Ancyronyx jhoanae sp. nov. (Coleoptera: Elmidae), A New Spider Riffle Beetle Species from Luzon, Philippines, and New Records for A. tamaraw Freitag, 2013 †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Taxon Sampling
2.2. Morphological Studies
2.3. Molecular Species Delimitation
3. Results
3.1. DNA Analyses
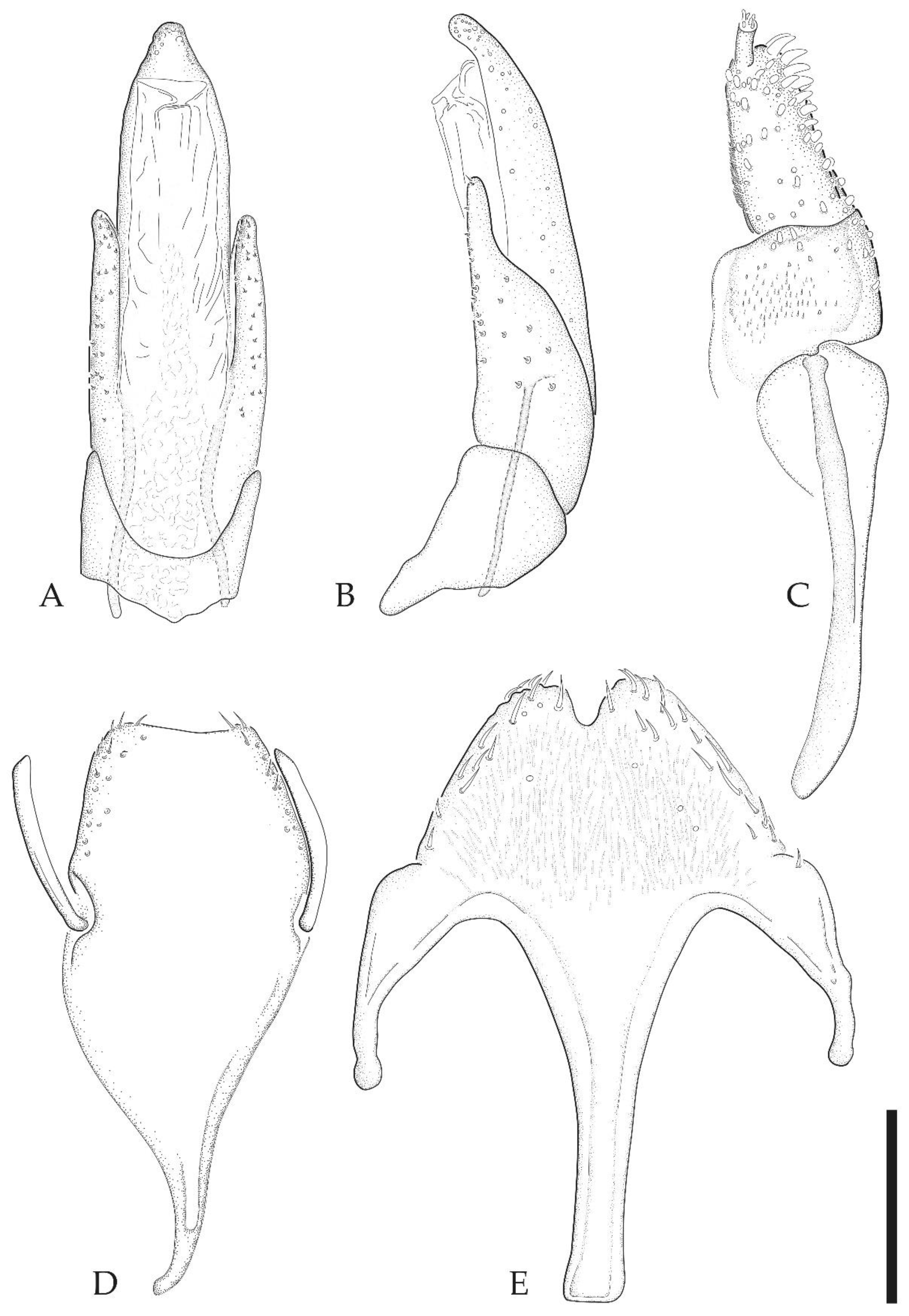
3.2. Taxonomy
- Ancyronyx patrolus species group (sensu Freitag & Jäch 2007)
- Ancyronyx jhoanae sp. nov.
- urn:lsid:zoobank.org:act:76007C04-F9FC-4554-A42E-441CE1E5F096
- Ancyronyx tamaraw Freitag, 2013
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seno, C.B.N.; Delocado, E.D.; Freitag, H. Three new species of Ancyronyx Erichson, 1847 (Coleoptera, Elmidae) from Mindanao, Philippines. Tijdschr. Voor Entomol. 2022, 165, 61–79. [Google Scholar] [CrossRef]
- Sabordo, M.R.; Delocado, E.; Freitag, H. Two new species of the genus Ancyronyx Erichson, 1847 from the island of Negros, Philippines (Insecta, Coleoptera, Elmidae). Tijdschr. Voor Entomol. 2020, 163, 13–30. [Google Scholar] [CrossRef]
- Jäch, M.A.; Kodada, J.; Brojer, M.; Shepard, W.D.; Čiampor, F. Coleoptera: Elmidae and Protelmidae. In World Catalogue of Insects; Brill: Leiden, The Netherlands, 2016; Volume 14, pp. 1–251. [Google Scholar]
- Freitag, H.; Jäch, M.A. The genus Ancyronyx Erichson, 1847 (Coleoptera, Elmidae) in Palawan and Busuanga, (Philippines) with descriptions of six new species. Zootaxa 2007, 1847, 37–59. [Google Scholar] [CrossRef]
- Ong, P.; Afuang, L.; Rosell-Ambal, R.G. The Philippine Biodiversity Conservation Priorities: A Second Iteration of the National Biodiversity Strategy and Action Plan. 2002. Available online: https://www.cbd.int/doc/world/ph/ph-nbsap-v2-en.pdf (accessed on 18 October 2022).
- Freitag, H.; Kodada, J. A taxonomic review of the genus Ancyronyx Erichson, 1847 from Sulawesi (Insecta: Coleoptera: Elmidae). J. Nat. Hist. 2017, 51, 561–606. [Google Scholar] [CrossRef]
- Kodada, J.; Jäch, M.A.; Čiampor, F., Jr. 19.2. Elmidae Curtis, 1830. In Handbook of Zoology; Walter de Gruyter: Berlin, Germany, 2016; Volume 4, pp. 561–589. [Google Scholar]
- Qiagen. DNeasy ® Blood & Tissue Handbook; Qiagen: Hilden, Germany, 2020. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Freitag, H. Ancyronyx Erichson, 1847 (Coleoptera, Elmidae) from Mindoro, Philippines, with description of the larvae and two new species using DNA sequences for the assignment of the developmental stages. Zookeys 2013, 321, 35–64. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kodada, J.; Jäch, M.A.; Freitag, H.; Čiamporová-Zaťovičová, Z.; Goffová, K.; Selnekovič, D.; Čiampor, F. Ancyronyx clisteri, a new spider riffle beetle species from Borneo, redescription of A. sarawacensis Jäch including a description of the larva and new distribution data for A. procerus Jäch using DNA barcodes (Coleoptera, Elmidae). Zookeys 2020, 2020, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Jäch, M.A. A taxonomic review of the Oriental species of the genus Ancyronyx Erichson, 1847 (Coleoptera, Elmidae). Rev. Suisse Zool. 1994, 101, 601–622. [Google Scholar] [CrossRef]
- Freitag, H.; Pangantihon, C.V.; Njunjić, I. Three new species of Grouvellinus champion, 1923 from Maliau Basin, Sabah, Borneo, discovered by citizen scientists during the first taxon expedition (Insecta, Coleoptera, Elmidae). Zookeys 2018, 2018, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Gardeux, V.; David, F.P.A.; Shajkofci, A.; Schwalie, P.C.; Deplancke, B. ASAP: A web-based platform for the analysis and interactive visualization of single-cell RNA-seq data. Bioinformatics 2017, 33, 3123–3125. [Google Scholar] [CrossRef] [PubMed]
- Freitag, H.; Jäch, M.A.; Wewalka, G. Diversity of aquatic and riparian Coleoptera of the Philippines: Checklist, state of knowledge, priorities for future research and conservation. Aquat. Insects 2016, 37, 177–213. [Google Scholar] [CrossRef]




| Species | Locality | Voucher | GenBank No. |
|---|---|---|---|
| A. berghaueri Sabordo, Delocado & Freitag, 2020 [2] | Negros | FR569 | MT568726 |
| A. clisteri Kodada et al., 2020 [13] | Borneo | FZ1640 | MK505421 |
| A. kalasan Seno, Delocado & Freitag, 2022 [1] | Mindanao | FR337 | OP609914 |
| A. manobo Seno, Delocado & Freitag, 2022 [1] | Mindanao | BS017 | OP609918 |
| A. minerva Freitag & Jäch, 2007 [4] | Palawan | FR467 | MT568723 |
| A. punkti Freitag & Jäch, 2007 [4] | Palawan | FR466 | MT568722 |
| A. schillhammeri Jäch, 1994 [14] | Mindanao | FR380 | OP609913 |
| A. jhoanae Seno & Freitag, sp. nov. | Luzon | FR238 | OQ259604 |
| FR338 | OQ259605 | ||
| A. tamaraw Freitag, 2013 [10] | Luzon | FR271 | MT568727 |
| A. zamboangaensis Seno, Delocado & Freitag, 2022 [1] | Mindanao | FR387 | OP609911 |
| G. quest Freitag, Pangantihon & Njunjić, 2018 [15] | Borneo | H17 | LR738834 |
| # | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A. jhoanae sp. nov. | ||||||||||||
| 2 | A. jhoanae sp. nov. | 1.21 | |||||||||||
| 3 | A. berghaueri | 4.38 | 4.98 | ||||||||||
| 4 | A. clisteri | 11.18 | 11.03 | 11.33 | |||||||||
| 5 | A. kalasan | 9.21 | 9.82 | 9.06 | 12.54 | ||||||||
| 6 | A. manobo | 10.57 | 10.12 | 10.12 | 12.84 | 11.48 | |||||||
| 7 | A. minerva | 12.99 | 12.84 | 12.08 | 13.29 | 13.90 | 13.44 | ||||||
| 8 | A. punkti | 13.60 | 13.14 | 13.29 | 14.35 | 15.41 | 13.90 | 8.46 | |||||
| 9 | A. sarawacensis | 11.48 | 11.33 | 11.78 | 8.76 | 12.99 | 13.29 | 13.90 | 13.29 | 13.90 | |||
| 10 | A. schillhammeri | 14.80 | 14.65 | 15.11 | 13.60 | 14.65 | 15.11 | 14.05 | 10.42 | 13.44 | 15.11 | ||
| 11 | A. tamaraw | 12.39 | 12.54 | 12.69 | 13.44 | 12.08 | 14.05 | 8.46 | 14.35 | 11.48 | 14.20 | 13.29 | |
| 12 | A. zamboangaensis | 9.67 | 9.06 | 9.37 | 12.39 | 6.19 | 9.67 | 13.60 | 15.56 | 17.98 | 18.13 | 17.52 | 16.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seno, C.B.N.; Freitag, H. Ancyronyx jhoanae sp. nov. (Coleoptera: Elmidae), A New Spider Riffle Beetle Species from Luzon, Philippines, and New Records for A. tamaraw Freitag, 2013. Taxonomy 2023, 3, 85-94. https://doi.org/10.3390/taxonomy3010008
Seno CBN, Freitag H. Ancyronyx jhoanae sp. nov. (Coleoptera: Elmidae), A New Spider Riffle Beetle Species from Luzon, Philippines, and New Records for A. tamaraw Freitag, 2013. Taxonomy. 2023; 3(1):85-94. https://doi.org/10.3390/taxonomy3010008
Chicago/Turabian StyleSeno, Christalle Beatriz N., and Hendrik Freitag. 2023. "Ancyronyx jhoanae sp. nov. (Coleoptera: Elmidae), A New Spider Riffle Beetle Species from Luzon, Philippines, and New Records for A. tamaraw Freitag, 2013" Taxonomy 3, no. 1: 85-94. https://doi.org/10.3390/taxonomy3010008
APA StyleSeno, C. B. N., & Freitag, H. (2023). Ancyronyx jhoanae sp. nov. (Coleoptera: Elmidae), A New Spider Riffle Beetle Species from Luzon, Philippines, and New Records for A. tamaraw Freitag, 2013. Taxonomy, 3(1), 85-94. https://doi.org/10.3390/taxonomy3010008







