Abstract
The Neotropical deer genus Mazama is characterized by homoplastic morphological characters, a high karyotypic diversity, and a polyphyletic condition. The species of the genus have been recovered into two multigeneric lineages, the subtribes Odocoileina and Blastocerina, of the tribe Odocoileini (New World deer) in the family Cervidae. Within the Blastocerina, gray brockets include two non-sister species, Subulo gouazoubira, occurring south of the Amazon region, and Passalites nemorivagus, occurring in the Guianas and in the Amazon region. We clarify the taxonomic status and phylogenetic position of Mazama americana citus Osgood, 1912 (referred to as either S. gouazoubira or P. nemorivagus by other authors). We collected a topotype of M. a. citus from the eastern shore of Lake Maracaibo, Venezuela, characterize it morphologically and cytogenetically (conventional banding and fluorescence in situ hybridization), and carry out a phylogenetic analysis of its whole mitogenome and Cytb alongside two additional specimens of M. a. citus from northwestern Venezuela. Our analyses reveal the topotype to be a large gray brocket with a cinnamon band above the eyes and 2n = 61 and FN = 70 karyotype. Using cattle whole chromosome painting and bacterial artificial chromosome X probes, we determined its karyotype to differ in at least 10 rearrangements from that of S. gouazoubira. Bayesian inference recovers M. a. citus within the Blastocerina subtribe, separated phylogenetically from other gray brockets (100% branch value), revealing the Osgood’s gray brocket to be a valid species that should be assigned to a new genus. We propose the generic name Bisbalus, with Bisbalus citus (Osgood, 1912) as the type species.
1. Introduction
Neotropical brockets are small to medium-sized (13 to 40 kg) deer, with short and unbranched antlers in males, inhabiting forests from southeastern Mexico to northern Argentina [1,2]. Traditional taxonomic reviews of the Neotropical deer possessing this morphotype have allocated them to the genus Mazama Rafinesque, 1817 [3], thought to include from 4 to 17 species and subspecies [4,5,6]. With the advance of genetic techniques, several authors have shown the genus Mazama to be polyphyletic and its species to be karyotypically diverse. These findings indicate that, owing to high levels of homoplasy, external and cranial morphologies alone are not sufficient to distinguish species and to establish phylogenetic relationships in these deer [7,8,9]. Phylogenies based on mitochondrial genes have shown species traditionally allocated to Mazama to represent at least three genera distributed in the two subtribes, Odocoileina and Blastocerina, of the tribe Odocoileini (New World deer) in the family Cervidae [7,8,9,10].
The taxonomy of deer traditionally assigned to the genus Mazama is in a state of rapid flux. Within the subtribe Odocoileina, red brockets have been deemed to represent the true Mazama, with Mazama americana (Erxleben, 1777) [11] as the type species [7]. Also, within this subtribe, Mazama bricenii Thomas, 1908 [12] was synonymized with Mazama rufina Pucheran, 1851 [13,14]; the Yucatan brown brocket deer, formerly Mazama pandora Merriam, 1901 [15], was transferred to the genus Odocoileus Rafinesque, 1832 [7,16]; Mazama rufa (Illiger, 1815) [17] was validated [18]; and Mazama bororo Duarte, 1996 [19] was synonymized with Mazama jucunda Thomas, 1913 [20,21]. Within the subtribe Blastocerina, the monophyletic Azara’s gray brocket, Mazama gouazoubira (Fischer, 1814) [22], has been proposed to belong to the resurrected genus Subulo Smith, 1827 [23], thus its updated name would be Subulo gouazoubira [24]; and the Amazonian gray brocket, formerly Mazama nemorivaga (Cuvier, 1817) [25] and recently updated to Passalites nemorivagus (Cuvier, 1817) [25], has been proposed to represent another genus within the Blastocerina given that it does not share a common ancestor with S. gouazoubira [7,8,26]). Much is yet to be learned regarding the alpha and beta taxonomy of the genus Mazama, thus the analysis of taxa described from the type localities across the Neotropics needs to be continued.
One species needing a clarification of its phylogenetic relationships is Mazama americana citus Osgood, 1912 [27], whose type locality is “El Panorama, Rio Aurare, eastern shore of Lake Maracaibo, Venezuela”. The specific allocation of this putative subspecies is based on the assumption that Cervus nemorivagus Cuvier, 1817 [25] is a synonym of Cervus americanus Erxleben, 1777 [5,20,21,22,23,24,25,26,27]. The description of specimens characterizes them as paler and more grayish than those of typical “M. americana”, with upperparts grizzled cinnamon that gradually become paler on the sides and underparts, white spots on the tarsal glands and on each side of the rhinarium and above each eye, a slightly larger body, notably larger cheek teeth, and differences in other minor cranial features [27].
Contrary to Osgood [27], Allen [4] deemed M. nemorivaga (Cuvier, 1817) [25] a valid species distinct from M. americana. Moreover, he deemed M. americana citus a valid species on its own, for which he used the name Mazama cita. He divided this species into two subspecies, M. cita cita, the form described by Osgood [27], and M. cita sanctaemartae, which he described. Allen [4] referred to M. nemorivaga and M. cita as the M. nemorivaga group, which he differentiated from other congeners based on the possession of a short (about 50% of condylobasal length) preorbital portion of the skull. According to Allen [4], compared with M. nemorivaga from the Guianas, M. cita differs in being slightly larger, in possessing a heavier dentition, and in showing a “very considerably” distinct coloration. Osgood [27] did not provide etymologies. However, in the same paper he described nine additional species and subspecies of mammals, for which he proposed epithets clearly based on Latin. If citus is also based on Latin, it represents the perfect passive participle (verbal adjective) of the verb cieō, and means “put in motion”, or “swift”. In Latin, this tense must be masculine, feminine, or neuter. This explains why most subsequent authors have used cita instead of citus to match gender with the feminine name Mazama.
Tate [28] grouped Mazama into two “Divisions”, the first of which (“A”) he distinguished from the second (“B”) based on the possession of elongated and narrow (as opposed to short and thick) antlers and large (as opposed to small) teeth and on geographic distribution, with members of Division “A” (M. americana group, large brockets) occurring in South America and eastern Panama, and members of Division “B” (M. simplicicornis (Illiger, 1815) group, small brockets) occurring in South and Central America and Mexico. Tate [28] included M. cita in his Division “A”. Previously, Lydekker [29] deemed M. cita a subspecies of Azara’s gray brocket, namely M. simplicicornis citus; whereas, subsequently, others [5,30,31] deemed it a subspecies of the southern gray brocket, namely M. gouazoubira cita. In his review of the genus Mazama in Venezuela, Bisbal [32,33] applied the name Mazama gouazoubira (Fischer, 1814) to all the gray or brown brockets from Venezuela, and recognized two subspecies: M. g. nemorivaga, occurring south of the Orinoco (Amazonas and Bolívar states), and M. g. cita, occurring in northern Venezuela (Zulia and Falcón states, central coastal region of the country).
Based on novel morphological, classical cytogenetic, molecular cytogenetic, and mitogenomic data, the present study aims to resolve the species level and generic status within the subtribe Blastocerina of Osgood’s gray brocket Mazama cita.
2. Materials and Methods
2.1. Specimens and Samples
To search in the field for deer specimens in northwestern Venezuela, where the type locality of Mazama cita is located, we used a collecting permit (SISTRA No. 20356) issued by the Ministerio del Poder Popular para el Ecosocialismo. We secured an adult male topotype that had been killed by a local hunter near El Consejo de Ciruma, Zulia state, ca. 35 km ESE from the type locality. The topotype was assigned the identification number T410 of the NUPECCE (Museu do Núcleo de Pesquisa e Conservação de Cervídeos, São Paulo, Brazil) and the catalog number CVULA 9109 (Colección de Vertebrados de la Universidad de Los Andes, Mérida, Venezuela). In addition, muscle tissue samples (VEN04 and VEN05) of two gray brockets were obtained for molecular analysis in nearby Falcon state. We provisionally adopted the binomial “Mazama cita” to refer to Osgood´s gray brocket.
2.2. Morphological Description
The freshly collected topotype was photographed, and its external body measurements (cm) and body mass (kg) were determined using measuring tape, a digital caliper, and a pendulum scale. The following measurements were taken: body length, head length and width, ear length, between-the-eyes width, mandible length, neck circumference, thorax circumference, shoulder height, metacarpus length, tail length, metatarsus length, and body mass (Table S1). The skin was removed and treated with a tanning solution. Aspects of the general coat color, chromogenetic fields of the body (neck, dorsal line of the body, ventral region of the body, tail, and front and hind feet), pigmentation patterns, and length of the hair in different regions of the body were assessed. The tarsal regions were examined for the presence of strips of anteverted hair and rounded hair tufts. Additionally, the chromogenetic fields of the head [34] were analyzed. A total of 38 standard cranial measurements for cervids [35] (see Table S2) were taken. The available skull was photographed at different angles to complement the documentation and description of the specimens. The body and cranial measurements of the collected topotype were compared to those of adult specimens of the gray brockets P. nemorivagaus and S. gouazoubira in the NUPECCE database.
2.3. Cytogenetic Analysis
Skin fragments (5 × 2 cm) from the inguinal region of the topotype were collected and preserved in liquid nitrogen, as described by Duarte et al. [36]. Then, the chromosomes were obtained by fibroblast in vitro culture following the procedure of Verma and Babu [37].
The chromosomal preparations were subjected to conventional Giemsa staining, G-banding [38] (modified), C-banding [39], and Ag-NOR [40]. The chromosomes were classified as acrocentric, submetacentric, or metacentric according to their arm ratio [41], separated into groups based on their relative lengths [9], and numbered as in the case of the S. gouazoubira (SGO) karyotype [24].
2.4. Fluorescence In Situ Hybridization
Fluorescence in situ hybridization was performed by applying cattle whole chromosome painting (WCP) probes to the karyotype of the Mazama cita topotype. We determined the homologies between the karyotype of M. cita topotype and that of S. gouazoubira, which retains the ancestral karyotype of the Cervidae [24]. For the preparation of probes, whole cattle chromosomes were isolated either by flow cytometry, using the MoFlo XDP Cell Sorter (Beckman Coulter, Brea, CA, USA), or by microdissection, using the PALM Microlaser system (Carl Zeiss MicroImaging GmbH, Munich, Germany), as described in Frohlich et al. [42]. Both techniques were employed to overcome the challenges associated with flow cytometry, particularly in distinguishing certain acrocentric autosomes during FISH probe preparation, as they tend to form groups in the flow karyogram. To address this issue, laser microdissection, an alternative and reliable method, was used to prepare probes specific for these chromosomes. The amplification was performed using the degenerate oligonucleotide primed polymerase chain reaction (DOP-PCR) [43] and probe labeling during the second PCR with Green-dUTP or Orange-dUTP (Abbott Park, IL, USA) [44]. Five BAC clones were selected from the CHORI-240 library based on the NCBI ARS-UCD1.2 assembly data obtained from BACPAC Genomics (Emeryville, CA, USA) (Table S3). For DNA extraction, we used a protocol adapted from the method outlined for the Wizard® Plus SV Minipreps DNA Purification Systems. BAC DNA was labeled with Green-DdUTP (Abbott, IL, USA), biotin 16-dUTP, or digoxigenin-11-dUTP (Roche, Mannheim, Germany) using BioPrime® Array CGH Genomic Labeling (Invitrogen, Carlsbad, CA, USA). The selected BAC clones were selected to be distributed along p- and q-arms of the submetacentric X chromosome of cattle. For the pseudo-autosomal region (PAR) probe, two BAC clones were selected and used together as a single probe. FISH was performed as described in Vozdova et al. [45]. A Zeiss Axio Image Z2 (Carl Zeiss Microimaging GmbH, Jena, Germany) fluorescence microscope equipped with appropriate fluorescence filters for the visualization of FISH results was used.
2.5. DNA Extraction and Sequencing
Genomic DNA extraction from tissue samples of Mazama cita topotype was performed using the Qiagen DNeasy® Blood Qiamp Tissue kit following the manufacturer’s instructions. Approximately 1 μg of DNA from each specimen was sent to NGS Soluções Genômicas (Piracicaba, São Paulo, Brazil for massive parallel DNA sequencing. First, the DNA samples were fragmented by Covaris sonication to an average size of 300 base pairs. Then, these fragmented DNA samples were used as input for genomic library preparation using the TruSeq Nano DNA sample preparation kit (Illumina, USA), following the manufacturer’s instructions. All genomic libraries were pooled and sequenced on the Illumina HiSeq 2500 Platform (Illumina, San Diego, CA, USA) using 100 bp paired-end sequencing. Unmerged reads were aligned to the S. gouazoubira mitogenome (GenBank accession number KJ772514) as a reference by using Bowtie2 v2.4.1 [46] on the Galaxy online server [47]. Annotation was performed on the MITOS [48] web-based platform (http://mitos.bioinf.uni-leipzig.de/index.py, accessed on 1 July 2022) and manually adjusted based on comparisons to the reference mitogenome using GENEIOUS (Biomatters; http://www.geneious.com/, accessed on 5 July 2022). To carry out phylogenetic analyses, we downloaded from the GenBank mitogenome sequences of S. gouazoubira, P. nemorivagus, Mazama americana, M. jucunda, M. nana, M. rufa, M. temama, Blastocerus dichotomus, Hippocamelus antisensis, Odocoileus virginianus, Ozotoceros bezoarticus, and Pudu puda, and used the sequence of Alces alces as the outgroup (see Table 1 for details).

Table 1.
Mitochondrial gene sequences used for phylogenetic analysis.
We also extracted genomic DNA from hair and muscle samples of two gray brocket deer from northwestern Venezuela identified as M. cita using a modified protocol based on the methodology described by Sambrook et al. [53]. The partial cytochrome b (Cytb 1040 bp) fragment was amplified, and the PCR products were purified with the Wizard SV gel and PCR Clean-Up System kit (Promega TM, Madison, WI, USA). This was followed by sequencing using the ABI BigDye Terminator kit (Applied Biosystems, San Francisco, CA, USA) in an ABI 3130xl automatic sequencer (Applied Biosystems). Thereafter, we aligned with other sequences for Neotropical deer available in the GenBank (Table 1).
2.6. Alignment and Bayesian Phylogenetic Inference
Both datasets, complete mitogenome and Cytb gene, were aligned separately using the Clustal X program [54]. For the mitochondrion genome, we excluded the control region from the alignment because of the tandem repeats located inside them and the high incidence of potential homoplasy. The optimal partitioning scheme was selected by means of the Partitionfinder 2.0 tool [55] on the CIPRES Science Gateway [56] based on the Bayesian information criterion. For the Cytb data set, we selected the best molecular evolution model using the jModelTest v. 0.1.1 [57], following the corrected Akaike information criterion, AICc [58].
Bayesian inference (BI) was performed using the multiple four chain Metropolis-coupled analysis (with default heating) available in MRBAYES 3 [59], with 10,000,000 generations and sampling every 1000 generations, until a variance of <0.01 was obtained. The first 25% of the sampled trees was discarded as burn-in, and the remaining trees were used to construct a 50% majority-rule consensus tree. The consensus tree was edited using FigTree v.1.4.0 [60].
3. Results
3.1. Morphological Description
The topotype of Mazama cita Osgood, 1912 has a general grayish-brown coloration (light brown laterally and whitish ventrally). The dorsal region of the body, head, and neck are dark brown, gradually paler, and with yellowish hairs in the submandibular region. There is a white-yellowish nasal patch, a dark lateral rostral band, an inferior orbital band with yellowish hairs, and a superior orbital band with a pale yellowish coloration that extends in the direction of the rostral band. The base of the ears and the anterior auricular region are whitish. The posterior auricular border is dark brown. Long white hairs are present on the inner auricular surface. The outer auricular surface is cinnamon brown. There is a pale brown mental patch, a grayish mandibular band, and a yellowish buccal region and gular patch. A tuft with dark brown and yellowish hairs is observed in the frontal region of the head. The opening of the preorbital pouch is pale brown. The front legs are grayish-brown in the proximal lateral region, pale brown in the distal lateral region, and whitish in the ventral region. Proximally, the hind legs are grayish-brown in the lateral region and whitish in the ventral region. Distally, the hind legs are dark brown both in the lateral and ventral region. A white spot on the tarsal gland is absent. The tail is white below and pale cinnamon above. The inguinal region is whitish. Short fine hairs cover the whole body. The auditory bulla is small and flattened. Two separate lacrimal holes are present in the orbit. The supraorbital foramen is short and deep. The lacrimal fossa is deep. The preorbital fossa has a triangular and elongated shape (Figure 1 and Figure 2).
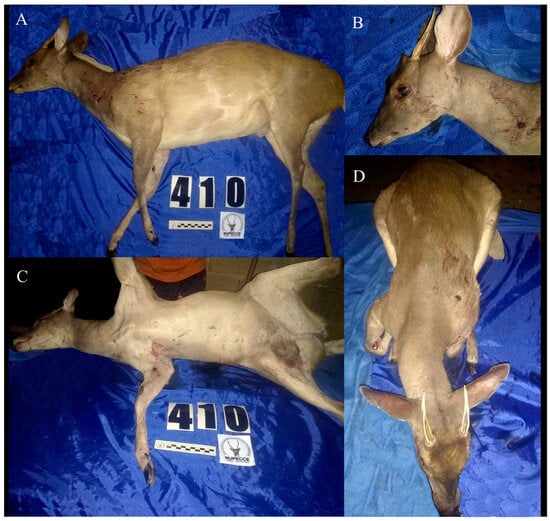
Figure 1.
Male topotype of Mazama cita (Osgood, 1912) collected in El Consejo de Ciruma, Zulia, Venezuela. (A) Lateral view of the body. (B) Close up of the head. (C) Ventral view of the body. (D) Dorsal view of the body.
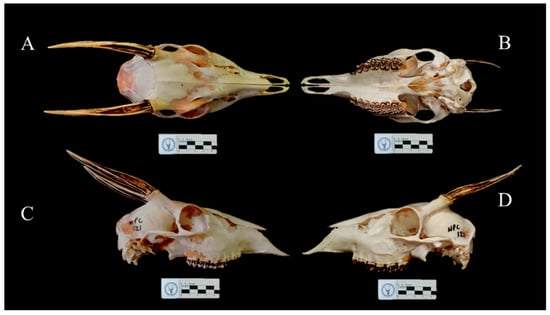
Figure 2.
Skull of the male topotype of Mazama cita (Osgood, 1912) collected in El Consejo de Ciruma, Zulia, Venezuela (A) Dorsal view. (B) Ventral view. (C) Right lateral view. (D) Left lateral view.
Locality: El Consejo de Ciruma, eastern shore of Lake Maracaibo, Zulia state, Venezuela.
Coordinates: 10°29′33.3″ N, 71°08′46.6″ W.
Specimen housed in: Colección de Vertebrados de la Universidad de Los Andes, Mérida, Venezuela (CVULA).
Catalog number: CVULA 9109 (full skeleton and skull, taxidermized skin).
Tissue sample deposited in: NUPECCE Tissue and Cell banking. TESE 410.
DNA sequence Deposit Number: OQ198443 (complete mitochondrion).
Karyotype: 2n = 61/FN = 70.
The topotype of M. cita had a body length of 850 mm, a skull length of 189.39 mm, a condylobasal length of 185.60 mm, a basal length of 166.29 mm, a zygomatic breadth of 81.27 mm, and a cheek tooth row length of 58.16 mm.
3.2. Cytogenetic Description
The Giemsa-stained karyotype of the M. cita (MCI) topotype showed a diploid number (2n) of 61, a fundamental number (FN) of 70, 30 pairs of autosomal chromosomes, and a simple XY sexual system (Figure 3). The X chromosome was submetacentric, and the Y chromosome was a small metacentric. The chromosomal pair MCI1 was included in group A (large biarmed autosome), the chromosomal pairs MCI2 to 4 were included in group C (small biarmed autosomes), and all acrocentric chromosomes were included in group E. The presence of supernumerary chromosomes (Bs) was observed as a variable from 0 to 5 in different metaphases.
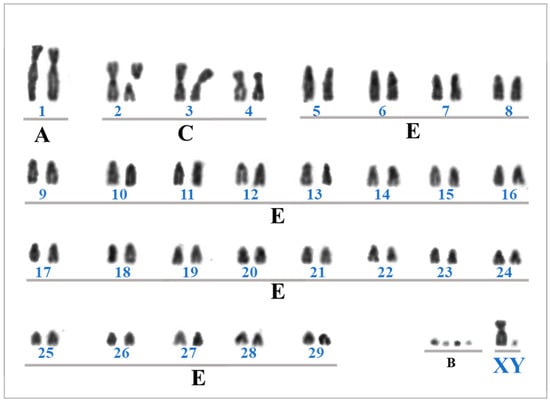
Figure 3.
Conventional Giemsa-stained karyotype of the male topotype of Mazama cita (Osgood, 1912). Groups of chromosomal relative lengths: A—large biarmed autosome; C—small biarmed autosomes; E—small acrocentric autosome; B—Supernumerary chromosomes; XY—Simple sexual system. Number of each chromosomal pair from 1 to 29.
The Ag-NOR stained karyotype shows nucleolus organizing regions in the telomeric area of the longer arms (q-arm) of the two chromosomes of pair 1 and in the telomeric region of the q-arm of the two chromosomes of pair 7 (Figure 4). C-banding revealed constitutive heterochromatin blocks in the pericentromeric region of all autosomes and in the X chromosome (Figure 4).
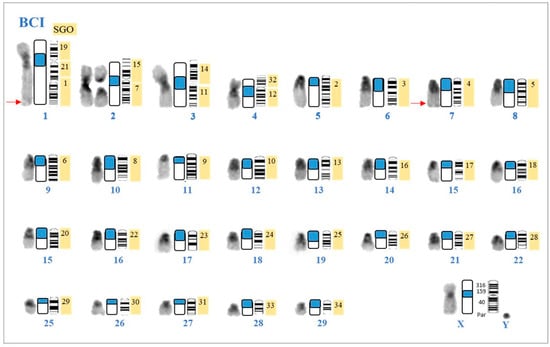
Figure 4.
Karyotype of a male topotype of Mazama cita (Osgood, 1912) (MCI) and homologies with the karyotype of Subulo gouazoubira (SGO). Left to right: C-band, schematic representation of C-band and G-band of MCI, and homology with Subulo gouazoubira SGO [24], adapted. Localization of Ag-NOR staining is indicated by arrows. Positions of bovine BAC probes on the MCI X chromosome are shown. PAR corresponds to pseudo-autosomal region probe, comprising clones BAC 453C5 and 326B13. Number of each chromosomal pair from 1 to 29.
The bovine WCP chromosomal painting demonstrated that M. cita (MCI) (2n = 61 and FN = 70) underwent chromosomal rearrangements with respect to the hypothetical ancestral deer karyotype retained by Subulo gouazoubira (2n = 70 and FN = 70) (Figure S1). The q-arm of the large submetacentric MCI1 was the result of tandem fusion of the chromosomes corresponding to SGO21 and SGO1 (BTA5 proximal region and BTA3). The shorter arm (p-arm) of the MCI1 corresponding to SGO19 (BTA17) was fused by centric fusion with the q-arm. The MCI2 was heterozygotic for a centric fusion involving SGO15 (BTA19) and SGO7 (BTA7) (Figure 4). We also observed a homozygotic centric fusion in MCI3 corresponding to SGO 14/11 (BTA21/16) and a homozygotic centric fusion in MCI4 corresponding to SGO 32/12 (BTA25/13), which formed the largest chromosomes in group C (Figure 4). The X chromosome of the M. cita topotype showed a submetacentric morphology with a centromeric shift when compared to the acrocentric X chromosome of S. gouazoubira, in which the proximal region of SGO X was inverted in the p-arm of the MCI X, as shown by the hybridization pattern of BAC clones. The q-arm preserved the same hybridization pattern as those shown for the distal region of the X chromosome in SGO (Figure 4).
3.3. Phylogenetic Analysis
The BI tree of the mitogenomes (Figure 5A) shows M. americana, M. rufa, M. nana, M. jucunda, M. temama, and O. virginianus as members of the large clade known as the Odocoileina subtribe, with a 100% posterior probability. The topotype of M. cita was recovered within the Blastocerina subtribe (100% pp) and was the first lineage that diverged in the tree, followed by P. puda. Also, in the Blastocerina clade, P. nemorivagus was recovered as a monophyletic species having B. dichotomus as its sister taxon. The monophyletic O. bezoarticus formed a separate clade together with H. antisensis and S. gouazoubira as sister taxa. A similar result (two main clades) was obtained with the partial Cytb BI tree (Figure 5B). The first corresponded to the Odocoileina subtribe (100% pp) and included M. americana, M. jucunda, M. nana, O. virginianus, M. rufa, and M. temama. The other corresponded to the Blastocerina subtribe, including M. cita, P. nemorivagus, S. gouazoubira, B. dichotomus, and O. bezoarticus. This clade was divided into three subclades, the M. cita subclade (100% pp), the monophyletic P. nemorivagus subclade (100% pp), and the monophyletic S. gouazoubira subclade (97% pp), which also included B. dichotomus and O. bezoarticus as successive sister taxa. The topotype of M. cita and the two specimens from the northwestern Venezuelan state of Falcon were the first branch that diverged within the Blastocerina subtribe, forming a separate monophyletic clade (100% pp). Within the M. cita clade, the individuals from Paraguana Pensinsula (Falcon state) were more closely related to each other than to the topotype from Zulia state. The P. nemorivagus clade was split into two clades with strong support, one of them formed by gray brockets from Bolivar state in Venezuela south of Orinoco, Guyana, and French Guiana (98% pp), and separated from the other clade, including brockets from Peru and Brazil (100% pp).
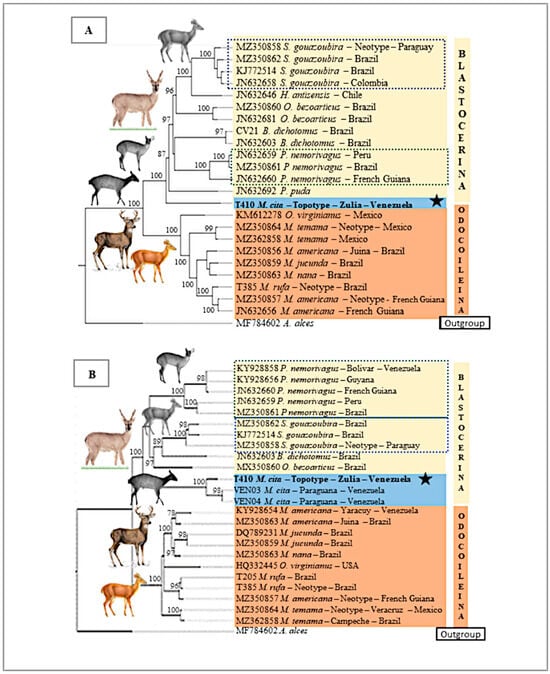
Figure 5.
Bayesian Inference (BI) of the mtDNA of several species of Neotropical deer. (A) Complete mitochondrion. (B) Partial Cytb 1040 bp. The values above clade nodes represent posterior probabilities. Blastocerina subtribe (light yellow square). Mazama cita (Osgood, 1912) clade (blue), topotype (black star). Odocoileina subtribe (melon red square). S. gouazoubira (blue dashed lines). P. nemorivagus (green dashed line). Outgroup, A. alces.
4. Discussion
4.1. Morphological Characterization
The description of the gray brocket deer from northwestern Venezuela, Mazama cita, is based on morphological features deemed unique of the type series [27] in comparison with other gray brockets. The pale brownish and yellowish-gray coloration of the topotype matches the previous description of M. cita, with the only exception of the absence of a white spot on the tarsal gland, which is present in the holotype. However, this character has been reported to be variable in other brocket species, such as S. gouazoubira [24,61]. The general coloration of M. cita, together with the yellowish spot over its eyes and the white color around the muzzle, have been proposed to be useful characters to distinguish the species from other gray brockets [4]. However, other authors have considered these characters to be variable, arguing that body and skull size are more important than coloration to differentiate the species [32,62]. The body and skull measurements of the topotype are close to those published for the gray brockets from northern Venezuela [4,27,32]. This is especially true of the greatest length of the skull, greatest nasal length, maxillary toothrow, zygomatic breadth, width of head, height at shoulder, and chest circumference.
Most measurements are greater in the M. cita topotype than reported [24,26] for other gray brockets. Owing to the availability for morphometric analyses of a single specimen of M. cita, we could not statistically test the difference. However, supporting this result, Bisbal [32], despite deeming both taxa morphometrically indistinguishable, provided measurements showing that, cranially, “M. gouazoubira cita” is larger than “M. gouazoubira nemorivaga”. Our measurements, considered together with those of Osgood [27] and Bisbal [32], suggest that M. cita is indeed larger than P. nemorivagus and S. gouazoubira, both in body and skull size. Tate [28] already assumed that this is the case by assigning M. cita to his large-brocket deer group. A descriptive study of specimens from localities across northern Venezuela is needed for a better morphometric diagnosis of M. cita.
4.2. Cytogenetic Description
The cytogenetic characteristics of the gray brockets S. gouazoubira and P. nemorivagus have been reported by several authors [24,25,26]. Neitzel [63] was the first to describe the karyotype of S. gouazoubira as possessing a 2n and an FN both equal to 70, which was recently corroborated based on topotypes found to possess a 2n = 69 and an FN = 70 owing to the presence of a centric fusion in a heterozygous state [24]. Karyotypical studies of P. nemorivagus from Brazil showed a 2n range from 67 to 69 and an FN = 70, with a submetacentric X chromosome unlike the acrocentric X chromosome of S. gouazoubira [26]. A P. nemorivagus collected in the type locality in French Guiana had a 2n = 69 and an FN = 72 karyotype, with a submetacentric X chromosome [26]. In contrast, our cytogenetic results for the gray brocket topotype collected in northwestern Venezuela show a 2n = 61 and an FN = 70 karyotype, with 2 submetacentric pairs, 1 metacentric pair, 1 heterozygotic fusion, and 25 acrocentric pairs. We uncover a simple sexual system, with submetacentric X and metacentric Y chromosomes.
Subulo gouazoubira retains the hypothetical ancestral karyotype of the Cervidae [64]. Fluorescence in situ hybridization (FISH) shows the karyotype of the M. cita topotype to share the fusion in heterozygosis between autosomes 7 and 15 reported for S. gouazoubira [24]. However, such a karyotype differs from that of the latter species by showing tree-centric fusions, one tandem fusion in the autosomal chromosomes, and an X chromosome with a submetacentric morphology as opposed to an acrocentric morphology. Interestingly, the X chromosome of the M. cita topotype is similar to the X chromosome of Capreolus capreolus and P. nemorivagus, with the same order and position of each X probe used in the proximal region and in the qproximal and qdistal regions [26,42]. The karyotypes of the topotype of M. cita and the individual of P. nemorivagus from French Guiana [26] show the same difference in fusions with respect to the karyotype of S. gouazoubira, except for the absence of the fusion in heterozygosis corresponding to BTA7 and BTA19 absent in P. nemorivagus. Conversely, the individual from French Guiana has a heterozygous fusion corresponding to BTA7 and BTA23 not observed in M. cita and S. gouazoubira.
The substantial karyotypic differences between M. cita and the other gray brockets S. gouazoubira and P. nemorivagus could be deemed efficient reproductive barriers should their populations be sympatric [65,66,67], which they are not. Therefore, based on the biological species concept, we confirm that M. cita is a distinct species from S. gouazoubira [24] and P. nemorivagus [26]. Moreover, we note that the differentiated diploid number of M. cita is highly unique for the taxon in the light of the findings of chromosomal studies involving other brockets, such as M. temama, M. rufa, and M. americana [9,18,68].
4.3. Phylogenetic Analysis
Mitochondrial DNA has been widely used to reconstruct the phylogenetic relationships of Neotropical deer [49,50,69,70]. Studies based on cytochrome b and the complete mitogenome have proven to be efficient in recovering relationships and enhancing the understanding of the evolutionary history of this complex group [7,8,10]. Our BI analyses of mtDNA confirm the findings of previous studies with respect to the polyphyly of Mazama and support the conclusion that different or new generic names are needed for some members of the Blastocerina subtribe [7,24].
Notwithstanding the limited number of mitogenome sequences available for Neotropical deer, based both on the Cytb and complete mitogenome trees, the Osgood´s gray brocket topotype is recovered as a separate monophyletic lineage with respect to the rest of the Blastocerina, thus M. cita can be inferred to have diverged early in the course of the evolutionary radiation of the subtribe. The Cytb tree, including sequences of additional M. cita individuals, confirms this relationship by showing the gray brocket specimens from northwestern Venezuela forming a monophyletic clade apart from all other Blastocerina. Clearly, M. cita does not share exclusive common ancestors with the other gray brockets (P. nemorivagus (type locality Cayenne, French Guiana) and Subulo gouazoubira (type locality Asunción, Paraguay)) [7,24]. Moreover, our results not only indicate that M. cita is a distinct species but also that it belongs to a separate undescribed genus.
In the Cytb analysis, the two M. cita from Paraguana Peninsula are closely related to the topotype from Zulia state in Venezuela. Based on the morphological peculiarities of one specimen of M. cita from Paraguana Peninsula when compared with samples of M. cita and P. nemorivagus from the Venezuelan mainland, Bisbal [32] raises the possibility of speciation in this population. However, he also recognizes the insufficiency of the sample size to discuss this hypothesis. As noted in other Neotropical deer studies [18,24], only an integrative review (ideally including multi-loci analyses with more samples from Paraguana and other areas of occurrence in Venezuela) may confirm this hypothesis.
4.4. Species-Level Validation of Osgood’s Gray Brocket
Taxonomic reviews based on integrative approaches have been advocated in the literature as an efficient method for taxa designation [71]. Our M. cita topotype was assessed based on the description of Osgood [27] of specimens collected near the Aurare river on the eastern shore of Lake Maracaibo, Venezuela. The morphology of the topotype agreed with the descriptions of Osgood [27] and Allen [4], which refer to a large gray brocket with a paler brown and more grayish coloration than those of the gray brockets previously described as P. nemorivagus (Cuvier, 1817) [25] and S. gouazoubira (Fischer, 1814) [22]. The cytogenetic assessment of M. cita revealed a unique karyotype when compared with other brockets, with 2n = 61 and FN = 70, a simple sexual system with a submetacentric X chromosome, and exclusive chromosomal rearrangements in the chromosome pairs 1 to 4. A mitochondrial phylogeny allocated the topotype to the Blastocerina subtribe, separated from the P. nemorivagus and S. gouazoubira clades, and also from the other Neotropical deer, including the genera Hippocamelus, Pudu, Ozotoceros, Blastocerus, and Odocoileus. A partial Cytb phylogeny with more M. cita samples from Paraguana Peninsula in Falcón state, also in northwestern Venezuela, confirmed a monophyletic clade with a high posterior probability, confirming the evolutionary separation of M. cita from other gray deer species. Thus, the morphological, cytogenetic, and phylogenetic results complement the description of the Osgood´s gray brocket and support the recognition of M. cita as a valid species within the Blastocerina.
4.5. A New Genus of Neotropical Deer
Mitochondrial phylogenies indicate that the genus Mazama, as traditionally defined, is polyphyletic since it includes at least three unrelated clades [7,8,10]. This has led to a revised taxonomy, according to which the application of this generic name is restricted to the brocket deer of the clade that includes the type species M. americana, a member of the subtribe Odocoileina [7,24]. As a consequence of this new taxonomic paradigm, it has become necessary to reassign members of the subtribe Blastocerina to other genera, as has been amply discussed by several authors [7,24]. In this context, with the support of genetic analyses, M. gouazoubira has been transferred to the resurrected genus Subulo Smith, 1827 [23,24]. Because the Amazonian brown brocket Mazama nemorivaga does not share a common ancestor with Subulo, it has recently been transferred to the revalidated genus Passalites Gloger, 1941 [7,8,24,26,72]. By monotypy, Jasper et al. [73] and Morales-Donoso et al. [26] list Cervus nemorivagus Cuvier, 1817 [25] as the type species of Passalites.
The subtribe Blastocerina also includes the genera Hippocamelus, Blastocerus, Ozotoceros, and Pudu. The recovery in our phylogeny of M. cita as the first lineage to have diverged within the Blastocerina clade indicates that this species cannot be placed in the brocket deer genus Subulo or in any other genera mentioned above. Four of the existing generic names available for Mazama (Homelaphus Gray, 1872 [74]; Doryceros Fitzinger, 1873 [75]; Nanelaphus Fitzinger, 1873 [75]; Coassus Gray, 1843 [76]) could not have been applied to the unique lineage represented by the gray brockets from northern Venezuela because they were proposed long before any material of M. cita was known to science. Only two names postdate the discovery of M. cita: Doratoceros Lydekker, 1915 [62], attributed to Fitzinger, which is a lapsus evidente for Doryceros Fitzinger, 1873 [73,75], which as we have just noted is too old a name to have been applied to M. cita; and Azarina Larrañaga, 1923 [77], type species Azarina fusca Larrañaga, 1923 [77] (based on Azara 1802 [78]), type locality Paraguay (far away from northwestern Venezuela). The genus name Mazama and Homelaphus are associated with M. americana [73]. As we have noted, M. cita has been deemed a synonym or subspecies of P. nemorivagus or S. gouazoubira [5,30,79]. The genus names Doryceros (=Doratoceros), Nanelaphus, and Coassus are associated with the latter two species [24,26], none of which share an exclusive common ancestor with M. cita in our phylogeny. Therefore, M. cita needs to be reallocated to a new monotypic genus, which we name:
Bisbalus gen. nov.
† zoobank: urn:lsid:zoobank.org:pub:59C68712-C4CA-49FB-B936-44D2243EB504
Type species by designation: Bisbalus citus Osgood, 1912.
Etymology: The new genus, a masculine noun in the genitive case, is named in honor of Francisco Javier Bisbal Enrich, 28 January 1953–13 July 2020, a Venezuelan biologist and naturalist, in recognition for his studies on the distribution, taxonomy, and biology of brocket deer (“matacán”), including the Osgood’s gray brocket, for his contributions to the knowledge of Venezuelan vertebrates, and for his lifelong dedication to the development and care of the largest zoological collection in Venezuela, which he led, housed at the Museo de la Estación Biológica Rancho Grande in Maracay.
Diagnosis: Because Bisbalus citus (Osgood, 1912), new name combination, is the only known member of the earliest diverging clade within the subtribe Blastocerina, this diagnosis is valid both for the genus and the species. B. citus possesses a convergent brocket deer morphotype. It is a large brocket, with body length greater than 850 mm and skull length greater than 180 mm. Its post-canine cheek teeth are big (total length greater than 58 mm). Its general pelage coloration is pale brownish-gray, with a yellowish spot over the eyes and white around the muzzle. Its antlers are relatively long and simple. Its karyotype is unique, with a diploid number of 60 to 62, a fundamental number of 70, a submetacentric X chromosome, and a metacentric Y chromosome.
Comparative description: The general gray body coloration differentiates Bisbalus citus from Mazama species. The yellowish spot over the eyes and white color around the muzzle distinguish the species from other gray brockets. In addition, the triangular and elongated preorbital fossa shape is a differential characteristic of B. citus compared with the inverted triangular and rectangular shapes described for Subulo gouazoubira and Passalites nemorivagus, respectively.
Included species: Bisbalus citus (Osgood, 1912). New combination.
Synonymy. Mazama americana citus refers to Cervus nemorivaga (Cuvier, 1817) (Osgood, 1912:43). Type locality eastern shore of Lake Maracaibo near to Aurare River in Venezuela.
Mazama cita cita Allen, 1915:550. Name combination [4];
Mazama simplicicornis citus Lydekker, 1915:212. Name combination [62];
Mazama gouazoubira citus Whitehead, 1994:493. Name combination [30];
Mazama nemorivaga cita Hummelink, 1940:135. Name combination 79];
Mazama gouazoubira cita Cabrera, 1961:338. Name combination [5];
Mazama gouazoubira Handley, 1976:62. Name combination [80];
Mazama gouazoubira cita Bisbal, 1991:90. Name combination [32];
Mazama cita Groves and Grubb 2011:79. Name combination [81].
5. Conclusions
In the present study, we provide morphological, cytogenetic, and phylogenetic evidence supporting Mazama americana citus, Osgood, 1912 to be a monophyletic lineage within the Blastocerina subtribe separated from other Neotropical deer, especially from other brocket deer species. Thus, we recognize the validity of this species and propose the new genus name Bisbalus, with Bisbalus citus (Osgood, 1912) as the type species. The generic description and the nomenclature rearrangement proposed here contribute to disentangling the complex taxonomic puzzle of Neotropical deer. Our description should be followed by a more complete review of the species applying the criteria of Peres et al. [18]. In this context, it is necessary to carry out a comprehensive molecular (including nuclear markers and a population approach) and cytogenetic characterization of more B. citus specimens from throughout the species’ range in Venezuela (including the Paraguaná Peninsula) and Colombia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/taxonomy4010002/s1, Figure S1: Whole chromosomes probes (WCP). (A) Homologies of chromosomess 1 to 4 between Mazama cita (MCI) and B. taurus (BTA). (B) Chromosomal correspondence between Mazama cita (BCI), Subulo gouazoubira (SGO) and B. taurus (BTA); Table S1: Body measurements of a male Mazama cita (Osgood, 1912), in centimeters (cm) following Driesch (1976); Table S2: Skull measurements of a male Mazama cita (Osgood, 1912), in millimeters (mm) following Driesch (1976); Table S3: Bovine BAC clones used for the chromosome X analysis in Mazama cita (Osgood, 1912), and their position in the cattle genome (ARS-UCD1.2 Assembly).
Author Contributions
Conceptualization, E.D.P.S. and J.M.B.D.; data curation, E.D.P.S. and J.M.; funding acquisition, M.V. and J.M.B.D.; investigation, E.D.P.S., W.J., J.M., M.V., H.C., S.K., A.M.B., and R.C.; methodology, E.D.P.S., M.V., H.C., S.K., A.M.B., and R.C.; project administration, J.M.B.D.; supervision, W.J. and J.M.B.D.; writing—original draft, E.D.P.S.; writing—review and editing, E.D.P.S., W.J., J.M., M.V., H.C., R.C., and J.M.B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) process n° 2017/07014-8 and 2019/06940-1, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code n° 001, and National Research Council (CNPq) process number 302368/2018-3.
Data Availability Statement
The data presented in this study are available in GenBank. Sequences obtained from tissue samples were recorded with the accession numbers OQ198443, OQ198444, OQ658775 and OQ658776.
Acknowledgments
We thank the Oficina Nacional de Diversidad Biológica del Ministerio de Ecosocialismo y Aguas for permission to collect the topotype. We would like to give special thanks to Carliz E. Díaz de Moreno for her help and support of this study. We thank El Consejo de Ciruma, estado Zulia, Venezuela, and Jefferson Diomar Duque Castro for assistance in the field to collect the specimen. We thank João Airton Boer for his support in the laboratory work at the NUPECCE/UNESP. We also thank Belkis Alicia Rivas Rodríguez from the University of Los Andes Vertebrate Collection (CVULA).
Conflicts of Interest
The authors declare that there are no competing interests.
References
- Eisenberg, J.F.; Redford, K.H. Mammals of the Neotropics: The Central Neotropics; University Chicago Press: Chicago, IL, USA, 1999; 609p. [Google Scholar]
- Weber, M.; Gonzalez, S. Latin American Deer Diversity and Conservation: A Review of Status and Distribution. Ecoscience 2003, 10, 443–454. [Google Scholar] [CrossRef]
- Rafinesque, C.S. New species of Mammifers. Am. Mon. Mag. 1817, 1, 363–364. [Google Scholar]
- Allen, A. Notes on American deer of the genus Mazama. Bull. Am. Mus. Nat. Hist. 1915, 34, 521–553. [Google Scholar]
- Cabrera, A. Catálogo de los mamíferos de América del Sur. Rev. Mus. Argent. Cienc. Nat. 1960, 4, 309–732. [Google Scholar]
- Czernay, S. Die Spiesshirsche und Pudus: Die Gattungen Mazama und Pudu A; Die Neue Brehm-Bucherei 581; Ziemsen: Wittenberg, Germany, 1987; 84p. [Google Scholar]
- Gutiérrez, E.E.; Helgen, K.M.; McDonough, M.M.; Bauer, F.; Hawkins, M.T.R.; Escobedo-Morales, L.A.; Patterson, B.D.; Maldonado, J.E. A gene-tree test of the traditional taxonomy of American deer: The importance of voucher specimens, geographic data, and dense sampling. ZooKeys 2017, 697, 87–131. [Google Scholar] [CrossRef] [PubMed]
- Heckeberg, N.S. The systematics of the Cervidae: A total evidence approach. PeerJ 2020, 8, e8114. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Rincón, A.; Morales-Donoso, J.A.; Sandoval, E.D.P.; Tomazella, I.M.; Mantellatto, A.M.B.; Thoisy, B.; Duarte, J.M.B. Designation of a neotype for Mazama americana (Artiodactyla, Cervidae) reveals a cryptic new complex of brocket deer species. ZooKeys 2020, 958, 143–164. [Google Scholar] [CrossRef]
- Heckeberg, N.S.; Erpenbeck, D.; Wörheide, G.; Rössner, G.E. Systematic relationships of five newly sequenced cervid species. PeerJ 2016, 4, e2307. [Google Scholar] [CrossRef]
- Erxleben, J.C.P. Systema Regni Animalis per Classes, Ordines, Genera, Species, Varietates: Cum Synonymia et Historia Animalium: Classis I. Mammalia; Imprensis Weigandianis: Lipsiae, Germany, 1777; pp. 1–764. [Google Scholar] [CrossRef]
- Thomas, O. A new deer of the brocket group from Venezuela. Ann. Mag. Nat. Hist. 1908, 1, 349–350. [Google Scholar] [CrossRef][Green Version]
- Pucheran, J. Note sur une espèce nouvelle de Cerf (Cervus rufinus Bourc. et Puch). Rev. Mag. Zool. 1851, 2, 561–565. [Google Scholar]
- Gutiérrez, E.E.; Maldonado, J.E.; Radosavljevic, A.; Molinari, J.; Patterson, B.D.; Martínez, C.J.M.; Rutter, A.R.; Hawkins, M.T.R.; Garcia, F.J.; Helgen, K.M. The Taxonomic Status of Mazama bricenii and the Significance of the Táchira Depression for Mammalian Endemism in the Cordillera de Mérida, Venezuela. PLoS ONE 2015, 10, e0129113. [Google Scholar] [CrossRef] [PubMed]
- Merriam, C.H. Seven new mammals from Mexico, including a new genus of rodents. Proc. Biol. Soc. Wash. 1901, 3, 559–563. [Google Scholar]
- Rafinesque, C.S. Description of some of the fossil teeth in a cave in Pennsylvania. Atl. J. Friend Knowl. 1832, 1, 109–110. [Google Scholar]
- Illiger, J.K.W. Uebelblick der Säugethiere nach ihrer Vertheilung über die Welttheile; Abhandlungen der Königlichen Akademie der Wissenschaften: Berlin, Germany, 1815; pp. 39–159. [Google Scholar]
- Peres, P.H.F.; Luduvério, D.J.; Bernegossi, A.M.; Galindo, D.J.; Nascimento, G.B.; Oliveira, M.L.; Sandoval, E.D.P.; Vozdova, M.; Kubickova, S.; Cernohorska, H.; et al. Revalidation of Mazama rufa (Illiger 1815) (Artiodactyla: Cervidae) as a Distinct Species out of the Complex Mazama americana (Erxleben 1777). Front. Genet. 2021, 12, 742870. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.M.B. Guia de Identificação de Cervídeos Brasileiros; FUNEP: Jaboticabal, Brazil, 1996; 14p. [Google Scholar]
- Thomas, O. The smaller south American cervidae. Ann. Mag. Nat. Hist. 1913, 11, 587. [Google Scholar] [CrossRef]
- Mantellatto, A.M.B.; González, S.; Duarte, J.M.B. Molecular Identification of Mazama Species (Cervidae: Artiodactyla) from Natural History Collections. Genet. Mol. Biol. 2020, 43, e20190008. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G. Zoognosia Tabulis Synopticis Illustrata. Typis Nicolai Sergeidis Vsevolozky. Hirsche (Cervi); Sitzungsbericht Konning Akademie Wissenschaften: Vienna, Austria, 1814. [Google Scholar]
- Smith, H.C. The seventh order of the Mammalia. The Ruminantia. In The Animal Kingdom Arranged in Conformity with Its Organization, by the Baron Cuvier, with Additional Descriptions of All the Species Hitherto Named, and Many Not before Noticed, by Edward Griffith and Others, 5th ed.; Griffith, E., Smith, C.H., Pidgeon, E., Eds.; Geo, B. Whitaker: London, UK, 1827; pp. 298–376. [Google Scholar]
- Bernegossi, A.M.; Borges, C.H.S.; Sandoval, E.D.P.; Cernohorska, H.; Svatava, K.; Vozdova, M.; Caparroz, R.; González, S.; Duarte, J.M.B. Resurrection of the genus Subulo Smith, 1827 for the gray brocket deer, with designation of a neotype. J. Mammal. 2023, 104, 619–633. [Google Scholar] [CrossRef]
- Cuvier, C.F. Dictionnarie des Sciences Naturelles; Zoologie, Mammiferes; FG Levrault: Paris, France, 1817; 534p. [Google Scholar]
- Morales-Donoso, J.A.; Vacari, G.Q.; Bernegossi, A.M.; Sandoval, E.D.P.; Peres, P.H.F.; Galindo, D.J.; de Thoisy, B.; Vozdova, M.; Kubickova, S.; Duarte, J.M.B. Revalidation of Passalites Gloger, 1841 for the Amazon brown brocket deer P. nemorivagus (Cuvier, 1817) (Mammalia, Artiodactyla, Cervidae). ZooKeys 2023, 1167, 241–264. [Google Scholar] [CrossRef]
- Osgood, W.H. Mammals from Western Venezuela and Eastern Colombia; Field Museum of Natural History, Zoology: Jena, Germany, 1912; pp. 33–68. [Google Scholar] [CrossRef]
- Tate, G. The mammals of the Guiana region. Bull. Am. Mus. Nat. Hist. 1939, 76, 151–229. [Google Scholar]
- Lydekker, R. The Deer of All Lands; Rowland Ward: London, UK, 1898; 329p. [Google Scholar]
- Whitehead, G.K. The Whitehead Encyclopedia of Deer; Swan Hill Press: Shrewsbury, UK, 1994; 493p. [Google Scholar]
- Pinder, L.; Leeuwenberg, F. Veado-catingueiro. In Biologia e conservação de Cervídeos sul-americanos: Blastocerus, Ozotoceros e Mazama; Duarte, J.M.B., Ed.; Funep: Jaboticabal, Brazil, 1997; pp. 59–68. [Google Scholar]
- Bisbal, F.J. Distribución y taxonomía del venado matacán (Mazama sp.) en Venezuela. Acta Biol. Venez. 1991, 13, 89–104. [Google Scholar]
- Bisbal, F.J. Biología poblacional del venado matacán (Mazama spp.) (Artiodactyla: Cervidae) en Venezuela. Rev. Biol. Trop. 1994, 42, 305–313. [Google Scholar]
- Hershkovitz, P. Neotropical deer (Cervidae): Part I. Pudus, Genus Pudu Gray; Fieldiana Zoology, New Series, No. 11; Field Museum of Natural History: Chicago, IL, USA, 1982; 86p. [Google Scholar] [CrossRef]
- von den Driesch, A. A Guide to the Measurement of Animal Bones from Archaeological Sites; Peabody Museum Bulletin 1; Harvard University: Cambridge, MA, USA; Peabody Museum of Archaeology and Ethnology: Cambridge, MA, USA, 1976; 148p. [Google Scholar]
- Duarte, J.M.B.; Boer, J.A.; Sandoval, E.D.P.; Bernegossi, A.M.; Tomazella, I.M. Skin Freezing Technique for Living Cell Bank. GenProtocols. Available online: https://genprotocols.genengnews.com/protocols/skin-freezing-technique-for-living-cell-bank/1041 (accessed on 2 August 2022).
- Verma, R.S.; Babu, A. Human Chromosomes: Principles and Techniques, 2nd ed.; McGraw Hill: New York, NY, USA, 1995; 419p. [Google Scholar]
- Seabright, M.A. A rapid banding technique for human chromosomes. Lancet 1971, 1, 9971–9972. [Google Scholar] [CrossRef] [PubMed]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Howell, W.W.; Black, D.A. Controlled silver staining of nucleous organizer regions with a protective colloidal developer: A 1-step method. Experientia 1980, 36, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Levan, A.; Fredga, K.; Sandberg, A. Nomenclature for centromeric position of chromosomes. Hereditas 1964, 52, 201–219. [Google Scholar] [CrossRef]
- Frohlich, J.; Kubickova, S.; Musilova, P.; Cernohorska, H.; Muskova, H.; Vodicka, R.; Rubes, J. Karyotype relationships among selected deer species and cattle revealed by bovine FISH probes. PLoS ONE 2017, 12, e0187559. [Google Scholar] [CrossRef]
- Telenius, H.; Carter, N.P.; Bebb, C.E.; Nordenskjold, M.; Ponder, B.A.J.; Tybbackuffe, A. Degenerate oligonucleotide-primed PCR: General amplification of target DNA by a single degenerate primer. Genomics 1992, 46, 718–725. [Google Scholar] [CrossRef]
- Kubickova, S.; Cernohorska, H.; Musilova, P.; Rubes, J. The use of laser microdissection for the preparation of chromosome-specific painting probes in farm animals. Chromosome Res. 2002, 46, 143–147. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Cernohorska, H.; Fröhlich, J.; Vodicka, R.; Rubes, J. Comparative Study of the Bush Dog (Speothos venaticus) Karyotype and analysis of satellite DNA sequences and their chromosome distribution in six species of Canidae. Cytogenet. Genome Res. 2019, 159, 88–96. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: Update. Nucleic Acids Res. 2018, 46, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo Metazoan Mitochondrial Genome Annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Caparroz, R.; Mantellatto, A.M.B.; Bertioli, D.J.; Figueiredo, M.G.; Duarte, J.M.B. Characterization of the complete mitochondrial genome and a set of polymorphic microsatellite markers through next-generation sequencing for the brown brocket deer Mazama gouazoubira. Gen. Mol. Biol. 2015, 38, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.; Delsuc, F.; Ropiquet, A.; Hammer, C.; Van Vuuren, B.J.; Matthee, C.; Ruiz-Garcia, M.; Catzeflis, F.; Areskoug, V.; Nguyen, T.T.; et al. Pattern and timing of diversification of Cetartiodactyla (Mammalia; Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. C. R. Biol. 2012, 335, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Ambriz, P.; De La Rosa, X.F.; Sifuente, A.M.; Parra, M.; Villa, A.; Chassin, O.; Arellano, W. The complete mitocondrial genomes of nine subspecies of mexican White tailed deer. J. Mammal. 2015, 97, 234–245. [Google Scholar] [CrossRef]
- Seabury, C.M.; Bhattarai, E.K.; Taylor, J.F.; Viswanathan, G.G.; Cooper, S.M.; Davis, D.S.; Dowd, S.E.; Lockwood, M.L.; Seabury, P.M. Genome-wide polymorphism and comparative analyses in the white-tailed deer (Odocoileus virginianus): A model for conservation genomics. PLoS ONE 2011, 6, e15811. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Press: New York, USA, 1989. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Cognato, A.I.; Vogler, A.P. Exploring data interaction and nucleotide alignment in a multiple gene analysis of Ips (Coleoptera: Scolytinae). Syst. Biol. 1997, 50, 758–780. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environment Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Huelsenbeck, J.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, version 1.4.0. Computer Program and Documentation. University of Oxford: Oxford, UK, 2012.
- Rossi, R.V. Taxonomia de Mazama Rafinesque, 1817 do Brasil (Artiodactyla, Cervidae). Master’s Thesis, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 2000; 147p. [Google Scholar]
- Lydekker, R. Catalogue of the Ungulate Mammals in the British Museum (Natural History); British Museum: London, UK, 1915. [Google Scholar]
- Neitzel, H. Chromosome evolution in der Familie der Hirshe (Cervidae). Bongo 1979, 3, 27–38. [Google Scholar]
- Neitzel, H. Chromosome evolution of Cervidae: Karyotypic and molecular aspects. In Cytogenetics, Basic and Applied Aspects; Obe, G., Basler, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 90–112. [Google Scholar]
- Garagna, S.; Page, J.; Fernandez-Donoso, R.; Zuccotti, M.; Searle, J. The Robertsonian phenomenon in the house mouse: Mutation, meiosis and speciation. Chromosoma 2014, 123, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Lavrenchenko, L.A.; Bulatova, N.S. The role of hybrid zones in speciation: A case study on chromosome races of the house mouse Mus domesticus and common shrew Sorex araneus. Biol. Bull. Rev. 2016, 6, 232–244. [Google Scholar] [CrossRef]
- Galindo, D.J.; Martins, G.S.; Vozdova, M.; Cernohorska, H.; Kubickova, S.; Bernegossi, A.M.; Kadlcikova, D.; Rubes, J.; Duarte, J.M.B. Chromosomal polymorphism and speciation: The case of the genus Mazama (Cetartiodactyla; Cervidae). Genes 2021, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Jorge, W.; Benirschke, K. Centromeric heterochromatin and G-banding of the red brocket deer, Mazama americana temama (Cervoidea, Artiodactyla) with a probable non-Robertsonian translocation. Cytologia 1977, 42, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Purdue, J.R.; Oleksyk, T.K.; Smith, M.H. Independent occurrences of multiple repeats in the control region of mitochondrial DNA of white-tailed deer. J. Hered. 2006, 97, 235–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Escobedo-Morales, L.A.; Castañeda-Rico, S.; Mandujano, S.; León-Paniagua, L.; Maldonado, J.E. First description of the mitochondrial genomes of the Central American brocket deer Mazama temama (Kerr, 1792) and the Yucatán Peninsula brocket deer Odocoileus pandora Merriam, 1901. Mol. Biol. Rep. 2023, 50, 4851–4863. [Google Scholar] [CrossRef]
- Fujita, M.K.; Leaché, A.D.; Burbrink, F.T.; McGuire, J.A.; Moritz, C. Coalescent-based Species Delimitation in an Integrative Taxonomy. Trends Ecol. Evol. 2012, 27, 480–488. [Google Scholar] [CrossRef]
- Gloger, C.W.L. Gemeinnütziges Hand-und Hilfsbuch der Naturgeschichte. Für gebildete Leser aller Stände, Besonders für die Reifere Jugend und Ihre Lehrer; U. Schulz und Co.: Wroclaw, Poland, 1841; 160p. [Google Scholar]
- Jasper, J.G.; Lee, T.E., Jr.; Zabel, C.J.; Twohy, C.L.; Lane, K.K.; Robertson, C.S. Mazama rufina (Artiodactyla: Cervidae). Mamm. Species 2022, 54, 212–219. [Google Scholar] [CrossRef]
- Gray, J.E. Catalogue of Ruminant MAMMALIA (Pecora, Linnaeus) in the British Museum; British Museum: London, UK, 1872. [Google Scholar]
- Fitzinger, L. J Die Gattungen der Familie der Hirsche (Cervi) Nach Ihrer Natürlichen Verwandtschaft; Sitzungsbericht Konning Akademie Wissenschaften Wien; Kaiserlich-Königlichen Hof- und Staatsdruckerei: Wien, Austria, 1873; pp. 332–362. [Google Scholar]
- Gray, J.E. List of the Specimens of Mammalia in the Collection of the British Museum; British Museum: London, UK, 1843. [Google Scholar]
- Larrañaga, D.A. Escritos de D. Dámaso A. Larrañaga; Insituto Histórico y Geográfico del Uruguay: Montevideo, Uruguay, 1923; Volume 2, p. 348. [Google Scholar]
- Azara, F. Apuntamientos Para la Historia Natural de los Cuadrúpedos del Paraguay y Río de la Plata, 1y 2; Imprenta de la Viuda de Ibarra: Madrid, Spain, 1802. [Google Scholar]
- Hummelinck, W. Mammals of the genera Odocoileus and Sylvilagus: Studies on the fauna of Curaçao, Aruba, Bonaire and the Venezuelan islands. Martinus Nijhoff, The Hague. Arch. Néerl. Zool. T 1940, 4, 135. [Google Scholar]
- Handley, C.O. Mammals of the Smithsonian Venezuelan Project. Bright Young Univ. Sci. Bull. 1976, 20, 1. [Google Scholar] [CrossRef]
- Groves, C.; Grubb, P. Ungulate Taxonomy; Johns Hopkins University Press: Baltimore, MD, USA, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).