A Novel CuAlMnFe/CeO2 Composite Alloy: Investigating the Wear and Corrosion Features
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
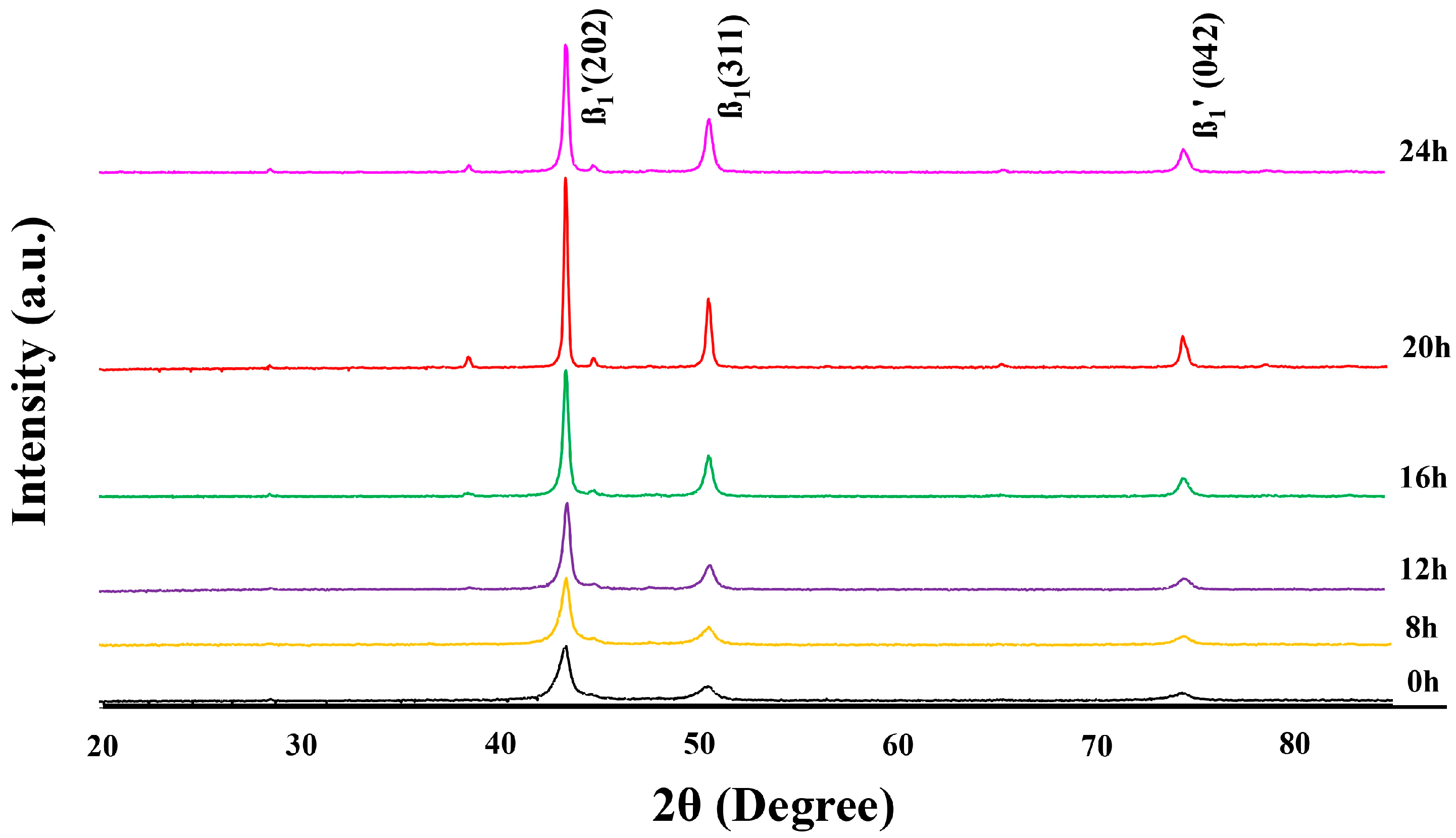
3.1. Phase Composition
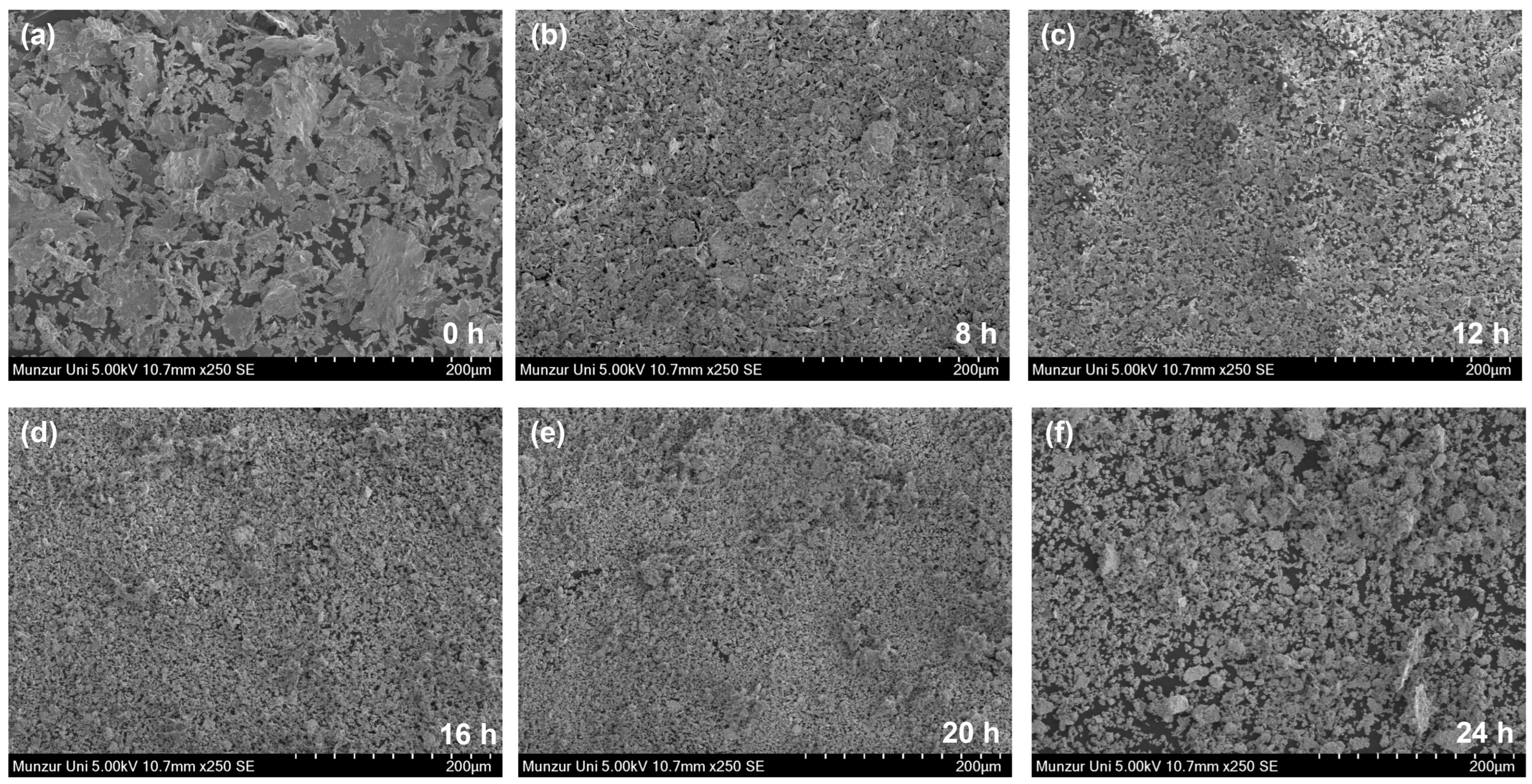
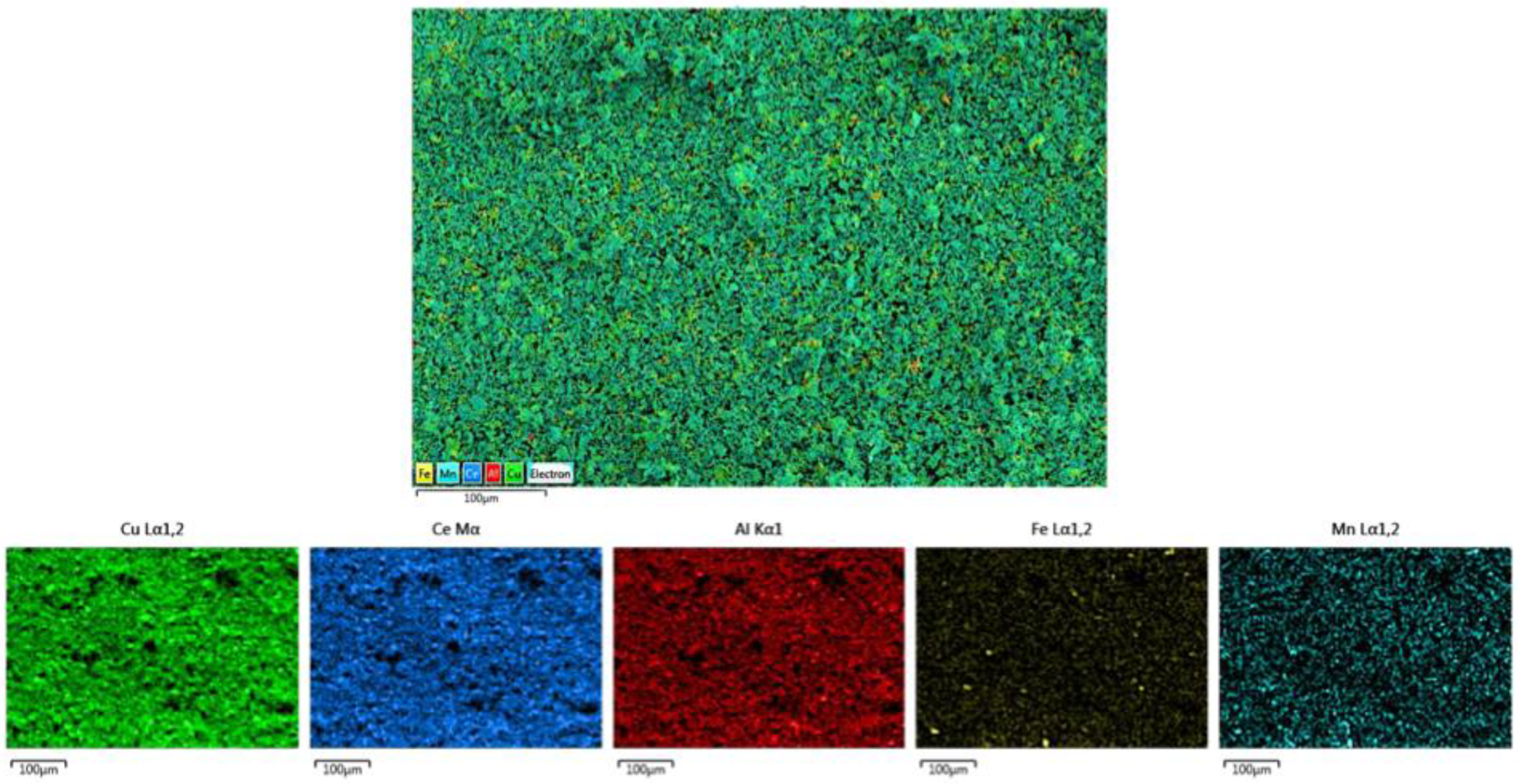
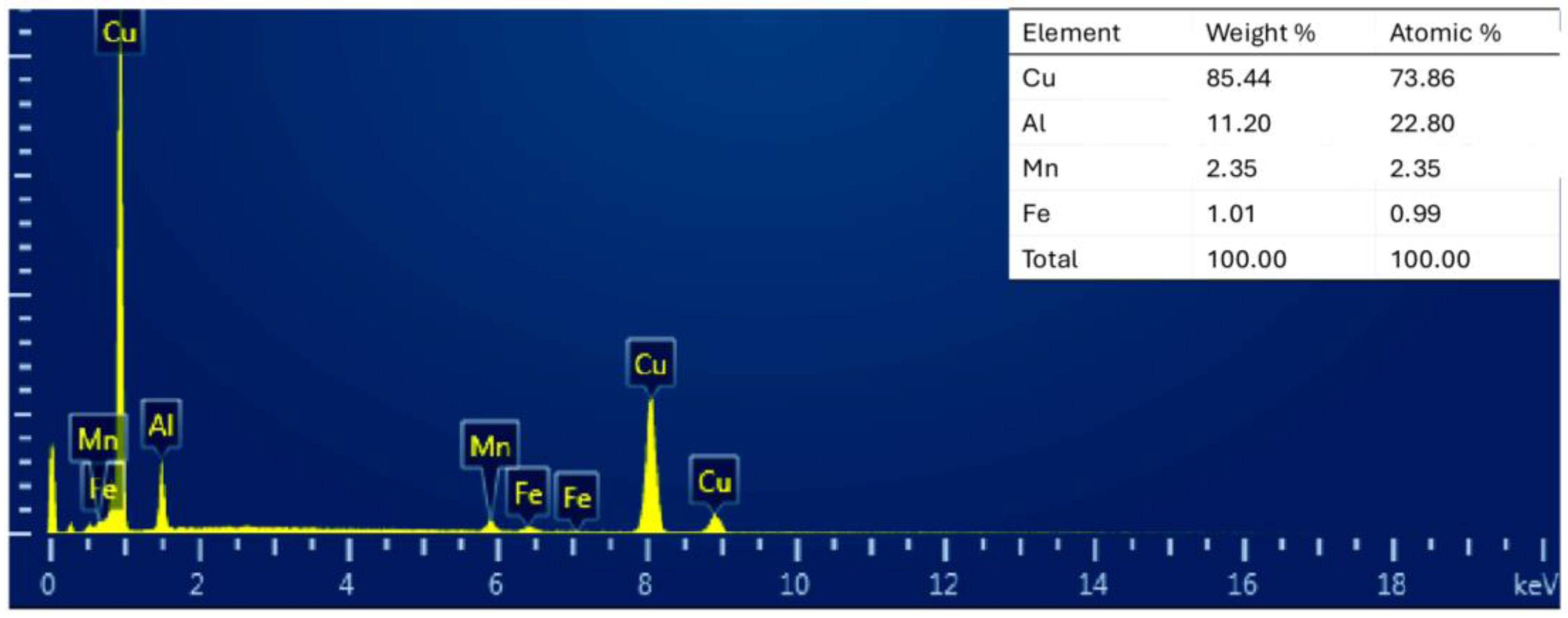
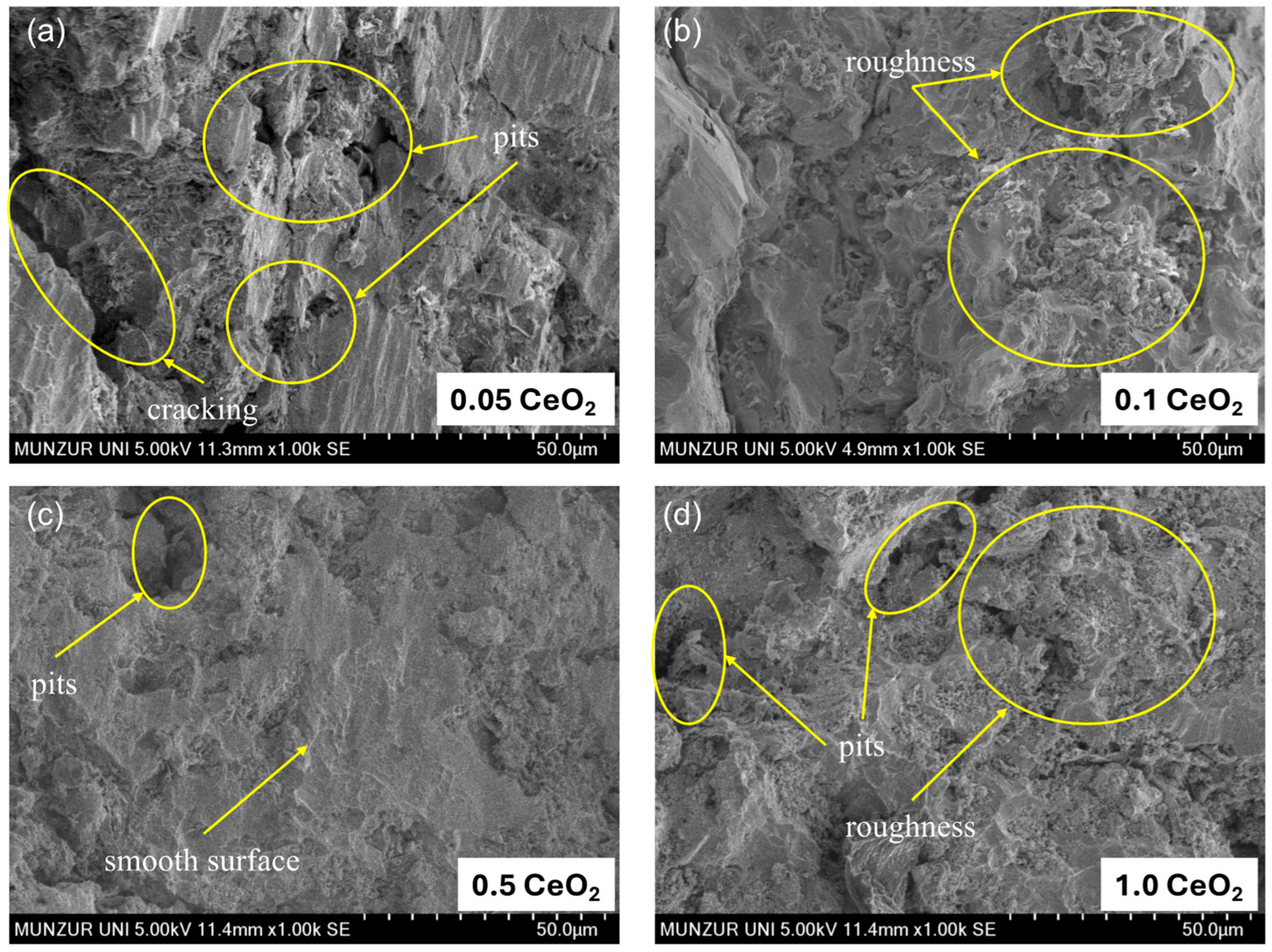
3.2. Study of Surface Morphology
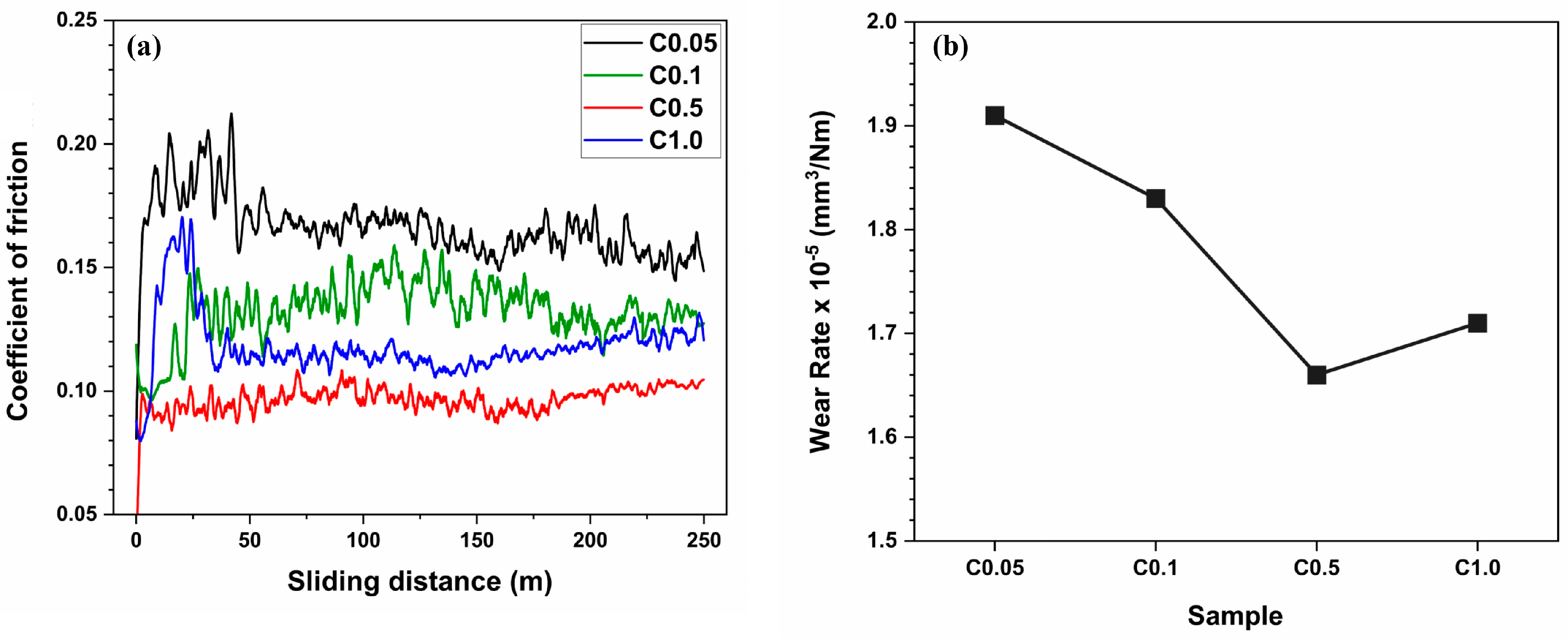
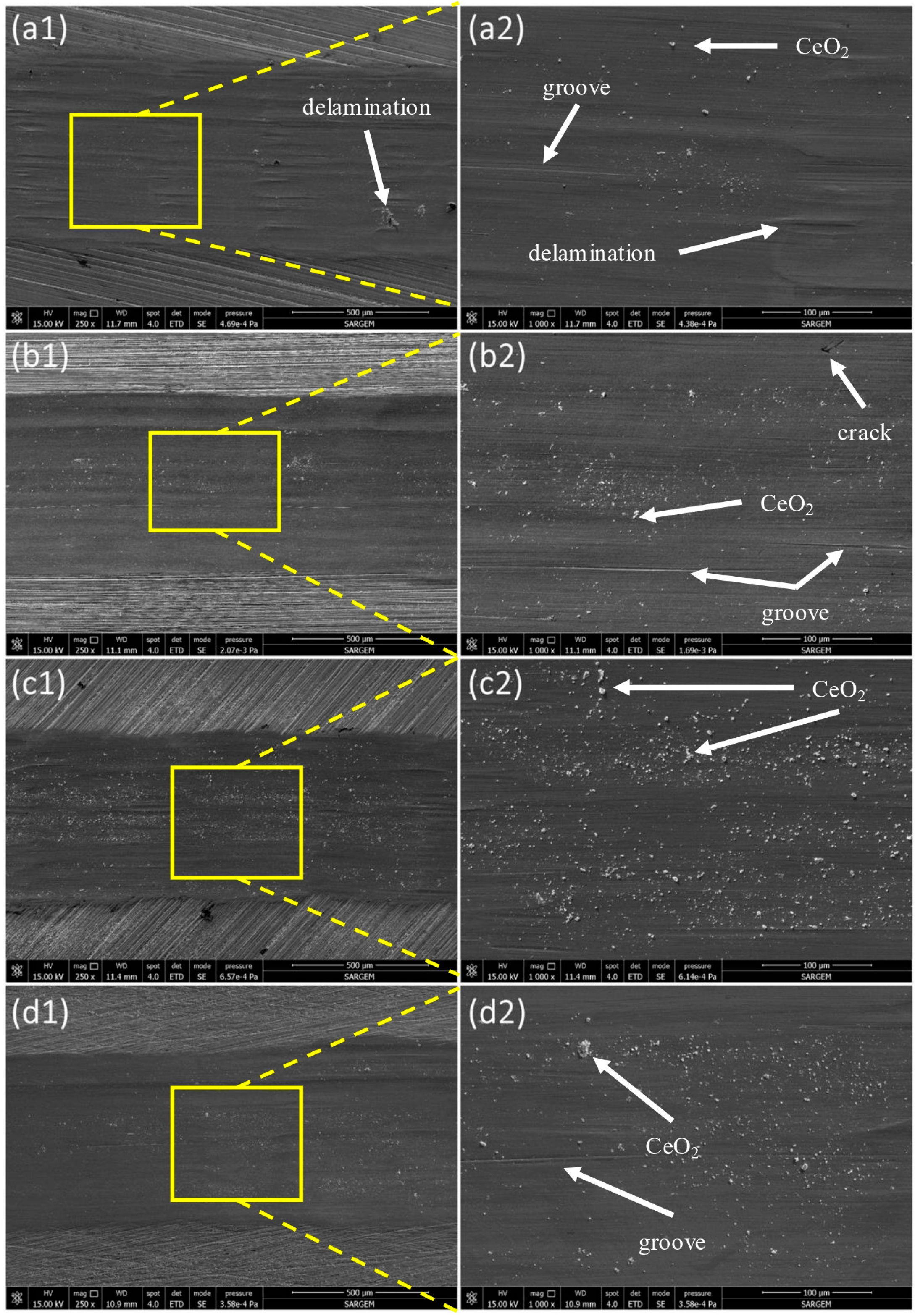
3.3. Hardness and Wear Behavior
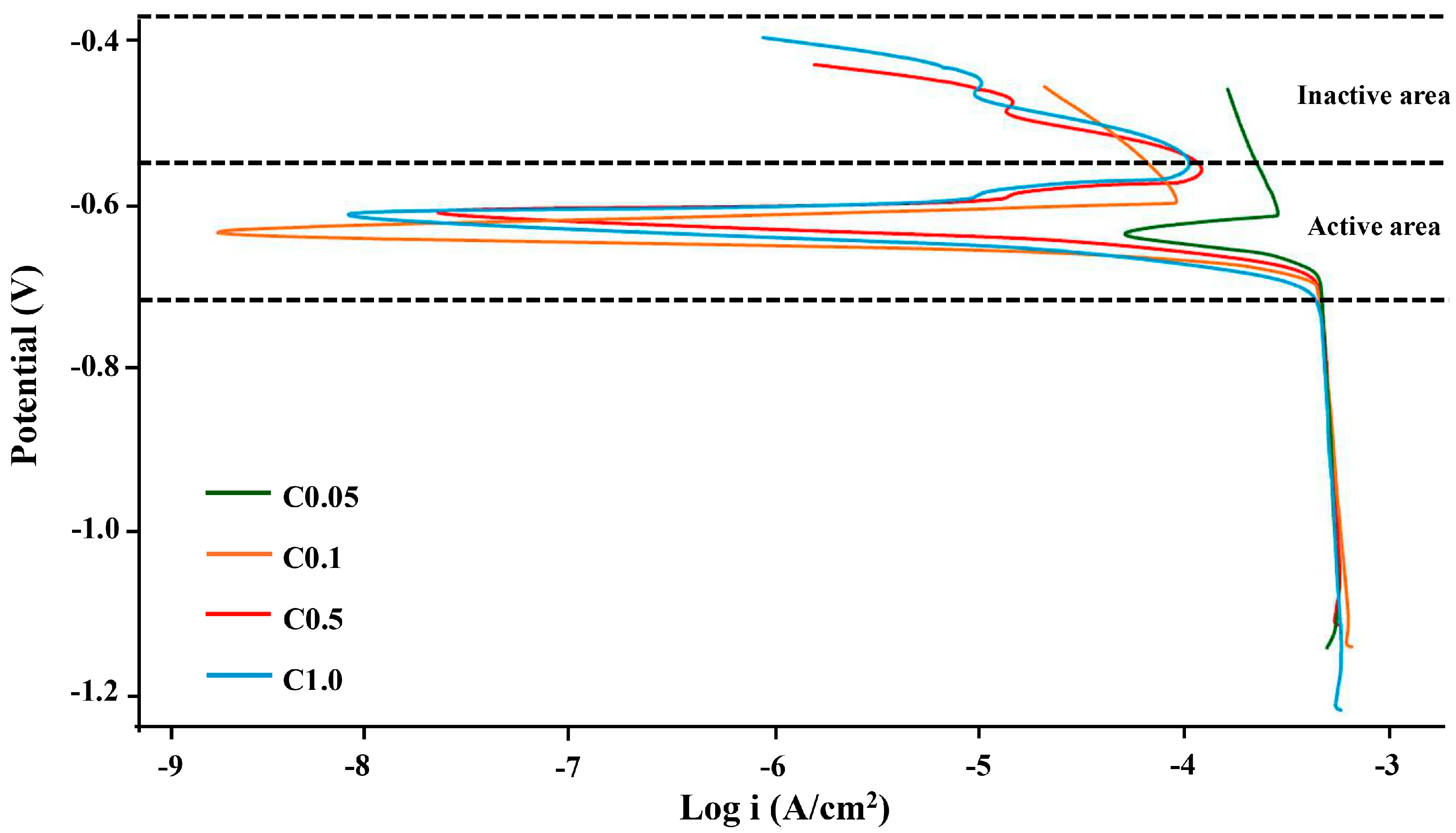
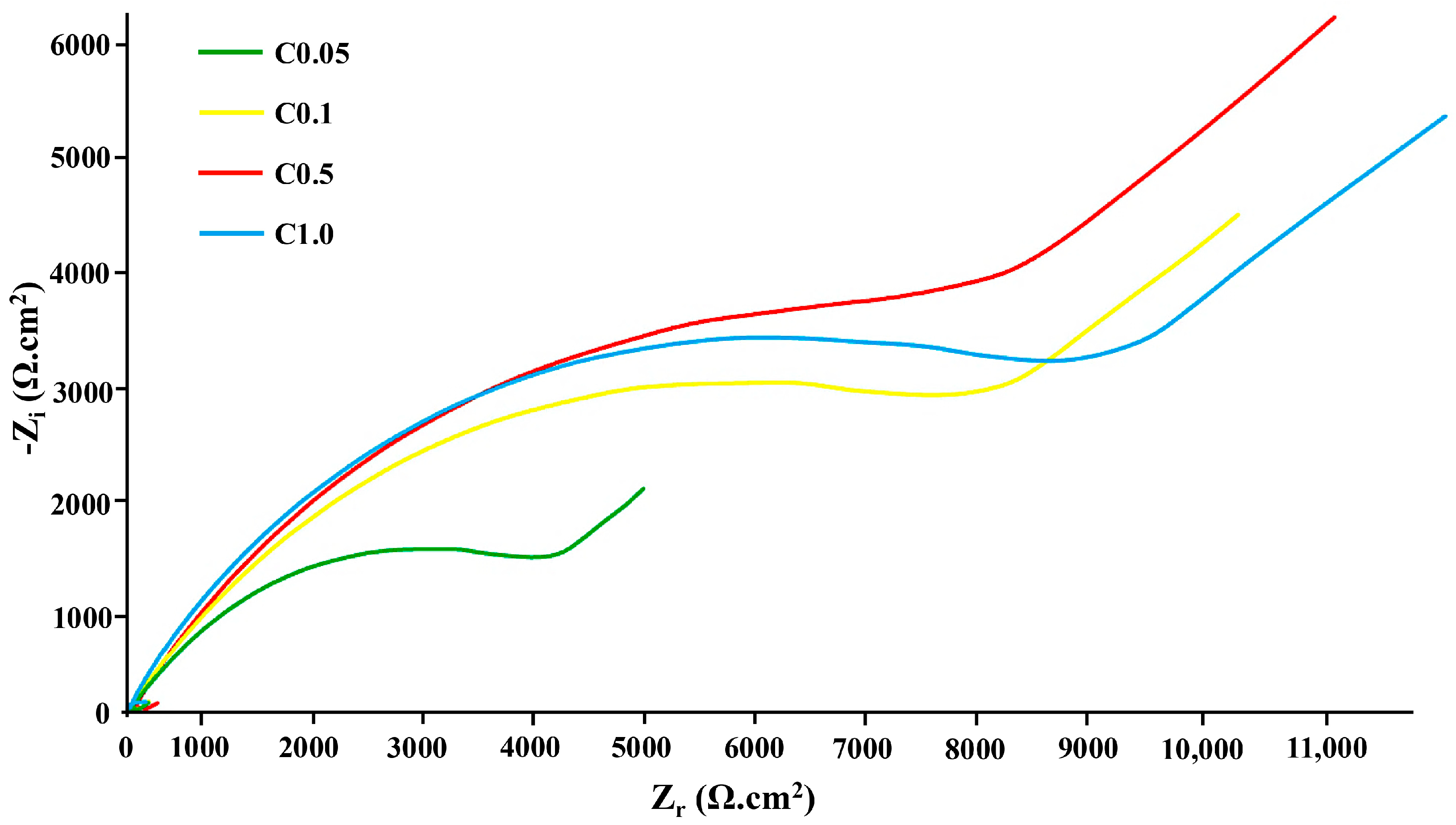
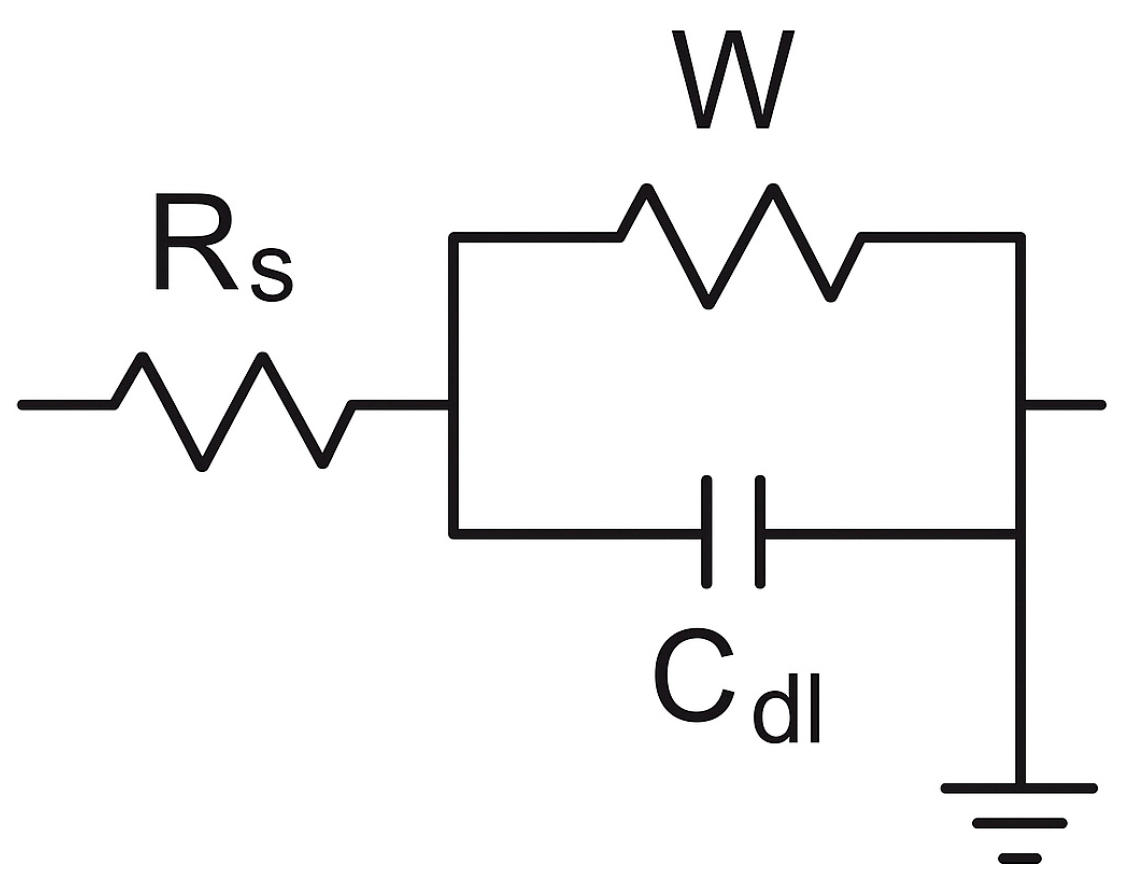
3.4. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sattari, M.; Kadkhodaei, M.; Akbarzadeh, S.; Gholami, R.; Beheshti, A. Wear in superelastic shape memory alloys: A thermomechanical analysis. Wear 2022, 488–489, 204139. [Google Scholar] [CrossRef]
- Sattari, M.; Esfahani, H.A.; Kadkhodaei, M.; Akbarzadeh, S.A. Mechanical contact model for superelastic shape memory alloys. J. Intell. Mater. Syst. Struct. 2020, 32, 208–218. [Google Scholar] [CrossRef]
- Guotai, L.; Tianyu, Y.; Pan, W.; Mingjun, C. Molecular dynamics simulation of phase transformation and wear behavior of Ni35Al30Co35 high temperature shape memory alloy. Wear 2023, 522, 204849. [Google Scholar]
- Mohammad, H.Y.; Kadkhodaei, M.; Salehan, M. Fully coupled thermomechanical modeling of shape memory alloys under multiaxial loadings and implementation by finite element method. Contin. Mech. Thermodyn. 2019, 31, 1683–1698. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y. Effect of Deformation Mode on the Wear Behavior of NiTi Shape Memory Alloys. Shap. Mem. Superelasticity 2016, 2, 204–217. [Google Scholar] [CrossRef]
- Canbay, C.A.; Tataroğlu, A.; Dere, A.; Al-Sehemi, A.G.; Karabulut, A.; Bektaş, A.; Al-Ghamdi, A.A.; Yakuphanoglu, F. The photo-electrical performance of the novel CuAlMnFe shape memory alloy film in the diode application. Mater. Sci. Eng. B 2021, 264, 114931. [Google Scholar] [CrossRef]
- Trehern, W.; Ozcan, H.; Franco, B.; Hite, N.; Malone, N.; Loveall, B.; Morrison, T.D.; Benafan, O.; Karaman, I. Exploring thermomechanical functionality of CuAlMn as an extreme low temperature shape memory alloy. Mater. Lett. 2022, 308, 131246. [Google Scholar] [CrossRef]
- Moskvichev, E.; Shamarin, N.; Smolin, A. Structure and Mechanical Properties of Cu–Al–Mn Alloys Fabricated by Electron Beam Additive Manufacturing. Materials 2023, 16, 123. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.S. Influence of Alloying Element Addition on Cu–Al–Ni High-Temperature Shape Memory Alloy without Second Phase Formation. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 884–888. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, R.; Hu, S.; Tu, F.; Jin, W. Research combining experiment and FEM analysis on sliding wear behaviors and mechanisms of TiNi alloy. Wear 2017, 386–387, 218–222. [Google Scholar] [CrossRef]
- Huang, X.; Kang, N.; Coddet, P.; El Mansori, M. Analyses of the sliding wear behavior of NiTi shape memory alloys fabricated by laser powder bed fusion based on orthogonal experiments. Wear 2023, 534–535, 205130. [Google Scholar] [CrossRef]
- Baroni, L.F.S.; Silva, R.; Vacchi, G.S.; Sordi, V.L.; Rovere, C.A.D. Influence of Ce on the corrosion properties of Fe-Mn-Si-based shape memory stainless steel. Mater. Today Commun. 2020, 25, 101649. [Google Scholar] [CrossRef]
- Hammadi, D.F.; Ahmed Adnan, R.S.; Al-Sarraf, M.A. The effect of silicon carbide addition on the transformation temperatures and wear resistance of Cu-based shape memory alloys. Mater. Today Proc. 2022, 62, 6068–6076. [Google Scholar] [CrossRef]
- Ercan, E. Analysis of Dry Wear. Microstructure and Thermodynamic Parameters of CuAlTa Bulk Material. Trans Indian Inst Met. 2021, 74, 2193–2202. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, Y.; Xu, P. Effect of La2O3 on Microstructure and Properties of Laser Cladding SMA Coating on AISI 304 Stainless Steel. Coatings 2022, 12, 1004. [Google Scholar] [CrossRef]
- Lu, B.; Cui, X.; Ma, W.; Dong, M.; Fang, Y.; Wen, X.; Jin, G.; Zeng, D. Promoting the heterogeneous nucleation and the functional properties of directed energy deposited NiTi alloy by addition of La2O3. Addit. Manuf. 2020, 33, 101150. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, Y.; Liu, Y.; Yu, Z.; Zhang, Z.; Ren, L. The martensitic transformation behavior and shape memory effect of laser powder bed fusion NiTi alloys influenced by rare earth addition. Mater. Sci. Eng. A 2022, 848, 143350. [Google Scholar] [CrossRef]
- Lu, B.; Cui, X.; Jin, G.; Dong, M.; Fang, Y.; Wen, X.; Ma, W. Effect of La2O3 addition on mechanical properties and wear behaviour of NiTi alloy fabricated by direct metal deposition. Opt. Laser Technol. 2020, 129, 106290. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, G.; Li, C.; Jin, Z. Influence of Cerium, Titanium and Nitrogen on Shape Memory Effect of Fe-Mn-Si-Cr-Ni Alloys. Scr. Mater. 1998, 38, 1163–1168. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; He, C.; Qi, F.; Wang, D.; Peng, S.; Shuai, C. Rare earth improves strength and creep resistance of additively manufactured Zn implants. Compos. Part B Eng. 2021, 216, 108882. [Google Scholar] [CrossRef]
- Yanan, L.; Ronglu, S.; Wei, N.; Tiangang, Z.; Yiwen, L. Effects of CeO2 on microstructure and properties of TiC/Ti2Ni reinforced Ti-based laser cladding composite coatings. Opt. Lasers Eng. 2019, 120, 84–94. [Google Scholar] [CrossRef]
- Hussain, S.; Pandey, A.; Dasgupta, R. Nano-CeO2 Doped Cu–Al–Ni SMAs with Enhanced Mechanical as well as Shape Recovery Characteristics. Met. Mater. Int. 2021, 27, 1478–1482. [Google Scholar] [CrossRef]
- Yang, S.; Omori, T.; Wang, C. A jumping shape memory alloy under heat. Sci. Rep. 2016, 6, 21754. [Google Scholar] [CrossRef]
- Pandey, A.; Jain, A.K.; Hussain, S. Effect of Nano CeO2 Addition on the Microstructure and Properties of a Cu-Al-Ni Shape Memory Alloy. Met. Mater. Trans. B. 2016, 47, 2205–2210. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Yang, Y.; Chen, W. The effects of ceria on the mechanical properties and thermal shock resistance of thermal sprayed NiAl intermetallic coatings. Intermetallics 2008, 16, 682–688. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, M.J.; Gao, F.; Liang, S.X.; Zhang, X.L.; Cui, H.X. Effects of aging treatment on the intergranular corrosion behavior of Al–Cu–Mg–Ag alloy. J. Alloys Compd. 2015, 639, 263–267. [Google Scholar] [CrossRef]
- Mahton, Y.; Kamde, M.A.; Chavan, A.; Saha, P. Influence of step-by-step cerium-based conversion and polydopamine duplex coating on stir cast AlCuMg alloys mitigating saltwater corrosion. Surf. Coat. Technol. 2024, 489, 131041. [Google Scholar] [CrossRef]
- Luo, B.; Lu, K.; Zhang, F. Effect of Aging Time on the Corrosion Resistance of the As-cast Al-Cu-Mg-Mn Alloy. JOM 2022, 74, 3616–3624. [Google Scholar] [CrossRef]
- Tian, W.; Hu, M.; Chen, X.; Zhou, H.; Sun, Y.; Lu, Q.; Wan, M. Effect of Ce addition on microstructure, mechanical properties and corrosion behavior of Al-Cu-Mn-Mg-Fe alloy. Mater. Res. Express 2020, 7, 036532. [Google Scholar] [CrossRef]
- Chen, J.; Bao, W.; Chen, H.; Ding, N.; Yang, X.; Yu, B.; Hong, T.; Cai, Z.; Xie, G. Dual phase reinforced CuCrZr alloy: Synergistic improvement of mechanical properties and corrosion resistance via metallic glass and rare earth oxides. Mater. Des. 2025, 251, 113686. [Google Scholar] [CrossRef]
- Liu, J.-S.; Yu, W.-X.; Chen, D.-Y.; Wang, S.-W.; Song, H.-W.; Zhang, S.-H. A Review of Studies on the Influence of Rare-Earth Elements on the Microstructures and Properties of Copper and Copper Alloys and Relevant Applications. Metals 2025, 15, 536. [Google Scholar] [CrossRef]
- Plakhova, T.V.; Romanchuk, A.Y.; Yakunin, S.N.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.G.; Shuh, D.K.; Tyliszczak, T.; Shiryaev, A.A.; et al. Solubility of Nanocrystalline Cerium Dioxide: Experimental Data and Thermodynamic Modeling. J. Phys. Chem. C. 2016, 120, 22615–22626. [Google Scholar] [CrossRef]
- Yavuzer, B.; Bicakci, Ü.; Simsek, D. Effect of Alloying Time and Heat Treatment on Microstructure and Tribological Properties of Mechanical Alloyed Cu-Al-Ni Shape Memory Alloy. J. Mater. Eng. Perform. 2024, 33, 11176–11187. [Google Scholar] [CrossRef]
- Yang, S.; Hong, S.; Li, M. Microstructure, martensitic transformation and shape memory effect of polycrystalline Cu-Al-Mn-Fe alloys. Sci. China Technol. Sci. 2021, 64, 400–406. [Google Scholar] [CrossRef]
- Dasgupta, R.; Jain, A.K.; Kumar, P.; Hussain, S.; Pandey, A. Role of alloying additions on the properties of Cu-Al-Mn shape memory alloys. J. Alloys Compd. 2015, 620, 60–66. [Google Scholar] [CrossRef]
- Mallik, U.S.; Sampath, V. Influence of quaternary alloying additions on transformation temperatures and shape memory properties of Cu-Al-Mn shape memory alloy. J. Alloys Compd. 2009, 469, 156–163. [Google Scholar] [CrossRef]
- Johnsy, A.G.; Agilan, S.; Jebasingh, S.; Vijay, J.S. Effect of sintering temperature on the mechanical properties of vacuum sintered Co-(Zn)-Ni alloys. Sci. Sinter. 2020, 52, 329–338. [Google Scholar] [CrossRef]
- Yi, X.; Wang, H.; Sun, K.; Shen, G.; Meng, X.; Gao, Z.; Cai, W. Tailoring martensitic transformation and mechanical properties of Ti-Ni composite reinforced by network structure of in-situ TiB and La2O3 phase. Vacuum 2021, 184, 109894. [Google Scholar] [CrossRef]
- Farajollahi, R.; Aval, H.J.; Jamaati, R. Effects of Ni on the microstructure, mechanical and tribological properties of AA2024-Al3NiCu composite fabricated by stir casting process. J. Alloys Compd. 2021, 887, 161433. [Google Scholar] [CrossRef]
- Perez, A.; Sanchette, F.; Billard, A.; Rébéré, C.; Berziou, C.; Touzain, S.; Creus, J. Comparison of the intrinsic properties of EBPVD Al-Ti and Al-Mg coatings. Mater. Chem. Phys. 2012, 132, 154–161. [Google Scholar] [CrossRef]
- Badiâ, A.; Hamadellah, A.; Bouayad, A.; Akili, C. Review of Grain Refinement Performance Of Aluminium Cast Alloys. Metall. taMater. Eng. 2023, 29, 1–15. [Google Scholar]
- Delaney, B.C.; Wang, Q.J.; Aggarwal, V.; Chen, W.; Evans, R.D. A Contemporary Review and Data-Driven Evaluation of Archard-Type Wear Laws. Appl. Mech. Rev. March 2025, 77, 022101. [Google Scholar] [CrossRef]
- Sedlaček, M.; Zupančič, K.; Šetina Batič, B.; Kosec, B.; Zorc, M.; Nagode, A. Influence of Precipitation Hardening on the Mechanical Properties of Co-Cr-Mo and Co-Cr-W-Mo Dental Alloys. Metals 2023, 13, 637. [Google Scholar] [CrossRef]
- Bhuyan, M.; Borah, A. Microstructure and Mechanical Properties Correlation of Al-4.28Cu-1.60 Mg Alloy Micro-Alloyed with Titanium. J. Mater. Eng. Perform. 2023, 32, 11085–11106. [Google Scholar] [CrossRef]
- Styles, M.J.; Hutchinson, C.R.; Chen, Y.; Deschamps, A.; Bastow, T.J. The coexistence of two S (Al2CuMg) phases in Al-Cu-Mg alloys. Acta Mater. 2012, 60, 6940–6951. [Google Scholar] [CrossRef]
- Li, A.; Pang, J.; Zhao, J. Crystallographic texture evolution and tribological behavior of machined surface layer in orthogonal cutting of Ti-6Al-4V alloy. J. Mater. Res. Technol. 2019, 8, 4598–4611. [Google Scholar] [CrossRef]
- Sharma, A.; Ahn, B. Brazeability, Microstructure, and Joint Characteristics of ZrO2/Ti-6Al-4V Brazed by Ag-Cu-Ti Filler Reinforced withCerium Oxide Nanoparticles. Adv. Mater. Sci. Eng. 2019, 2019, 8602632. [Google Scholar] [CrossRef]
- Guan, R.G.; Tie, D. A Review on Grain Refinement of Aluminum Alloys: Progresses, Challenges and Prospects. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 409–432. [Google Scholar] [CrossRef]
- Sun, M.; Yang, D.; Zhang, Y.; Mao, L.; Li, X.; Pang, S. Recent Advances in the Grain Refinement Effects of Zr on Mg Alloys: A Review. Metals 2022, 12, 1388. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, J.; Hu, D.; Li, J. Microstructure and wear resistance of direct laser-deposited TiC-enhanced aluminum-based composite coating for brake discs. Surf. Coat. Technol. 2023, 455, 129193. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, B.; Qiao, N.; Soomro, R.A.; Zhang, R.; Xu, B. Stabilized Ti3C2Tx-doped 3D vesicle polypyrrole coating for efficient protection toward copper in artificial seawater. Appl. Surf. Sci. 2024, 642, 158639. [Google Scholar] [CrossRef]
- Pantić, M.; Jovanović, S.; Djordjevic, A.; Petrović Savić, S.; Radenković, M.; Šarkoćević, Ž. Predicting Wear Rate and Friction Coefficient of Li2Si2O5 Dental Ceramic Using Optimized Artificial Neural Networks. Appl. Sci. 2025, 15, 1789. [Google Scholar] [CrossRef]
- Uniyal, P.; Gaur, P.; Yadav, J.; Khan, T.; Ahmed, O.S. A Review on the Effect of Metal Oxide Nanoparticles on Tribological Properties of Biolubricants. ACS Omega 2024, 9, 12436–12456. [Google Scholar] [CrossRef]
- Hazra, B.; Baranwal, P.; Bera, S.; Show, B.K. Improvement in dry sliding wear resistance of Al-17Si-5Cu alloy after an enhanced heat treatment process. Trans. Nonferrous Met. Soc. China. 2018, 28, 1705–1713. [Google Scholar] [CrossRef]
- Brandaleze, E.; Romanyuk, M.; Barrirero, J.; Mücklich, F.; Schell, N.; Brokmeier, H.G.; Ávalos, M.; Bolmaro, R. High deformation and delamination mechanisms explained through mesoscale and nanoscale phenomena in pearlitic steel wires. Metall. Res. Technol. 2024, 121, 111. [Google Scholar] [CrossRef]
- Wang, L.; Dong, B.; Qiu, F.; Geng, R.; Zou, Q.; Yang, H.; Li, Q.; Xu, Z.; Zhao, Q.; Jiang, Q. Dry sliding friction and wear characterization of in situ TiC/Al-Cu3.7-Mg1.3 nanocomposites with nacre-like structures. J. Mater. Res. Technol. 2020, 9, 641–653. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, L.; Wang, W.; Jin, Z.; Zhang, H. Effects of Ni content on microstructure and wear behavior of Al-13Si-3Cu-1Mg-xNi-0.6Fe-0.6Mn alloys. Wear 2022, 500–501, 204365. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Y.; Liang, S.; Xie, W. The effects of non-isothermal aging on the strength and corrosion behavior of AlZnMgCu alloy. J. Alloys Compd. 2016, 681, 57–65. [Google Scholar] [CrossRef]
- Chai, Z.; Jiang, C.; Zhao, Y.; Wang, C.; Zhu, K.; Cai, F. Microstructural characterization and corrosion behaviors of Ni-Cu-Co coatings electrodeposited in sulphate-citrate bath with additives. Surf. Coat. Technol. 2016, 307, 817–824. [Google Scholar] [CrossRef]
- Doğan, F.; Duru, E.; Akbulut, H.; Aslan, S. How the duty cycle affects wear and corrosion: A parametric study in the Ni-B-TiN composite coatings. Results Surf. Interfaces. 2023, 11, 100112. [Google Scholar] [CrossRef]
- Doğan, F.; Uysal, M.; Algül, H.; Duru, E.; Akbulut, H.; Aslan, S. Optimization of pulsed electro co-deposition for Ni-B-TiN composites and the variation of tribological and corrosion behaviors. Surf. Coat. Technol. 2020, 400, 126209. [Google Scholar] [CrossRef]
- Du, J.; Ding, D.; Zhang, W.; Xu, Z.; Gao, Y.; Chen, G.; Chen, W.; You, X.; Chen, R.; Huang, Y.; et al. CeLa enhanced corrosion resistance of Al-Cu-Mn-Mg-Fe alloy for lithium battery shell. Appl. Surf. Sci. 2017, 422, 221–227. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Wang, Z.; Xu, X.; Chen, M.; Xie, J. Corrosion mechanism and corrosion behavior prediction of Cu-10Ni-X alloys in NaCl solution combining DFT calculation and experiments. Corros. Sci. 2024, 227, 111671. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Ikeuba, A.I. AFM and EIS investigation of the influence of pH on the corrosion film stability of Al4Cu2Mg8Si7 intermetallic particle in aqueous solutions. Appl. Surf. Sci. Adv. 2022, 11, 100291. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.C.; Kim, J.M.; Choi, J.Y.; Yoon, W.S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
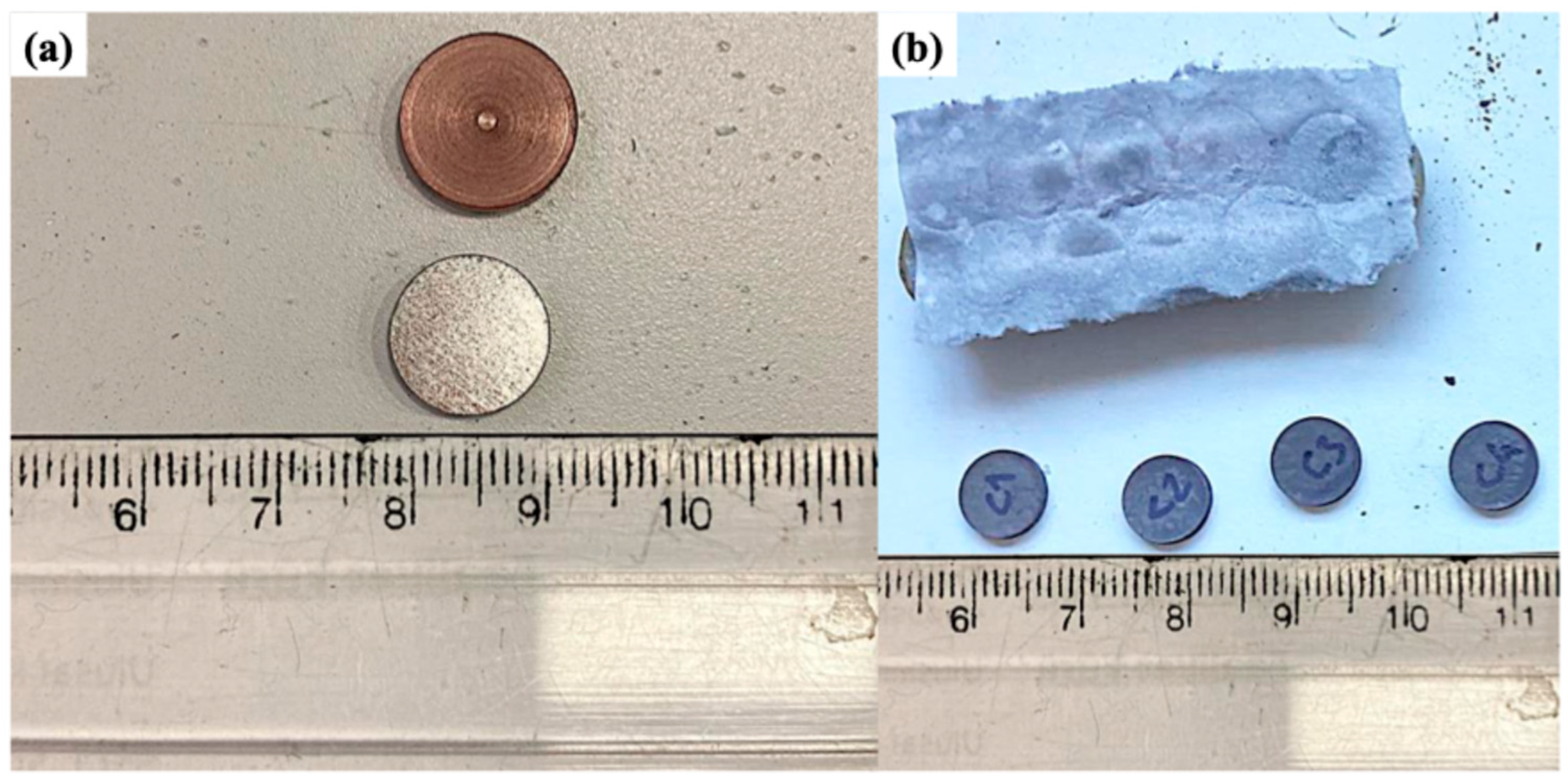


| Sample Codes | Total Weight (g) | Cu (wt.%) | Al (wt.%) | Mn (wt.%) | Fe (wt.%) | CeO2 (wt.%) |
|---|---|---|---|---|---|---|
| C0.05 | 10 | 80.95 | 12 | 5 | 2 | 0.05 |
| C0.1 | 10 | 80.9 | 12 | 5 | 2 | 0.1 |
| C0.5 | 10 | 80.5 | 12 | 5 | 2 | 0.5 |
| C1.0 | 10 | 80 | 12 | 5 | 2 | 1.0 |
| Sample | Ecorr (V) | icorr (A/cm2) |
|---|---|---|
| C0.05 | −0.73 | 1.43 × 10−8 |
| C0.1 | −0.68 | 5.41 × 10−8 |
| C0.5 | −0.58 | 9.03 × 10−6 |
| C1.0 | −0.70 | 6.99 × 10−7 |
| Sample | Rs (Ω·cm2) | Rct (Ω·cm2) | CPEdl (μF·cm−2) | Warburg Coefficient (σ, Ω·s−½·cm2) |
|---|---|---|---|---|
| C0.05 | 251 | 2105 | 58 | 47 |
| C0.1 | 312 | 3213 | 36 | 61 |
| C0.5 | 524 | 5474 | 13 | 105 |
| C1.0 | 453 | 4519 | 29 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doğan, F.; Duru, E. A Novel CuAlMnFe/CeO2 Composite Alloy: Investigating the Wear and Corrosion Features. Solids 2025, 6, 43. https://doi.org/10.3390/solids6030043
Doğan F, Duru E. A Novel CuAlMnFe/CeO2 Composite Alloy: Investigating the Wear and Corrosion Features. Solids. 2025; 6(3):43. https://doi.org/10.3390/solids6030043
Chicago/Turabian StyleDoğan, Fatih, and Erhan Duru. 2025. "A Novel CuAlMnFe/CeO2 Composite Alloy: Investigating the Wear and Corrosion Features" Solids 6, no. 3: 43. https://doi.org/10.3390/solids6030043
APA StyleDoğan, F., & Duru, E. (2025). A Novel CuAlMnFe/CeO2 Composite Alloy: Investigating the Wear and Corrosion Features. Solids, 6(3), 43. https://doi.org/10.3390/solids6030043






