Systematics
Family Bufonidae Gray, 1825
Genus Rhinella Fitzinger, 1826
Rhinella moralesi sp. nov. Lehr, Cusi, Rodrigues, Venegas, García-Ayachi and Catenazzi, 2021
LSID:urn:lsid:zoobank.org:pub:BE76DE75-AF3A-4B99-9351-B11A65D0AC29
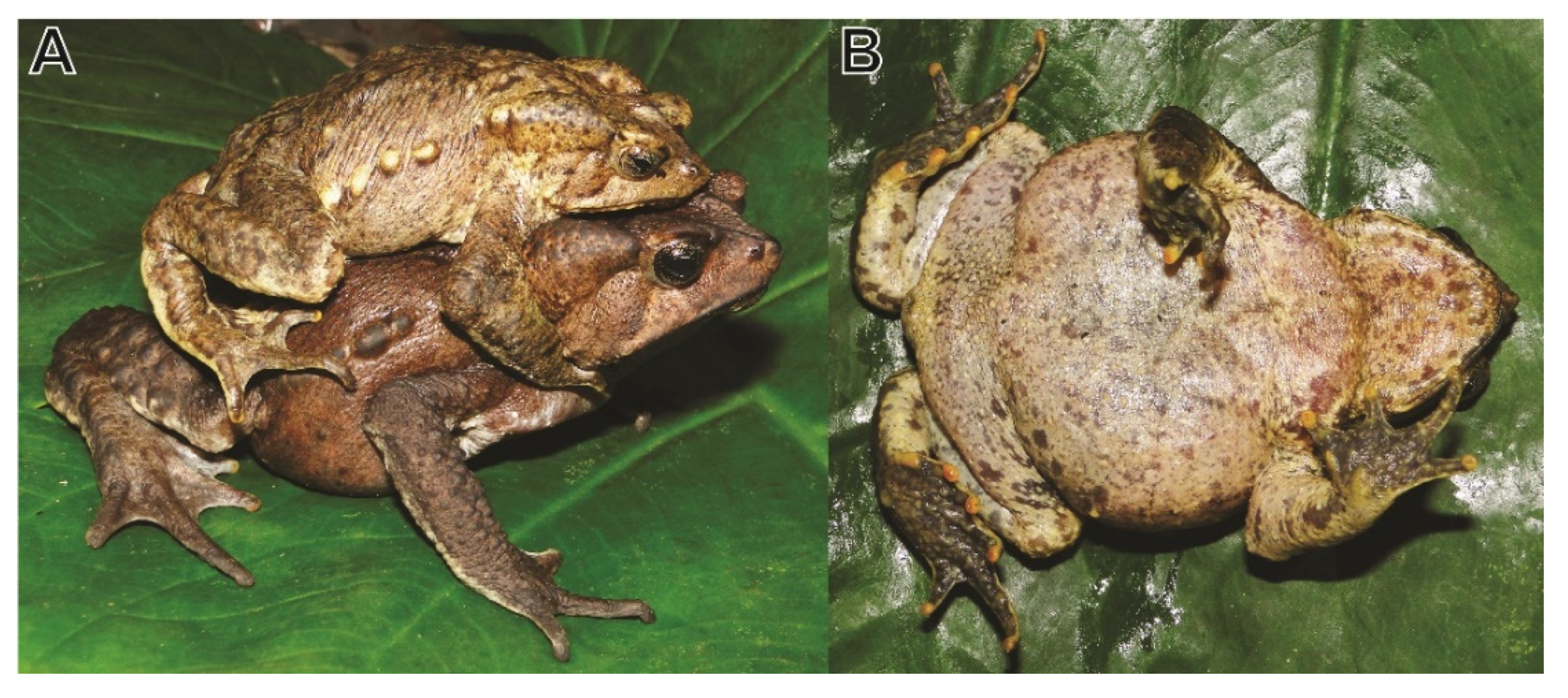
Holotype (
Figure 3A and
Figure 4A,B): MUSM 15959 (field number AMNH 11993), an adult male, collected in the Río Abiseo Valley, collected within a radius of 1 km around Jucusbamba (7°53′17.002″ S, 77°11′49.009″ W, WGS84 GPS coordinates, not to be confused with Jucusbamba in Departamento La Libertad) at ca. 1830 m a.s.l., Río Abiseo National Park, Departamento San Martín, Peru, on 11 July 1999 by L.O. Rodriguez and A. Catenazzi.
Paratypes (3 males, 3 females,
Figure 4C,D,
Figure 6 and
Figure 7): MUSM 15958 (field number AMNH 11992), adult male, collected in the Río Abiseo Valley, near Jucusbamba (7°53′17.002″ S, 77°11′49.009″ W, WGS84 GPS coordinates) at 2010 m a.s.l., Río Abiseo National Park, Departamento San Martín, Peru, on 11 July 1999 by L.O. Rodriguez and A. Catenazzi. CORBIDI 713, adult female, collected in Laguna Negra (6°53′29.303″ S, 77°23′18.29″ W, WGS84 GPS coordinates) at 1788 m a.s.l., Province Mariscal Cáceres, Departmento San Martín, Peru, on 6 February 2008 by P.J. Venegas. CORBIDI 20268, male, collected in Posic (6°26′58.031″ S, 77°14′11.349″ W, WGS84 GPS coordinates) at 1902 m a.s.l., Province Mariscal Cáceres, Departmento San Martín, Peru, on 7 December 2018 by P.J. Venegas. CORBIDI 20371, adult male, CORBIDI 20372, adult female, collected in Nuevo Chirimoto (6°34′5.716″ S, 77°15′26.49″ W, WGS84 GPS coordinates) at 2019 m a.s.l., Province Mariscal Cáceres, Departmento San Martín, Peru, on 10 December 2018 by L.A. García-Ayachi. CORBIDI 20837, a subadult female, collected in Uriarte (5°29′52.389″ S, 78°21′30.799″ W, WGS84 GPS coordinates) at 2305 m a.s.l., Province Bagua, Departmento Amazonas, Peru, on 15 August 2019 by A. Marchelie.
Referred specimens (12 juveniles,
Figure 6C,D,G,H): CORBIDI 18862, collected near Yambrasbamba (5°41′54.514″ S, 77°59′15.355″ W, WGS84 GPS coordinates) at 2072 m a.s.l., Province Bongara, Departmento Amazonas, Peru, 5 September 2017 by P.J. Venegas. CORBIDI 20280–281, collected in Posic (6°26′58.031″ S, 77°14′11.349″ W, WGS84 GPS coordinates) at 1902 m a.s.l., Province Mariscal Cáceres, Departmento San Martín, Peru, on 7 December 2018 by P.J. Venegas. CORBIDI 20300–20305, 20383–20384, collected in Nuevo Chirimoto (6°34′5.716″ S, 77°15′26.49″ W, WGS84 GPS coordinates) at 2019 m a.s.l., Province Mariscal Cáceres, Departmento San Martín, Peru, on 10 December 2018 by L.A. García-Ayachi. CORBIDI 20748, a juvenile, collected in Cataratas de Nueva Esperanza (6°34′7.111″ S, 77°15′5.671″ W, WGS84 GPS coordinates) at 1989 m a.s.l., Province Bagua, Departmento Amazonas, Peru, on 11 August 2019 by P.J. Venegas.
Diagnosis: A large species of Rhinella tentatively assigned to the festae species Group due to having preorbital crest absent, supraorbital crest weak, nuptial pads dark, and tarsal fold absent, characterized by the following combination of characters: (1) SVL 56.1–70.8 mm in males (n = 4), SVL 90.6–91.6 in females (n = 2); (2) snout protruding beyond the margin of lip, slightly pointed in dorsal view, inclined posteroventrally in profile; (3) nostrils protuberant, directed dorsolaterally, anterior half exceeding anterior margin of lower jaw; (4) canthal crest distinct, slightly elevated, supraorbital and postorbital crests weakly defined, covered with tubercles, supratympanic crests indistinguishable, pretympanic crest absent; (5) tympanic membrane and tympanic annulus absent; (6) bony protrusion at angle of jaw absent; (7) neural crest of vertebrae absent; (8) parotoid glands long, prominent, from posterior margin of upper eyelid to posterior level of arm insertion, outside nearly straight, inside bulbous, incorporated into lateral row of tubercles; (9) lateral row of tubercles diagonally from posterior margin of parotoid gland slightly exceeding middle of flanks; tubercles large, rounded to subconical, and widely spaced; (10) skin on dorsum smooth with large scattered round to ovoid subconical tubercles; (11) skin on dorsal surfaces of limbs smooth, tubercular with small scattered round to ovoid subconical tubercles; (12) first finger slightly longer than the second; (13) palmar tubercle large, ovoid, two times size of ovoid thenar tubercle; (14) inner metatarsal tubercle ovoid, barely elevated, two times size of outer round to ovoid subconical metatarsal tubercle; (15) modal webbing on foot: I 1—1+ II 1—3+ III 1—4– IV 4—2+ V in males; (16) subarticular tubercles prominent, round to oval; supernumerary tubercles round, slightly smaller than the former; (17) subgular vocal sac and vocal slits absent, brown nuptial excrescences present on first and second finger in males; (18) in life, dorsum uniform dark purple brown or tan, flanks pale purple brown to tan; venter light grey with brown mottling; iris golden with irregular dark brown mottling.
Comparisons: Morphologically,
R. moralesi sp. nov. differs from all members of the
festae species Group by having larger males (largest SVL in males of
R. moralesi sp. nov. 70.8 mm,
R. chavin 52.0 mm [
8],
R. multiverrucosa 47.7 mm [
16],
R. lilyrodriguezae 46.4 mm (
n = 5),
R. chullachaki 44.2 mm [
14],
R. macrorhina 43.4 mm [
10],
R. acrolopha 41.9 mm [
10],
R. yanachaga 41.6 mm [
13],
R. paraguas 41.7 mm [
18],
R. rostrata 41 mm [
12],
R. tenrec 40.2 mm [
21],
R. ruizi 39.4 mm [
19],
R. arborescandens 35.3 mm [
4],
R. festae 33.9 mm [
32],
R. manu 32.3 mm [
5],
R. nicefori 32 mm [
10],
R. tacana 30.6 mm [
20], and
R. lindae 29.9 mm [
15]; males of
R.
nesiotes, and
R. truebae are unknown, but the SVL of females is smaller, 29.9 mm [
11] and 65.9 mm [
21], respectively vs. 91.6 mm in
R. moralesi sp. nov., and by having the outer dorsolateral tarsus surface with a subconical ridge of fused tubercles.
Furthermore, R. moralesi sp. nov. differs from nine members of the R. festae species Group (R. chavin, R. lilyrodriguezae, R. lindae, R. manu, R. multiverrucosa, R. nesiotes, R. tacana, R. truebae, and R. yanachaga) that have an externally visible tympanic annulus and tympanic membrane, whereas it shares with 10 members (R. acrolopha, R. arborescandens, R. chulluchaki, R. festae, R. macrorhina, R. nicefori, R. paraguas, R. rostrata, R. ruizi, and R. tenrec) the absence of an externally visible tympanic annulus and tympanic membrane.
From the latter 10 species,
R. moralesi sp. nov. can be distinguished as follows:
Rhinella moralesi sp. nov. and
R. acrolopha have males that lack vocal slits, but males of
R. moralesi sp. nov. are much larger (70.8 mm vs. 41.9 mm) [
10]. Furthermore,
R. moralesi sp. nov. has prominent elongate parotid glands in contact with the eye (small, ovoid, not in contact with eye).
Rhinella moralesi sp. nov. differs from
R. arborescandens by having parotoid glands elongate and in contact with the eye (ovoid, not in contact), and males with finger one and two with nuptial pads (distinct keratinous spines on finger one) [
4].
Rhinella moralesi sp. nov. differs from
R. chullachaki by having parotoid glands elongate and in contact with the eye (ovoid, not in contact), and pretympanic crests absent (well defined) [
14].
Rhinella moralesi sp. nov. and
R. festae have males that lack vocal slits. However,
R. moralesi sp. nov. differs from
R. festae by having a short protuberant snout (long) and large dorsal tubercles (minute) [
10].
Rhinella moralesi sp. nov. differs from
R. macrorhina by having males without vocal slits (present), by having a short protuberant snout (long), a dorsolateral row of widely spaced large, rounded to subconical tubercles (continuous row of small and depressed tubercles), and pretympanic crest absent (present) [
10].
Rhinella moralesi sp. nov. differs from
R. nicefori by having males without vocal slits (present) and supraorbital crests weakly defined (distinct) [
17].
Rhinella moralesi sp. nov. differs from
R. paraguas by having parotoid glands in contact with the eye (not in contact) and pretympanic crests absent (well defined) [
18].
Rhinella moralesi sp. nov. shares with
R. rostrata a row of large dorsolateral tubercles but differs by having long prominent parotoid glands (small, sub-triangular), pretympanic crest absent (present), hands and feet with relatively short and stubby digits (long and slender), and first finger slightly longer than second (first finger about half the size of the second) [
12].
Rhinella moralesi sp. nov. differs from
R. ruizi by having males with nuptial pads (absent), parotoid glands elongate and in contact with the eye (triangular, not in contact), and long fingers and toes (short) [
19].
Rhinella moralesi sp. nov. differs from
R. tenrec by lacking a snout with a proboscis (proboscis present), parotoid glands long, prominent, and in contact with the eye (small, triangular, not in contact), and dorsum smooth with large scattered, round to ovoid tubercles (smooth with low tubercles) [
21].
Ten species (including the new species) of the
Rhinella festae Group are currently recognized in Peru. From the seven species that have an externally visible tympanum,
R. moralesi sp. nov. can be distinguished as follows:
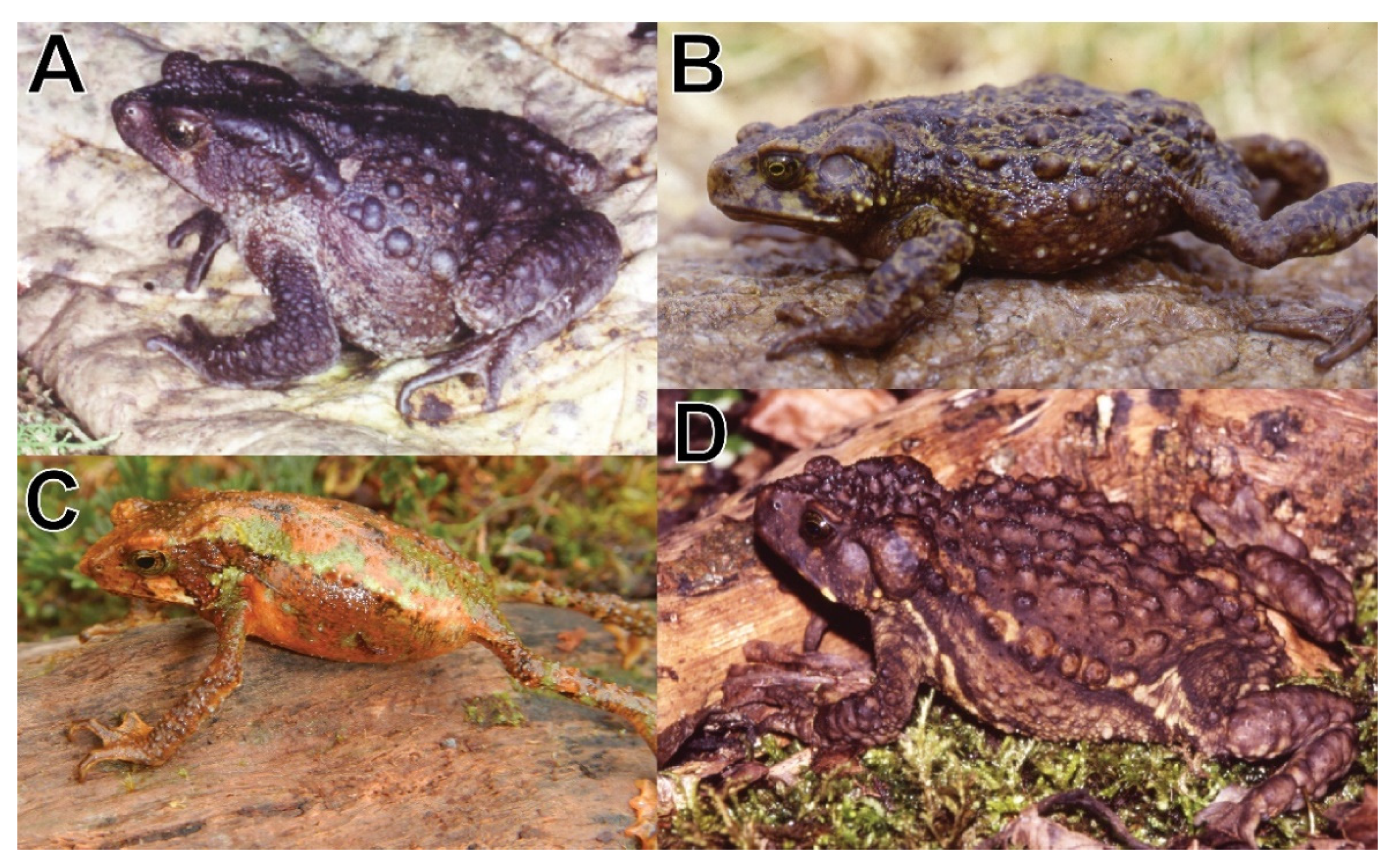
Rhinella moralesi sp. nov. and
R. chavin (
Figure 3A,B) share a stout body with relatively short extremities and short digits, large glands on the dorsum and a lateral row of enlarged, elevated glands, males with nuptial pads on the first and second finger and absence of vocal slits [
8]. However,
R. moralesi sp. nov. has a protuberant, slightly pointed, and posteroventrally inclined snout (truncate in dorsal view, rounded in profile, not protuberant), has the parotoid gland in contact with the eye (narrowly separated), lacks large and elongate glands on the forearm and tibia (present), and lacks keratin-tipped tubercles (present).
Rhinella moralesi sp. nov. and
R. multiverrucosa (
Figure 3A,D) share a stout body with relatively short extremities and short digits, parotoid glands in contact with the eye, large glands on the dorsum, and a lateral row of enlarged, elevated glands, males with nuptial pads on the first and second finger and vocal slits absent [
16]. However,
R. moralesi sp. nov. has elongated parotoid glands (subtriangular), lacks large glands on the head between parotoid glands (two large glands present), dorsum without keratin-tipped tubercles (present), lacks large glands on forearm and tibia (present), has a protuberant, slightly pointed, and posteroventrally inclined snout (snout truncate in dorsal view, rounded in profile, not protuberant).
Rhinella moralesi sp. nov. and
R. yanachaga (
Figure 3A,C) both have a slightly pointed snout in dorsal view, which is protruding in lateral view, a distinct canthus rostralis, and males have nuptial pads on first and second fingers and a protuberant cloaca with its opening directed ventrally [
13]. However,
R. moralesi sp. nov. is larger with an SVL of 70.8 mm (41.6 mm), has elongate parotoids in contact with the eye (subtriangular, not in contact with the eye), dorsolateral tubercle row distinct (weakly defined), dorsum in males smooth with large scattered round to ovoid subconical tubercles (numerous small keratin-tipped tubercles), and males lack vocal slits (present).
Rhinella moralesi sp. nov. is easily distinguished from
R. lilyrodriguezae by having relatively short and stubby extremities (long and slender in
R. lilyrodriguezae), having a short snout (long), lacking pretympanic crests (present), and dorsum with large glands (small glands) [
7].
Rhinella moralesi sp. nov. and
R. manu have males without vocal slits and a slightly pointed and protruding snout [
5]. However,
R. moralesi sp. nov. has the parotoid gland contacting the eye (not in contact), nuptial pads on fingers one and two (distinct keratinous spines on finger one), and dorsum with large glands (with numerous small glands).
Rhinella moralesi sp. nov. can be distinguished from
R. tacana by lacking a visible tympanum (present), males without vocal slits (present), dorsum with large glands (absent), and a maximum SVL in males of 70.8 mm (30.7 mm) [
20].
Rhinella moralesi sp. nov. and
R. nesiotes have the snout pointed in dorsal view and projecting. However,
R. moralesi sp. nov. has a lateral row of tubercles (absent), cranial crests present (absent), and the parotoid gland is elongated and contacting the eye (ovoid, not contacting) [
11].
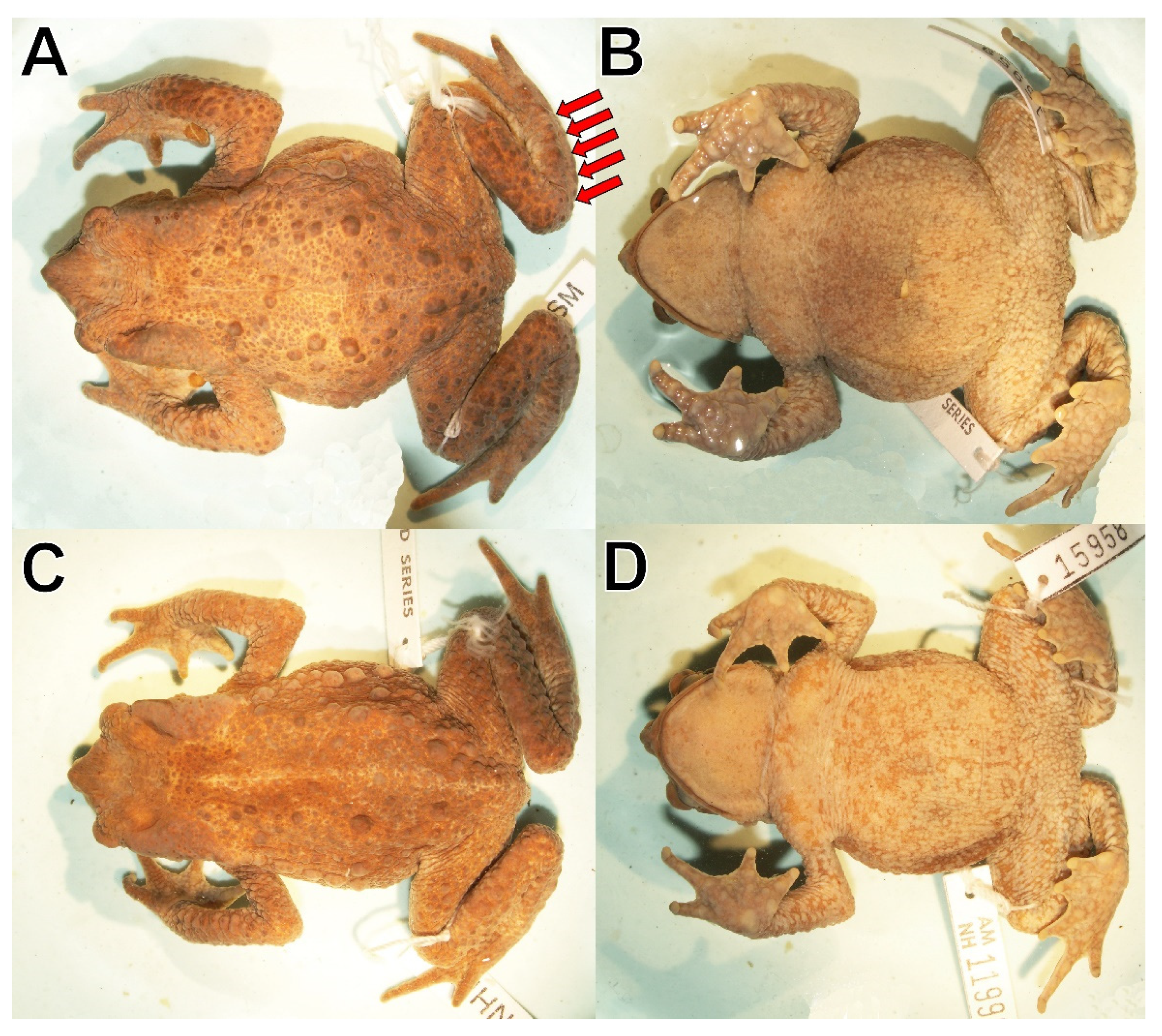
Description of holotype: Adult male; body robust; SVL 67.6 mm; head about as wide as long; snout slightly pointed in dorsal view, protruding beyond the margin of lip, rounded above and inclined posteroventrally in profile (
Figure 5B), with a broad fleshy vertical keel; canthus rostralis nearly straight in dorsal view, angular in profile; canthal crest distinct, slightly elevated, supraorbital and postorbital crests weakly defined, covered with tubercles, supratympanic crests indistinguishable, pretympanic crest absent; dorsum of head flat, skin co-ossified with underlying cranial bones; interorbital distance slightly larger than eyelid width; horizontal eye diameter slightly larger than distance between nostril and anterior corner of eye; internarial area concave; nostrils protuberant, directed laterally, anterior half exceeding anterior margin of lower jaw; loreal region strongly concave with tiny subconical granules; lips rounded bearing tiny subconical granules; small V-shaped notch at symphysis of upper jaw; tympanic membrane and tympanic annulus absent; tympanic area with tubercular folds and tiny subconical granules; corner of upper jaws with two prominent conical tubercles on each side (
Figure 5B); bony protrusion at angle of jaw absent; neural crest of vertebrae absent; skin on dorsum and flanks smooth with large scattered round to ovoid subconical tubercles lacking keratinized tips; parotoid glands long, prominent, from posterior margin of upper eyelid to posterior level of arm insertion, outside nearly straight, inside bulbous; posterior end of parotoid gland incorporated into lateral row of widely spaced rounded to subconical tubercles extending diagonally and slightly exceeding middle of flanks (
Figure 3A); skin on dorsal surfaces of limbs smooth, tubercular with scattered round to ovoid subconical tubercles slightly smaller than those on dorsum; cranial crests and parotoid glands smooth; upper eye lids with a prominent conical outer ridge and round to ovoid subconical tubercles; skin on throat, chest, belly, and ventral surfaces of forelimbs and hind limbs coarsely areolate to warty (
Figure 4B); forelimb relatively long; fingers with lateral fringes; tips of digits rounded, terminating in indistinct discs; relative length of fingers I > II < IV< III; palmar tubercle large, ovoid, two times size of ovoid thenar tubercle; subarticular tubercles most prominent at the base of fingers, round to oval, distal subarticular tubercles on Finger II on left hand and Finger IV on right hand bifid; supernumerary tubercles numerous, about half size of subarticular tubercles; basal webbing between fingers; brown nuptial excrescences present on dorsal surfaces of fingers I and II (
Figure 5C,D); foot longer than tibia; relative length of toes I < II < III = V < IV; inner tarsal fold absent; outer dorsolateral tarsus surface with a subconical ridge of fused tubercles (
Figure 4A); inner metatarsal tubercle ovoid, barely elevated, two times size of outer round to ovoid subconical metatarsal tubercle; subarticular tubercles most prominent at the base of toes, round to oval, distal subarticular tubercles often irregularly shaped or bifid; supernumerary tubercles round, slightly smaller than subarticular tubercles; toes with moderate webbing, I 1–1+ II 1––3+ III 1–4– IV 4––2+ V; lateral fringes broad; tips of digits rounded, terminating in indistinct discs (
Figure 5E); tongue elongate, two thirds attached to mouth floor; choanae small, oval; dentigerous processes of vomers and maxillary teeth absent; vocal slits absent; subgular vocal sac absent.
Measurements (in mm) of the holotype: SVL 67.6; TL 26.8; FL 28.2; HL 23.7; HW 23.9; ED 5.6; IOD 6.9; EW 6.7; IND 4.6; E-N 5.2; PL 17.7; PW 6.4.
Coloration of holotype in life (
Figure 3A): Dorsum dark purple-brown, flanks pale purple-brown to tan; iris golden with irregular dark brown mottling.
Coloration of holotype in alcohol (
Figure 4A,B, and
Figure 5): Dorsal surfaces pale hazel brown with tubercles and parotid glands grayish brown. Middorsal tan hairline from snout to cloaca. Nuptial pads hazel brown and distinct from the grayish tan coloration of hands (
Figure 4D). Lateral surfaces of head (
Figure 4B) tan with pale hazel brown mottling. Flanks colored as dorsum. Throat, belly, and ventral surfaces of legs grayish tan with pale brown mottling. Ventral surfaces of hands and feet pale gray (
Figure 5C–E).
All paratypes (
Figure 3C,D,
Figure 6 and
Figure 7) are similar to the holotype regarding morphology and proportions. Females are larger than males. All adult males have brown nuptial pads and lack a subgular vocal sac. Adults have a ridge of fused tubercles on the outer dorsolateral surface of the tarsus (
Figure 6A and
Figure 7A). The smallest juvenile (CROBIDI 20281) has an SVL of 17.7 mm, the largest 29.3 mm (CORBIDI 20305). The coloration for a male (MUSM 15958,
Figure 3C,D) in life was noted by AC as dark tan dorsally, without patterns, and light grey with brown mottling ventrally. In alcohol, the paratype is colored like the holotype except for a slightly broader middorsal hairline (
Figure 3C). Dorsal coloration in adults in life is dark brown (CORBIDI 20372) or pale brown (CORBIDI 713) and the venter is pale grayish cream with brown (CORBIDI 20372) or gray (CORBIDI 713) marmoration. Hand and toe surfaces are dark gray (CORBIDI 713, 20371) or pale gray (CORBIDI 20372) with toe and fingertips ranging from dark gray (CORBIDI 713) to brownish orange (CORBIDI 20371). One juvenile is blackish brown (CORBIDI 18862) dorsally and laterally and has the parotoid gland with pale olive flecks and the lateral line of tubercles is pale olive (
Figure 6G), the venter is slightly paler with blackish-brown blotches. One juvenile (CORBIDI 18862) is dorsally, laterally, and ventrally dark brown and orange-brown marmorated. The parotoid gland, lateral line of tubercles, and groin are tan.
Etymology: We dedicate the new species to our late colleague and friend Professor Victor Morales in recognition of his contributions to Neotropical herpetology.
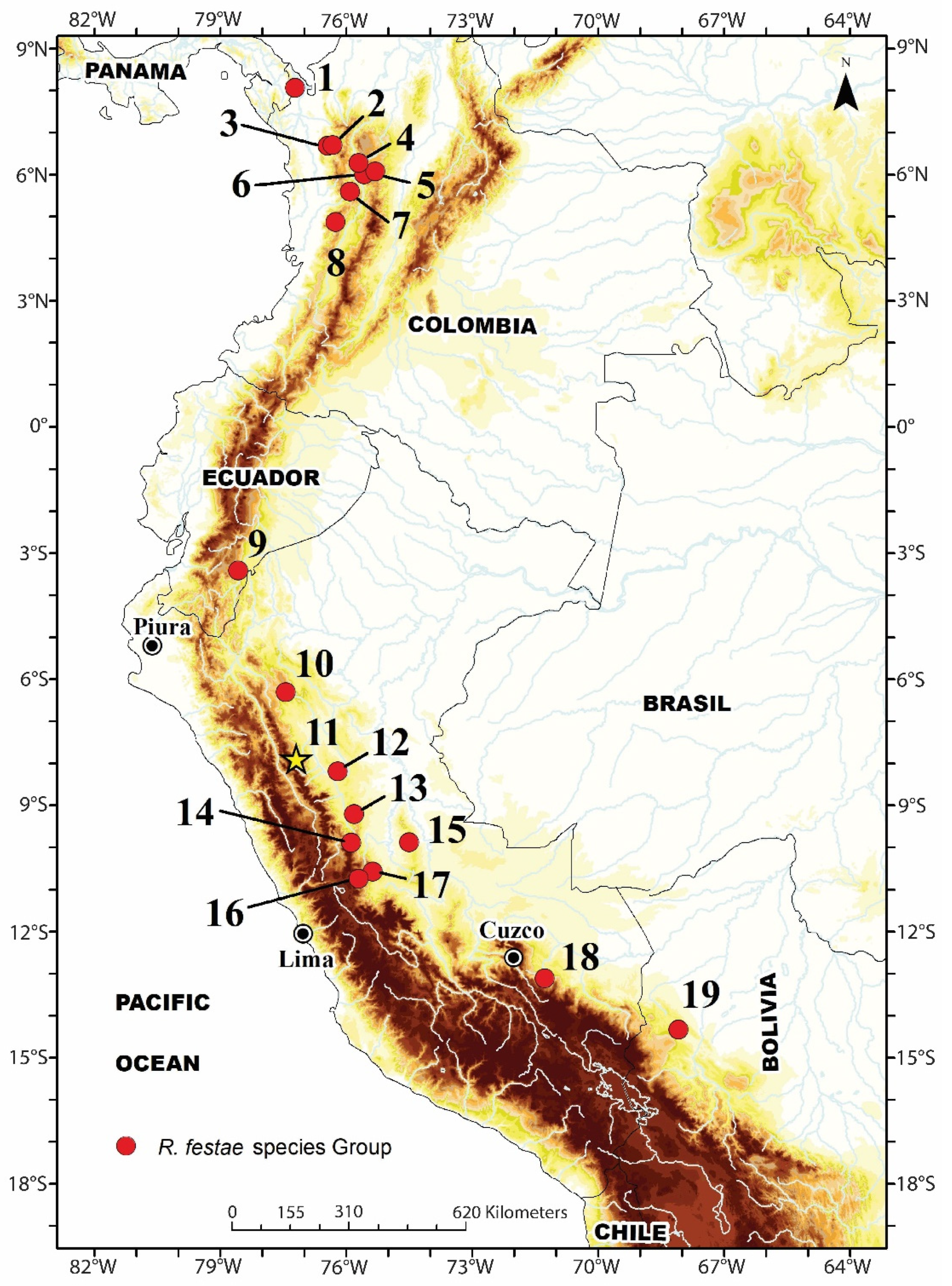
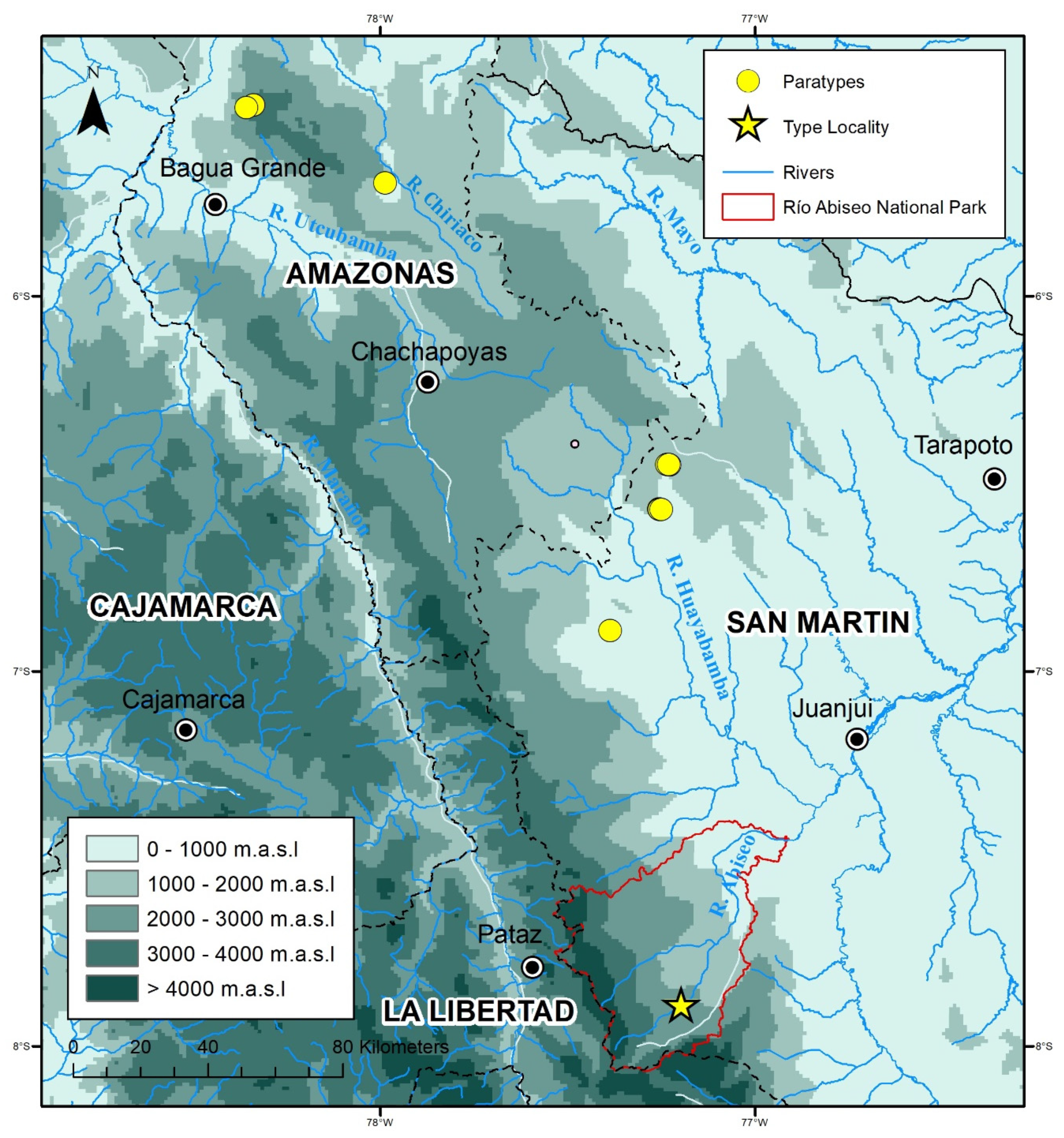
Distribution, ecology, and threat status:
Rhinella moralesi sp. nov. is known from seven montane forest localities between 1788 and 2305 m a.s.l. in Departamentos Amazonas and San Martín (
Figure 1 and
Figure 2). The distance between the northernmost and southernmost distribution records for
R. moralesi sp. nov. is about 294 km (airline). At Río Abiseo National Park, both specimens were found in proximity: the holotype was found in a terrestrial Sherman trap, and the male paratype (MUSM 15958) was found by botanists under unknown circumstances. The type locality was characterized by very humid montane forest, with some clearings and disturbed areas. There were signs of past and current burning along most of the Río Abiseo Valley sites that AC and LOR visited, including Jucusbamba. Eight 10 × 10 m
2 leaf litter plots searched at elevations from 1800 to 2000 m a.s.l. around Jucusbamba and the nearby locality of Granadilla, and two nocturnal transects in Jucusbamba (1730–1800 m a.s.l.) did not reveal any amphibians. At Granadilla (1990–2070 m a.s.l.), amphibians (possibly sympatric with
R. moralesi sp. nov.) observed during four nocturnal transects included
Gastrotheca sp. gr.
testudinea,
Pristimantis cf.
corrugatus, and
Pristimantis sp.
At other sites in northern Peru, one adult female specimen (CORBIDI 713) was found inactive under a log during the day at 1100 h. Two specimens (male CORBIDI 20268, subadult female CORBIDI 20837) were found walking on the leaf litter by night between 2000 and 2200 h. One couple (male CORBIDI 20371, female CORBIDI 20372,
Figure 7A) were found in amplexus walking on the leaf litter at 1000 h on 10 December 2018. All juveniles were found at night perched on leaves between 10 or 30 cm above ground or on the leaf litter. Specimens collected in Laguna Negra, Cataratas de Nueva Esperanza, and Uriarte (CORBIDI 713, 20748, 20837) were collected in primary montane forest, while specimens from Yambrasbamba, Posic, and Nuevo Chirimoto were collected in patches of montane forest near cropland of corn (
Zea mays) and pastures for cattle ranching. Subadult and adult specimens of
R. moralesi sp. nov. did not show saltatorial locomotion when captured or when handled for photography, while some juveniles showed weak jumps attempting to escape.
In Laguna Negra, Posic, and Nuevo Chirimoto, R. moralesi sp. nov. was collected in sympatry with Gastrotheca testudinea, Hemiphractus sp., Pristimantis nephophilus, and Rhinella margaritifera complex; at Yambrasbamba in sympatry with P. galdi, P. nephophilus, P. schultei, Hyloscirtus phyllognathus, and Nymphargus posadae; at Cataratas de Nueva Esperanza and Uriarte in sympatry with Pristimantis serendipitus, P. katoptroides, P. schultei, P. sp., Lynchius sp., and Noblella sp.
We detected infection by the pathogenic chytrid fungus (Bd) in the only sampled individual (CORBIDI 20748), captured at 1695 m a.s.l. in Nueva Esperanza (Bagua Province, Amazonas Department) on 11 August 2019. The level of infection ZE = 14 zoospores is low and not typically associated with diseased individuals. The presence of Bd in northern Peru has previously been reported, and the oldest confirmed record for the country is from northern Peru, during the 1999 mass die-off of
Atelopus patazensis in Pataz, Department of La Libertad [
33]. The distribution records of
R. moralesi sp. nov. cover a polygon area of 10661.84 km
2 (EOO) and the AAO is 32 km
2 revealing an IUCN conservation status of either “Vulnerable” or “Endangered”. Based on habitat destruction outside of protected areas resulting from agricultural activities and a Bd record, we consider the threat status for
R. moralesi sp. nov. as “Vulnerable” according to the IUCN red list criteria and categories [
34].