Zamia magnifica (Zamiaceae, Cycadales): A New Rupicolous Cycad Species from Sierra Norte, Oaxaca, Mexico
Abstract
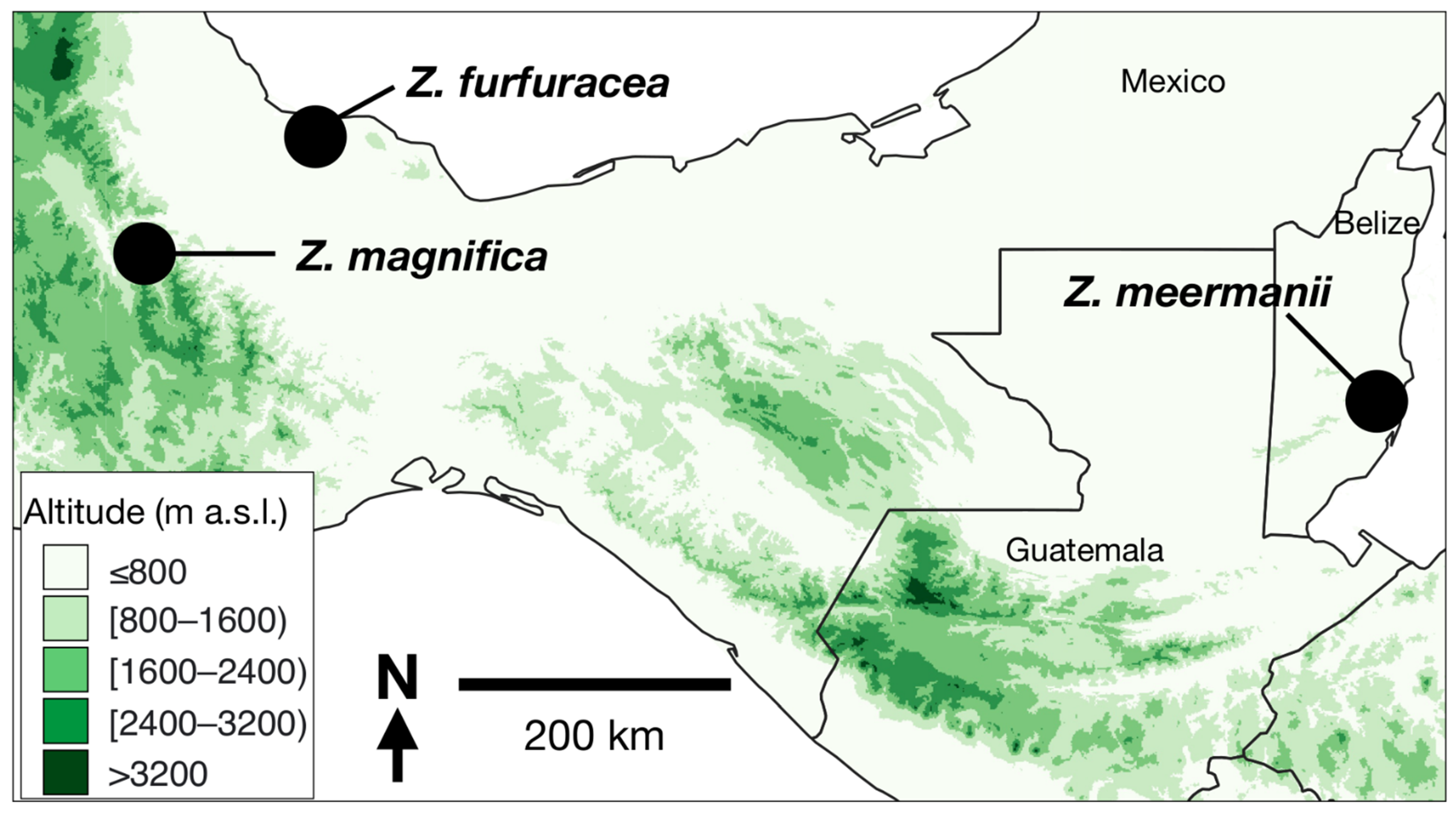
1. Introduction
2. Materials and Methods
3. Results
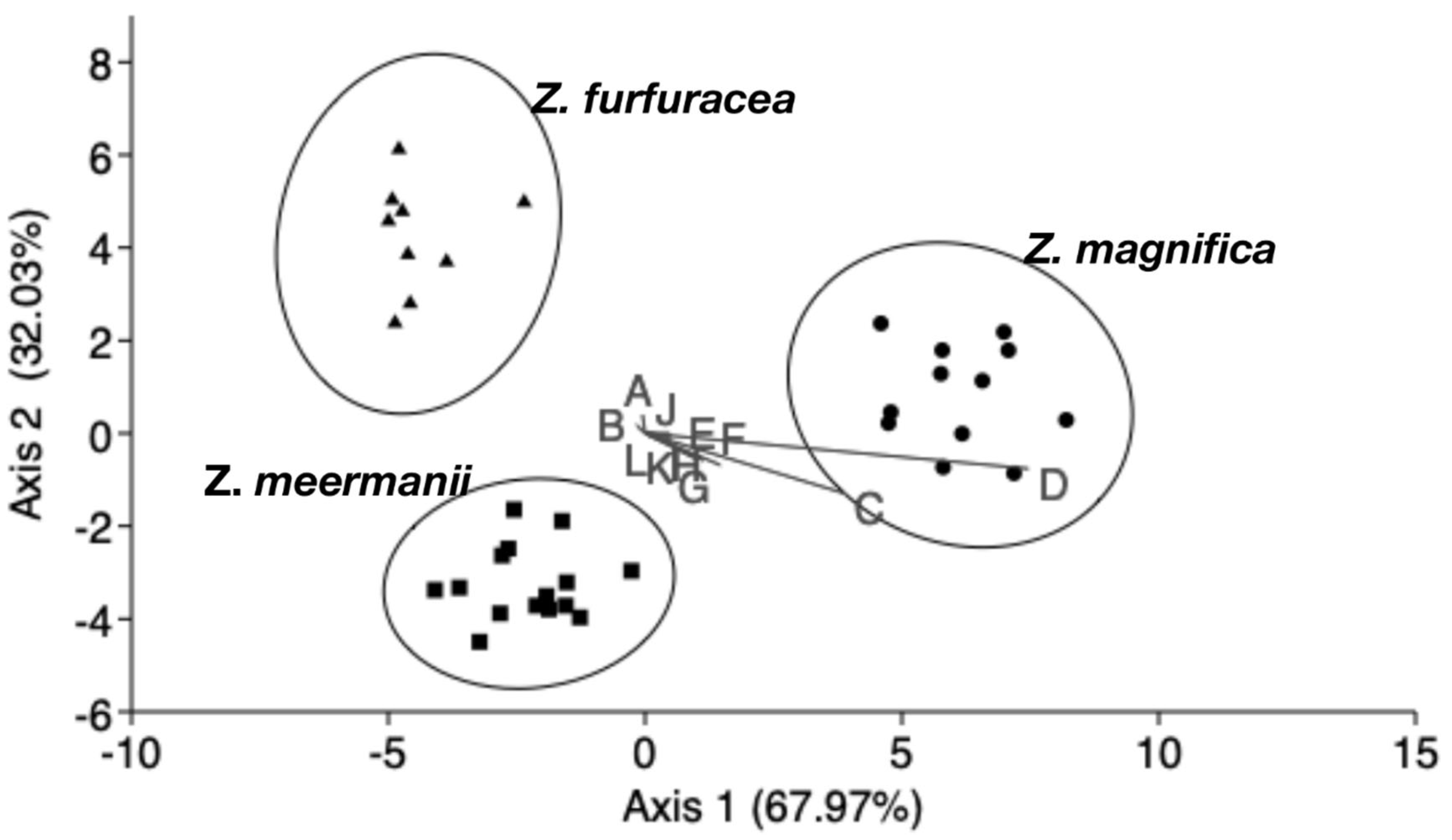
3.1. Morphometric Analysis
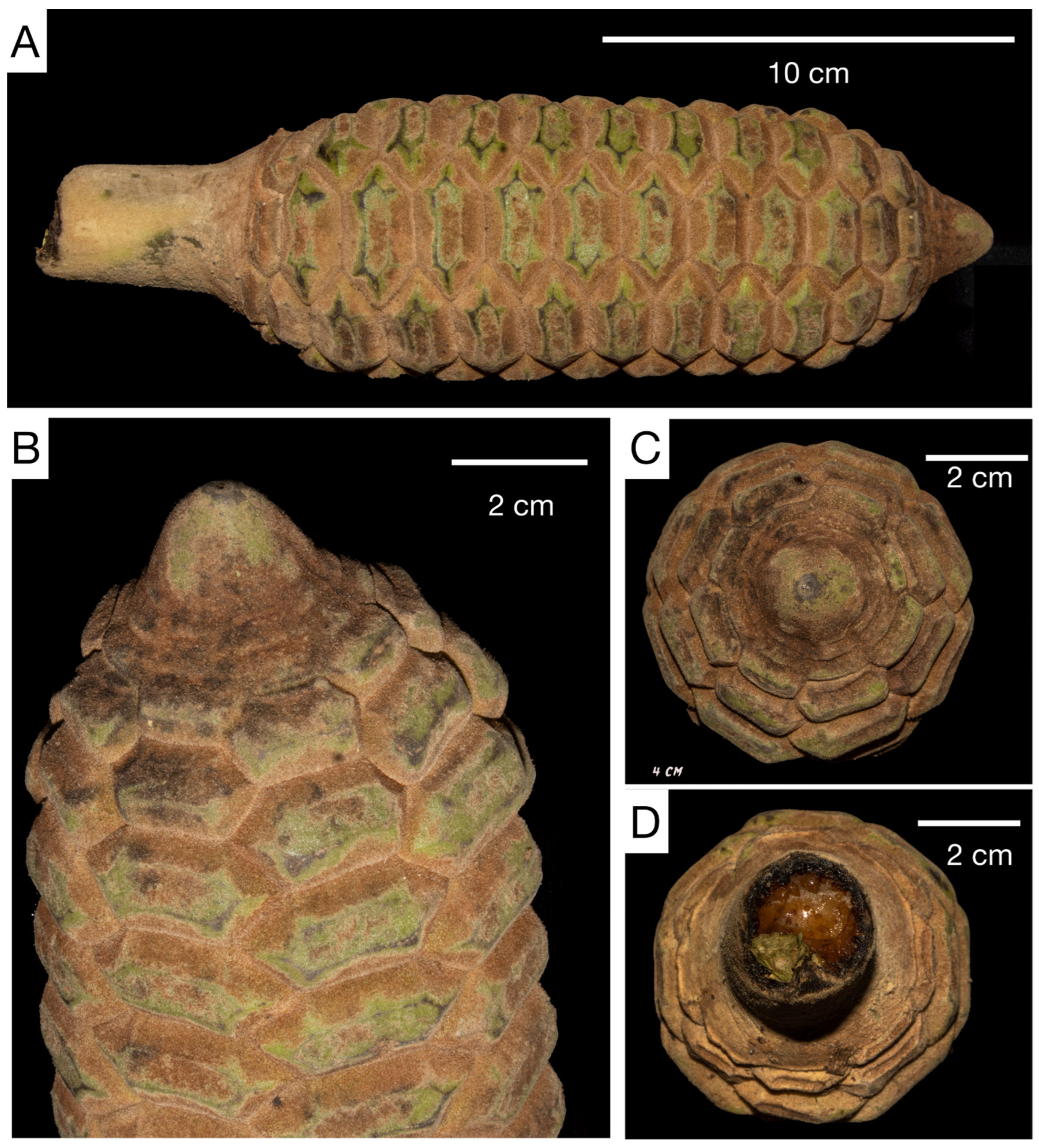
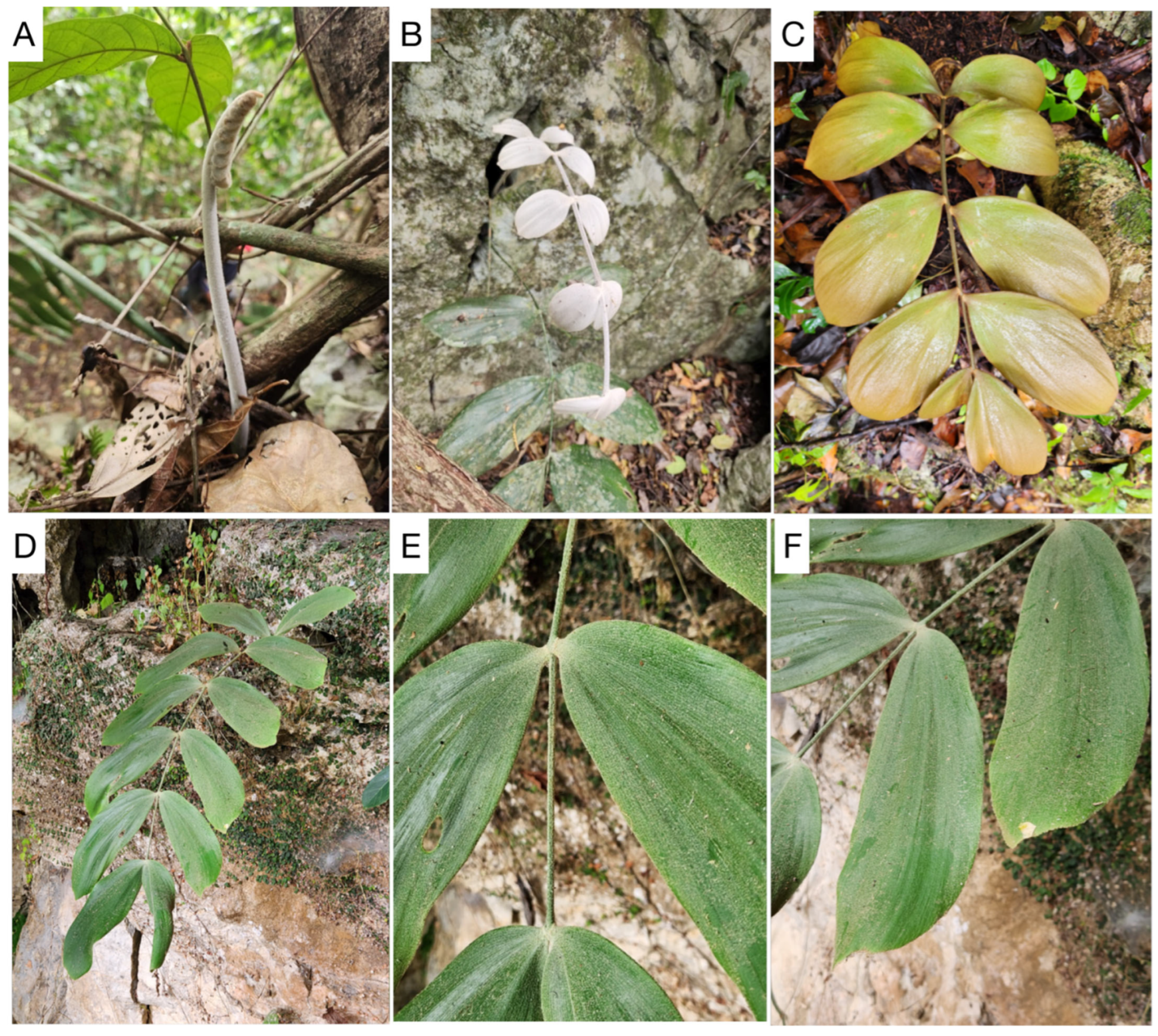
3.2. Taxonomic Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norstog, K.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997; p. 384. [Google Scholar]
- Calonje, M.; Vovides, A.P.; Gutiérrez-Ortega, J.S. An overview of cycadales in Mesoamerica and the Caribbean: Biology, distribution, and conservation. In Under the Shade of Thipaak: The Ethnoecology of Cycads in Mesoamerica and the Caribbean; Carrasco, M.D., Cibrián Jaramillo, A., Bonta, M., Englehardt, J.D., Eds.; University Press of Florida: Gainesville, FL, USA, 2022; pp. 27–61. [Google Scholar]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G.; Carmona, A.; Arana, M.; Mercado-Gómez, J.D. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Cienc. 2022, 94, e20211167. [Google Scholar] [CrossRef] [PubMed]
- Calonje, M.; Stevenson, D.W.; Osborne, R. The World List of Cycads, Online Edition. 2023. Available online: http://cycadlist.org (accessed on 23 April 2023).
- Calonje, M.; Meerow, A.W.; Griffith, M.P.; Salas-Leiva, D.; Vovides, A.P.; Coiro, M.; Francisco-Ortega, J. A time-calibrated species tree phylogeny of the New World cycad genus Zamia L. (Zamiaceae, Cycadales). Int. J. Plant Sci. 2019, 180, 286–314. [Google Scholar] [CrossRef]
- Calonje, M.; Stevenson, D.W.; Calonje, C.; Ramos, Y.A.; Lindstrom, A. A new species of Zamia from Choco, Colombia (Cycadales, Zamiaceae). Brittonia 2010, 62, 80–85. [Google Scholar] [CrossRef]
- Calonje, M.; Esquivel, H.E.; Stevenson, D.; Calonje, C.; Pava, D. A new arborescent species of Zamia from the Central Cordillera of Tolima, Colombia (Cycadales, Zamiaceae), with comments on the Z. poeppigiana species complex. Brittonia 2011, 63, 442–451. [Google Scholar] [CrossRef]
- Calonje, M.; Esquivel, H.E.; Morales, G.; Mora-Lizcano, Y.A.; Stevenson, D. A new arborescent species of Zamia (Cycadales, Zamiaceae) from the department of Huila, Eastern Cordillera of Colombia. Caldasia 2012, 34, 8. [Google Scholar]
- Calonje, M.; Betancur, J.; Lindstrom, A.; Lopez-Gallego, C.; Castro, J.; Castro, C.; Miguel Niño, S.; Barraez, D.C. Zamia orinoquiensis (Zamiaceae, Cycadales), a new species from the western Orinoquía region of Colombia. Phytotaxa 2022, 556, 119–135. [Google Scholar] [CrossRef]
- Lindstrom, A.J.; Idarraga, A. Zamia incognita (Zamiaceae): The exciting discovery of a new gymnosperm from Colombia. Phytotaxa 2009, 2, 6. [Google Scholar] [CrossRef]
- Segalla, R.; Calonje, M. Zamia brasiliensis, a new species of Zamia (Zamiaceae, Cycadales) from Mato Grosso and Rondônia, Brazil. Phytotaxa 2019, 404, 1–11. [Google Scholar] [CrossRef]
- Stevenson, D.W.; López, D.C.; Arboleda, N.C. A new Zamia (Zamiaceae) from Colombia. Brittonia 2018, 70, 364–368. [Google Scholar] [CrossRef]
- Calonje, M.; Taylor-Blake, A.S.; Stevenson, D.; Holzman, G.; Ramos, Y.A. Zamia lindleyi: A misunderstood species from the highlands of western Panama. Mem. N. Y. Bot. Gard. 2012, 106, 419–437. [Google Scholar]
- Calonje, M.; Morales, G.; López-Gallego, C.; Roldán, F. A taxonomic revision of Zamia montana and Zamia oligodonta, with notes on their conservation status. Phytotaxa 2015, 192, 279–289. [Google Scholar] [CrossRef]
- Lindstrom, A.J. Typification of some species names in Zamia L. (Zamiaceae), with an assessment of the status of Chigua D. Stev. Taxon 2009, 58, 265–270. [Google Scholar] [CrossRef]
- Nicolalde-Morejón, F.; Vovides, A.P.; Stevenson, D.W. Taxonomic revision of Zamia in mega-Mexico. Brittonia 2009, 61, 301–335. [Google Scholar] [CrossRef]
- Calonje, M. A new cliff-dwelling species of Zamia (Zamiaceae) from Belize. J. Bot. Res. Inst. Texas 2009, 3, 23–29. [Google Scholar]
- Calonje, M.; Meerman, J.; Griffith, M.P.; Hoese, G. A new species of Zamia (Zamiaceae) from the Maya Mountains of Belize. J. Bot. Res. Inst. Texas 2009, 3, 31–41. [Google Scholar]
- Pérez-Farrera, M.Á.; Vovides, A.P.; Martínez-Camilo, R.; Martínez-Meléndez, N.; Gómez-Domínguez, H.; Galicia-Castellanos, S. Zamia grijalvensis sp. nov. (Zamiaceae, Cycadales) from Chiapas, Mexico with notes on hybridization and karyology. Nord. J. Bot. 2012, 30, 565–570. [Google Scholar] [CrossRef]
- Nicolalde-Morejón, F.; Martínez-Domínguez, L.; Stevenson, D.W.; Vergara-Silva, F. Disentangling the identity of Zamia from Mexican Pacific seaboard, with a description of a new species. Nord. J. Bot. 2019, 37. [Google Scholar] [CrossRef]
- Calonje, M.; Meerman, J. What is Zamia prasina (Zamiaceae: Cycadales)? J. Bot. Res. Inst. Texas 2009, 3, 43–49. [Google Scholar]
- Pérez-Farrera, M.Á.; Vovides, A.; Ruíz Castillejos, C.; Galicia, S.; Cibrian-Jaramillo, A.; López, S. Anatomy and morphology suggest a hybrid origin of Zamia katzeriana (Zamiaceae). Phytotaxa 2016, 270, 161–181. [Google Scholar] [CrossRef]
- Gutiérrez-Ortega, J.S.; Pérez-Farrera, M.A.; López, S.; Vovides, A.P. Demographic history and species delimitation of three Zamia species (Zamiaceae) in south-eastern Mexico: Z. katzeriana is not a product of hybridization. Bot. J. Linn. Soc. 2023, boac062. [Google Scholar] [CrossRef]
- Pérez-Farrera, M.A.; Vovides, A.P.; Martinez-Camilo, R.; Martinez Meléndez, N.; Iglesias, C. A reassessment of the Ceratozamia miqueliana species complex (Zamiaceae) of southeastern Mexico, with comments on species relationships. System. Biodiv. 2009, 7, 433–443. [Google Scholar] [CrossRef]
- Pérez-Farrera, M.A.; Vovides, A.P.; Avendaño, S. Morphology and leaflet anatomy of the Ceratozamia norstogii species complex (Zamiaceae, Cycadales). Int. J. Plant Sci. 2014, 175, 110–121. [Google Scholar] [CrossRef]
- Gutiérrez-Ortega, J.S.; Jiménez-Cedillo, K.; Pérez-Farrera, M.A.; Martínez, J.F.; Molina-Freaner, F.; Watano, Y.; Kajita, T. Species definition of Dioon sonorense (Zamiaceae, Cycadales), and description of D. vovidesii, a new cycad species from northwestern Mexico. Phytotaxa 2018, 369, 107–114. [Google Scholar] [CrossRef]
- Gutiérrez-Ortega, J.S.; Pérez-Farrera, M.A.; Vovides, A.P.; Chávez-Cortázar, A.; López, S.; Santos-Hernández, N.G.; Ruíz-Roblero, S.K. Ceratozamia sanchezae (Zamiaceae): A new cycad species from Chiapas Highlands (Mexico). Phytotaxa 2021, 500, 201–216. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Breedlove, D.E. Flora of Chiapas. Part 1. Introduction to the Flora of Chiapas; California Academy of Sciences: San Francisco, CA, USA, 1981; p. 35. [Google Scholar]
- Ferrusquia-Villafranca, I. La Geología de México: Una Synopsis. In La Biodiversidad de México; Ramamoorthy, T.P., Bye, R., Lot, A., Fa, J., Eds.; UNAM, Instituto de Biología. D.F.: Mexico City, Mexico, 1998; pp. 1–107. [Google Scholar]
- INEGI [Instituto Nacional de Estadística y Geografía]. Prontuario de Información Geográfica Municipal de los Estados Unidos Mexicanos. Available online: https://www.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/07/07115.pdf (accessed on 4 May 2023).
- Toledo, V.M. Pleistocene Changes of Vegetation in Tropical Mexico. In Biological Diversification in the Tropics; Prance, G.T., Ed.; Columbia University Press: New York, NY, USA, 1982; pp. 93–111. [Google Scholar]
- Meave, J.A.; Rincón-Gutiérrez, A.; Ibarra-Manríquez, G.; Gallardo-Hernández, C.; Romero-Romero, M.A. Checklist of the vascular flora of a portion of the hyper-humid region of La Chinantla, Northern Oaxaca Range, Mexico. Bot. Sci. 2017, 95, 722–759. [Google Scholar] [CrossRef]
- Villaseñor, J.L.; Meave, J.A. Floristics in Mexico today: Insights into a better understanding of biodiversity in a megadiverse country. Bot. Sci. 2022, 100, 14–33. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación de México. 1ra Edición digital, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Limusa, Mexico. 2006. Available online: https://www.biodiversidad.gob.mx/publicaciones/librosDig/pdf/VegetacionMx_Cont.pdf (accessed on 4 May 2023).
- Rzedowski, J.; Palacios-Chávez, R. El bosque de Engelhardtia (Oreomunnea) mexicana en la región de la Chinantla (Oaxaca, México). Una reliquia del Cenozoico. Bot. Sci. 1977, 36, 93–127. [Google Scholar] [CrossRef]
- Gutiérrez-Ortega, J.S.; Salinas-Rodríguez, M.M.; Ito, T.; Pérez-Farrera, M.A.; Vovides, A.P.; Martínez, J.F.; Molina-Freaner, F.; Hernández-López, A.; Kawaguchi, L.; Nagano, A.J.; et al. Niche conservatism promotes speciation in cycads: The case of Dioon merolae (Zamiaceae) in Mexico. New Phytol. 2020, 227, 1872–1884. [Google Scholar] [CrossRef]
- Pérez-Farrera, M.A.; Gutiérrez-Ortega, J.S.; Vovides, A.P.; Calonje, M.; Díaz-Jiménez, P. Ceratozamia dominguezii (Zamiaceae): A New Cycad Species from Southeastern Mexico. Taxonomy 2021, 1, 345–359. [Google Scholar] [CrossRef]
- Glos, R.A.; Salzman, S.; Calonje, M.; Vovides, A.P.; Coiro, M.; Gandolfo, M.A.; Specht, C.D. Leaflet anatomical diversity in Zamia (Cycadales: Zamiaceae) shows little correlation with phylogeny and climate. Bot. Rev. 2022, 88, 437–452. [Google Scholar] [CrossRef]
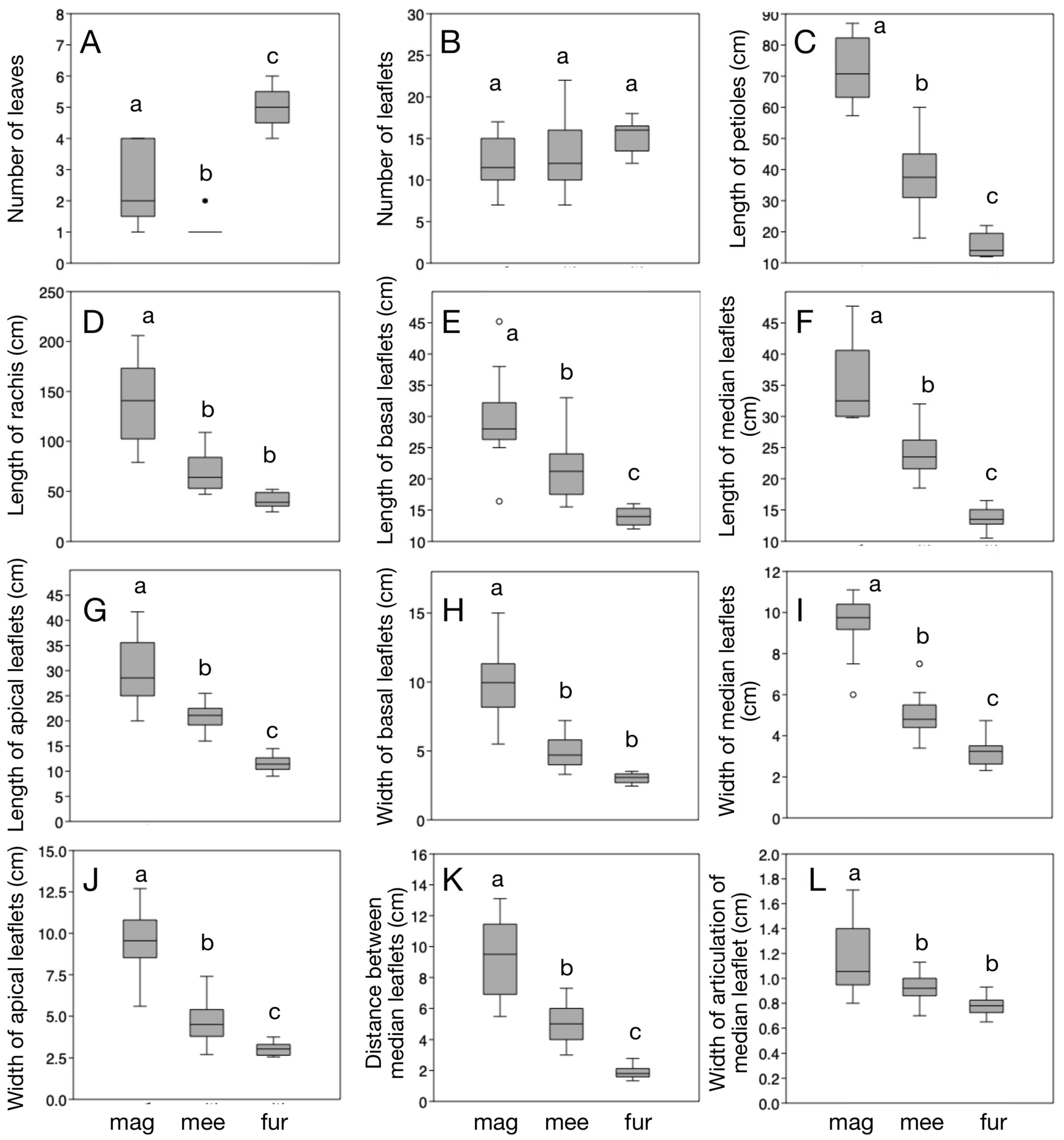





| Key | Trait | Welch’s F | D.f. | p |
|---|---|---|---|---|
| A | Number of leaves | 104.1 | 13.05 | <0.001 |
| B | Number of leaflets | 4.036 | 21.96 | <0.05 |
| C | Length of petioles (cm) | 154.6 | 20.75 | <0.001 |
| D | Length of rachis (cm) | 42.13 | 19.61 | <0.001 |
| E | Length of basal leaflets (cm) | 36.37 | 17.78 | <0.001 |
| F | Length of median leaflets (cm) | 88.63 | 20.31 | <0.001 |
| G | Length of apical leaflets (cm) | 81.41 | 19.07 | <0.001 |
| H | Width of basal leaflets (cm) | 60.22 | 18.91 | <0.001 |
| I | Width of median leaflets (cm) | 82.84 | 20.5 | <0.001 |
| J | Width of apical leaflets (cm) | 72.89 | 19.47 | <0.001 |
| K | Distance between median leaflets (cm) | 86.12 | 19.17 | <0.001 |
| L | Width of articulation of median leaflets (cm) | 13.42 | 20.16 | <0.001 |
| Key | mag vs. mee | mag vs. fur | mee vs. fur |
|---|---|---|---|
| A | 5.966 *** | 8.489 *** | 15.46 *** |
| B | 0.9178 ns | 2.713 ns | 1.994 ns |
| C | 13.74 *** | 20.07 *** | 8.365 *** |
| D | 10.09 *** | 12.11 *** | 3.396 ns |
| E | 5.448 *** | 9.419 *** | 4.912 *** |
| F | 9.546 *** | 15.71 *** | 7.661 *** |
| G | 7.622 *** | 13.47 *** | 7.3 *** |
| H | 11.46 *** | 13.96 *** | 4.076 ns |
| I | 14.72 *** | 18.3 *** | 5.619 *** |
| J | 13.41 *** | 15.09 *** | 3.87 * |
| K | 9.617 *** | 14.7 *** | 6.537 *** |
| L | 4.641 *** | 6.724 *** | 2.769 ns |
| Z. magnifica | Z. meermanii | Z. furfuracea | Total | |
|---|---|---|---|---|
| Z. magnifica | 12 | 0 | 0 | 12 |
| Z. meermanii | 0 | 15 | 0 | 15 |
| Z. furfuracea | 0 | 0 | 9 | 9 |
| Total | 12 | 15 | 9 | 36 |
| Z. magnifica | Z. meermanii | Z. furfuracea | |
|---|---|---|---|
| Z. magnifica | <0.001 | <0.001 | |
| Z. meermanii | 90.604 | <0.05 | |
| Z. furfuracea | 169.31 | 15.892 |
| Trait | Z. magnifica | Z. meermanii | Z. furfuracea |
|---|---|---|---|
| Habitat | Karstic terrain | Karstic terrain | Sand dunes |
| Trunk | Branching with age | Typically solitary | Branching with age |
| Color emergence of leaves | Pink | Reddish-brown | Ochre to light brown |
| Texture of leaflets | Subcoriaceous with caducous tomentum | Coriaceous with caducous tomentum | Coriaceous with persistent tomentum |
| Leaflets | Widely obovate | Obovate to oblanceolate or narrowly oblong | Obovate to oblanceolate |
| Peduncle of ovulate strobilus | Thick and short | Thick and short | Thin and long |
| Color of mature ovulate strobilus | Light orange | Light brown | Yellowish green |
| Color of mature pollen strobilus | Light orange | Cream to brown | Light brown |
| Ovulate strobilus apex shape | Acute or obtuse | Apiculate | Apiculate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Farrera, M.A.; Gutiérrez-Ortega, J.S.; Martínez-Martínez, M.G.; Calonje, M. Zamia magnifica (Zamiaceae, Cycadales): A New Rupicolous Cycad Species from Sierra Norte, Oaxaca, Mexico. Taxonomy 2023, 3, 232-249. https://doi.org/10.3390/taxonomy3020017
Pérez-Farrera MA, Gutiérrez-Ortega JS, Martínez-Martínez MG, Calonje M. Zamia magnifica (Zamiaceae, Cycadales): A New Rupicolous Cycad Species from Sierra Norte, Oaxaca, Mexico. Taxonomy. 2023; 3(2):232-249. https://doi.org/10.3390/taxonomy3020017
Chicago/Turabian StylePérez-Farrera, Miguel Angel, José Said Gutiérrez-Ortega, Mauricio Gerónimo Martínez-Martínez, and Michael Calonje. 2023. "Zamia magnifica (Zamiaceae, Cycadales): A New Rupicolous Cycad Species from Sierra Norte, Oaxaca, Mexico" Taxonomy 3, no. 2: 232-249. https://doi.org/10.3390/taxonomy3020017
APA StylePérez-Farrera, M. A., Gutiérrez-Ortega, J. S., Martínez-Martínez, M. G., & Calonje, M. (2023). Zamia magnifica (Zamiaceae, Cycadales): A New Rupicolous Cycad Species from Sierra Norte, Oaxaca, Mexico. Taxonomy, 3(2), 232-249. https://doi.org/10.3390/taxonomy3020017






