthly basis in the shallow lake Sakadaš (45°36′32.2” N, 18°48′05.5” E), which is located in the protected floodplain of the Middle Danube known as Kopački Rit Nature Park (Croatia, Europe). The area is under a temperate continental climate. The depth of the lake is highly variable (approx. 3–8 m) and depends on the income of floodwaters from the Danube River. Stević et al. [30] and Mihaljević et al. [31] provide a detailed description of the study area.
To assess the vertical distribution of R. raciborskii, samples were taken from the central part of the lake at intervals of 1 m from the surface to the bottom. The samples were fixed with Lugol’s solution.
The water temperature (WT), dissolved oxygen (DO), conductivity (Cond) and pH were measured using a WTW Multi 340i portable measuring device (Wissenschaftlich-Technische Werkstätten, Weilheim, Germany); the water depth (WD) was measured using a rope; and the transparency (SD) was measured using a Secchi disc. Nutrient concentrations were analyzed in the laboratory according to standard methods for ammonium (NH4-N [32]), nitrates (NO3-N [33]), nitrites (NO2-N [34]), total nitrogen (TN [35]) and total phosphorus (TP [36]).
The phenotypic diversity of R. raciborskii species was determined according to [1,5,11,22]. The abundance of trichomes was determined according to [37] using an inverted microscope (Axiovert 25, Carl Zeiss®, Inc., Göttingen, Germany). The following morphological forms of R. raciborskii were separately counted: primary filaments (young trichomes with acuminate ends) and secondary filaments—trichomes with a heterocyte at one end, trichomes with a heterocyte at one end and one akinete, trichomes with heterocytes at both ends, trichomes with heterocytes at both ends and one akinete, trichomes with heterocytes at both ends and two akinetes and trichomes with a heterocyte at one end and two akinetes. The dimensions of the trichomes of each morphological form as well as the heterocytes and akinetes were measured using the Motic Images Plus 2.0 ML program (Motic China Group Co., Ltd., Xiamen, China) in 2011.
A hierarchical cluster analysis (CA) was used to determine the similarity (Bray–Curtis similarity) within the studied months based on R. raciborskii abundance [38]. A redundancy analysis [39] was used to explain the abundance relationships between the R. raciborskii morphological forms and physicochemical factors. The abundance data were transformed (square root) and standardized. The statistical software CANOCO v4.5 (Microcomputer Power, Ithaca, NY, USA) was used for the analyses.
3. Results
3.1. Environmental Characteristics
Environmental parameters were measured from May to October in 2007 and 2011 to determine the conditions that preceded the development of R. raciborskii (Figure 1). The WD was lower in 2007 (3.3–6.0 m) than in 2011 (5.4–7.5 m); the SD ranged from 0.6 to 1.8 m in 2007 and from 0.7 to 1.4 m in 2011. WT at the surface ranged from 11.2 to 26.1 °C in 2007 and from 16.9 to 25.9 °C in 2011. The lowest WT at the bottom (10.2 °C) was recorded in May 2011; the highest WT at the bottom (22 °C) was in August 2007, when the lake depth was only 3.3 m. The concentrations of NH4-N and TN were higher at the bottom than at the surface, with very high values in August and September 2011 (up to 5.54 mg L−1 of NH4-N and up to 8.01 mg L−1 of TN). The TP concentration was also higher at the bottom, reaching 1.45 mg L−1 in 2007 and 1.43 mg L−1 in 2011. The concentrations of NO2-N and NO3-N were higher in 2011 than in 2007, with a peak of NO2-N in May 2007 (0.26 mg L−1) and NO3-N in September 2007 (1.15 mg L−1). In contrast, TN/TP was higher in 2007 than in 2011, with a peak value of 24.9 in July 2007.
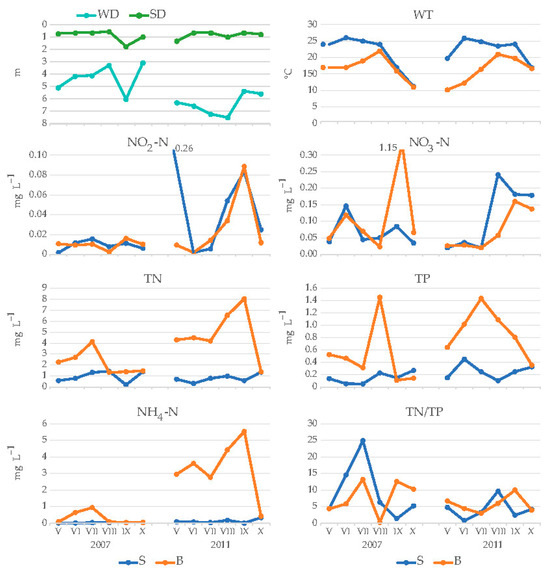
Figure 1.
Values of physical and chemical properties of the water of Lake Sakadaš during the research period (2007 and 2011). Legend: surface (S), bottom (B), water depth (WD), transparency (SD), water temperature (WT), ammonium (NH4-N), nitrites (NO2-N), nitrates (NO3-N), total nitrogen (TN), total phosphorus (TP) and ratio of total nitrogen and total phosphorus (TN/TP).
3.2. Dimensions of Trichomes, Heterocytes and Akinetes
The median length of the trichomes was between 76 and 135.1 µm (Figure 2a), while the median width of the trichomes was between 1.3 µm and 1.6 µm (Figure 2b). Primary filaments and trichomes with one heterocyte had a lower median length than other trichomes. In addition, the median width of primary filaments was significantly smaller than that of other trichomes.
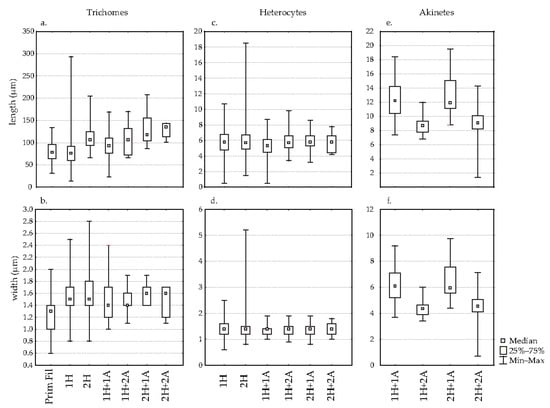
Figure 2.
Box–whisker diagram of the length (a) and width (b) of R. raciborskii trichomes (n = 716), the length (c) and width (d) of R. raciborskii heterocytes (n = 839) and length (e) and width (f) of R. raciborskii akinetes (n = 94) in Lake Sakadaš in August and September of 2011. Legend: PrimFila—primary filaments (young trichomes with acuminate ends); 1H—trichomes with heterocytes at one end; 1H + 1A—trichomes with heterocytes at one end and one akinete; 2H—trichomes with heterocytes at both ends; 2H + 1A—trichomes with heterocytes at both ends and an akinete; 2H + 2A—trichomes with heterocytes at both ends and two akinetes; 1H + 2A—trichomes with heterocytes at one end and two akinetes.
The median length of the heterocytes ranged from 5.3 to 5.8 µm (Figure 2c) while the median width of the heterocytes was 1.4 µm and there were no differences between the different morphological forms (Figure 2d).
The median akinete length was between 8.7 and 12.2 µm (Figure 2e), while the median width of the akinetes was between 4.4 and 6.1 µm (Figure 2f). The length and width of the akinetes were greater when only one akinete was present in the trichome. In contrast, they were smaller in trichomes with two akinetes, regardless of the number of heterocytes.
3.3. Abundance and Morphology of R. raciborskii
The variation in abundance of R. raciborskii was in the range of 19.08 × 106 ind. L−1 (at 4 m in June)—74.64 × 106 ind. L−1 (at 1 m depth in August) in 2007 and from 0.07 × 106 ind. L−1 (at 3–5 m in August) to 27.81 × 106 ind. L−1 (at the surface in September) in 2011 (Figure 3). In general, the abundance of this species was higher in the upper layers than in the deeper layers of the water column. In 2007, R. raciborskii was found from the surface to the bottom but, at that time, the water depth was low (up to 4 m). In 2011, the water depth was greater (up to 7.5 m) and the studied species was not found at depths below 5 m.
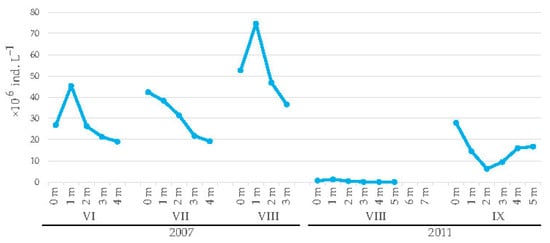
Figure 3.
Vertical distribution of the total abundance of R. raciborskii in the periods June–August 2007 and August–September 2011.
The development of different trichome morphologies of R. raciborskii was noted. We found only straight primary filaments (young trichomes with acuminate ends) and six different secondary filaments (trichomes with one heterocyte, trichomes with two heterocytes, trichomes with one heterocyte and one akinete, trichomes with two heterocytes and two akinetes, trichomes with one heterocyte and two akinetes and trichomes with two heterocytes and one akinete) (Figure 4).
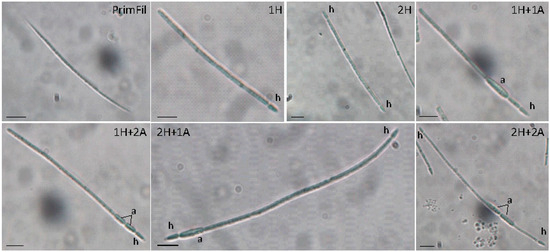
Figure 4.
Different morphological forms of R. raciborskii in Lake Sakadaš in 2007 and 2011. For the morphological forms of R. raciborskii, see the legend in Figure 2; h—heterocyte, a—akinete. Scale bars = 10 µm.
Young trichomes with one or two acuminate ends and without heterocytes (the primary filaments) were the most abundant in 2007, with maximum development in August (34.73 × 106 ind. L−1 at 1 m) (Figure 5). Short fragmented trichomes with heterocytes at one end (the secondary filaments) were also well developed, from 4.87 × 106 ind. L−1 at the bottom in June to 36.70 × 106 ind. L−1 at 1 m in August, when this morphological form was the most dominant. Other morphological forms developed at less than 10% of R. raciborskii abundance (Figure 6).
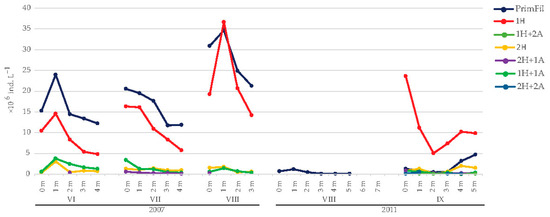
Figure 5.
Abundance of morphological forms of R. raciborskii in Lake Sakadaš in 2007 and 2011. For the morphological forms of R. raciborskii, see the legend in Figure 2.
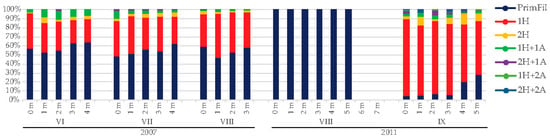
Figure 6.
Relative abundance of different morphological forms of R. raciborskii in Lake Sakadaš in 2007 and 2011. For the morphological forms of R. raciborskii, see the legend in Figure 2.
In 2011, at the beginning of development (August), only one morphological form (primary filament) of R. raciborskii was detected with a low number of individuals (0.07–1.21 × 106 ind. L−1) (Figure 5). This form was present from the surface to a depth of five meters. In September, a massive development of R. raciborskii was detected (27.81 × 106 ind. L−1 at the surface). In addition to the primary filament, there were six other morphological forms of secondary filaments. The predominant morphological form at all depths in September was the trichome with one heterocyte. Its abundance ranged from 5.02 × 106 to 23.60 × 106 ind. L−1. The higher relative abundance of this morphological form was found in the surface water layer, while a lower relative abundance was found in the bottom layer (Figure 6). The primary filament was also present in September, with a higher abundance at the bottom (4.72 × 106 ind. L−1). Other morphological forms occurred at all investigated depths but with a relative abundance of less than 12% (Figure 6).
3.4. Statistical Analysis
The hierarchical cluster analysis included data on the quantitative composition of the different morphological forms of R. raciborskii during the research period from June to August 2007 and August to September 2011 at Lake Sakadaš. The dendrogram (Figure 7) showed that the data were separated into two main groups, with a Bray–Curtis similarity of about 70%. The first group included samples from August 2011, at which time only the primary filament was developed without the secondary morphological forms of R. raciborskii. In the second group, there were two subgroups with a Bray–Curtis similarity of about 80%. The first subgroup contained samples from September 2011 to a depth of 4 m, when the predominant morphological form was the trichome with one heterocyte. The second subgroup included all samples from 2007 and samples from the bottom layer in September 2011, at which time the primary filament was significantly represented.
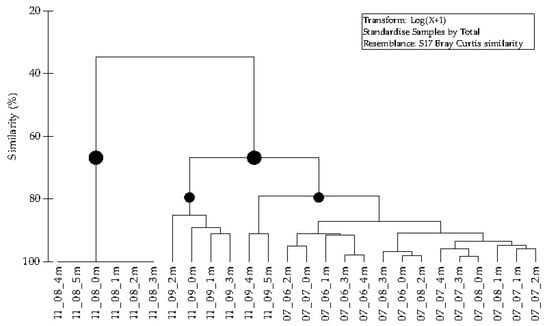
Figure 7.
A hierarchical dendrogram showing the group-average clustering of Bray–Curtis similarity indices based on abundance data of morphological forms of R. raciborskii. Legend: 11_08_4m (year_month_depth).
In the RDA, we used physical and chemical parameters from May to October 2007 and 2011 to determine which environmental parameters were most significant for the development of different morphological forms of R. raciborskii. The eigenvalues for RDA axis 1 (0.7) and axis 2 (0.04) explained 72.3% of the variance in the species data. According to the analysis, among the 12 environmental variables (WD, SD, WT, DO, pH, Cond, NH4-N, NO2-N, NO3-N, TN, TP and TN/TP) included in analysis, only WT was identified as a significant environmental variable by the forward selection procedure (Figure 8).
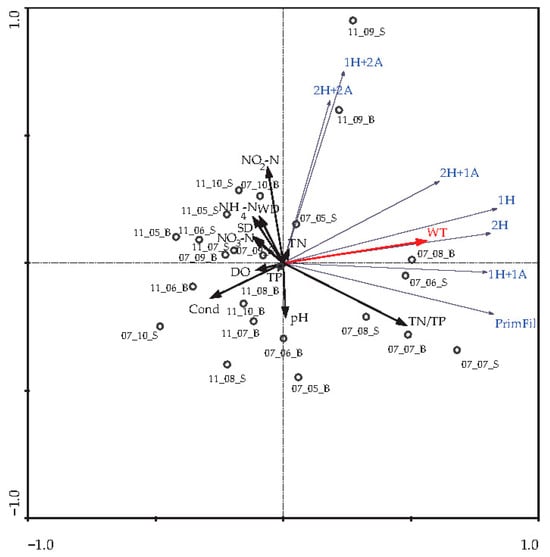
Figure 8.
Triplot of environmental parameters and morphological forms of R. raciborskii from May to October of 2007 and 2011. Statistically significant environmental variable (WT) is marked in a red color. Legend: 11_08_4m (year_month_depth); for the morphological forms of R. raciborskii, see legend in Figure 2.
4. Discussion
The morphology of R. raciborskii was highly variable during the blooming period in 2007 and 2011 for the studied floodplain lake. In both years, the developed populations exclusively consisted of straight filaments. Similarly, this morphological filament type has been found to be dominant in different ecological conditions, from temperate lakes in Hungary [22] and France [40] to subtropical lakes (for example, in Uruguay [24]) and tropical lakes in Australia [41]. The bloom started to develop in summer but at different times, in June 2007 and in August 2011, when favorable hydrological conditions prevailed. Our previous studies [42] have shown that cyanobacteria, especially R. raciborskii, are particularly sensitive to the income of flood waters into the lake, leading to the disappearance of the cyanobacterial bloom. For this reason, the bloom lasted until August 2007 and September 2011.
At the beginning of the bloom in 2011, only homocytous straight trichomes appeared in the community, with morphological characteristics fully consistent with the definition of primary filaments. According to Padisak [22] (p. 389) “primary filaments (those that derive directly from the akinetes) possess the following morphological features: the trichomes have one or two acuminate ends; they are narrower (2.4–2.6 µm) than older filaments, their lengths vary in a wide range (from 40 to 300 µm); some of them are straight but others are slightly coiled even in populations that later consist exclusively of straight filaments; cell walls between the cells are not or only hardly visible; the filaments have a fine granulation but no gas-vacuoles, polyphosphate- or any other contrasting bodies are seen”. Later, in a mature community, secondary filaments developed, i.e., heterocytic filaments with a different number (usually 1 or 2) of heterocytes and spores. However, the primary filaments remained predominant in the community throughout the entire period of blooming, while the filaments with one heterocyte were the most numerous of the secondary filaments.
A similar distribution of the different trichome morphotypes was found by Lamia et al. [43] in a shallow Mediterranean lake, where primary filaments dominated the annual life cycle, while secondary filaments with heterocytes and akinetes occurred at a low abundance. Also, the majority (more than 90%) of the populations of R. raciborski found in a eutrophic urban subtropical lake in southern Brazil consisted of straight and slightly curved trichomes composed only of vegetative cells, i.e., primary filaments [13]. The authors emphasized that a low number of trichomes with heterocytes was also common in other Brazilian waters and explained this by the fact that, under conditions where there is no nitrogen deficiency, nitrogen fixation is not necessary as a competitive strategy for R. raciborskii and, consequently, heterocyte formation is not required. Saker and Neilan [41] showed that different nitrogen sources can lead to significant differences in the morphology of R. raciborskii trichomes, with the loss of heterocysts and tapering being most notable in cultures supplied with NH4+.
The primary filaments, as homocytous trichomes without heterocytes and akinetes, may look like a species of another genus, as described by Komárek [44] for that found in a Cuban lagoon in 1980, but the population most likely consisted of sterile filaments of R. raciborskii [7]. Similar to this misconception, one could speculate about the historical finding of a Raphidiopsis mediterranea bloom in the studied lake in the early seventies of the last century [45]. Now it looks as if it might also have been the primary filament of R. raciborskii. As Komárková et al. [7] note, it is likely that many identifications of Raphidiopsis, especially when akinetes were not present, were made without taking into account the variability of R. raciborskii growing or surviving in different natural conditions.
Significant differences were found in the dimensions of the filaments as well as in the dimensions of the heterocytes and akinetes and their number along the filaments. The total length of primary filaments varied (13.6 to 293.5 µm), similar to that in Lake Balaton (40–300 µm [22]), while filaments twice as long (42.4–430 µm) could develop in tropical lakes [7]. The gradual increase in trichome length from the sediments to the surface observed both in this lake and in tropical lakes [46] could be influenced by thermal stratification. In addition, with an increase in temperature, the growth rate of R. raciborski increases, which may lead to a decrease in trichome length, as confirmed by [47] under natural conditions in a tropical lake. The length of the filament could also be influenced by light intensity, as experimentally confirmed by [48], who observed longer trichomes cultivated at higher light intensities. Moreover, due to their shorter length, trichomes can move more freely through the water column and capture the light better, which could explain why R. raciborskii does not form surface blooms [21]. In contrast, Wojciechowski et al. [49] attributed the enlargement of trichomes to an adaptation to optimize light absorption under limiting conditions.
The ability to form heterocytes is a phylogenetically important qualitative trait of Raphidiopsis and an important morphological feature [6]. According to the definition provided by Komárek et al. [50] heterocytes (ovoid or conical, sometimes slightly curved, unipored and terminal) develop from the terminal cells, which transform into proheterocytes after asymmetrical cross-division of the terminal cell in a young trichome. In this study, heterocytes developed in both years in the mature populations at one or both ends of the primary filament, usually one and rarely two. According to Komárková [12], the apical heterocytes in Cylindrospermopsis do not usually simultaneously develop at both trichome ends but successively develop only from terminal, constricted cells. From this point of view, the most closely related genus is Anabaenopsis, in which the heterocytes also divide from smaller cells after asymmetrical division [12]. As a rule, heterocytes mainly develop from the terminal cells of the trichome and intercalary heterocytes are rarely found [25]. The terminal position of the heterocytes poses a challenge for R. raciborskii to transfer the N fixed in the two terminal heterocytes to 50 or more associated vegetative cells [51]. The ends of the trichome attenuate when no heterocytes are present [52].
The development and frequency of heterocytes depend on the environmental conditions. It is not inconceivable that heterocytes form in filaments at depth during a bloom, where necessary soluble nutrients and trace elements are available in excess [53]. In the lake studied, the largest number of filaments with one heterocyte was found in the near-surface or surface water layers (up to a depth of 3 m) where NH4-N and TN concentrations were significantly lower than in the near-bottom water layers. Therefore, it is possible that a lack of inorganic N in the water contributed to the formation of heterocytes, as found in many studies [7,54,55]. However, our results show that heterocytes can also form under conditions with very high N concentrations, as was the case in the summer of 2011. It can, therefore, be assumed that heterocytes occur in this lake regardless of nitrogen availability. Yema et al. [56] emphasized that the nitrogen concentration is not important for the occurrence of heterocytes but that heterocytes only become functional in the absence of nitrogen. In addition, experiments performed by Marques et al. [16] showed that the development of heterocytes does not always depend on nitrogen limitations in the environment.
R. raciborskii begins to produce a number of akinetes when the population reaches its maximum [57]. The akinetes facultatively develop next to or slightly distant from the heterocytes, solitarily, in pairs or in short series. Of the other related genera, the genus Aphanizomenon has subsymmetrical trichomes but the heterocytes intercalary develop at several places from one vegetative cell, whereas the heterocytes in Cylindrospermum develop at both trichome ends and the akinetes develop symmetrically next to the heterocytes [12].
In our study, one or two akinetes developed in trichomes slightly distant from the heterocytes, which is a peculiarity of the species [5]. Akinetes were almost twice as small in filaments with two akinetes (median 8–10 × 3.9–5 µm) than in filaments with only one akinete (median 10.5–15 × 5–7.5 µm). In addition, filaments with one or two akinetes accounted for only a very small proportion (less than 2%) of the total abundance of R. raciborskii. This could indicate that the populations were far from their climax in both years because intense flooding abruptly interrupted their development. A lack of akinete formation in R. raciborskii is common in high-latitude lakes. For example, Werner et al. [13] reported that the trichomes with akinetes were rare in a subtropical lake in Brazil, while akinetes were never observed in a small urban lake in Australia, suggesting that R. raciborskii may overwinter as vegetative cells rather than akinetes [53]. This trend is consistent with strains from tropical Australia, where it persists throughout the year as vegetative cells and rarely produces akinetes [58].
In temperate lakes, akinete production is a survival strategy under unfavorable environmental conditions [19] such as temperature shock [25]. It has been observed [59] that the concentration of akinetes increases with an increase in the magnitude of temperature fluctuations. For example, in a shallow eutrophic lake in northeastern Germany, akinete production started when R. raciborskii populations reached their maximum cell density and water temperatures started to decrease [60].
Akinetes are inocula for the next year’s growth and, in temperate habitats, their germination is influenced by spring warming [4]. In the lake studied in 2007, germination began as early as June at a water temperature of 23.5 °C, whereas in 2011 it did not begin until August at a temperature of 22 °C. In the same temperature range (22–23.5 °C), R. raciborskii showed a high percentage of germination in Lake Balaton [22]. Germination can also start at much lower temperatures, for example, in German waters at a temperature of 17 °C [61] or even lower, at 15 °C [62]. As the size of the population for the next summer depends on the size of the inoculum [22,60], a low production of akinetes probably had an influence on the decreasing trend of the R. raciborski bloom in the studied lake [63].
5. Conclusions
In the development cycle of the natural population of R. raciborski in a studied floodplain lake of a temperate region, important morphological features could be identified that were of significance for the taxonomic and ecological valorization of the species. At the beginning of the blooming period, the so-called primary filaments had developed, the basic characteristics of which were that they had homocytic, straight trichomes (without heterocytes and akinetes) with one or two acuminate ends and a length that could vary greatly from a few tens to a few hundred µm. This could be confusing in the taxonomic identification of R. raciborskii when the entire population is at this stage, as was the case at the beginning of the bloom in the studied lake. Primary filaments can easily be mistaken for a similar filamentous species that does not form heterocytes throughout its life development. Such misconceptions in the taxonomic identification of R. raciborskii can easily occur, as has been publicly acknowledged by eminent algologists.
Later, in a mature community, secondary filaments (i.e., heterocytic filaments with a different number (usually 1 or 2) of heterocytes and spores) could be found in different representations of the population, together with primary filaments. The position of the heterocytes was terminal in the filament, which is a characteristic of the species, and no deviations were found. The specific ecological conditions of the studied lake—namely, major disturbances caused by flooding—could have influenced the sudden disappearance of the population and the formation of akinetes as the main developmental stage of this species did not occur as expected. The correct taxonomic identification of R. raciborski, this extremely invasive and potentially highly toxic species, is of exceptional ecological and social importance.
Author Contributions
F.S.: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation, review and editing, and visualization; M.M.: conceptualization, methodology, validation, investigation, writing—original draft preparation, review and editing, and funding acquisition; D.Š.M. methodology, validation, investigation, data curation, and writing—review and editing; T.Ž.P. validation, data curation, and writing—review and editing; V.Z. investigation and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded partly by the Ministry of Science, Education and Sports of the Republic of Croatia (project no. 285-0000000-2674) and the Josip Juraj Strossmayer University of Osijek, Department of Biology (Institutional project No. 3105-1).
Data Availability Statement
Raw data that support the outcomes of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to Ana Milić for help with the laboratory analysis and microscopy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hauer, T.; Komárek, J. CyanoDB 2.0—On-Line Database of Cyanobacterial Genera. World-Wide Electronic Publication. Available online: http://www.cyanodb.cz/ (accessed on 24 January 2025).
- Padisak, J. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an Expanding, Highly Adaptive Cyanobacterium: Worldwide Distribution and Review of Its Ecology. Arch. Hydrobiol. 1997, 107, 563–593. [Google Scholar]
- Antunes, J.T.; Leão, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: Review of the Distribution, Phylogeography, and Ecophysiology of a Global Invasive Species. Front. Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Shi, J. Overview of the Distribution and Adaptation of a Bloom-Forming Cyanobacterium Raphidiopsis raciborskii: Integrating Genomics, Toxicity, and Ecophysiology. J. Oceanol. Limnol. 2022, 40, 1774–1791. [Google Scholar] [CrossRef]
- Komárek, J.; Komárková, J. Phenotype Diversity of the Cyanoprokaryotic Genus Cylindrospermopsis (Nostocales); Review 2002. Czech Phycology 2003, 3, 1–30. [Google Scholar]
- Komarek, J. Quo Vadis, Taxonomy of Cyanobacteria (2019). Fottea 2020, 20, 104–110. [Google Scholar] [CrossRef]
- Komárková, J.; Laudares-Silva, R.; Senna, P. Extreme Morphology of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in the Lagoa Do Peri, a Freshwater Coastal Lagoon, Santa Catarina, Brazil. Algol. Stud. 1999, 94, 207–222. [Google Scholar] [CrossRef]
- Dvorak, P.; Hasler, P. Occurrence and Morphological Variability of Cylindrospermopsis raciborskii (WOLOSZ.) SEENAYYA et SUBBA RAJU (Cyanophyta, Nostocales) near Olomouc in 2006. Fottea 2007, 7, 39–42. [Google Scholar] [CrossRef]
- Lind, O.; Dávalos-Lind, L.; López, C.; López, M.; Bressie, J. Seasonal Morphological Variability in an In Situ Cyanobacteria Monoculture: Example from a Persistent Cylindrospermopsis Bloom in Lake Catemaco, Veracruz, Mexico. J. Limnol. 2016, 75, 66–80. [Google Scholar] [CrossRef]
- Saker, M.L.; Neilan, B.A.; Griffiths, D.J. Two Morphological Forms of Cylindrospermopsis raciborskii (Cyanobacteria) Isolated from Solomon Dam, Palm Island, Queensland. J. Phycol. 1999, 35, 599–606. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Modern Approach to the Classification System of Cyanophytes 4—Nostocales. Arch. Hydrobiol. Suppl. B Monogr. Beiträge 1990, 82, 247–345. [Google Scholar]
- Komárková, J. The Tropical Planktonic Genus Cylindrospermopsis (Cyanophytes, Cyanobacteria). An. IV Congr. Lat. Am. Ficologia 1998, 1, 327–340. [Google Scholar]
- Werner, V.; Tucci, A.; Silva, L.; Yunes, J.; Neuhaus, E.; Berthold, D.; Laughinghouse IV, H. Morphological, Ecological and Toxicological Aspects of Raphidiopsis raciborskii (Cyanobacteria) in a Eutrophic Urban Subtropical Lake in Southern Brazil. Iheringia Ser. Bot. 2020, 75, e2020018. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Kormas, K.; Vardaka, E., (Savi); Katsiapi, M.; Gkelis, S. Raphidiopsis mediterranea Skuja Represents Non-Heterocytous Life-Cycle Stages of Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju in Lake Kastoria (Greece), Its Type Locality: Evidence by Morphological and Phylogenetic Analysis. Harmful Algae 2009, 8, 864–872. [Google Scholar] [CrossRef]
- Aguilera, A.; Berrendero, E.; Kaštovský, J.; Echenique, R.; Salerno, G. The Polyphasic Analysis of Two Native Raphidiopsis Isolates Supports the Unification of the Genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Marques, L.C.B.; Lima, J.E.; Pimentel, J.d.S.M.; Giani, A. Heterocyte Production, Gene Expression, and Phylogeography in Raphidiopsis (=Cylindrospermopsis) raciborskii. FEMS Microbiol. Ecol. 2022, 98, fiac052. [Google Scholar] [CrossRef]
- AlgaeBase: Listing the World’s Algae. Available online: https://www.algaebase.org/ (accessed on 28 January 2025).
- Komarek, J.; Kaštovský, J.; Mares, J.; Johansen, J. Taxonomic Classification of Cyanoprokaryotes (Cyanobacterial Genera) 2014, Using a Polyphasic Approach. Preslia Praha 2014, 86, 295–335. [Google Scholar]
- Bittencourt-Oliveira, M. do C. Increase in Straight and Coiled Cylindrospermopsis raciborskii (Cyanobacteria) Populations Under Conditions of Thermal De-Stratification in a Shallow Tropical Reservoir. J. Water Resour. Res. 2011, 3, 245–252. [Google Scholar] [CrossRef]
- McGregor, G.B.; Fabbro, L.D. Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) in Queensland Tropical and Subtropical Reservoirs: Implications for Monitoring and Management. Lakes Reserv. Res. Manag. 2000, 5, 195–205. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Putt, E.; Falconer, I.; Humpage, A. Phenotypical Variation in a Toxic Strain of the Phytoplankter, Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) During Batch Culture. Environ. Toxicol. 2001, 16, 460–467. [Google Scholar] [CrossRef]
- Padisák, J. Estimation of Minimum Sedimentary Inoculum (Akinete) Pool of Cylindrospermopsis raciborskii: A Morphology and Life-Cycle Based Method. Hydrobiologia 2003, 502, 389–394. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Y.; Li, R.; Yang, Y.; Jiang, Y. Recent Advances in the Ecology of Bloom-Forming Raphidiopsis (Cylindrospermopsis) raciborskii: Expansion in China, Intraspecific Heterogeneity and Critical Factors for Invasion. Int. J. Environ. Res. Public Health 2023, 20, 1984. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Kruk, C. Cylindrospermopsis raciborskii (Cyanobacteria) Extends Its Distribution to Latitude 34°53′ S: Taxonomical and Ecological Features in Uruguayan Eutrophic Lakes. Panam. J. Aquat. Sci. 2008, 3, 142–151. [Google Scholar]
- Shafik, H.M.; Vörös, L.; Sprőber, P.; Présing, M.; Kovács, A.W. Some Special Morphological Features of Cylindrospermopsis raciborskii in Batch and Continuous Cultures. Hydrobiologia 2003, 506, 163–167. [Google Scholar] [CrossRef]
- Galvanese, E.F.; Padial, A.A.; Aubriot, L. Acclimation at High Temperatures Increases the Ability of Raphidiopsis raciborskii (Cyanobacteria) to Withstand Phosphate Deficiency and Reveals Distinct Strain Responses. Eur. J. Phycol. 2019, 54, 359–368. [Google Scholar] [CrossRef]
- McGregor, G.B.; Sendall, B.C.; Hunt, L.T.; Eaglesham, G.K. Report of the Cyanotoxins Cylindrospermopsin and Deoxy-Cylindrospermopsin from Raphidiopsis mediterranea Skuja (Cyanobacteria/Nostocales). Harmful Algae 2011, 10, 402–410. [Google Scholar] [CrossRef]
- Li, R.; Wilhelm, S.W.; Carmichael, W.W.; Watanabe, M.M. Polyphasic Characterization of Water Bloom Forming Raphidiopsis Species (Cyanobacteria) from Central China. Harmful Algae 2008, 7, 146–153. [Google Scholar] [CrossRef]
- Moore, D.; McGregor, G.; Shaw, G. Morphological Changes during Akinete Germination in Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria). J. Phycol. 2004, 40, 1098–1105. [Google Scholar] [CrossRef]
- Stević, F.; Mihaljević, M.; Špoljarić Maronić, D. Changes of Phytoplankton Functional Groups in a Floodplain Lake Associated with Hydrological Perturbations. Hydrobiologia 2013, 709, 143–158. [Google Scholar] [CrossRef]
- Mihaljević, M.; Stević, F.; Špoljarić, D.; Pfeiffer, T.Ž. Spatial Pattern of Phytoplankton Based on the Morphology-Based Functional Approach along a River–Floodplain Gradient. River Res. Appl. 2015, 31, 228–238. [Google Scholar] [CrossRef]
- ISO 7150-1:1998 ISO; Water Quality—Determination of Ammonium—Part 1: Manual Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- HRN ISO 7890-3:1998 ISO; Water Quality—Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid. International Organization for Standardization: Geneva, Switzerland, 1998.
- HRN EN 26777:1998 ISO; Water Quality—Determination of Nitrite—Molecular Absorption Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- HRN ISO 5663:2001 ISO; Water Quality—Determination of Total Nitrogen—Method after Mineralization with Selenium. International Organization for Standardization: Geneva, Switzerland, 2001.
- HRN ISO 6878:2008 ISO; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 2008.
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Theor. Angew. Limnol. Mitteilungen 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Clarke, K. Nonparametric Multivariate Analyses of Changes in Community Structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; Volume 3, ISBN 978-0-521-89108-0. [Google Scholar]
- Briand, J.; Leboulanger, C.; Humbert, J.; Bernard, C.; Dufour, P. Cylindrospermopsis raciborskii (Cyanobacteria) Invasion at Mid-Latitudes: Selection, Wide Physiological Tolerance, or Global Warming? J. Phycol. 2004, 40, 231–238. [Google Scholar] [CrossRef]
- Saker, M.L.; Neilan, B.A. Varied Diazotrophies, Morphologies, and Toxicities of Genetically Similar Isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia. Appl. Environ. Microbiol. 2001, 67, 1839–1845. [Google Scholar] [CrossRef]
- Stević, F. Složenost Utjecaja Poplava na Strukturu i Dinamiku Fitoplanktona Poplavnog Područja; Poslijediplomski Sveučilišni Interdisciplinarni Znanstveni Studij Zaštita Prirode i Okoliša: Osijek, Zagreb, 2011. [Google Scholar]
- Lamia, S.; Guellati, F.; Touati, H.; Bensouilah, M. Advanced Description of the Morphology of Cylindrospermopsis raciborskii in a Shallow Mediterranean Lake. Aquat. Ecol. 2024, 59, 247–262. [Google Scholar] [CrossRef]
- Komárek, J. Sobre Las Cianoficeas de Cuba: (3) Especies Planctónicas Que Forman Florecimientos de Las Aguas. Acta Bot. Cub. 1984, 19, 25–31. [Google Scholar]
- Gucunski, D. The phytoplankton of Bijelo jezero in the summer of 1977. Acta Bot. Croat. 1982, 41, 65–76. [Google Scholar]
- Fabbro, L.; Duivenvoorden, L. Profile of a Bloom of the Cyanobacterium Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju in the Fitzroy River in Tropical Central Queensland. Mar. Freshw. Res. 1996, 47, 685–694. [Google Scholar] [CrossRef]
- Soares, M.; Lurling, M.; Huszar, V. Growth and Temperature-Related Phenotypic Plasticity in the Cyanobacterium Cylindrospermopsis raciborskii. Phycol. Res. 2013, 61, 61–67. [Google Scholar] [CrossRef]
- Bonilla, S.; Aubriot, L.; Soares, M.C.S.; González-Piana, M.; Fabre, A.; Huszar, V.L.M.; Lürling, M.; Antoniades, D.; Padisák, J.; Kruk, C. What Drives the Distribution of the Bloom-Forming Cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiol. Ecol. 2012, 79, 594–607. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Fernandes, L.; Fonseca, F. Morpho-Physiological Responses of a Subtropical Strain of Cylindrospermopsis raciborskii (Cyanobacteria) to Different Light Intensities. Acta Bot. Bras. 2016, 30, 232–238. [Google Scholar] [CrossRef][Green Version]
- Komárek, J. “Diversita a Moderní Klasifikace Sinic (Cyanoprocaryota) [Diversity and Modern Classification of Cyanobacteria (Cyanoprokaryota)]”. Inaugural Dissertation, Scientific Research Publishing. Available online: https://www.scirp.org/reference/referencespapers?referenceid=964491 (accessed on 25 February 2025).
- Marques, L.C.B. Taxonomy and Nitrogen Fixation in Raphidiopsis raciborskii (Woloszynska) Aguilera, Berrendero Gómez, Kastovsky, Echenique & Salerno. Taxonomia e Fixação de Nitrogênio em Raphidiopsis raciborskii (Woloszynska) Aguilera, Berrendero Gómez, Kastovsky, Echenique & Salerno; Universidade Federal de Minas Gerais: Belo Horizonte, Brazil, 2020. [Google Scholar]
- Baker, P.; Fabbro, L. A Guide to the Identification of Common Blue-Green Algae (Cyanoprokaryotes) in Australian Freshwaters; CQUniversity: Sydney, Australia, 1999; ISBN 978-1-876144-48-7. [Google Scholar]
- Everson, S.; Fabbro, L.; Kinnear, S.; Wright, P. Extreme Differences in Akinete, Heterocyte and Cylindrospermopsin Concentrations with Depth in a Successive Bloom Involving Aphanizomenon ovalisporum (Forti) and Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju. Harmful Algae 2011, 10, 265–276. [Google Scholar] [CrossRef]
- Briand, J.F.; Robillot, C.; Quiblier-Llobéras, C.; Humbert, J.F.; Couté, A.; Bernard, C. Environmental Context of Cylindrospermopsis raciborskii (Cyanobacteria) Blooms in a Shallow Pond in France. Water Res. 2002, 36, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.W.; Sauter, S. Distribution and Abundance of Cylindrospermopsis raciborskii in Indiana Lakes and Reservoirs; Indiana University, School of Public and Environmental Affairs: Bloomington, IN, USA, 2005; p. 54. [Google Scholar]
- Yema, L.; Litchman, E.; de Tezanos Pinto, P. The Role of Heterocytes in the Physiology and Ecology of Bloom-Forming Harmful Cyanobacteria. Harmful Algae 2016, 60, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Padisák, J.; Istvánovics, V. Differential Response of Blue-Green Algal Groups to Phosphorus Load Reduction in a Large Shallow Lake: Balaton, Hungary. SIL Proc. 1922–2010 1997, 26, 574–580. [Google Scholar] [CrossRef]
- Saker, M.L.; Griffiths, D.J. Occurrence of Blooms of the Cyanobacterium Cylindrospermopsis raciborskii (Woloszynska) Seenayya and Subba Raju in a North Queensland Domestic Water Supply. Mar. Freshw. Res. 2001, 52, 907–915. [Google Scholar] [CrossRef]
- Moore, D.; O’donohue, M.; Garnett, C.; Shaw, G.; Critchley, C. Factors Affecting Akinete Differentiation in Cylindrospermopsis raciborskii (Nostocals, Cyanobacteria). Freshw. Biol. 2005, 50, 345–352. [Google Scholar] [CrossRef]
- Rücker, J.; Tingwey, E.; Wiedner, C.; Anu, C.; Nixdorf, B. Impact of the Inoculum Size on the Population of Nostocales Cyanobacteria in a Temperate Lake. J. Plankt. Res. 2009, 31, 1151–1159. [Google Scholar] [CrossRef]
- Mischke, U. Cyanobacteria Associations in Shallow Polytrophic Lakes: Influence of Environmental Factors. Acta Oecologica 2003, 24, S11–S23. [Google Scholar]
- Tingwey, E.I. Studies on the Life Cycle of Akinete Forming Cyanobacterium Cylindrospermopsis raciborskii in the Temperate Region; Faculty of Environmental Sciences and Process Engineering, Brandenburg University of Technology in Cottbus: Cottbus, Germany, 2009. [Google Scholar]
- Stević, F.; Mihaljević, M.; Špoljarić Maronić, D.; Žuna Pfeiffer, T.; Zahirović, V. The Decreased Incidence of Raphidiopsis Raciborskii Bloom in a Temperate Floodplain Lake in the Middle Danube Affected by Extreme Hydrological Events. Water 2025, 17, 309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).