A New Species of Terrestrial-Breeding Frog, Genus Pristimantis (Anura: Strabomantidae), from the Peruvian Yungas of Central Peru †
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Research and Ethics
2.2. Morphology
2.3. Molecular Analyses
2.4. Bioacoustics
2.5. Species Delimitation
3. Results
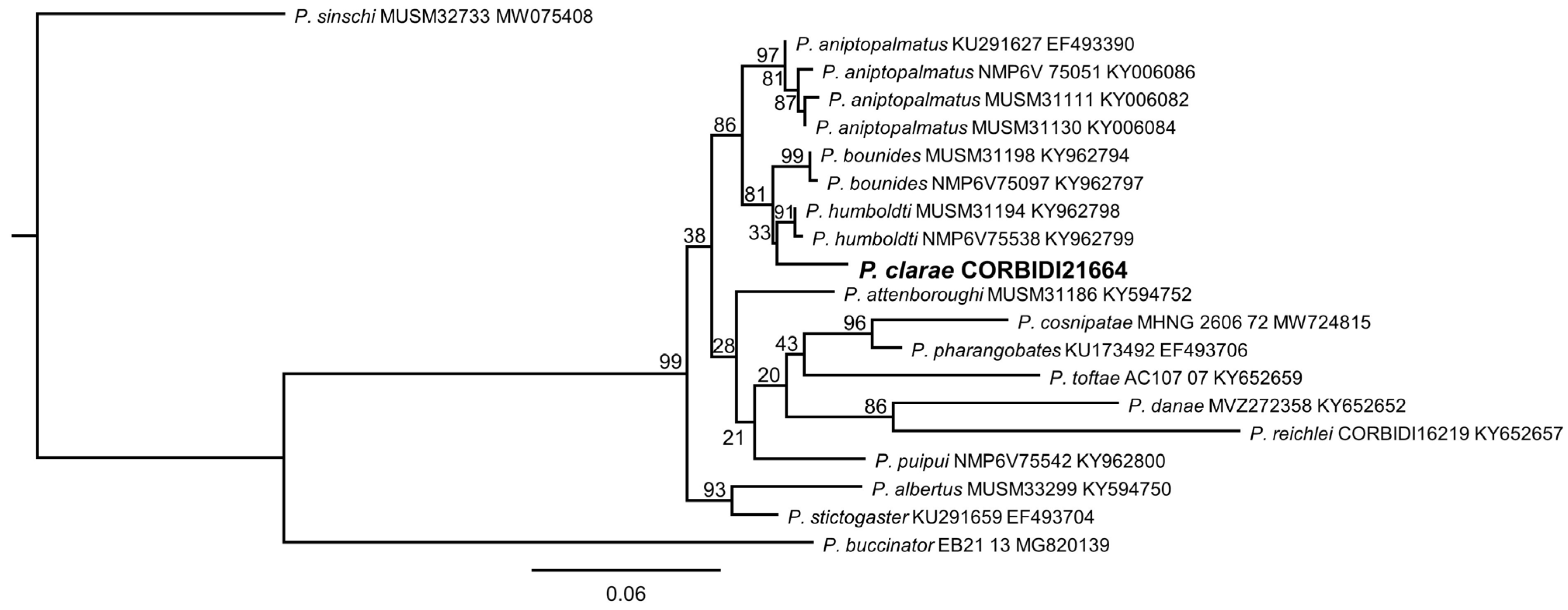
3.1. Systematics
3.2. Taxonomy
3.2.1. Holotype
3.2.2. Paratypes
3.2.3. Diagnosis
3.2.4. Comparison with Other Species
3.2.5. Description of the Holotype
3.2.6. Intraspecific Variation
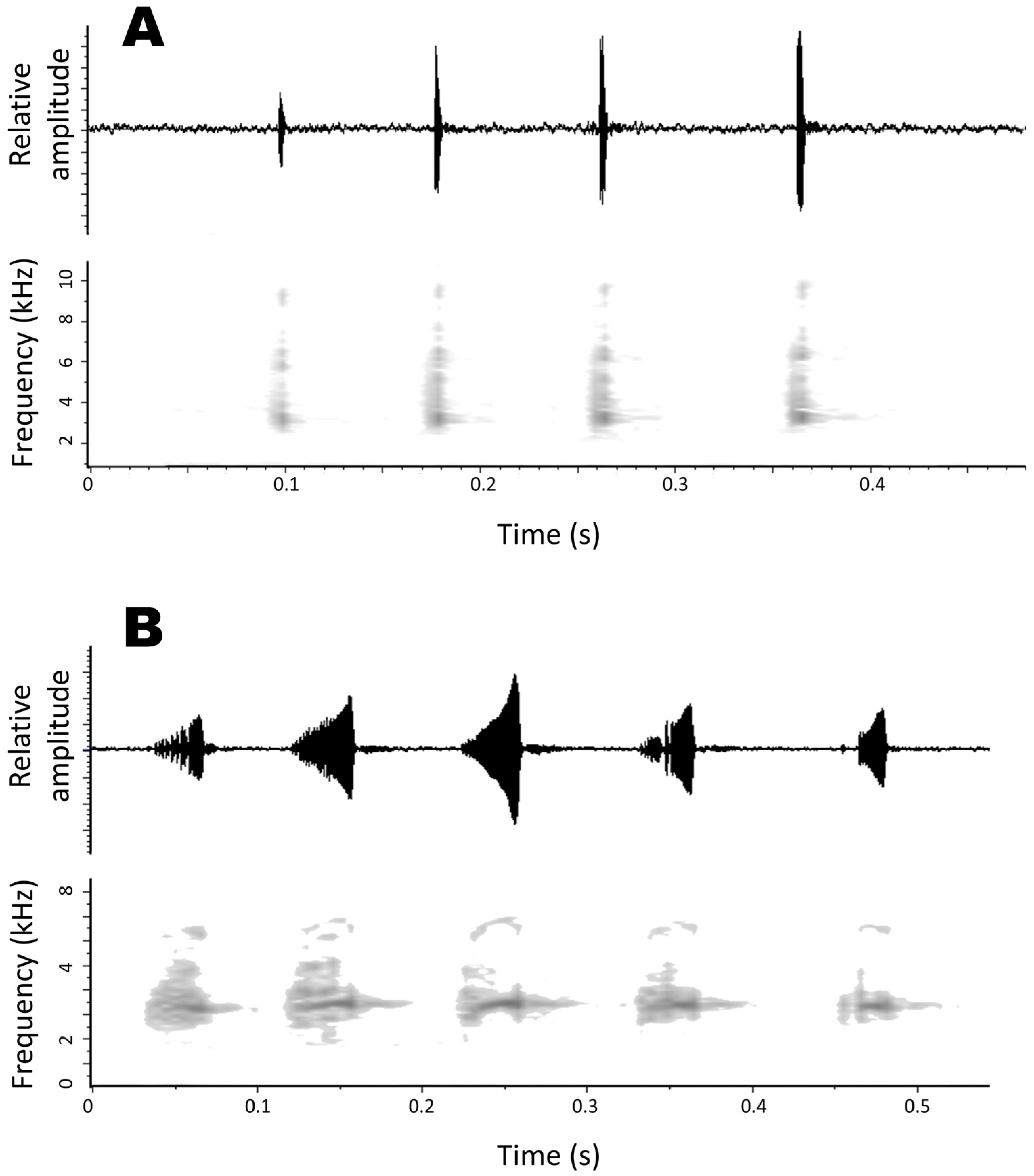
3.2.7. Advertisement Call
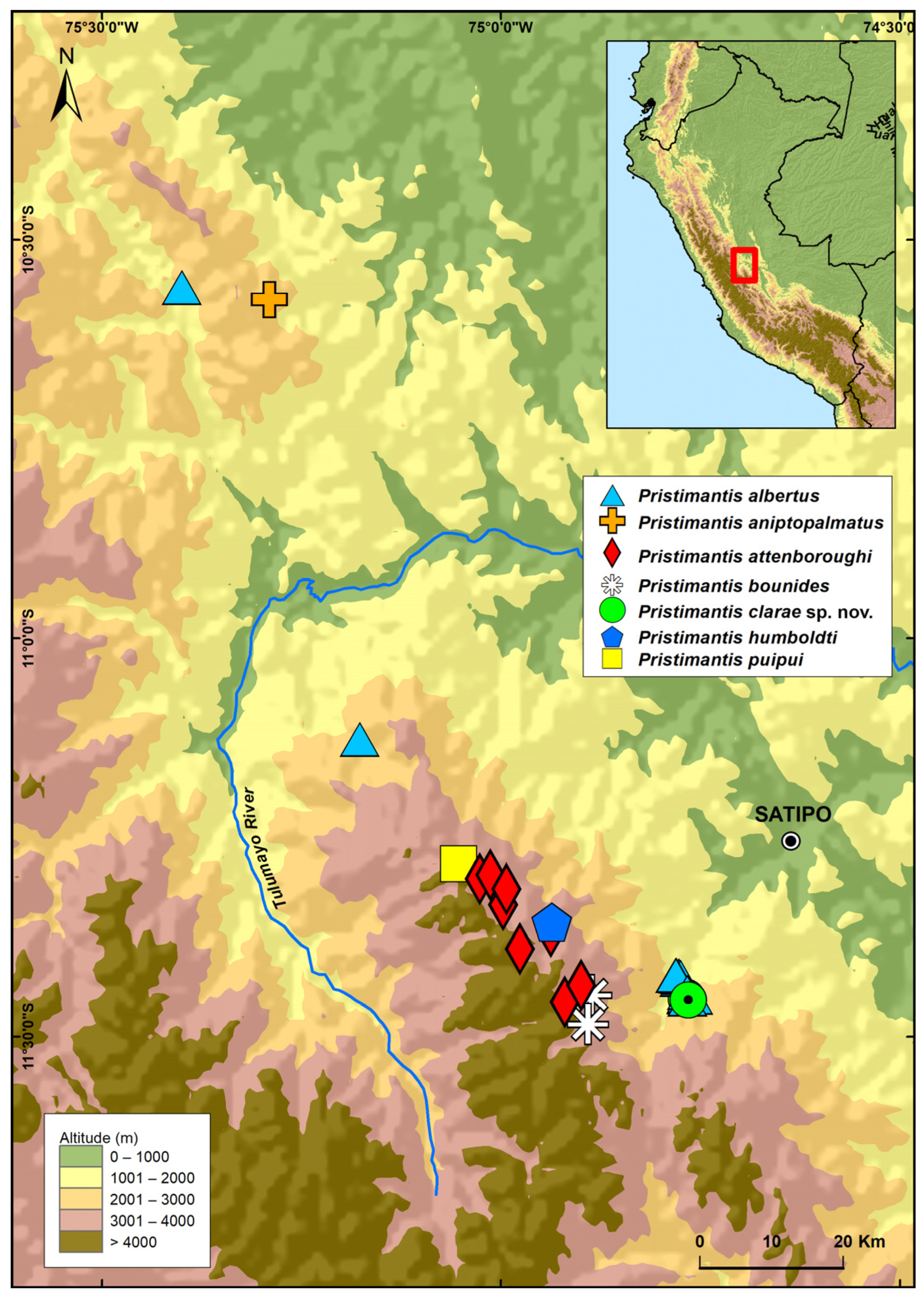
3.2.8. Distribution and Ecology
3.2.9. Etymology
3.3. Advertisement Call of Pristimantis albertus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- AmphibiaWeb. Available online: https://amphibiaweb.org (accessed on 8 February 2023).
- Duellman, W.E.; Lehr, E. Terrestrial Breeding Frogs (Strabomantidae) in Peru; Natur und Tier-Verlag GmbH: Munster, Germany, 2009; p. 382. [Google Scholar]
- Hedges, S.B.; Duellman, W.E.; Heinicke, M.P. New World direct-developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa 2008, 1737, 1–182. [Google Scholar] [CrossRef]
- Campbell, J.A.; Lamar, W.W. The Venomous Reptiles of the Western Hemisphere; Comstock Pub. Associates: Ithaca, NY, USA, 2004; 1032p, Volume 1. [Google Scholar]
- Lehr, E. Amphibien und Reptilien in Peru: Die Herpetofauna Entlang des 10. Breitengrades von Peru: Arterfassung, Taxonomie, Okologische Bemerkungen und Biogeographische Beziehungen; Natur und Tier-Velag: Munster, Germany, 2002; p. 208. [Google Scholar]
- Duellman, W.E.; Hedges, S.B. Eleutherodactyline frogs (Anura: Leptodactylidae) from the Cordillera Yanachaga in central Peru. Copeia 2005, 2005, 526–538. [Google Scholar] [CrossRef]
- Duellman, W.E.; Hedges, S.B. Three new species of Pristimantis (Lissamphibia, Anura) from montane forests of the Cordillera Yanachaga in Central Peru. Phyllomedusa 2007, 6, 119–135. [Google Scholar] [CrossRef]
- Boano, G.; Mazzotti, S.; Sindaco, R. A new peculiar frog species of the genus Pristimantis from Yanachaga-Chemillén National Park, Peru. Zootaxa 2008, 1674, 51–57. [Google Scholar] [CrossRef]
- Chávez, G.; Catenazzi, A. A new species of frog of the genus Pristimantis from Tingo Maria National Park, Huanuco Department, central Peru (Anura, Craugastoridae). Zookeys 2016, 610, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Chávez, G.; García-Ayachi, L.A.; Catenazzi, A. Beauty is in the eye of the beholder: Cruciform eye reveals new species of direct-developing frog (Strabomantidae, Pristimantis) in the Amazonian Andes. Evol. Syst. 2021, 5, 81–92. [Google Scholar] [CrossRef]
- Duellman, W.E.; Chaparro, J.C. Two distinctive new species of Pristimantis (Anura: Strabomantidae) from the Cordillera Oriental with a distributional synopsis of strabomantids in Central Peru. Zootaxa 2008, 1918, 12–25. [Google Scholar] [CrossRef]
- Lehr, E.; Aguilar, C.; Duellman, W.E. A striking new species of Eleutherodactylus from Andean Peru (Anura: Leptodactylidae). Herpetologica 2004, 60, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lehr, E.; Lundberg, M.; Aguilar, C.; Von May, R. New species of Eleutherodactylus (Anura: Leptodactylidae) from the eastern Andes of central Peru with comments on central Peruvian Eleutherodactylus. Herpetol. Monogr. 2006, 20, 105–128. [Google Scholar] [CrossRef]
- Lehr, E.; Moravec, J. A new species of Pristimantis (Amphibia, Anura, Craugastoridae) from a montane forest of the Pui Pui Protected Forest in central Peru (Región Junín). Zookeys 2017, 645, 85–102. [Google Scholar] [CrossRef]
- Lehr, E.; Moravec, J.; Cusi, J.C.; Gvoždík, V. A new minute species of Pristimantis (Amphibia: Anura: Craugastoridae) with a large head from the Yanachaga-Chemillén National Park in central Peru, with comments on the phylogenetic diversity of Pristimantis occurring in the Cordillera Yanachaga. Eur. J. Taxon. 2017, 325, 1–22. [Google Scholar] [CrossRef]
- Lehr, E.; von May, R. A new species of terrestrial-breeding frog (Amphibia, Craugastoridae, Pristimantis) from high elevations of the Pui Pui Protected Forest in central Peru. Zookeys 2017, 660, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Moravec, J.; Lehr, E.; Kodejs, K. A new species of Pristimantis (Amphibia, Anura, Strabomantidae) from the Pui Pui Protected Forest (central Peru), with comments on Pristimantis albertus Duellman & Hedges, 2007. Zookeys 2020, 994, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Lehr, E.; Von May, R.; Moravec, J.; Cusi, J.C. Three new species of Pristimantis (Amphibia, Anura, Craugastoridae) from Upper Montane Forests and High Andean Grasslands of the Pui Pui Protected Forest in Central Peru. Zootaxa 2017, 4299, 301–336. [Google Scholar] [CrossRef]
- Lehr, E.; Lyu, S.; Catenazzi, A. A new, critically endangered species of Pristimantis (Amphibia: Anura: Strabomantidae) from a mining area in the Cordillera Occidental of northern Peru (Región Cajamarca). Salamandra 2021, 57, 15–26. [Google Scholar]
- Páez, N.B.; Ron, S.R. Systematics of Huicundomantis, a new subgenus of Pristimantis (Anura, Strabomantidae) with extraordinary cryptic diversity and eleven new species. Zookeys 2019, 868, 1–112. [Google Scholar] [CrossRef]
- Venegas, P.J.; Duellman, W.E. Two syntopic new species of the Pristimantis orestes Group (Anura: Strabomantidae) from Northwestern Peru. Zootaxa 2012, 3249, 47–59. [Google Scholar] [CrossRef]
- Catenazzi, A.; von May, R. Conservation status of amphibians in Peru. Herpetol. Monogr. 2014, 28, 1–23. [Google Scholar] [CrossRef]
- Pinheiro, H.T.; Moreau, C.S.; Daly, M.; Rocha, L.A. Will DNA barcoding meet taxonomic needs? Science 2019, 365, 873–874. [Google Scholar] [CrossRef]
- Scott, N.J. Complete species inventories. In Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Heyer, W.R., Donnelly, M.A., McDiarmid, R.W., Hayek, L.C., Foster, M.S., Eds.; Smithsonian Institution Press: Washinton, DC, USA, 1994; pp. 78–84. [Google Scholar]
- Lynch, J.D.; Duellman, W.E. Frogs of the Genus Eleutherodactylus (Leptodactylidae) in Western Ecuador: Systematic, Ecology, and Biogeography; Natural History Museum, The University of Kansas: Lawrence, KS, USA, 1997; p. 236. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, K. The general lineage concept of species, species criteria, and the process of speciation. In Endless Forms: Species and Speciation; Howard, D.J., Berlocher, S.H., Eds.; Oxford University Press: Oxford, UK, 1998; pp. 57–75. [Google Scholar]
- de Queiroz, K. Species concepts and species delimitation. Syst. Biol. 2007, 56, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.R.; Kluge, A.G. A consideration of epistomology in Systematic Biology, with special reference to species. Cladistics 1994, 10, 259–294. [Google Scholar] [CrossRef]
- Padial, J.M.; Miralles, A.; De la Riva, I.; Vences, M. The integrative future of taxonomy. Front. Zool. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Crawford, A.J.; Lips, K.R.; Bermingham, E. Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. Proc. Natl. Acad. Sci. USA 2010, 107, 13777–13782. [Google Scholar] [CrossRef]
- Ortiz, D.A.; Hoskin, C.J.; Werneck, F.P.; Réjaud, A.; Manzi, S.; Ron, S.R.; Fouquet, A. Historical biogeography highlights the role of Miocene landscape changes on the diversification of a clade of Amazonian tree frogs. Org. Divers. Evol. 2022, 23, 395–414. [Google Scholar] [CrossRef]
- Reyes-Puig, C.; Mancero, E. Beyond the species name: An analysis of publication trends and biases in taxonomic descriptions of rainfrogs (Amphibia, Strabomantidae, Pristimantis). ZooKeys 2022, 1134, 73–100. [Google Scholar] [CrossRef]
- Chavez, G.; Cosmopolis, C.H.; Lujan, L. Annotated checklist and ecological notes of anurans from the southern region of Yanachaga Chemillen National Park, central Andes of Peru. Herpetotropicos 2012, 8, 23–28. [Google Scholar]


| Pristimantis clarae sp. nov. | ||
|---|---|---|
| Females (n = 2) | Males (n = 11) | |
| SVL | 17.6–19.3 | 12.9–15.6 (14.2 ± 0.8) |
| TL | 9.7–10.3 | 7.3–8.0 (7.7 ± 0.2) |
| FL | 8.3–9.0 | 6.2–7.5 (6.9 ± 0.4) |
| HL | 5.6–6.5 | 4.5–7.8 (5.4 ± 0.9) |
| HW | 6.6–6.9 | 4.9–5.8 (5.5 ± 0.3) |
| ED | 2.3–2.6 | 2.0–2.4 (2.2 ± 0.1) |
| IOD | 2.0–2.1 | 1.7–2.0 (1.8 ± 0.1) |
| EW | 0.8–1.7 | 1.3–1.5 (1.4 ± 0.1) |
| IND | 1.9–2.0 | 1.4–1.9 (1.6 ± 0.1) |
| E-N | 1.8–1.9 | 1.2–1.5 (1.4 ± 0.1) |
| TD | 0.9–1.0 | 0.9–1.2 (1.0 ± 0.1) |
| TL/SVL | 0.54–0.55 | 0.50–0.57 (0.54 ± 0.02) |
| FL/SVL | 0.46–0.47 | 0.47–0.51 (0.48 ± 0.01) |
| HL/SVL | 0.32–0.33 | 0.34–0.51 (0.38 ± 0.05) |
| HW/SVL | 0.36–0.38 | 0.37–0.42 (0.39 ± 0.02) |
| HW/HL | 1.07–1.19 | 0.72–1.11 (1.04 ± 0.11) |
| E–N/ED | 0.71–0.82 | 0.59–0.70 (0.64 ± 0.04) |
| EW/IOD | 0.38–0.84 | 0.71–0.82 (0.78 ± 0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venegas, P.J.; García-Ayachi, L.A.; Marchelie, A.; Ormeño, J.R.; Catenazzi, A. A New Species of Terrestrial-Breeding Frog, Genus Pristimantis (Anura: Strabomantidae), from the Peruvian Yungas of Central Peru. Taxonomy 2023, 3, 331-345. https://doi.org/10.3390/taxonomy3020019
Venegas PJ, García-Ayachi LA, Marchelie A, Ormeño JR, Catenazzi A. A New Species of Terrestrial-Breeding Frog, Genus Pristimantis (Anura: Strabomantidae), from the Peruvian Yungas of Central Peru. Taxonomy. 2023; 3(2):331-345. https://doi.org/10.3390/taxonomy3020019
Chicago/Turabian StyleVenegas, Pablo J., Luis A. García-Ayachi, Axel Marchelie, Jesús R. Ormeño, and Alessandro Catenazzi. 2023. "A New Species of Terrestrial-Breeding Frog, Genus Pristimantis (Anura: Strabomantidae), from the Peruvian Yungas of Central Peru" Taxonomy 3, no. 2: 331-345. https://doi.org/10.3390/taxonomy3020019
APA StyleVenegas, P. J., García-Ayachi, L. A., Marchelie, A., Ormeño, J. R., & Catenazzi, A. (2023). A New Species of Terrestrial-Breeding Frog, Genus Pristimantis (Anura: Strabomantidae), from the Peruvian Yungas of Central Peru. Taxonomy, 3(2), 331-345. https://doi.org/10.3390/taxonomy3020019







