Abstract
Two-dimensional (2D) crystals which present unconventional stoichiometries on graphene surfaces in ambient conditions, such as Na2Cl, Na3Cl, and CaCl, have attracted significant attention in recent years due to their electronic structures and abnormal cation–anion ratios, which differ from those of conventional three-dimensional crystals. This unconventional crystallization is attributed to the cation–π interaction between ions and the π-conjugated system of the graphene surface. Consequently, their physical and chemical properties—including their electrical, optical, magnetic, and mechanical characteristics—often differ markedly from those of conventional crystals. This review summarizes the recent progress made in the fabrication and analysis of the structures, distinctive features, and applications of these 2D unconventional stoichiometry crystals on graphene surfaces in ambient conditions. Their special properties, including their piezoelectricity, metallicity, heterojunction, and room-temperature ferromagnetism, are given particularly close attention. Finally, some significant prospects and further developments in this exciting interdisciplinary field are proposed.
1. Introduction
The discovery of new materials often drives significant industrial innovations while also advancing fundamental scientific understanding. New materials can be obtained through two primary approaches: (1) tailoring intrinsic material parameters, such as the chemical composition, dimensionality, and geometric size of the sample features; (2) inducing changes in the material through external parameters, such as temperature, pressure, epitaxial strain, or electric and magnetic fields [1,2]. Unconventional stoichiometric crystals are a prime example of a new type of material, exhibiting distinct structures and an outstanding performance. Current research on unconventional stoichiometric crystals primarily focuses on high-pressure phases (see Table 1), as such conditions can effectively alter interatomic distances and bonding patterns, thereby inducing structural transformations and stabilizing novel high-pressure phases [1,3]. With the development of high-pressure experimental techniques such as diamond anvil cells, experimental hydrostatic pressure has risen from a few hundred atmospheres to 1000 GPa [4,5,6]. The possibility of reaching higher pressures has spurred research into the synthesis of new compounds with unconventional stoichiometries. Recently, crystal structure prediction (CSP) methods based on first-principles calculations have flourished, owing to the dramatic advances in computer power and algorithmic progress [2,7]. These computational breakthroughs have shortened the theory–experiment validation cycle of new materials from several years to mere months, dramatically accelerating the discovery process of new materials, especially unconventional stoichiometric crystals observed under extreme conditions.
Research over the past three decades on unconventional stoichiometric crystals has shown their distinct two-phase growth pattern (see Figure 1). From 1996 to 2015, publications related to unconventional stoichiometric crystals showed a gradually increasing trend. However, since 2016, the publication rate of papers pertaining to this topic has accelerated dramatically. Between 2016 and 2025, the number of relevant publications exceeded the cumulative total of the previous 20 years, demonstrating that technological breakthroughs can transform fundamental exploration into a thriving research frontier. A large variety of atypical compounds, such as NaCln (n = 3, 7), NanCl (n = 2, 3), Na3Cl2 [8], LiHn (n > 10) [9], V2O [10], H3S [11], IrF8 [12], and CaH7 [13], have been predicted or synthesized under high-pressure conditions. Materials with unconventional stoichiometries often exhibit extraordinary attributes, such as unusual bonding and electronic properties, metallic properties [8,9,10], and superconductivity [11,13,14]. However, harsh preparation conditions and extreme dependence on high-pressure conditions severely limit the development and application of such materials. Hence, there is an urgent need to find new materials which exhibit unconventional stoichiometries under ambient conditions in order to further promote the development of materials science.
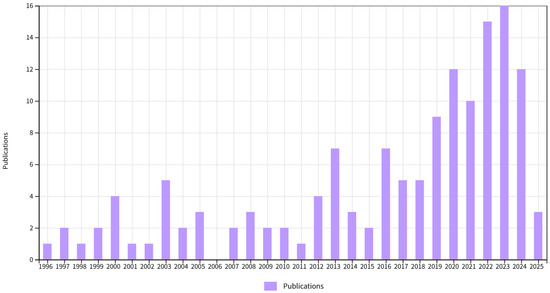
Figure 1.
The evolution of publication trends in the unconventional stoichiometric crystals field over time, according to Web of Science data.
In the 21st century, graphene [15,16] and carbon nanotubes [17] have emerged as rising stars in materials science, exhibiting exceptional mechanical properties, unique physical characteristics, and incomparable catalytic surfaces [18]. Two-dimensional (2D) graphene in particular has facilitated revolutionary advancements in various applications due to its exceptional properties. Recently, 2D Na2Cl, Na3Cl [19], and CaCl [20] crystals with unconventional stoichiometries have been identified on graphene oxide membranes (GOMs) or reduced graphene oxide membranes (rGOMs) at ambient conditions. These 2D crystals’ unique electronic structures endow them with novel attributes, including metallic properties, room-temperature ferromagnetism [20,21], a specific heterostructure, and piezoelectricity [20,22]. In this review, we discuss some recent advances in the fabrication, structural analysis, property exploration, and applications of 2D unconventional stoichiometric crystals on graphene surfaces at ambient conditions. First, we revisit the effect of hydrated cation-π interactions on the behavior of ions at the interface between the solution and the graphene-based materials from the perspective of statistical physics, followed by an introduction to the preparation methods of freestanding GOMs and rGOMs used in the experiments. Building upon this foundation, we discuss the fabrication and structure of 2D unconventional stoichiometric crystals on graphene surfaces at ambient conditions. Special attention is paid to their forming mechanism and the ways in which their properties contrast with those of conventional bulk crystals. Finally, we provide a perspective on future research directions and potential applications of these 2D materials with unconventional stoichiometry.


Table 1.
Examples of unconventional stoichiometric crystals.
Table 1.
Examples of unconventional stoichiometric crystals.
| Conditions | Crystals | Brief Description | Properties | Ref. |
|---|---|---|---|---|
| High pressure | NaCl3 | 1. The calculated phase diagram features unexpected compounds: NaCl3, which is stable above 20 GPa; NaCl7, which is stable above 142 GPa; and Na2Cl, Na3Cl, and Na3Cl2, which are stable above 100 GPa, 77 GPa, and 120 GPa, respectively. 2. High-pressure experiments were performed in a laser-heated diamond anvil cell at 10 to 80 GPa on the Na-Cl system, using excess chlorine and sodium to synthesize NaCl3 and Na3Cl. | Unusual bonding and electronic properties; metallic Na3Cl | [8] |
| NaCl7 | ||||
| Na2Cl | ||||
| Na3Cl | ||||
| Na3Cl2 | ||||
| LiHn, n = 2–8 | Every phase of LiHn (n > 1) is computed to become metallic and stable or metastable in the range of 100–165 GPa. | Metallicity | [9] | |
| V2O | It is identified to be thermodynamically stable in the pressure range of 80–200 GPa. | Metallic and superconducting properties | [10] | |
| H3S | It formed from H2S by decomposition under pressure. | High-Tc superconductivity | [11] | |
| IrF8 | Synthesis of IrF8 using IrF6 and F2 as precursors at a pressure above 39 GPa. | / | [12] | |
| GaH7 | Calculations show a high estimated Tc above 100 K at 200–300 GPa for GaH7. | Superconductivity | [13] | |
| Moderate pressure | PtN2 | PtN2, PtN4, PtN5, and Pt3N4 compounds are stabilized at a moderate pressure of 50 GPa by combining first-principles calculations and particle-swarm-optimized structure search methods. | / | [14] |
| PtN4 | / | |||
| PtN5 | / | |||
| Pt3N4 | Metallic and superconducting properties | |||
| Low pressure | LiCs | It is prepared from an approximately equimolar mixture in very low pressure conditions (<0.1 GPa). | / | [23] |
| Structure prediction | KN2 | Two stable non-stoichiometric 2D materials, KN2 and KN4 monolayers, are uncovered using a combination of swarm intelligence structural search and ab initio calculation. | Metallic properties | [24] |
| KN4 | Superconductivity | |||
| K3Cl2 | K3Cl2 is predicted based on a systematic PSO algorithm approach combined with DFT calculations. | Metallic properties | [25] | |
| Under ambient conditions | Na2Cl | The direct observation, under ambient conditions, of Na2Cl and Na3Cl as Na–Cl crystals on reduced graphene oxide membranes and on the surfaces of natural graphite powders from salt solutions far below the saturated concentration. | / | [19] |
| Na3Cl | / | |||
| CaCl | CaCl crystals are obtained by soaking ultrathin reduced graphene oxide membranes (thickness < 10 nm) in CaCl2 solution below the saturated concentration under ambient conditions. | Metallic properties, room-temperature ferromagnetism, heterojunction, and a piezoelectricity-like property | [20] | |
| Li2Cl | Li2Cl crystal in ultrathin reduced graphene oxide (rGO) membranes by simply soaking the rGO membrane in unsaturated LiCl solution under ambient conditions. | High areal capacitance of 220 mF cm−2, heterostructure property, and piezoelectricity | [22] | |
| K2Cl | K2Cl crystals are obtained by soaking reduced graphene oxide membranes (12 mm diameter) in 0.5 M KCl salt solution for 3 h under ambient conditions. | / | [26] | |
| NaCl2 | NaCl2 crystals are prepared under ambient conditions in graphene oxide membranes with controlled positive surface potential (p-GO). | Room-temperature ferromagnetism | [21] |
2. Hydrated Cation−π Interactions on the Graphene-Based Material Surface
Graphene-based materials feature π electron-rich hexagonal carbon ring structures [27]. Cation–π interaction—the non-covalent interaction between a cation and a π electron-rich carbon-based structure, which was first observed in the 1980s—has a significant impact on ion accumulation behavior on π electron-rich surfaces [28]. Both experimental and theoretical studies have demonstrated that cation–π interactions play important roles in various systems, such as controlling the structures and functions of microscale and nanoscale materials, macromolecules, and proteins [29,30,31,32,33,34]. In aqueous media, Dougherty noted that the cation−π interaction between a cation and a π electron-rich carbon-based structure is seriously weakened by aqueous solution due to cations’ hydrating effects [35]. However, recent theoretical and experimental studies have confirmed that such interactions exert statistical influence on interfacial dynamics, significantly influencing the behaviors and movement of hydrated cations as well as the reactivities and physical characteristics of carbon-based materials or biological macromolecules.
Based on the density functional theory (DFT) computations, Shi et al. incorporated cation–π interactions into the classic force field to study the behavior of ions on π electron-rich carbon-based surfaces in the aqueous solution (see Figure 2a) [36]. They identified a clear enrichment of Na+ on a π electron-rich carbon-based surface in NaCl solutions using molecular dynamics (MD) simulations (see Figure 2b). Interestingly, Cl− was also enriched to some extent on the surface due to the electrostatic interaction between Na+ and Cl−, although the hydrated Cl−-π interaction was weak. The difference in the numbers of Na+ and Cl− accumulated at the interface led to significant negatively charged behavior in the solution, especially in nanoscale systems. Using DFT computation, the analysis showed that the adsorption behavior of hydrated cations on a graphene surface is mainly attributed to the competition between the cation−π interactions and hydration effects: water-mediated cation–water–π interactions and water–cation–π interactions [37]. Meanwhile, Yang et al. revisited the effect of the hydrated cation−π interactions at the interface using statistical physics [38]. They conducted a comprehensive analysis of the statistical effect of hydrated cation–π interactions over time, revealing how hydrated cation−π interactions affect every component dynamically and cause a time-dependent statistical effect at the liquid–solid/soft interface. Thus, they offered an improved understanding of the attachment–detachment processes of cations at π electron-rich interfaces, shifting the perspective from static comparisons to statistical characterization of adsorption-state occupancy during dynamic processes. This provides a temporal perspective for understanding the behavioral mechanisms of various components at π electron-rich interfaces in water–salt systems. Take NaCl solution as an example in an MD simulation to showcase the real-time interfacial dynamics between a benzene molecule and NaCl solution. The results show that the sum of hydrated cation−π interactions over time during the dynamic attachment–departure processes of cations at various interfaces leads to Na+ being attached to the benzene surface for a significant amount of time. This dynamic process not only promotes the ion adsorption on the surface, facilitating the formation of crystals, but also helps to stabilize 2D Na–Cl crystals of stoichiometries with an excess of Na+ due to the stronger Na+–π interaction and charge transfer between Na+ and the aromatic rings in the graphitic surfaces. These findings advance our understanding of crystallization, indicating that crystals with unconventional stoichiometries can be thermodynamically stable in simple systems at ambient conditions. Such crystals have unique electron and spin distributions, and are thus expected to possess novel magnetic, optical, and mechanical properties with extensive applications.
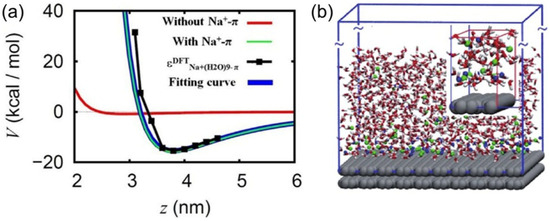
Figure 2.
(a) Theoretical computation of hydrated cation–π interactions. The blue curve represents the fitted Na+-π interaction potential on the graphite surface; the black curve shows the adsorption energies of Na+ with nine water molecules on the graphite surface at different distances (z, the vertical dimension between the Na+ and the surface) at the B3LYP/6–31 G(d) level and the fitting potential; the green curve depicts the Na+-graphene surface interaction potential simulated with the modified classical force field; the red curve displays the corresponding interaction potential simulated using the unmodified classical force field. (b) Snapshot of Na+ and Cl− in the NaCl solution on the graphite surface. Close-up of the lower-left corner in the upper-right inset. The gray structures depict the graphite sheets; water molecules and ions are shown with oxygen in red, hydrogen in white, Na+ in blue, and Cl− in green, respectively. Adapted with permission from Ref. [36]. Copyright 2025 Springer Nature.
3. Fabrication of Freestanding GOM and rGOM
The graphene oxide (GO) suspension is prepared from natural graphite through an improved version of the Hummers method, which mainly consists of three steps: pre-oxidation, strong oxidation exfoliation, and purification [39]. First, natural graphite powder is added to an 80 °C solution of concentrated H2SO4, K2S2O8, and P2O5 to form pre-oxidized graphite. After pre-oxidation, the product is diluted, centrifuged, and washed with deionized water, followed by vacuum drying at 60 °C to obtain pre-oxidized graphene. Next, the pre-oxidized graphite is mixed with concentrated H2SO4, and KMnO4 is gradually added; the temperature is maintained below 6 °C to prevent excessive reaction. Subsequently, deionized water is added stepwise to dilute the mixture while ensuring that the temperature remains below 20 °C to facilitate the formation of larger graphene oxide sheets. Finally, the product is further oxidized with 30% H2O2, then centrifuged and washed with a 1:10 HCl solution and deionized water to remove residual ions, yielding the GO suspension for further use.
Freestanding GOM is fabricated from GO suspension using the drop-casting method [40,41]. Firstly, 1 mL of GO suspension with a concentration of 5 mg/mL is dropped onto the polyethylene terephthalate (PET) substrate with a smooth surface to prepare the GOM [42]. In our experiments, to shorten the preparation time without compromising the quality of the GOM, it is dried at 60 °C for 12 h. Subsequently, the GOM is carefully peeled off, repeatedly washed, and soaked with deionized water to remove any adsorbed impurities. The washed GOM remains in a dry Petri dish for three days until it has dried. Next, the dried GOM undergoes a 1 h thermal reduction process to obtain rGOM. Both the prepared GOM and rGOM are stored in clean, dry containers for further use.
4. Two-Dimensional Na-Cl, K-Cl, and Li-Cl Crystals of Unconventional Stoichiometries on Graphene Surface at Ambient Conditions
4.1. Two-Dimensional Na–Cl Crystals
At ambient conditions, NaCl is the only known stable form of the Na–Cl crystal, whose stoichiometry is 1:1, forming a cubic crystal [43]. In 2018, Shi et al. experimentally observed the formation of two-dimensional (2D) Na2Cl and Na3Cl crystals on the rGOMs in a salt solution with concentrations far below saturation at ambient conditions [19]. These rGOMs were immersed in 3.0 mol L−1 (M) NaCl solutions in a polyethylene bag for 30 min, after which 2D Na-Cl crystals were directly observed on rGOMs, as shown in Figure 3a. The researcher provided four observations to demonstrate that the 2D Na–Cl crystals formed on rGOMs show unconventional stoichiometries: (1) a right shift in the XRD (200) peak at ~32° with respect to the peaks of normal NaCl crystals (see Figure 3c); (2) most (~72%) of the atomic percent of Na and Cl in the selected areas of rGOMs ranged from 1 to 3 via the energy-dispersive X-ray spectroscopy (EDS) element measurements (see Figure 3d); (3) prediction of stable 2D Na-Cl crystals, including Na2Cl and Na3Cl, on a graphitic surface and between two graphene sheets by DFT computation (see Figure 3b); (4) consistency between the right shifts and order of the XRD (200) peaks at ~32° observed experimentally and those predicted theoretically. The unconventional crystallization of 2D Na-Cl crystals, such as Na2Cl and Na3Cl, was attributed to the cation–π interaction between the ions and the π-conjugated system in the graphitic surface, which is suggested by MD and DFT calculations [36,37,38]. Further, there is a charge transfer between the unoccupied valence orbitals of Na+ and the delocalized π states of the aromatic ring structure in the graphene sheet by molecular orbitals and residuary Mulliken charges analyses [44,45]. This charge transfer assists in the stabilization of the 2D Na-Cl crystal structures of unconventional stoichiometries on the graphene sheet and between two graphene sheets.
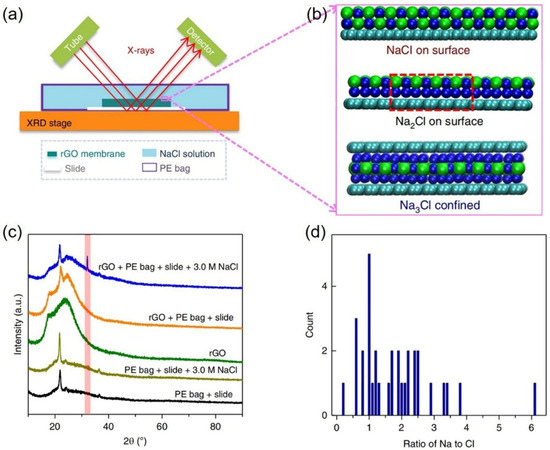
Figure 3.
Two-dimensional Na-Cl crystals from salt solutions far below the saturated concentration: (a) schematic picture of experimental setup; (b) structure snapshots from DFT calculations of Na+ and Cl− on a graphene surface and between two graphene sheets; (c) XRD pattern of rGOMs immersed in the 3.0 M NaCl solution sealed in a PE bag with a glass slide (blue line) together with the XRD patterns of four control systems; (d) ratios of Na to Cl in the 2D crystal structures as determined by EDS–TEM. a.u.—arbitrary units. Adapted with permission from Ref. [19]. Copyright 2025 Springer Nature.
During the current preparation of anomalous stoichiometric 2D crystals, both normal and anomalous stoichiometric crystals coexist, with the content of anomalous stoichiometric crystals in rGOM being less than 1%. To facilitate the exploration of the potential applications, it is urgent to improve synthetic procedures and achieve a high-yield synthesis of 2D abnormal crystals with unconventional stoichiometries on rGOM. By applying negative potential on rGOM, Xia et al. achieved a high-yield synthesis of Na2Cl crystals on rGOM [46]. As shown in Figure 4a, they utilized a three-electrode configuration to build the electric potential environment, with a potential of −0.6 V applied to rGOMs. The rGOM, as a working electrode with a negative potential, was incubated for 1 h in a saturated NaCl solution (see Figure 4a). Then, the rGOMs were dried with an infrared lamp for 30 min in air. Compared to that at 0.0 V, the atomic content of Na increased by one order of magnitude when a negative potential of −0.6 V was applied to rGOMs, as shown in Figure 4b. This high-atomic-content Na existed in the form of dense 2D Na-Cl crystals in rGOM, with a lateral size of tens of nanometers (inset in Figure 4a). DFT calculations revealed that, especially at the low negative potential of −0.6 V, the increase in negative charges on the surface of graphene led to stronger Na+–π interaction and weaker electrostatic repulsion between cations. The authors suggested that the negative potential on rGOM not only promoted the formation of 2D Na2Cl crystals but also helped to stabilize crystal structures. Furthermore, 2D Na2Cl crystals with a square structure exhibited an unexpected piezoelectric property, which was directly observed by piezoresponse force microscopy (PFM) and membrane bending experiments, as shown in Figure 4d. The output voltage of 2D Na2Cl crystals in the rGOMs increased with the degree of bending. When the rGOMs were quickly bent with a periodic bending angle of 150°, the output voltage could reach ≈ 180 mV, as shown in Figure 4c, meeting the voltage requirement of most nanodevices in realistic applications. This study presents a valuable exploration of successful high-yield synthesis of 2D crystals with unconventional stoichiometries through the application of negative potential to rGOM, which sheds light on potential improvements that could be applied to synthetic procedures.
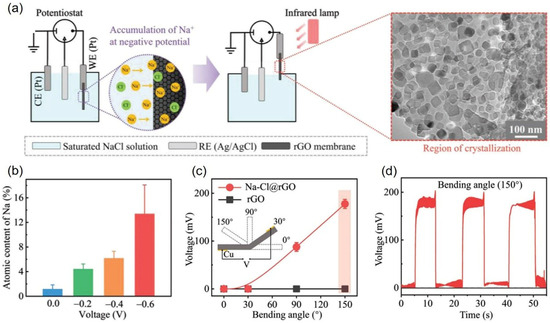
Figure 4.
Two-dimensional Na-Cl crystals in the rGO membranes (Na-Cl@rGO) by applying a negative potential: (a) schematic of synthesis of 2D Na-Cl crystals; (b) atomic content of Na in Na-Cl@rGO measured by EDS-SEM; (c) piezoelectric behavior of rGO, Na-Cl@rGO membranes bent at angles of 30°, 90°, and 150°, respectively; (d) voltage responses from rGO (black line) and Na-Cl@rGO membranes (red line). Adapted with permission from Ref. [46]. Copyright 2025 John Wiley and Sons.
Recently, Yi et al. experimentally synthesized a stable 2D anion-rich NaCl2 crystal with a sandwiched structure (see Figure 5a), confined in graphene oxide membranes with positive surface potential (p-GO), at ambient conditions [21]. p-GO suspension was prepared by adding polyethyleneimine (PEI) to the GO suspension, which functionalized the GO flakes and modulated the zeta (ζ) potential of the GO surface to positive potential from the original negative potential in a neutral environment. Freestanding p-GO membranes were fabricated by drop-casting the p-GO suspension and then drying them at 70 °C for 12 h. When the p-GO membranes were immersed in the NaCl solution, the surface of p-GO with a positive potential exhibited a more favorable attraction to Cl− than to Na+, forming a stable 2D anion-rich NaCl2 crystal, whose structure comprised a layer of positively charged sodium cations sandwiched by two layers of negatively charged chloride anions. The authors suggested that the unconventional structure of 2D anion-rich NaCl2 crystals induced ferromagnetism at room temperature (see Figure 5b). Magnetic property measurement system (MPMS-3, Quantum Design) results showed strongly enhanced ferromagnetism with clear hysteresis loops in Na-Cl@p-GO (see Figure 5c). The saturation magnetic moment (Ms) of Na-Cl@p-GO was 0.63 emu/g, approximately three times that of p-GO (0.20 emu/g). Theoretical calculations revealed room-temperature ferromagnetism originating from the spin-polarization of electrons in the Cl elements of these crystals. This was unexpected, as conventional compounds composed solely of Na and Cl are typically non-magnetic [47]. The spin-polarized band and density-of-state calculation [48] results showed that the spin-down channel near the Fermi level was fully occupied by the p electrons of the chloride anions, whereas the spin-up channel remained empty. This electronic configuration contrasts with the properties commonly observed in most transition metal chalcogenides.
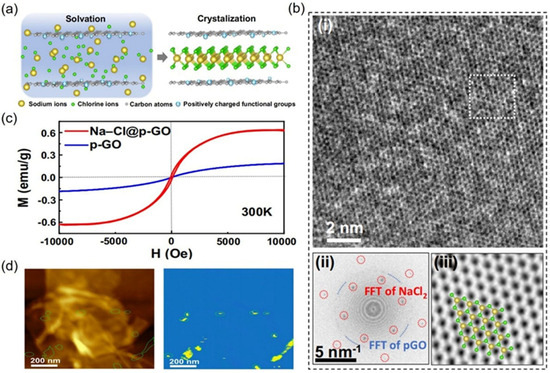
Figure 5.
Two-dimensional NaCl2 crystal with sandwiched structure in GOMs with p-GO: (a) schematic design of a p-GO membrane with a positively charged surface prepared by functionalizing GO with polyethyleneimine (PEI); (b) high-resolution TEM (HR-TEM) of a NaCl2 single crystal; (c) magnetization hysteresis loops of Na-Cl@p-GO membranes and p-GO membranes measured at room temperature; (d) atomic force microscopy (AFM) images (left) and MFM images (right) of Na–Cl@p-GO membrane. Reprinted with permission from Ref. [21]. Copyright 2025 Springer Nature.
4.2. Two-Dimensional Li-Cl Crystals
Under ambient conditions, LiCl is the only known stable form of the Li-Cl crystal, whose stoichiometry is 1:1, and the corresponding crystals have an insulating effect [49]. Li and Cl form highly ionic bonds due to the large difference in their electronegativity, making them suitable for use in batteries [50,51,52,53,54,55] and capacitors [56,57,58]. It is important to understand the Li-Cl configuration and its properties for the development of Li-based electrochemical energy storage and conversion devices. Recently, Li et al. successfully prepared a novel abnormal 2D Li2Cl crystal in ultrathin rGOMs by simply immersing the rGOM in a LiCl solution with concentrations far below saturation at ambient conditions [22]. These large, flat, stable 2D Li2Cl crystals could be directly observed on ultrathin rGOMs by freezing the crystals in LiCl solution to provide electronic imaging protection and in situ solution crystallization. The Li-Cl lattice exhibited a square lattice, characterized by four first-order maxima at ≈7.781 nm−1 by electron diffraction and fast Fourier transform (FFT) analyses, and the diffraction points of Li2Cl crystals displayed quite good consistency between experimental observations and theoretical predictions. In contrast to LiCl, these Li2Cl crystals exhibited metallic properties rather than insulating properties. The metallicity of 2D Li2Cl crystals was experimentally confirmed using conductive atomic force microscopy (C-AFM) on graphene sheets. Figure 6a,b show the AFM images and I–V characteristics of both pure graphene sheets and Li2Cl–graphene sheets on the Au-coated quartz substrates. Interestingly, the I–V curve of dried Li2Cl–graphene exhibited a resistance of 2.6 × 106 Ω, only 1/3 times lower than that of pure graphene, suggesting that the Li2Cl crystals possess metallic properties. DFT calculations were performed, and thus, the most stable geometry-optimized structure of Li2Cl was obtained, with Li ions on the bottom layer and Li ions and Cl ions on the top layer, ultimately forming a metallic Li2Cl crystal heterojunction configuration in the membrane. The authors suggested that the 2D Li2Cl crystals consist of alternately distributed Li and Cl elements, and the material containing these crystals should show piezoelectricity-like properties due to the differing electric effects of the two elements under compressive or tensile strain. As shown in Figure 6c, the output voltage of the dried Li2Cl-rGO membrane increased with the degree of bending, indicating a clear piezoelectricity-like characteristic. In addition, the dried Li2Cl-rGO membranes were highly sensitive to humidity and had a corresponding current at every humidity (see Figure 6d), indicating that the dried Li2Cl-rGO membrane can be used as a humidity sensor.
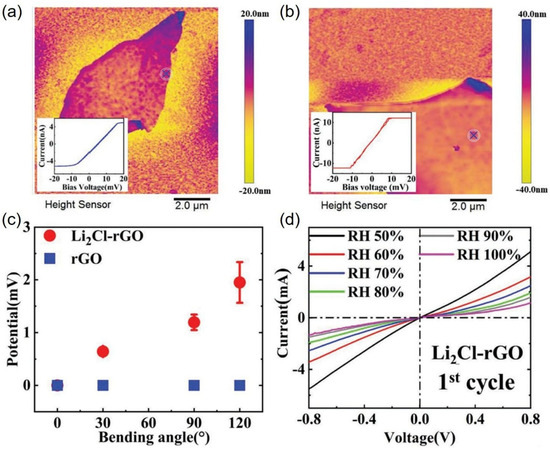
Figure 6.
Two-dimensional metallic Li-Cl crystals from salt solution below the saturated concentration: AFM images and I–V characteristics of (a) the pure dried graphene sheets and (b) dried graphene sheets with Li and Cl on Au-coated quartz substrates; (c) piezoelectricity-like property of the dried Li2Cl-rGO membrane; (d) LSV curves of dried Li2Cl-rGO for the first cycle for humidity sensing. Adapted with permission from Ref. [22]. Copyright 2025 John Wiley and Sons.
4.3. Two-Dimensional K-Cl Crystals
Limited cycling stability, low specific capacity, and poor K-ion intercalation performance limit the large-scale application of rechargeable potassium-ion batteries (PIBs) [59,60,61,62,63,64,65]. Zhan et al. synthesized a freestanding K-Cl–graphene paper electrode with abnormal stoichiometric KxCl crystals (KxCl-rGO) and proposed a novel universal design strategy using this KxCl crystal-decorated freestanding rGO as an electrode to create a highly efficient K-graphene battery [26]. As shown in Figure 7a, a freestanding GOM was prepared by drop-casting GO suspension onto a smooth paper substrate and then drying it. Then, the electrical conductivity of these GOMs was improved by annealing in air at 400 °C. To complete the K-ion decoration, GOMs were further immersed in 0.5 M KCl solution for 3 h, forming abnormal stoichiometric KxCl crystals, which generally exhibited good conductivity and potassium storage performance. The authors used the KxCl-rGO as an anode in half cells against potassium metal to explore the impact of the KxCl crystal decorations with unconventional stoichiometries on K-ion storage. Firstly, the storage behavior of K+ ions in both rGO and KxCl-rGO was investigated by cyclic voltammetry (CV), as shown in Figure 7b. For rGO, a cathodic peak was observed at approximately 0.60 V in the first cycle, while no cathodic peak was observed around the exact location for KxCl-rGO. Notably, the KxCl-rGO exhibited a broad and robust reduction peak at 0.06 V, attributed to the intercalation of K ions into the graphene layers. The authors suggested that the KxCl crystal decoration with unconventional stoichiometries not only promoted the insertion of K ions but also improved the capacity of the samples, which exhibited a critical difference in the energetics of KxCl-rGO versus rGO K-ion storage. In addition, the galvanostatic charge/discharge profiles of the KxCl-rGO electrode exhibited no apparent discharge plateau between 0.5 and 0.01 v in the first potassiation cycle at a current density of 20 mA g−1, which was consistent with the expectation that this unconventional stoichiometric KxCl crystal decoration can play a role in pre-potassium. This result indicated KxCl crystals with unconventional stoichiometries decorating rGO could store more K-ions than pure rGO. Figure 7d shows the galvanostatic charge/discharge profiles of the K-ion full batteries (KxCl-rGO as the anode and K2Fe[Fe(CN)6] as the cathode) at a current density of 20 mA g−1 with a voltage window of 1.0–4.0 V. The K2Fe[Fe(CN)6]//KxCl-rGO full batteries exhibited a charge/discharge capacity of 70.7/46.5 mAh g−1 with an initial CE of 65.8%. These results indicated that the K-ion storage performance of rGO can be significantly enhanced after the decoration with abnormal stoichiometric KxCl crystal.
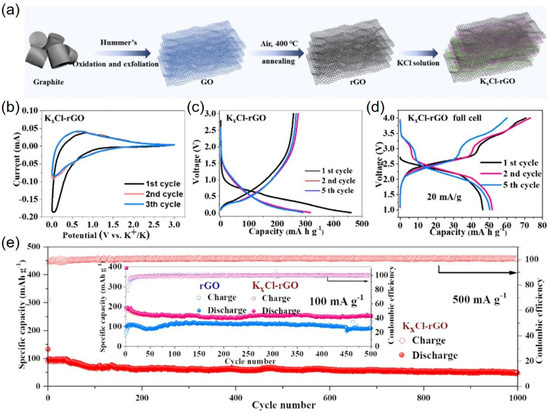
Figure 7.
(a) Schematic of the synthesis process of KxCl-rGO electrode; (b) CV curves of KxCl-rGO as PIB anodes in half cells at a scan rate of 0.1 mV s−1; (c) galvanostatic discharge and charge profiles for the first five cycles of the KxCl-rGO electrode at a current density of 20 mA g−1; (d) galvanostatic charge–discharge curves of the KxCl-rGO K-ion full batteries at a current density of 20 mA g−1; (e) cycling performance and corresponding Coulombic efficiency of freestanding KxCl-rGO electrode at 500 mA g−1. Adapted with permission from Ref. [26]. Copyright 2025 Elsevier.
5. Two-Dimensional Ca-Cl Crystals of Unconventional Stoichiometries on Graphene Surfaces at Ambient Conditions
Compared to monovalent cations, the cation–π interactions between divalent cations and π-conjugated systems are much stronger [20], which makes it much easier to obtain 2D crystals of unconventional stoichiometries at the same experimental conditions. Just by simply immersing ultrathin rGOMs (with a thickness < 10 nm) in CaCl2 solution with concentrations below saturation, Zhang et al. directly observed 2D Ca-Cl crystals on rGOMs under ambient conditions via cryo-electron microscopy (cryo-EM) [20], as shown in Figure 8a. Figure 8b(i) showed high-resolution cryo-EM images of 2D Ca-Cl crystals with a lattice spacing of 4.29 ± 0.14 Å, corresponding to a graphene-like honeycomb lattice with a side length of 2.86 ± 0.09 Å. The stoichiometries of crystals on rGOMs at different detection depths were analyzed by XPS combined with argon ion etching [66]. With increasing detection depth, the Ca:Cl ratios varied from a value below 0.6:1 at the membrane top surface to a stable ∼1.2:1 in the inner sheets (Figure 8c). It can be inferred from these results that the membrane surface exhibited regular CaCl2 because of evaporation of the adsorbing salt solution, but the inner sheets mainly exhibited CaCl crystals with a stable Ca:Cl ratio of ∼1:1. Figure 8d showed the magnetization hysteresis loops of the dried rGO and Ca–Cl@rGO membranes at 300 K. For the dried CaCl@rGO membrane, the saturation magnetic moment (Ms) was ∼2.1 emu/g, whereas the Ms of the dried rGOM was ∼0.4 emu/g. This result indicated that the magnetism of rGOM can be significantly enhanced by ∼400% by introducing as little as ∼5% calcium to the membrane. DFT calculations revealed that the possible origin of such strong ferromagnetism was the edge or defect effects of the CaCl crystals, where there was an unpaired valence electron in Ca+, and the saturation magnetic moment computed was consistent with the experimental observations. In addition, the metallicity, coexistence of piezoelectricity-like property, and heterojunction were also experimentally verified. It was proposed that such abnormal crystal materials are common in nature, since the strong cation–π interactions also existed between other metal cations (such as Mg2+, Fe2+, Co2+, Cu2+, Cd2+, Cr2+ and Pb2+) and graphitic surfaces [29]. In fact, CuCl crystals with unconventional stoichiometries could exist stably for several days under ambient conditions and exhibited similar room-temperature ferromagnetism, which would make it possible to overcome the challenge of the short lifetime of the +1 copper ion [67].
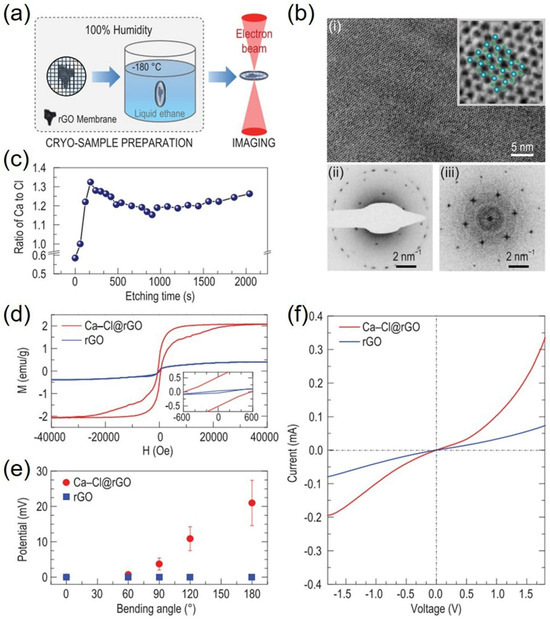
Figure 8.
Two-dimensional Ca-Cl crystals from salt solution below the saturated concentration: (a) schematic drawings of the sample preparation processes; (b) cryo-EM image of the Ca-Cl crystals in the ultrathin rGOM; (c) atomic ratio of Ca to Cl as a function of the etching time; (d) room-temperature ferromagnetism of the dried Ca-Cl@rGO membrane; (e) piezoelectricity-like property of the dried Ca-Cl@rGO membrane under ambient conditions; (f) heterojunction behavior of the dried Ca-Cl@rGO membrane. Reprinted with permission from Ref. [20]. Copyright 2025 Oxford University Press.
6. Two-Dimensional Na-Cl Crystals of Unconventional Stoichiometries on Metal Surfaces at Ambient Conditions
Since crystals of unconventional stoichiometries can form at the interface of carbon-based materials under ambient conditions, it is reasonable to infer that similar phenomena will occur at the interface of other materials, such as metals. He et al. found salt crystals of unconventional stoichiometries (i.e., Na2Cl and Na3Cl) formed from unsaturated solutions on an iron surface, which could enhance iron corrosion [68]. This finding helps to improve our understanding of fundamental physical and chemical views, including crystallization, metal corrosion, and electrochemical reactions. As shown in Figure 9a, some 2D Na2Cl crystals of unconventional stoichiometries formed on the iron surface in addition to the ordinary NaCl crystals. Based on EDS analysis using scanning electron microscopy (SEM), the atomic percentage of O and Fe for the iron surface with Na2Cl was approximately 0.76, which was about six times higher than that with ordinary NaCl (~0.12, see Figure 9b). This contradicts the conventional theory in which metal corrosion is typically attributed to chlorine-induced corrosion. DFT calculations suggested that the adsorption energy of Cl−–iron was significantly higher than that of Na+–iron. As shown in Figure 9e, the adsorption energy of Cl− rapidly decreased to zero (green line in Figure 9e), whereas the adsorption energy of Na+ decreased slowly (blue line in Figure 9e). Notably, the long-range interaction between Na+ and iron facilitated more rapid adsorption of Na+ on the iron surface, since the adsorption energy of Na+–iron was greater than that of Cl—iron when the distance between Na+ and iron exceeded 0.4 nm. Under such conditions, the abnormal Na2Cl crystal site had a higher corrosion rate than the NaCl site. These findings provide new insights into metallic corrosion.
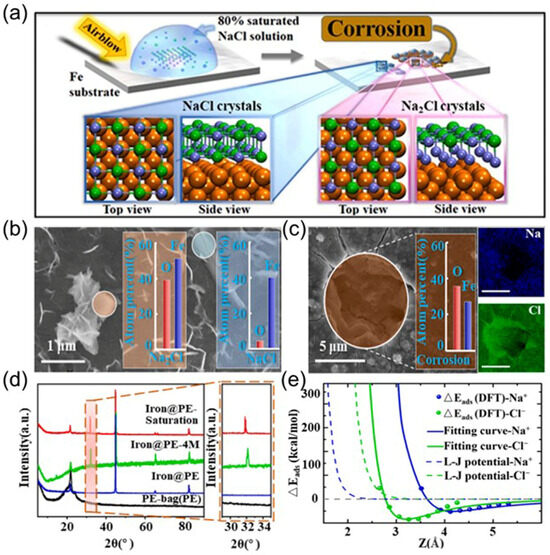
Figure 9.
SEM-EDS images of Na–Cl crystals with different concentrations of NaCl solutions on the surface of the Fe sample: (a) schematic diagram of the experimental procedure for growing Na2Cl crystals on metal; (b,c) SEM image of the corrosion condition of the crystal on the metal surface; (d) XRD pattern of iron sample immersed in 4.0 M and saturated with NaCl solution; (e) density functional theory (DFT) adsorption energies between Na+–(H2O)4/Cl−–(H2O)4 (dots) and iron. Adapted with permission from Ref. [68]. Copyright 2025 Elsevier.
7. Conclusions and Perspective
Recently, 2D crystals with unconventional stoichiometries on GOM/rGOM at ambient conditions, such as Na2Cl, Na3Cl, and CaCl, have attracted a great deal of attention in light of their unique electronic structures. The fabrication of these 2D crystals is remarkably straightforward, requiring only immersion of GOM/rGOM substrates in corresponding unsaturated solution at ambient conditions. For main-group elements such as Na, Ca, and Cl, whose inner electron shells are fully occupied, their unconventional stoichiometry crystals generally exhibit unique outer-layer (as a valence electron layer) electronic structures. These configurations, which are inherently unstable (previously observed only under extreme conditions such as high pressure), simultaneously manifest distinctive orbital electron arrangements that give rise to extraordinary physical properties. For instance, the 2D NaCl2 crystals exhibit room-temperature ferromagnetism with clear hysteresis loops and a transition temperature above 320 K, originating from the spin-polarization of electrons in the Cl elements; the 2D CaCl crystals on rGOM display unexpected metallicity, room-temperature ferromagnetism, and resultant graphene–CaCl heterojunction, coexistence of piezoelectricity-like properties and metallicity, together with distinct hydrogen storage and release capability under ambient conditions; and the KxCl crystal-decorated freestanding rGO electrodes exhibit outstanding K ion storage properties, including high capacity and good rate capability. These properties completely contradict the traditional understanding that only transition metals are ferromagnetic and have high expectations in applications of material science, electronic components, biology, chemistry, and physics.
At present, research on 2D crystals of unconventional stoichiometries at ambient conditions is in its infancy. It is believed that every metal element possesses unique properties, such as room-temperature ferromagnetism, through the formation of the corresponding abnormal 2D crystals containing metal ions with unpaired valence electrons. Furthermore, considering the widespread abundance of metallic cations and carbon on Earth, such nanoscale ‘special’ compounds with previously unrecognized properties could be ubiquitous in nature. It is imperative that synthesis-related issues are addressed to unlock the full application potential of these 2D crystals and their derivatives. Applying a negative potential to rGOM to achieve the high-yield synthesis of Na2Cl crystals represents a promising strategy for improving their size and yield. Nevertheless, further refinement of synthetic protocols is required to obtain larger, higher-yield 2D crystals. Another important issue is the development of repeatable and controllable synthesis methods, as the performance of these 2D crystals is highly dependent on the architecture of the nanoscale building blocks, meaning that in many cases, experiments of this nature cannot be successfully repeated. Whatever the future holds for research on 2D crystals with unconventional stoichiometries at ambient conditions, one can be assured that their unique properties will ensure a steady stream of compelling scientific inquiries. Looking ahead, advancing the established synthetic procedures will be the key to unlocking new opportunities for this material.
Funding
This work was supported by the National Natural Science Foundation of China (No. 12435001), the Natural Science Foundation of Shanghai, China (No. 23JC1401400), the Fundamental Research Funds for the Central Universities of East China University of Science.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, L.; Wang, Y.; Lv, J.; Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2017, 2, 17005. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Ma, Y. Materials by design at high pressures. Chem. Sci. 2022, 13, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Sun, Y.; Zurek, E.; Lin, H. Chemistry under high pressure. Nat. Rev. Chem. 2020, 4, 508–527. [Google Scholar] [CrossRef]
- Dubrovinsky, L.; Dubrovinskaia, N.; Prakapenka, V.; Abakumov, A. Implementation of micro-ball nanodiamond anvils for high-pressure studies above 6 Mbar. Nat. Commun. 2012, 3, 1163. [Google Scholar] [CrossRef] [PubMed]
- Dubrovinskaia, N.; Dubrovinsky, L.; Solopova, N.; Abakumov, A.; Turner, S.; Hanfland, M.; Bykova, E.; Bykov, M.; Prescher, C.; Prakapenka, V.; et al. Terapascal static pressure generation with ultrahigh yield strength nanodiamond. Sci. Adv. 2016, 2, e1600341. [Google Scholar] [CrossRef] [PubMed]
- McMillan, P. Pressing on: The legacy of Percy W. Bridgman. Nat. Mater. 2005, 4, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Gong, X.; Yin, W. Crystal structure prediction by combining graph network and optimization algorithm. Nat. Commun. 2022, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Oganov, A.; Goncharov, A.; Zhu, Q.; Boulfelfel, S.; Lyakhov, A.; Stavrou, E.; Somayazulu, M.; Prakapenka, V.; Konôpková, Z. Unexpected Stable Stoichiometries of Sodium Chlorides. Science 2013, 342, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Zurek, E.; Hoffmann, R.; Ashcroft, N.; Oganov, A.; Lyakhov, A. A little bit of lithium does a lot for hydrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 17640–17643. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, J.; Yu, H.; Lin, J.; Zhang, S.; Yang, G. Unconventional stable stoichiometry of vanadium peroxide. Phys. Chem. Chem. Phys. 2020, 22, 11460–11466. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.; Eremets, M.; Troyan, I.; Ksenofontov, V.; Shylin, S. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, Z.; Liu, C.; Zhang, J.; Du, X.; Yang, G.; Ma, Y. IrF8 Molecular Crystal under High Pressure. J. Am. Chem. Soc. 2019, 141, 5409–5414. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cui, W.; Shi, J.; Hao, J.; Li, Y. Superconducting H7 chain in gallium hydrides at high pressure. Phys. Chem. Chem. Phys. 2023, 25, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xiao, X.; Dai, W.; Sun, W.; Ding, K.; Lu, C. Predicted the structural diversity and electronic properties of Pt–N compounds under high pressure. J. Phys. Condens. Mattter 2023, 35, 285501. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Geim, A.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.; Grigorieva, I.; Firsov, A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Khan, U.; Blau, W.; Gun’ko, Y. Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Geim, A.; Novoselov, K. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Chen, L.; Yang, Y.; Li, D.; Qian, Z.; Liang, S.; Yan, L.; Li, L.H.; Wu, M.; Fang, H. Two-dimensional Na–Cl crystals of unconventional stoichiometries on graphene surface from dilute solution at ambient conditions. Nat. Chem. 2018, 10, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, G.; Peng, B.; Gao, P.; Chen, L.; Zhong, N.; Mu, L.; Zhang, L.; Zhang, P.; Gou, L.; et al. Novel 2D CaCl crystals with metallicity, room-temperature ferromagnetism, heterojunction, piezoelectricity-like property and monovalent calcium ions. Nat. Sci. Rev. 2021, 8, nwaa274. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Jiang, J.; Yang, Y.; Zhang, Y.; Gao, S.; Zhao, Y.; Hu, J.; Su, X.; Xia, X.; Peng, B.; et al. Two-dimensional anion-rich NaCl2 crystal under ambient conditions. Nat. Commun. 2025, 16, 464. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Liu, X.; Qiu, Y.; He, Z.; Zhang, S.; Fang, H.; Chen, L.; Zhang, L.; Shi, G. 2D metallic abnormal Li2Cl crystals with unique electronic characteristics applied in capacitor and humidity sensor. Adv. Mater. Interfaces 2023, 10, 11. [Google Scholar] [CrossRef]
- Desgreniers, S.; Tse, J.; Matsuoka, T.; Ohishi, Y.; Tse, J. Mixing unmixables: Unexpected formation of Li–Cs alloys at low pressure. Sci. Adv. 2015, 1, e1500669. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Y.; Jiang, X.; Wang, B.; Zhao, J. Unconventional stoichiometric two-dimensional potassium nitrides with anion-driven metallicity and superconductivity. J. Mater. Chem. C 2024, 12, 103–109. [Google Scholar] [CrossRef]
- Shao, L.; Huo, H.; Tian, S.; Zhao, X.; Chen, D.; Li, Y.; Ma, C.; Ye, H.; Su, C.; Du, Y. A new 2D metallic K3Cl2 nanosheet as a promising candidate of NO2 gas sensor and capturer. Appl. Surf. Sci. 2022, 604, 154554. [Google Scholar] [CrossRef]
- Zhan, J.; Lei, Z.; Liu, X.; Yang, M.; Li, M.; Fang, H.; Zhang, Y.; Wang, Y.; Shi, G. Abnormal-stoichiometric KxCl crystal decoration: A new strategy to improve K-Ion storage performance of graphene paper. Carbon 2020, 192, 93–100. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R. Graphene-Based Materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Sunner, J.; Nishizawa, K.; Kebarle, P. Ion-solvent molecule interactions in the gas phase. The potassium ion and benzene. J. Phys. Chem. 1981, 85, 1814–1820. [Google Scholar] [CrossRef]
- Mahadevi, A.; Sastry, G. Cation-π interaction: Its role and relevance in chemistry, biology, and material science. Chem Rev. 2013, 113, 2100–2138. [Google Scholar] [CrossRef] [PubMed]
- Daze, K.; Hof, F. The cation-π interaction at protein-protein interaction interfaces: Developing and learning from synthetic mimics of proteins that bind methylated lysines. Acc. Chem. Res. 2013, 46, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Song, B.; Shi, G.; Li, H.; Ji, G.; Hu, J.; Chen, X.; Fang, H. Cation⊗3π: Cooperative Interaction of a Cation and Three Benzenes with an Anomalous Order in Binding Energy. J. Am. Chem. Soc. 2012, 134, 12104–12109. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yang, J.; Zhao, J.; Fang, H. Intercalation and Diffusion of Lithium Ions in a Carbon Nanotube Bundle by ab initio Molecular Dynamics Simulations. Energy Environ. Sci. 2011, 4, 1379–1384. [Google Scholar] [CrossRef]
- Xiu, X.; Puskar, N.; Shanata, J.; Lester, H.; Dougherty, D. Nicotine binding to brain receptors requires a strong cation–π interaction. Nature 2009, 458, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Torrice, M.; Bower, K.; Lester, H.; Dougherty, D. Probing the Role of the Cation–π Interaction in the Binding Sites of GPCRs using Unnatural Amino Acids. Proc. Natl. Acad. Sci. USA 2009, 106, 11919–11924. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, A. The Cation-π Interaction. Acc. Chem. Res. 2013, 46, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, J.; Wang, C.; Song, B.; Tu, Y.; Hu, J.; Fang, H. Ion enrichment on the hydrophobic carbon-based surface in aqueous salt solutions due to cation-π interactions. Sci. Rep. 2013, 3, 3436. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Yang, Y.; Liu, J.; Du, W.; Chen, J.; Shi, G.; Fang, H. Hydrated cation-π interactions of π-electrons with hydrated Li+, Na+, and K+ cations. Phys. Chem. Chem. Phys. 2021, 23, 14662–14670. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, S.; Wu, H.; Shi, G.; Fang, H. Revisit the hydrated cation−π interaction at the interface: A new view of dynamics and statistics. Langmuir 2022, 38, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Kovtyukhova, N.; Ollivier, P.; Martin, B.; Mallouk, T.; Chizhik, S.; Buzaneva, E.; Gorchinskiy, A. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Dikin, D.; Stankovich, S.; Zimney, E.; Piner, R.; Dommett, G.; Evmenenko, G.; Nguyen, S.; Ruoff, R. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective ion penetration of graphene oxide membranes. ACS Nano 2013, 7, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Sai, H.; Li, Z.; Li, F. Formation of uniform reduced graphene oxide films on modified PET substrates using drop-casting method. Particuology 2014, 17, 66–73. [Google Scholar] [CrossRef]
- Roessler, D.; Walker, W. Electronic Spectra of Crystalline NaCl and KCl. Phys. Rev. 1968, 166, 599–606. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Huang, Y.; Peng, B.; Wang, T.; Yi, R.; Zhao, Y.; Jiang, J.; Dai, F.; Fan, Y.; Li, P.; et al. High-yield synthesis of sodium chlorides of unconventional stoichiometries. Adv. Mater. 2023, 35, 2303072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, D.; Si, M.; Zhu, Z.; Yang, G.; Shi, Z.; Xue, D. Origin of the unexpected room temperature ferromagnetism: Formation of artificial defects on the surface in NaCl particles. J. Mater. Chem. C 2013, 1, 6216–6222. [Google Scholar] [CrossRef]
- Serr, A.; Netznetz, R. Polarizabilities of hydrated and free ions derived from DFT calculations. Int. J. Quantum Chem. 2006, 106, 2960–2974. [Google Scholar] [CrossRef]
- Prencipe, M.; Zupan, A.; Dovesi, R.; Aprà, E.; Saunders, V. Ab initio study of the structural properties of LiF, NaF, KF, LiCl, NaCl, and KCl. Phys. Rev. B 1995, 51, 3391. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Goodenough, J. Layered lithium cobalt oxide cathodes. Nat. Energy 2021, 6, 323. [Google Scholar] [CrossRef]
- Whittingham, M. Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 2014, 114, 11414. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.; Freunberger, S.; Hardwick, L.; Tarascon, J. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798. [Google Scholar] [CrossRef] [PubMed]
- Assat, G.; Tarascon, J. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 2018, 3, 373. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Hu, W.; Hu, X. Thermotolerant separators for safe lithium-ion batteries under extreme conditions. J. Mater. Chem. A 2020, 8, 20294. [Google Scholar] [CrossRef]
- Shen, L.; Lv, H.; Chen, S.; Kopold, P.; Aken, P.; Wu, X.; Maier, J.; Yu, Y. Dual-functionalized double carbon shells coated silicon nanoparticles for high performance lithium-ion batteries. Adv. Mater. 2017, 29, 1700142. [Google Scholar] [CrossRef] [PubMed]
- Jezowski, P.; Crosnier, O.; Deunf, E.; Poizot, P.; Béguin, F.; Brousse, T. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt. Nat. Mater. 2018, 17, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode materials, electrolytes, and challenges in nonaqueous lithium-ion capacitors. Adv. Mater. 2018, 30, 1705670. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Bianchini, M.; Seo, D.; Rodriguez-Garcia, J.; Ceder, G. Recent Progress and Perspective in Electrode Materials for K-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702384. [Google Scholar] [CrossRef]
- Share, K.; Cohn, A.; Carter, R.; Rogers, B.; Pint, C. Role of nitrogen-doped graphene for improved high-capacity potassium ion battery anodes. ACS Nano 2016, 10, 9738–9744. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Syu, J.; Zhao, Y.; Lo, C.; Varma, A.; Pol, V. Binder-free N- and O-rich carbon nanofiber anodes for long cycle life K-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 17872–17881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, W.; Xie, J.; Lei, H.; Zhu, Y.; Huang, W.; Xu, X.; Zhao, Z.; Mai, W. Sulphur-doped reduced graphene oxide sponges as high-performance free-standing anodes for K-ion storage. Nano Energy 2018, 53, 415–424. [Google Scholar] [CrossRef]
- Ruan, J.; Zhao, Y.; Luo, S.; Yuan, T.; Yang, J.; Sun, D.; Zheng, S. Fast and stable potassium-ion storage achieved by in situ molecular self-assembling N/O dual-doped carbon network. Energy Storage Mater. 2019, 23, 46–54. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, Y.; Zhang, Q.; Luo, C.; Li, H.; Wu, Y.; Zhang, H.; Wang, X.; Liu, H.; He, X.; et al. Designing and understanding the superior potassium storage performance of nitrogen/phosphorus Co-doped hollow porous bowl-like carbon anodes. Adv. Funct. Mater. 2020, 31, 2007158. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Yi, Z.; Zhou, J.; Pan, Z.; Tian, J.; Wang, Y.; Sun, S.; Lin, N.; Qian, Y. Revealing the double-edged behaviors of heteroatom sulfur in carbonaceous materials for balancing K-storage capacity and stability. Adv. Funct. Mater. 2020, 31, 2006875. [Google Scholar] [CrossRef]
- Lu, Y.; Li, D.; Liu, F. Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching. Materials 2022, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.; Goswami, A.; Felpin, F.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R. Cu and Cu-based nanoparticles: Synthesis and applications in review catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, X.; Li, Y.; Yang, H.; Ding, Z.; Luo, Y.; Shi, G. Unexpected iron corrosion by excess sodium in two-dimensional Na-Cl crystals of abnormal stoichiometries at ambient conditions. J. Colloid Interface Sci. 2023, 648, 102–107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).