Integrative Taxonomy of Metarhabditis Associated with Parasitic Otitis in Dairy Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Light Microscopy (LM)
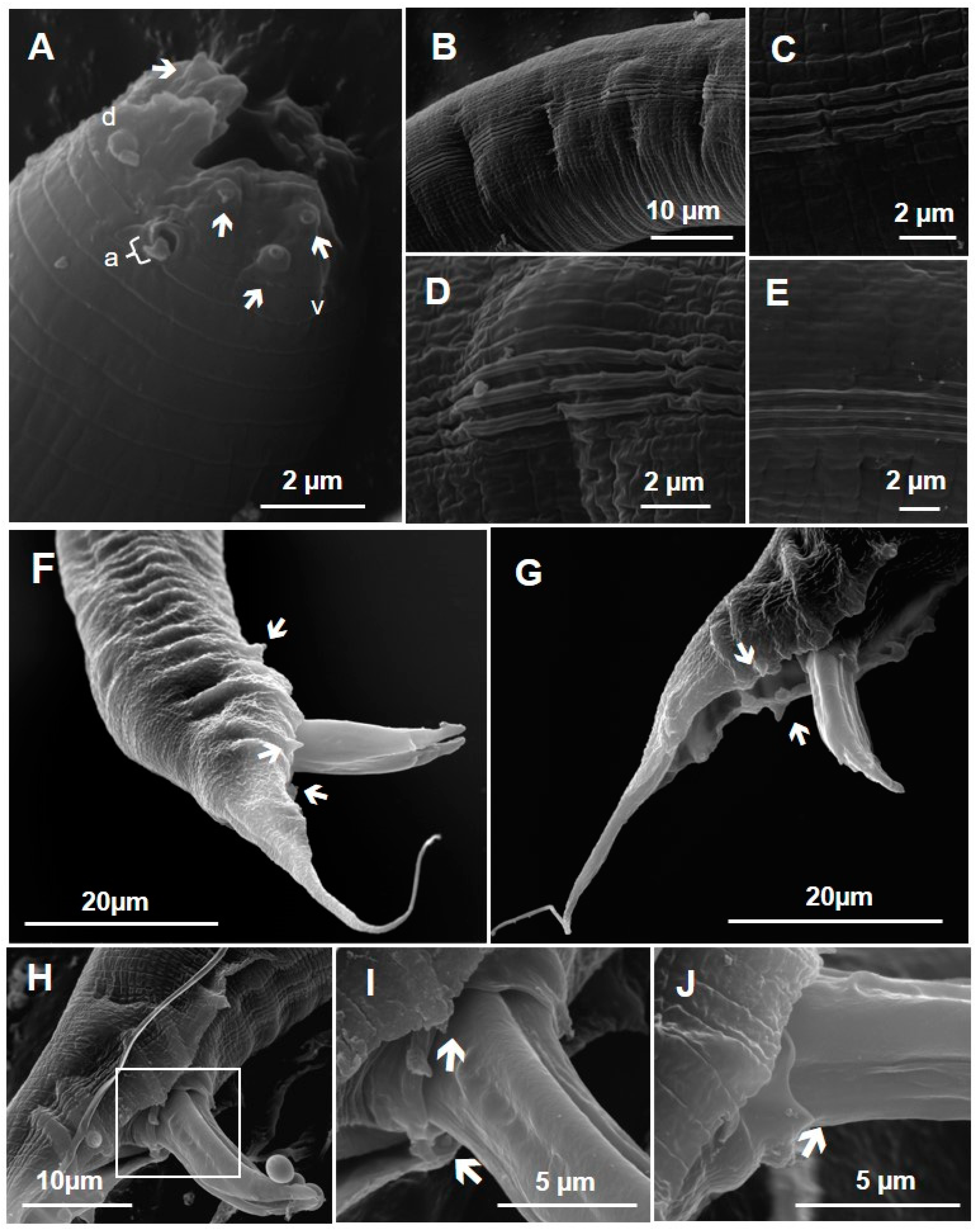
2.3. Scanning Electron Microscopy (SEM)
2.4. Genetic Characterization
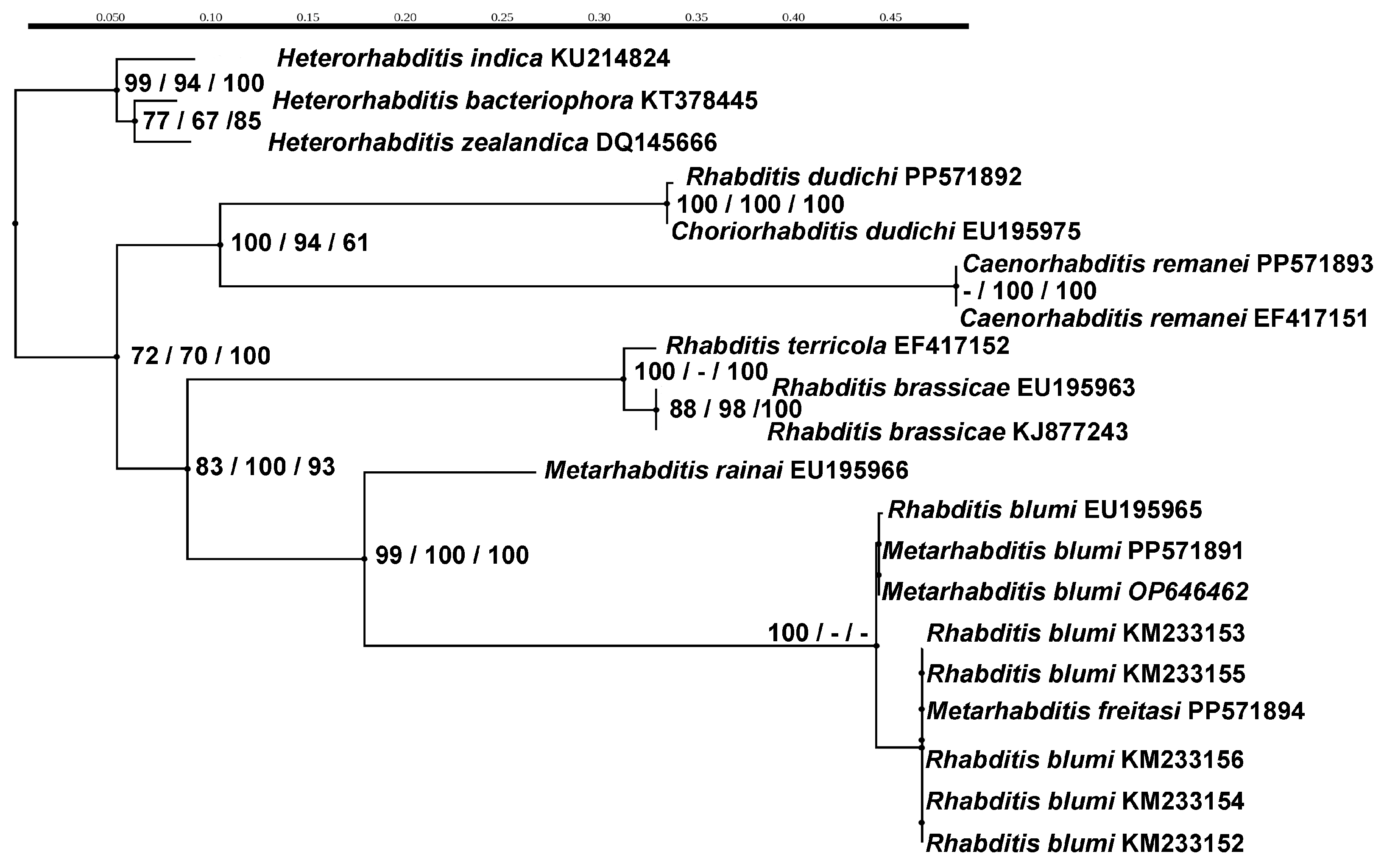
2.5. Phylogenetic Analyses
3. Results
3.1. Taxonomy Identification
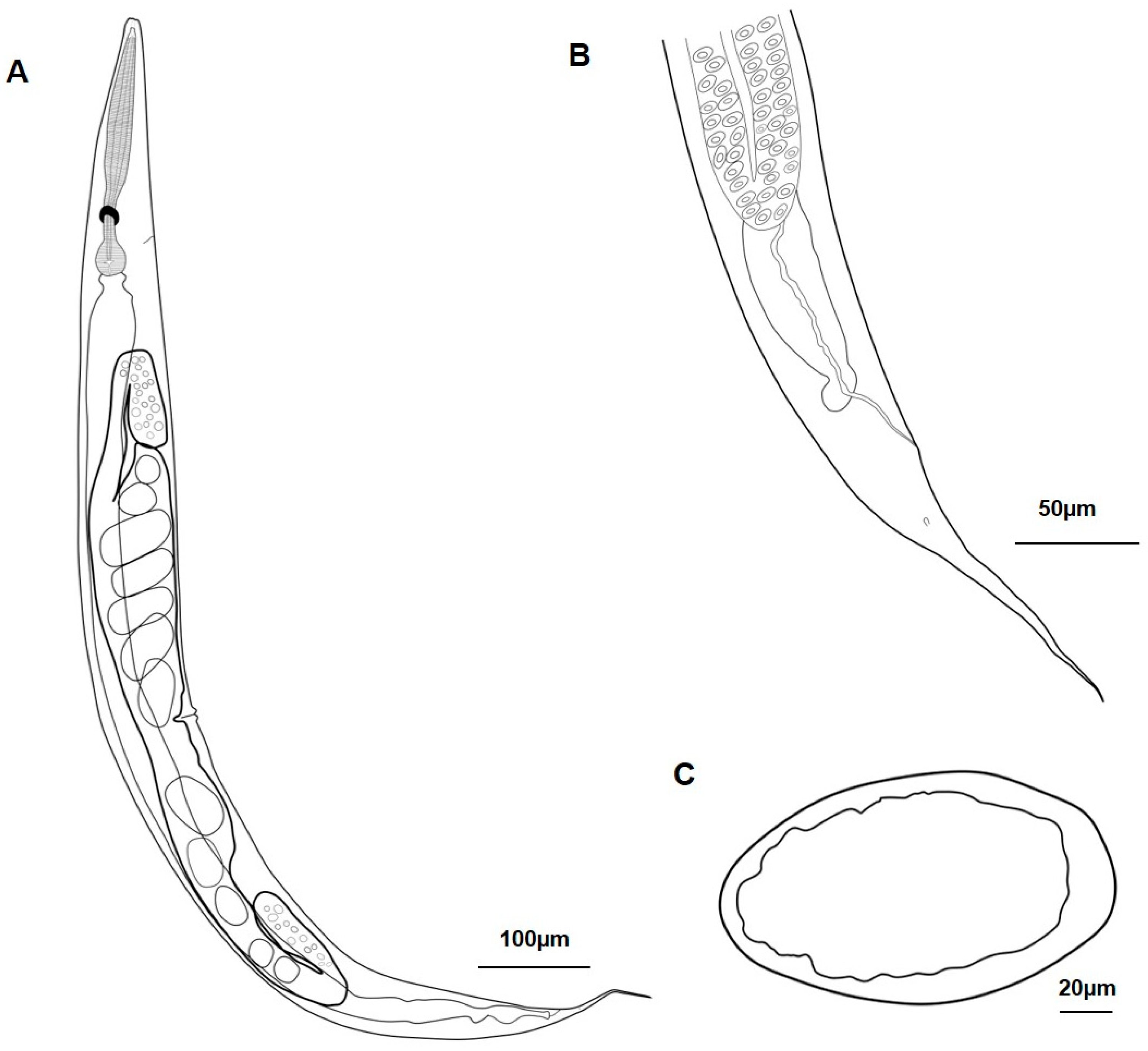
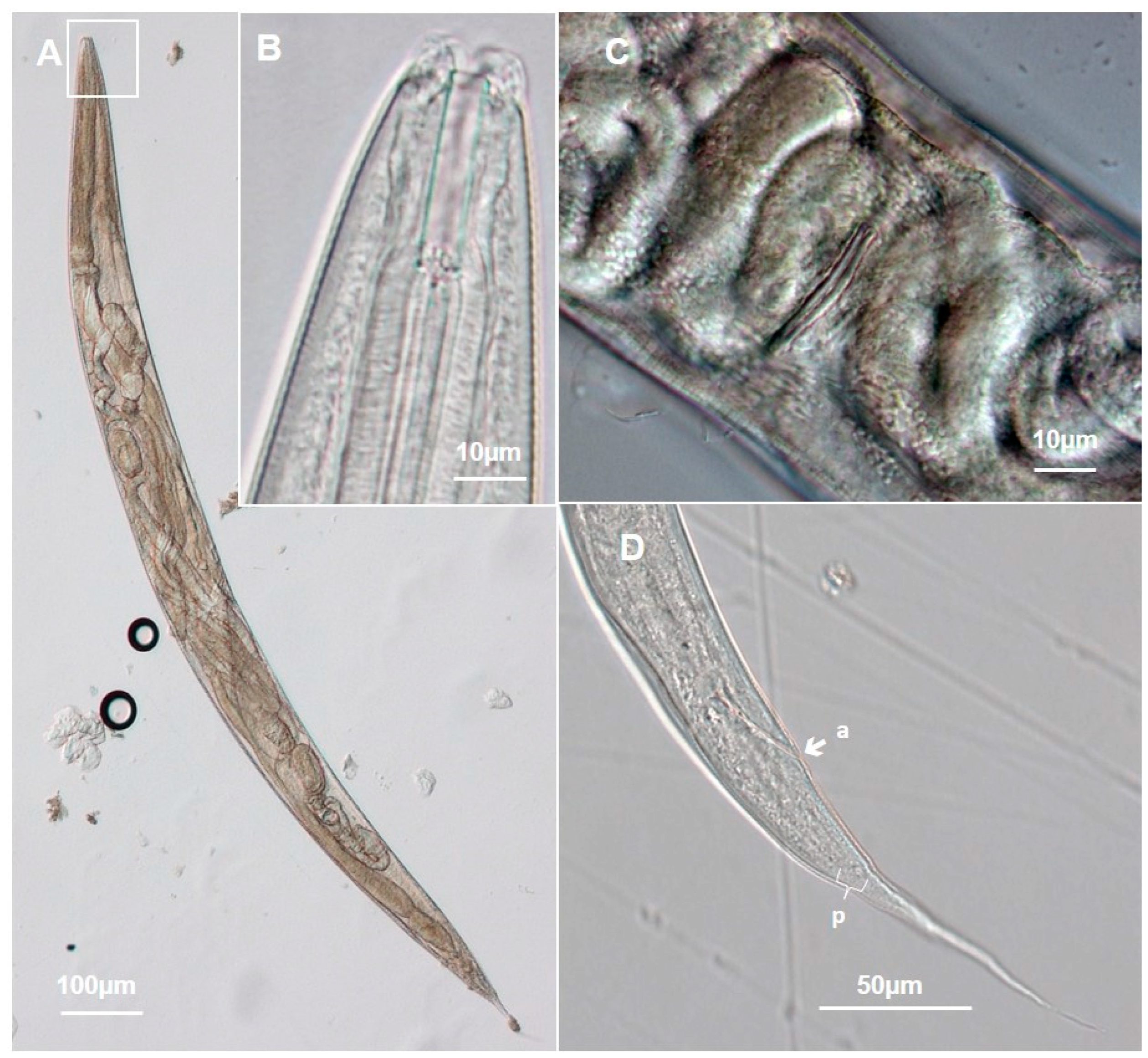
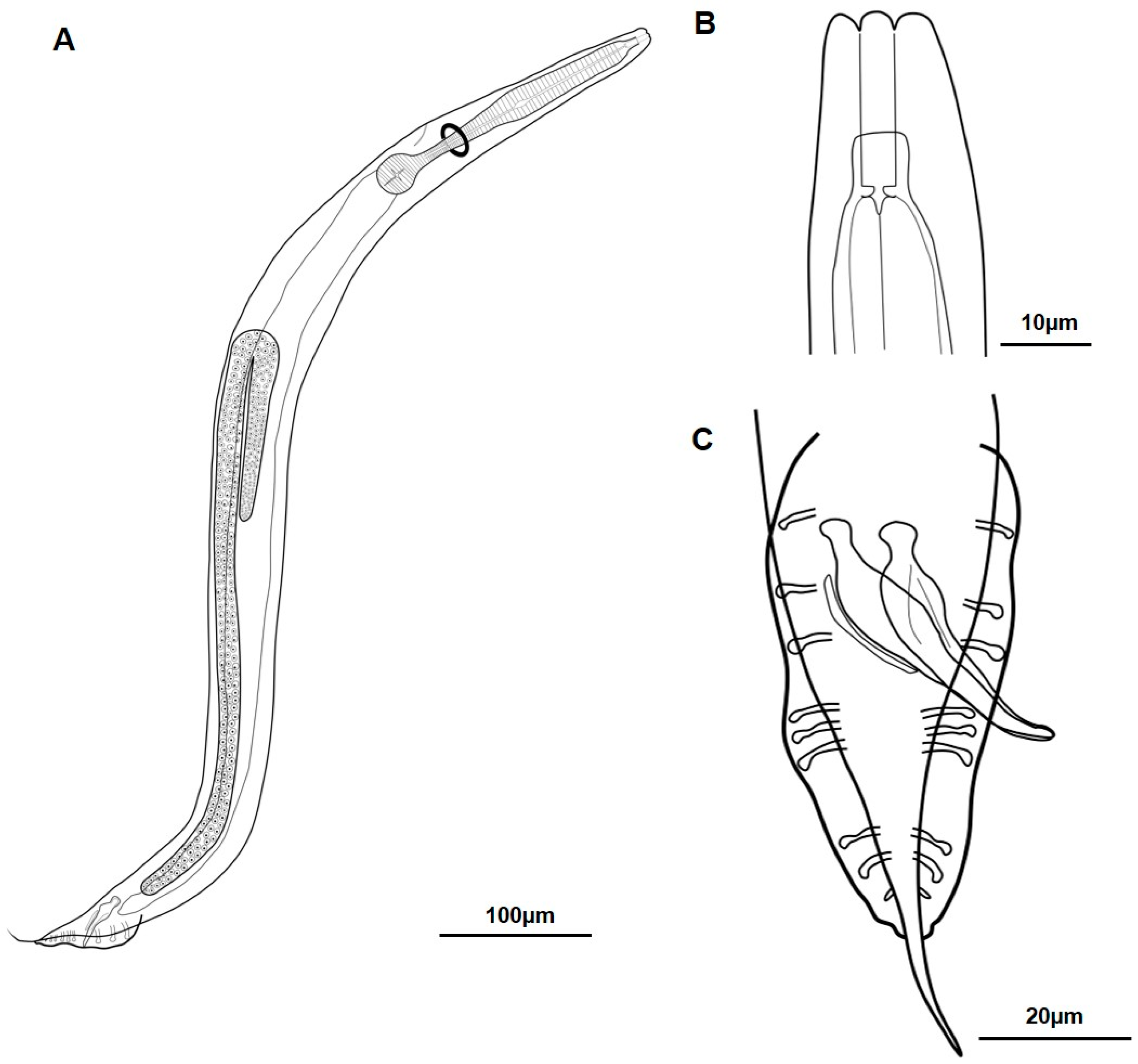
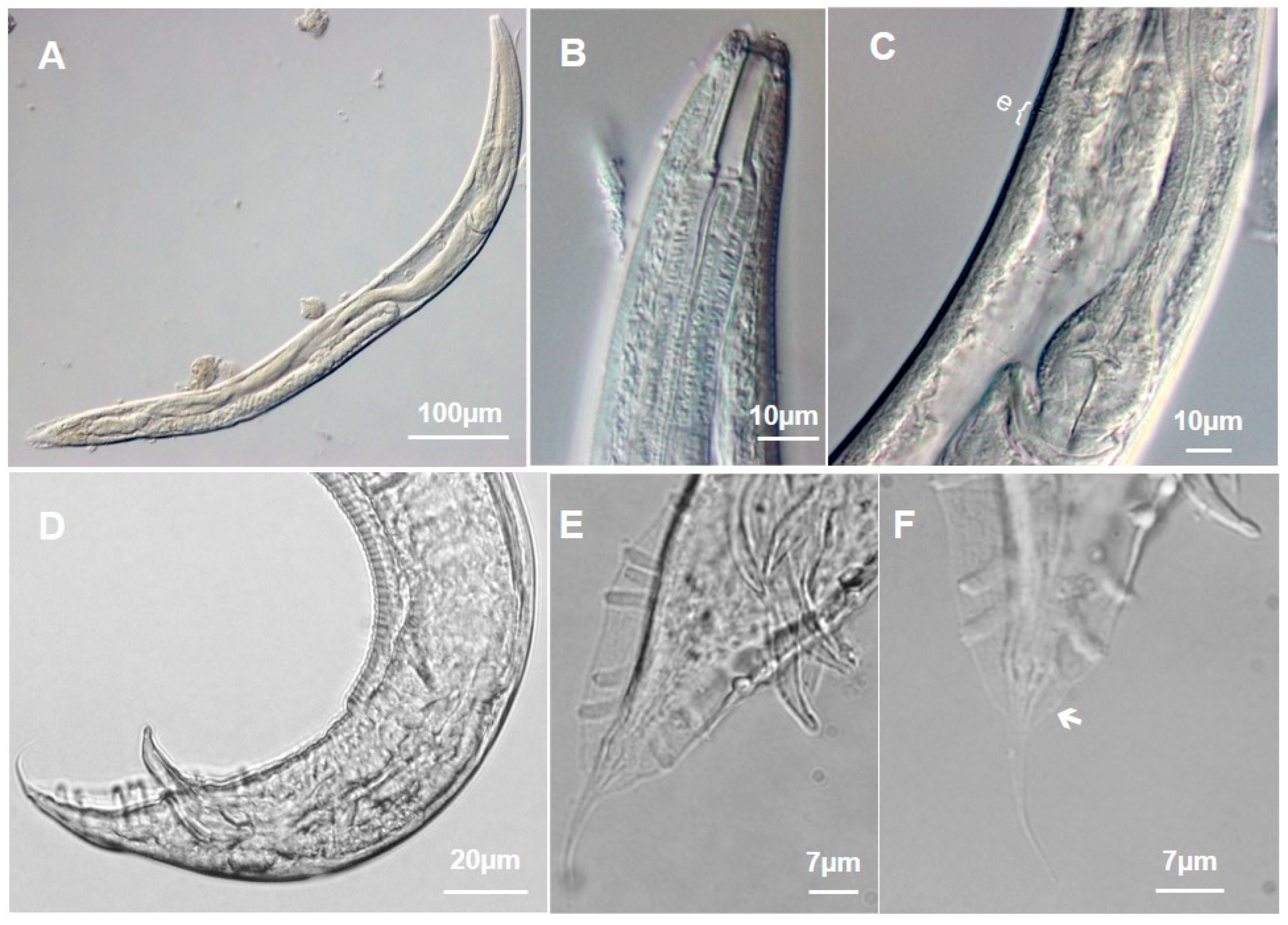
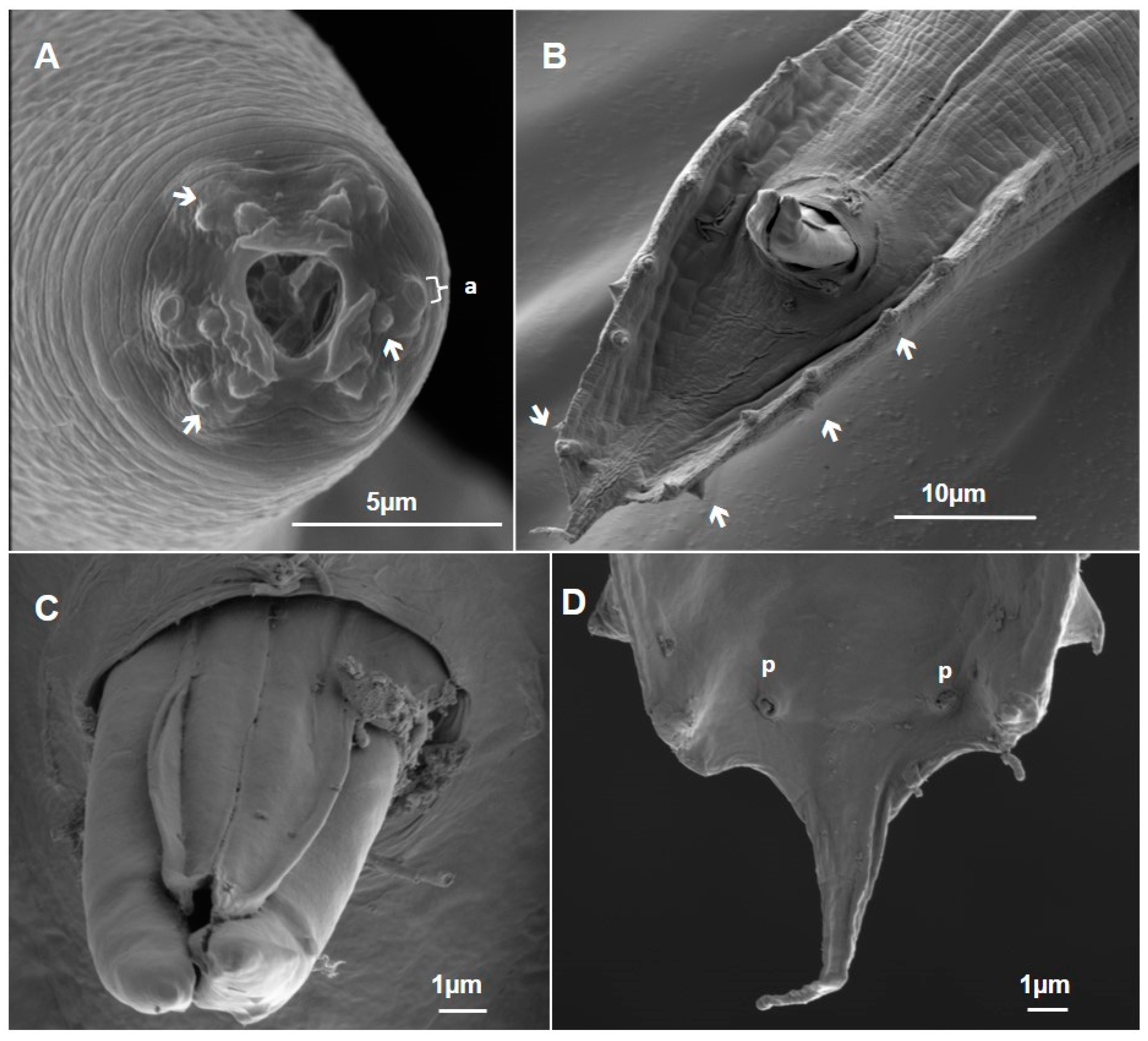
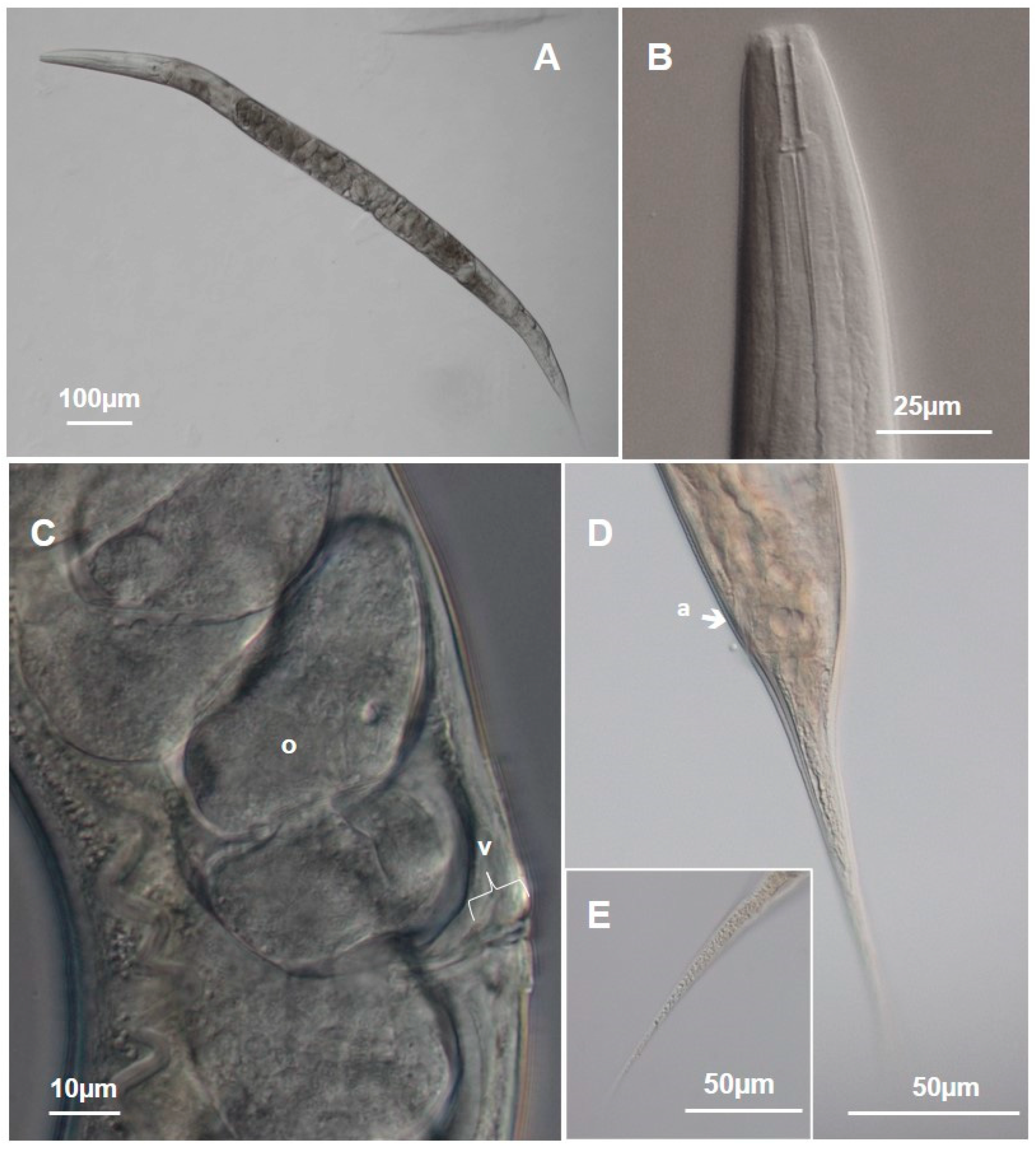
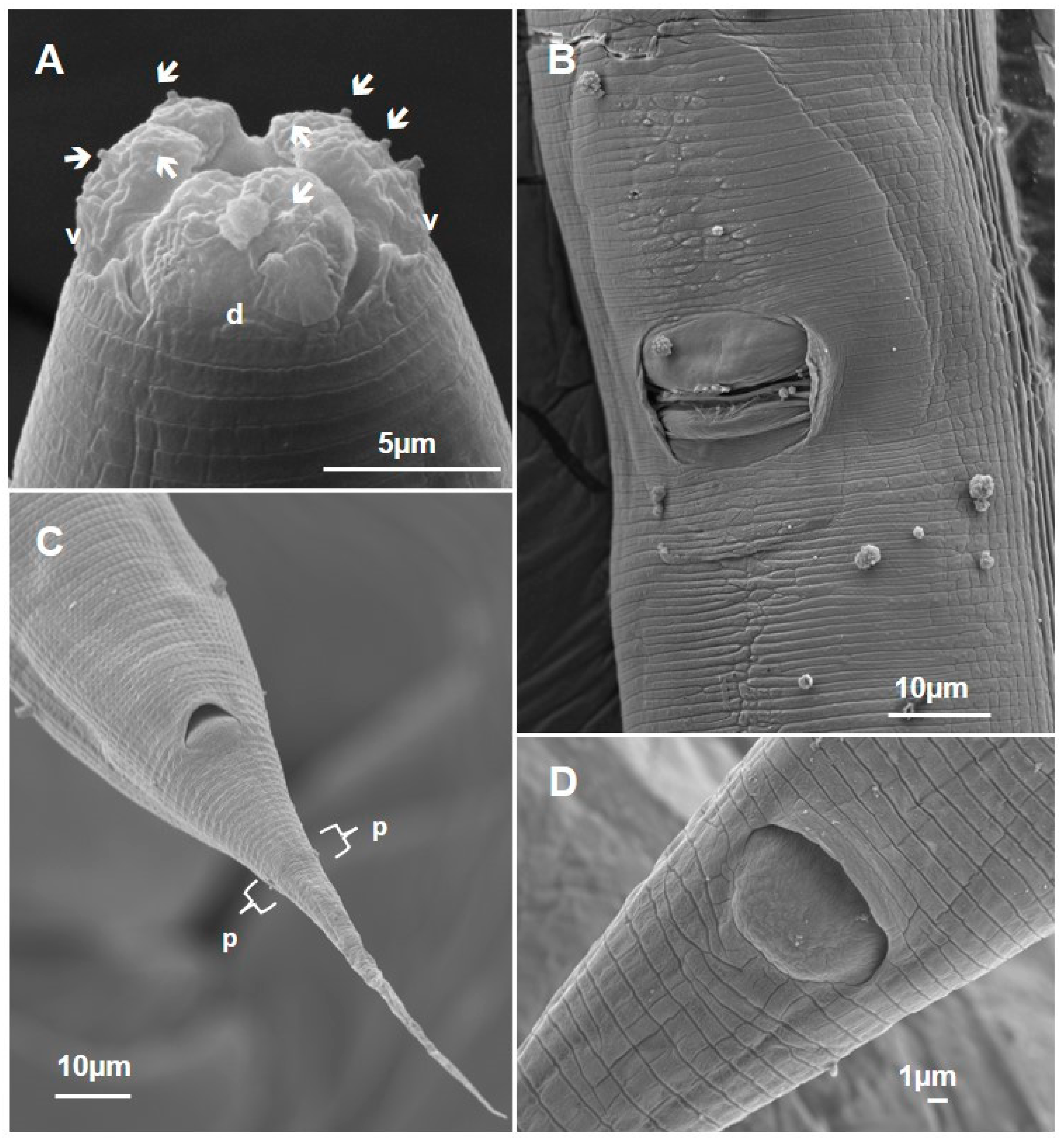
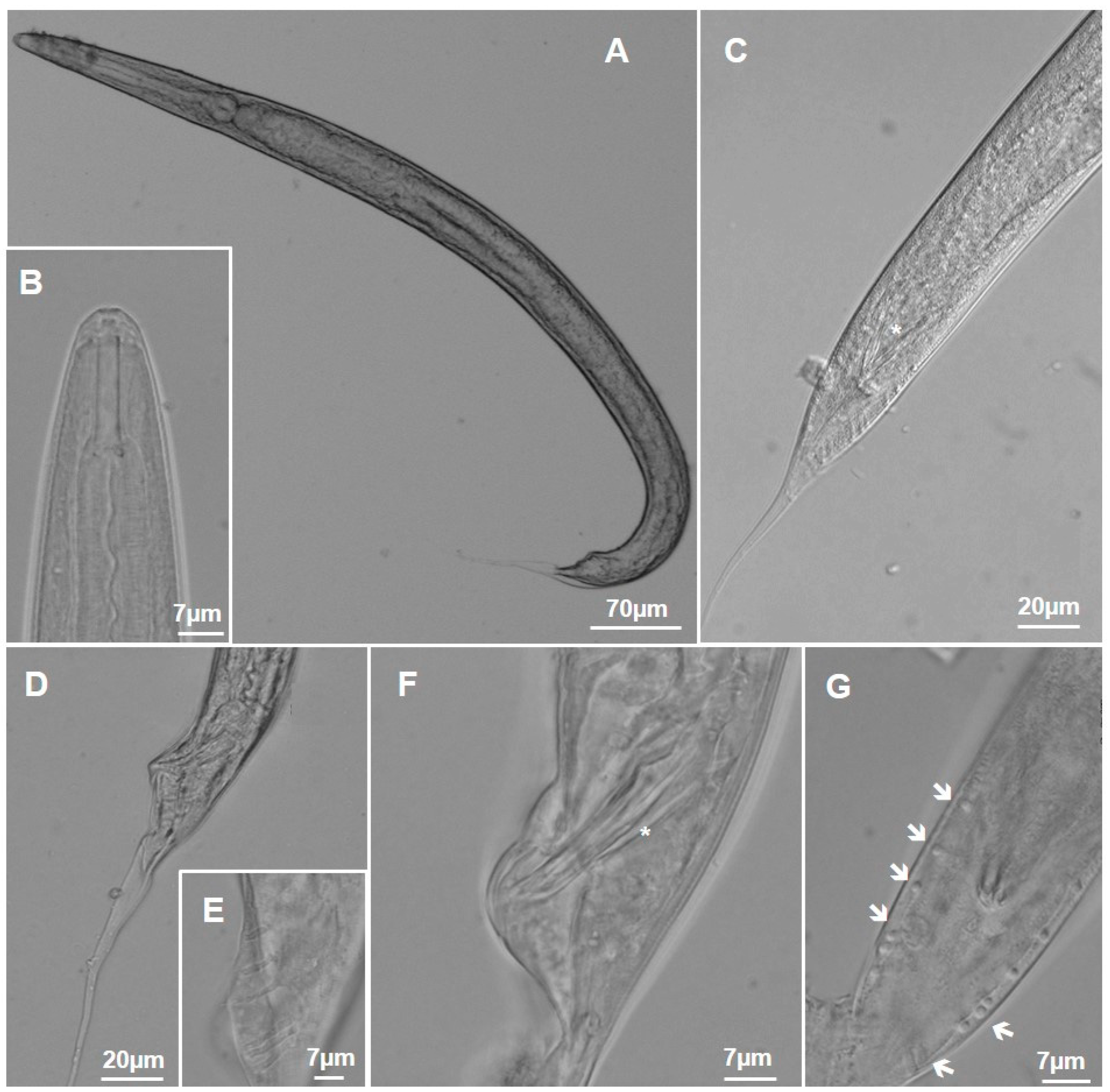
3.1.1. Metarhabditis costai (Martins, 1985), Sudhaus, 2011
3.1.2. Metarhabditis freitasi (Martins, 1985), Sudhaus, 2011
3.1.3. Metarhabditis blumi (Sudhaus, 1974)
3.1.4. Metarhabditis sp.
3.2. Genetic Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verocai, G.G.; Fernandes, J.I.; Correia, T.R.; Melo, R.M.P.S.; Alves, P.A.M.; Scott, F.B. Otite Parasitária Bovina Por Nematoides Rhabditiformes em Vacas Gir no Estado do Rio de Janeiro, Brasil. Rev. Bras. Parasitol. Vet. 2007, 16, 105–107. [Google Scholar] [PubMed]
- Leite, P.V.B.; Cunha, L.M.; Leite, L.B.; Leite, R.C. Farmers’ Perception About Parasitic Otitis in Gyr Breed from Three States of Brazil. Pesq. Vet. Bras. 2012, 32, 855–858. [Google Scholar] [CrossRef][Green Version]
- Cardona, J.A.; González, M.; Álvarez, J. Frequency of clinical parasitic otitis due to Rhabditiform nematodes (Rhabditis sp) in six Gyr breed cattle farms in Cordoba, Colombia. Rev. Colomb. Cienc. Pecu. 2012, 25, 417–421. [Google Scholar]
- Bulletin of the International Dairy Federation 446/2010—World Dairy Situation 2010. Available online: https://www.fil-idf.org/wp-content/uploads/woocommerce_uploads/Publications/Sold/Bulletins/446-2010-The-World-Dairy-Situation-2010.pdf (accessed on 8 March 2024).
- Douphrate, D.I.; Hagevoort, G.R.; Nonnenmann, M.W.; Lunner Kolstrup, C.; Reynolds, S.J.; Jakob, M.; Kinsel, M. The dairy industry: A brief description of production practices, trends, and farm characteristics around the world. J. Agromed. 2013, 18, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Charlier, J.; Dijk, J.V.; Morgan, E.R.; Geary, T.; Samson-Himmelstjerna, G.V.; Edwin Claerebout, E. Control of helminth ruminant infections by 2030. Parasitology 2018, 145, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Sobral, S.; Almeida, L.S.; Ferraz, C.M.; Junior, O.L.F.; Lopes, A.D.C.G.; Langoni, H.; Moreira, T.F.; Lima, J.A.C.; Vilela, V.L.R.; Braga, F.R. Infestações por Rhabditis spp.: Uma revisão. Vet. Zoot. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Duarte, E.R.; Hamdan, J.S. Otitis in cattle, an etiological reveiw. J. Vet. Med. Bras. Infect. 2004, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leite, P.V.B.; Cunha, A.P.; Silva, M.X.; Bello, A.C.P.P.; Domingues, L.N.; Junior, A.L.; Leite, R.C. Clinical aspects and dynamics of auricular parasitoses in Gir cattle. Pesq. Vet. Bras. 2013, 33, 319–325. [Google Scholar] [CrossRef]
- Msolla, P.; Semuguruka, W.D.; Kasuku, A.A.; Shoo, M.K. Clinical Observations on Bovine parasitic otitis in Tanzania. Trop. Anim. Health. Prod. 1993, 25, 15–18. [Google Scholar] [CrossRef]
- Ushewokunze-Obatolu, U.; Pfukenyi, D.M.; USHE, T. A retrospective epidemiological study of parasitic otitis in cattle in the South-East Lowveld of Zimbabwe. Zimb. Vet. J. 1999, 30, 19–24. [Google Scholar] [CrossRef]
- Teschner, M.; Würfel, W.; Sedlacek, L.; Suerbaum, S.; De Tappe, D.; Mathias, M.W.; Hornef, W. Outer Ear Canal Infection with Rhabditis sp. Nematodes in a Human. J. Clin. Microbiol. 2014, 52, 1793–1795. [Google Scholar] [CrossRef]
- Andrássy, I. A Taxonomic Review of the Auborder Rhabiditina (Nematoda: Sercenentia); ORSTOM: Paris, France, 1983. [Google Scholar]
- Taboga, L. Rhabditis terrícola: An Opportunistic Nematode Parasite of Earthworms Cocoons. J. Invertebr. Pathol. 1981, 38, 22–25. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, Y.O.; Ha, J.; Youn, S.H.; Kim, H.H.; Bilgrami, A.L.; Shin, C.H. Effects of associated bacteria on the pathogenicity and reproduction of the insect-parasitic nematode Rhabditis blumi (Nematoda: Rhabditida). Can. J. Microbiol. 2011, 57, 750–758. [Google Scholar] [CrossRef]
- Lee, W.K.; Choi, W.Y.; Lee, O.R. Rhabditis elongata Schneider, 1866 from Students in Korea. Kisaengchunghak Chapchi 1978, 16, 113–116. [Google Scholar] [CrossRef]
- Ahn, Y.K. Rhabditis sp. infect cases in rural schoolchildren. Kor. J. Parasitol. 1985, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.M.B.; Araújo, J.L.B.; Viera, M.C.M.; Damasceno, F.; Barbosa, A.P. Um caso de parasitismo por Rhabditis sp em criança natural de Goiânia, Goiás, Brasil. Rev. Soc. Bras. Med. Trop. 2002, 35, 519–522. [Google Scholar] [CrossRef][Green Version]
- Tahseen, Q.; Hussain, A.; Tomar, V.; Shah, A.A.; Jairajpuri, M.S. Description of Metarhabditis andrassyana gen. n., sp. n. Nematoda: Rhabditidae from India. Int. J. Nematol. 2004, 14, 163–168. [Google Scholar]
- Sudhaus, W. Phylogenetic systematization and catalogue of paraphyletic “Rhabditidae” (Secernentea, Nematoda). J. Nematode Morphol. Syst. 2011, 14, 113–178. [Google Scholar]
- Sudhaus, W. An update of the catalogue of paraphyletic ’Rhabditidae’ (Nematoda) after eleven years. Soil. Org. 2023, 95, 95–116. [Google Scholar] [CrossRef]
- Martins, W., Jr.; Nunes, I.J.; Ribeiral, L.A.; Rosaz, C.E.E.; Nunes, V.A. Nota sobre a ocorrência da Rhabditidae (Nematoda, Rhabditida) relacionados com otite em bovinos na região geo-econômica de Brasília/DF. Ciênc. Cult. 1971, 23, 248–249. [Google Scholar]
- Round, M.C. The Helminths Parasites of Domesticated Animals in Kenya. J. Helminthol. 1962, 36, 375–449. [Google Scholar] [CrossRef] [PubMed]
- Martins, W., Jr. Rhabditis (Rhabditis) freitasi sp. n. e Rhabditis (Rhabditis) costai sp. n. (Nematoda-Rhabditidae) isolados de otite bovina. Mem. Inst. Oswaldo Cruz 1985, 80, 11–16. [Google Scholar] [CrossRef][Green Version]
- Leite, R.C.; Nunes, V.A.; Nunes, I.J.; Costa, A.L.; Facchini, J.L.H.; Lopes, C.W.G. Otite parasitária por nematóides rhabditiformes: Aspectos epidemiológicos e clínicos. Rev. Bras. Med. Vet. 1993, 15, 49–51. [Google Scholar]
- Abdalla, M.S.; Peixoto, T.C.; Alves, P.A.M.; França, T.N.; Brito, M.F. Aspectos Anátomo-Patológicos da otite Causada por Rhabditis sp. em Bovinos no Estado do Rio de Janeiro, Brasil. In Anais do Congresso Brasileiro de Veterinária; Sovergs: Gramado, Brazil, 2008. [Google Scholar]
- Bossi, P.V.; Consoli, E.A.; Rosa, J.M.O.; Leite, L.B.; Leite, R.C.; de Oliveira, C.M.G. Molecular identification and phylogenetic analysis of Metarhabditis blumi (Nematoda: Rhabditida). Vet. Parasitol. 2015, 214, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. The Online Review of C. elegans Biology. Available online: https://www.ncbi.nlm.nih.gov/books/NBK19649/ (accessed on 8 March 2024).
- Pessanha, T.S.; Herrera, H.M.; Jansen, A.M.; Iñiguez, A.M. “Mi Casa, Tu Casa”: The coati nest as a hub of Trypanosoma cruzi transmission in the southern Pantanal biome revealed by molecular blood meal source identification in triatomines. Parasites Vectors 2023, 16, 26. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL w: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids. Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.70. 2021. Available online: http://www.mesquiteproject.org (accessed on 8 March 2024).
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart model selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MRBayes 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Manna, B. Rhabditis suklai sp.ov. (Nematoda:Rhabditida) from Cervus unicolor from Alipore Zoological Garden, Kolkata, India. Flor. Fauna (Jhansi) 2014, 20, 313–319. [Google Scholar]
- Abolafia, J.; Peña-Santiago, R. Description of Metarhabditis giennensis sp. N. (Nematoda, Rhabditida, Rhabditidae) from decaying wood of a riverbank forest in the southern Iberian Peninsula. Zootaxa 2019, 4652, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Prassad, J.S.; Khan, R.; Somasekhar, N.; Tahseen, Q. A revision of the genus Metarhabditis (Nematoda: Rhabditidae) with description of three known species, a key to the identification of congeners and discussion of their relationship. J. Nat. Hist. 2013, 47, 2599–2622. [Google Scholar] [CrossRef]
- Carta, L.K.; Osbrinkink, W. Rhabditis rainai n. sp. (Nematoda: Rhabditida) associated with the Formosan subterranean termite, Coptotermes formosanus (Isoptera: Rhinotermitidae). Nematology 2005, 7, 863–879. [Google Scholar] [CrossRef]
- Barbosa, J.D.; Barbosa, J.; Henrique, D.; Henrique, L.; Araújo, V.; Loiola, L.; Alessandra, S.; Reis, B.; Masiero, F.; and Farias, M.D.E. Detecção e tratamento de otite por Rhabditis blumi em bovinos da região Norte do Brasil. Pesq. Vet. Bras. 2017, 36, 605–610. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr. Rhabditis adenobia sp. n. (Nematoda: Rhabditidae) from the Colleterial Glands of Oryctes monoceros L. and Other Tropical Dynastid Beetles (Coleoptera: Scarabaeidae). Proc. Helminthol. Soc. Wash. 1971, 38, 99–108. [Google Scholar]
- Bhat, A.H.; Srivastava, S.; Rana, A.; Chaubey, A.K.; Machado, R.A.R.; Abolafia, J. Morphological, morphometrical, and molecular characterization of Metarhabditis amsactae (Ali, Pervez, Andrabi, Sharma and Verma, 2011) Sudhaus, 2011 (Rhabditida, Rhabditidae) from India and proposal of Metarhabditis longicaudata as a junior synonym of M. amsactae. J. Nematol. 2020, 52, 1–23. [Google Scholar] [CrossRef]
- Moon, J.-H.; Indong, R.A.; Alcantara, A.V., Jr.; Yoon, K.-H.; Lee, J.I. Comparison of Life Traits in Two Bacterivorous Nematodes Suggest Different Ecological Strategies to Exploit Similar Habitats. Life 2022, 12, 1516. [Google Scholar] [CrossRef]
- Ali, S.S.; Prevez, R.; Andrabi, R.; Sharma, R.; Verma, V. Oscheius amsactae n. sp. (Nematoda: Rhabditida), a necromenic associate of red-hairy caterpillar, Amsacta moori (Lepidoptera: Arctiidae) from Kanpur district, India. Arch. Phytopathol. Plant Prot. 2011, 44, 871–881. [Google Scholar] [CrossRef]







| M. costai Present Study | M. costai Martins, 1985 | M. freitasi Present Study | M. freitasi Martins, 1985 | Metarhabditis blumi. Present Study | Metarhabditis blumi Sudhaus, 1974 | |
|---|---|---|---|---|---|---|
| Body length | 1059.2 ± 82.2 (993.7–1221) | 1012 ± 150 (844–1175) | 971.2 ± 87 (861.2–1070) | 1235 ± 135 (1035–1405) | 920 ± 117.1 (764.5–1045.4) | 995–1415 |
| Body diameter * | 52.2 ± 6.6 (43.5–63.5) | 64 ± 11 (51–79) | 59 ± 7.9 (51.3–74.2) | 73 ± 10 (54–88) | 57.6 ± 10.6 (45.4–80.6) | 49–68 |
| Stoma length | 23.8 ± 2.7 (19–27.2) | - | 26 ± 2.1 (24.3–30.6) | 19.7 ± 2.8 (17.4–23.9) | 24.8 ± 2.6 (21.4–30) | 25–27 |
| Stoma width | 4.2 ± 1 (3.2–5.6) | - | 4.2 0.6 (3.2–5) | - | 4.2 ± 0.6 (3.4–5.2) | - |
| Pharynx length | 190.1 ± 12 (183.1–213.7) | 192 ± 6 (183–198) | 205.8 ± 14.8 (172.6–215.3) | 163 ± 16 (146–193) | 204.9 ± 22.8 (175–232.7) | 226–276 |
| Pharynx length | 214.7 ± 12.3 (206.2–238.7) | - | 231.8 ± 15.9 (198.3–261.7) | - | 204.9 ± 22.8 (175–232.7) | - |
| Basal bulbus length | 34.6 ± 3 (31.9–39.5) | - | 34.41 ± 2.5 (31.9–38.3) | 34 ± 2.2 (26–35) | 34.9 ± 3.7(29–41) | - |
| Basal bulbus width | 29.4 ± 4 (22–33.2) | - | 28.2 ± 2.6 (22–31.2) | 27 ± 1.5 (24–30) | 27.2 ± 3.8 (22–35) | - |
| Nerve ring | 159.7 ± 7.1 (150–163.8) | - | 160 ± 19.9 (137.9–176.6) | - | 146.7 ± 16 (124.5–163.8) | - |
| Spicule length | 42 ± 5.4 (34.7–50.6) | 40 ± 6 (33–50) | 40.6 ± 3.3 (32.8–44.7) | 47 ± 4.3 (43–52) | 44.1 ± 3.4 (39–50) | 45–51 |
| Gubernaculum length | 26.2 ± 0.3 (26–26.5) | 21 ± 1.9 (20–24) | 24.6 ± 2.5 (21.8–26.6) | 20 ± 1.6 (17–22) | 17.5 ± 3.7 (15–21) | 16–22 |
| Tail length | 41.8 ± 1.1 (40.5–43.7) | 37 ± 2.8 (35–42) | 43.8 ± 4.8 (37.6–48.9) | 41 ± 7 (35–48) | 55.9 ± 10.2 (31.5–60) | 50–66 |
| Post-bursa tail | 1.4 ± 0.2 (1.1–1.7) | - | 7.8 ± 1.5 (6–9.8) | - | 17.5 ± 1.5 (14–22.6) | - |
| a | 20.4 ± 1.3 (19.2–22.8) | 16.1 ± 1.35 (14–17) | 16.7 ± 3 (12.7–21.8) | 17.2 ± 3.1 (14–25.5) | 16.4 ± 3.1 (10.4–19) | 17.8–22.3 |
| b | 4.9 ± 0.2 (4.7–5.1) | 5.3 ± 0.8 (4.4–6.7) | 4.2 ± 0.4 (3.6–4.8) | 7.6 ± 0.8 (6.7–9.1) | 4.5 ± 0.3 (4.0–5.0) | 4.2–5.9 |
| c | 25 ± 2.2 (22.8–29.2) | 27.1 ± 3.9 (23.2–34.2) | 22.3 ± 2.2 (18.8–27.2) | 25.3 ± 5.1 (23.9–39.5) | 16.9 ± 3.1 (14–25) | 17–27 |
| M. freitasi Present Study | M. freitasi Martins, 1985 | M. blumi Present Study | M. blumi Sudhaus, 1974 | |
|---|---|---|---|---|
| Body length | 1014.8 ± 124.5 (838.7–1118.6) | 1530 ± 155 (1253–1714) | 1180.4 ± 177.7 (948–1371.8) | 1324–1819 |
| Body diameter | 62.5 ± 7 (52.4–67.8) | 98 ± 13 (73–117) | 69.2 ± 13.2 (52–83.1) | 59–85 |
| Stoma length | 26.4 ± 3.3 (23.4–30.5) | 21 ± 2 (19–26) | 28 ± 4.7 (20–33.8) | 29–35 |
| Stoma width | 5 ± 0.3 (4.7–5.3) | 4.2 ± 6 (3.5–5.2) | 4.2 ± 0.8 (3–5.2) | - |
| Pharynx length | 200 ± 8 (192–210) | - | 252.2 ± 22.5 (180–256.9) | 244–298 |
| Pharynx length | 226.4 ± 10.4 (216–241) | 201 ± 25 (176–262) | 252.2 ± 22.5 (180–256.9) | - |
| Basal bulbus length | 34.2 ± 11.4 (30–37) | 32 ± 2.3 (29–35) | 37.3 ± 5.1 (31.5–44.2) | - |
| Basal bulbus width | 27.3 ± 3.2 (25–30) | 30 ± 4.1 (21–35) | 27.1 ± 3.9 (18.3–32) | - |
| Nerve ring | 161.7 ± 1.9 (149–172) | 151 ± 15 (132–165) | 163.4 ± 19.4 (15.3–193.1) | - |
| Tail length | 148.8 ± 8.1 (137–155) | 132 ± 15 (112–154) | 101.3 ± 16.6 (78–128.2) | 156–221 |
| Egg length | 54 ± 6.1 (49–60) | 50 ± 6.7 (44–66) | 49.5 ± 7 (39–58.4) | - |
| Egg width | 33.4 ± 4.1 (30–39) | 33 ± 3.3 (26–32) | 28.9 ± 5.9 (20.1–33.8) | - |
| a | 16.2 ± 0.2 (16–16.5) | 15.7 ± 1.7 (13.3–18) | 17.3 ± 2 (14.6–20) | 18–23.3 |
| b | 4.5 ± 0.5 (4–5) | 7.6 ± 1.9 (6.5–8.5) | 5.2 ± 0.6 (4.1–5.9) | 4.8–6.1 |
| c | 6.8 ± 0.6 (6.1–7.3) | 11.7 ± 1.7 (8.2–14.1) | 12.1 ± 1.9 (10–13.7) | 7.0–9.7 |
| v (%) | 56.4 ± 6.9 (47–63) | 52 ± 16 (50–56) | 54.6 ± 4.8 (47.8–63.3) | 48–52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caracciolo, M.E.; de Andrade-Silva, B.E.; Borba, V.H.; Castello-Branco, A.; Santos, H.A.d.; Iñiguez, A.M.; Lopes-Torres, E.J. Integrative Taxonomy of Metarhabditis Associated with Parasitic Otitis in Dairy Cattle. Taxonomy 2024, 4, 464-486. https://doi.org/10.3390/taxonomy4030023
Caracciolo ME, de Andrade-Silva BE, Borba VH, Castello-Branco A, Santos HAd, Iñiguez AM, Lopes-Torres EJ. Integrative Taxonomy of Metarhabditis Associated with Parasitic Otitis in Dairy Cattle. Taxonomy. 2024; 4(3):464-486. https://doi.org/10.3390/taxonomy4030023
Chicago/Turabian StyleCaracciolo, Makoto Enoki, Beatriz Elise de Andrade-Silva, Victor Hugo Borba, Ander Castello-Branco, Hudson Andrade dos Santos, Alena Mayo Iñiguez, and Eduardo José Lopes-Torres. 2024. "Integrative Taxonomy of Metarhabditis Associated with Parasitic Otitis in Dairy Cattle" Taxonomy 4, no. 3: 464-486. https://doi.org/10.3390/taxonomy4030023
APA StyleCaracciolo, M. E., de Andrade-Silva, B. E., Borba, V. H., Castello-Branco, A., Santos, H. A. d., Iñiguez, A. M., & Lopes-Torres, E. J. (2024). Integrative Taxonomy of Metarhabditis Associated with Parasitic Otitis in Dairy Cattle. Taxonomy, 4(3), 464-486. https://doi.org/10.3390/taxonomy4030023







