Performance Evaluation of Low-Grade Clay Minerals in LC3-Based Cementitious Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Activation
2.2. Characterization Techniques
2.3. Development of LC3
2.4. Experimental Setup
3. Results and Discussion
3.1. Reactivity of the Clays
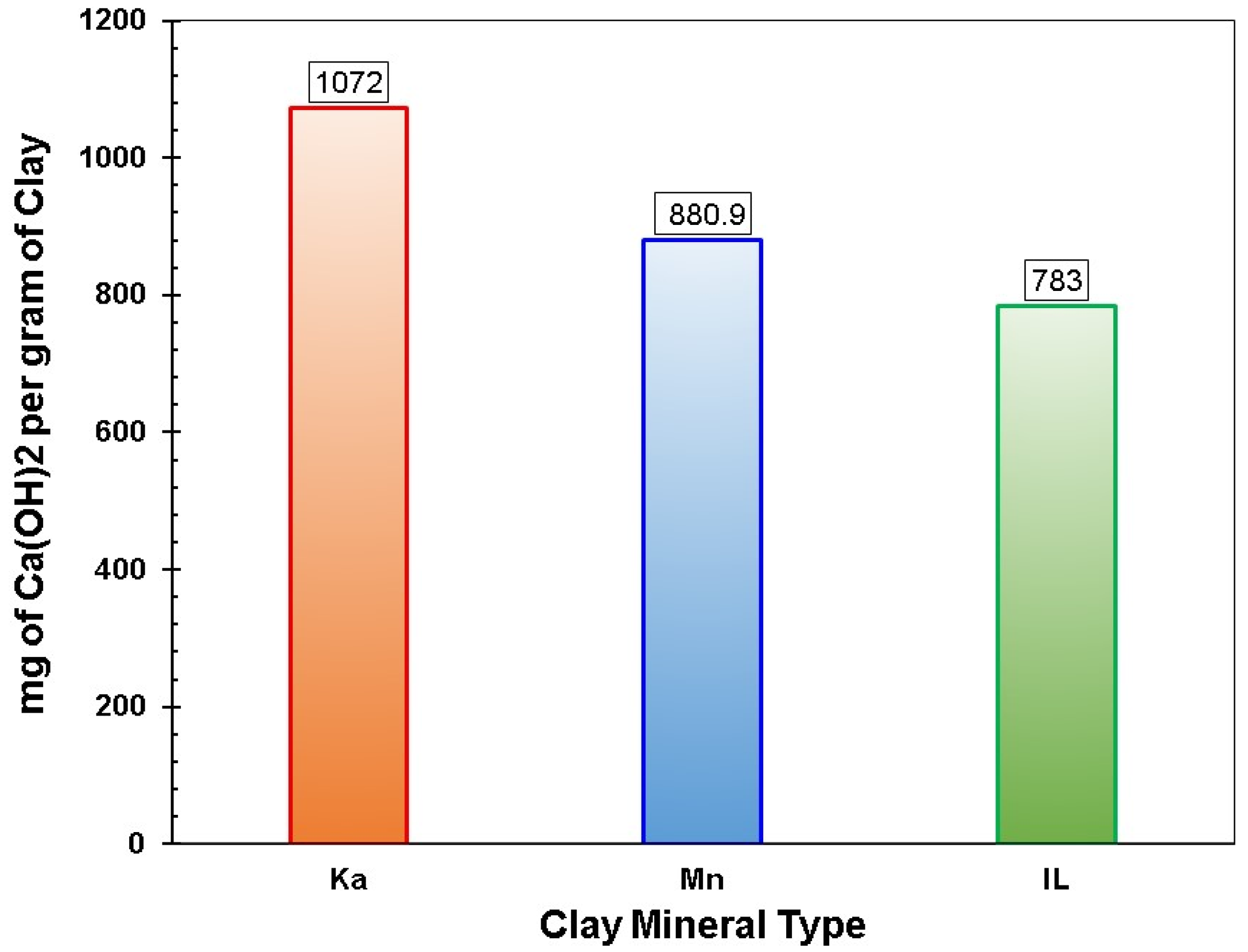
3.1.1. The Modified Chapelle Test
3.1.2. Mineralogical Evaluation of Calcined Clays
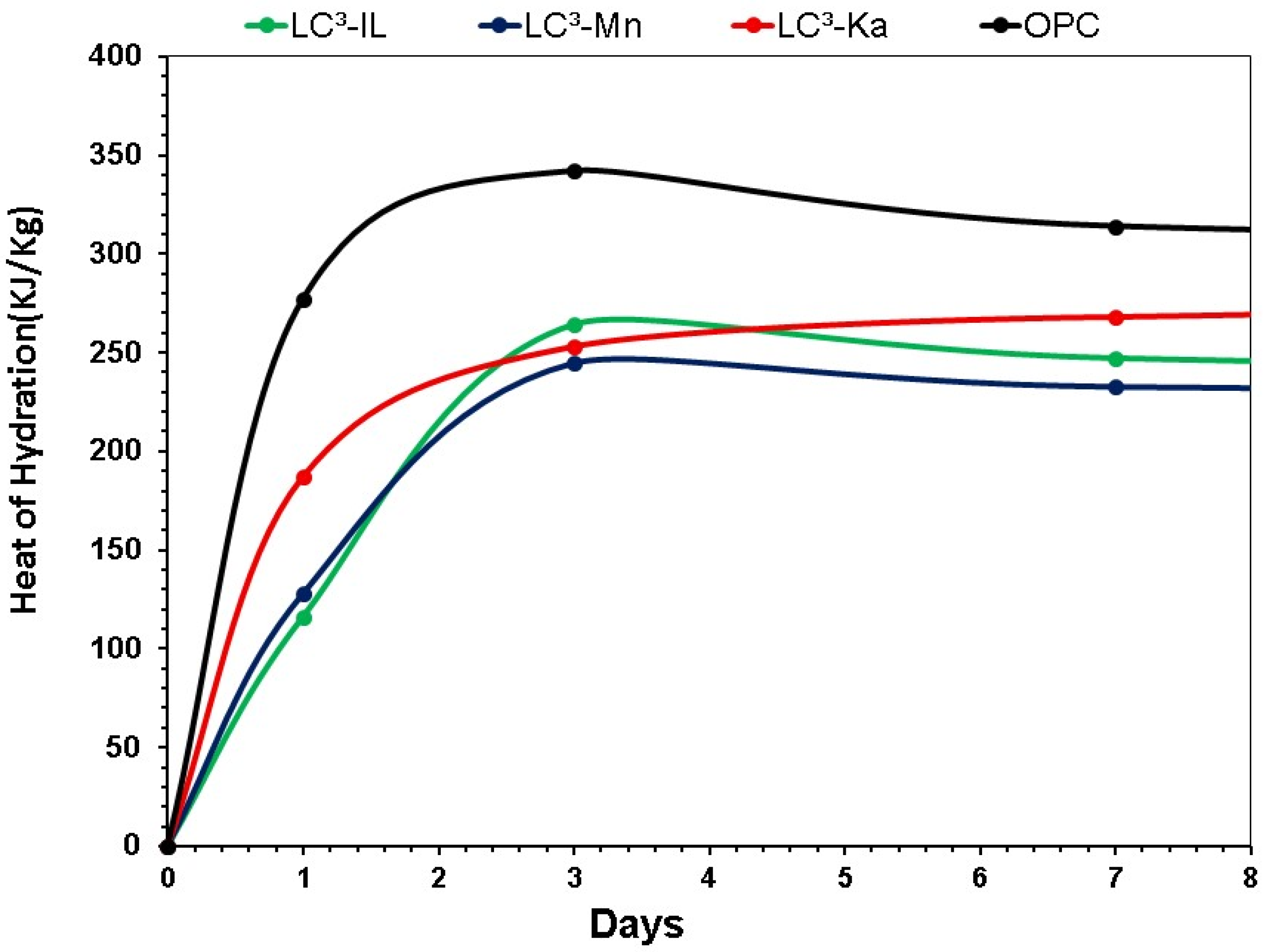
3.2. Heat of Hydration
3.3. Hydration Products
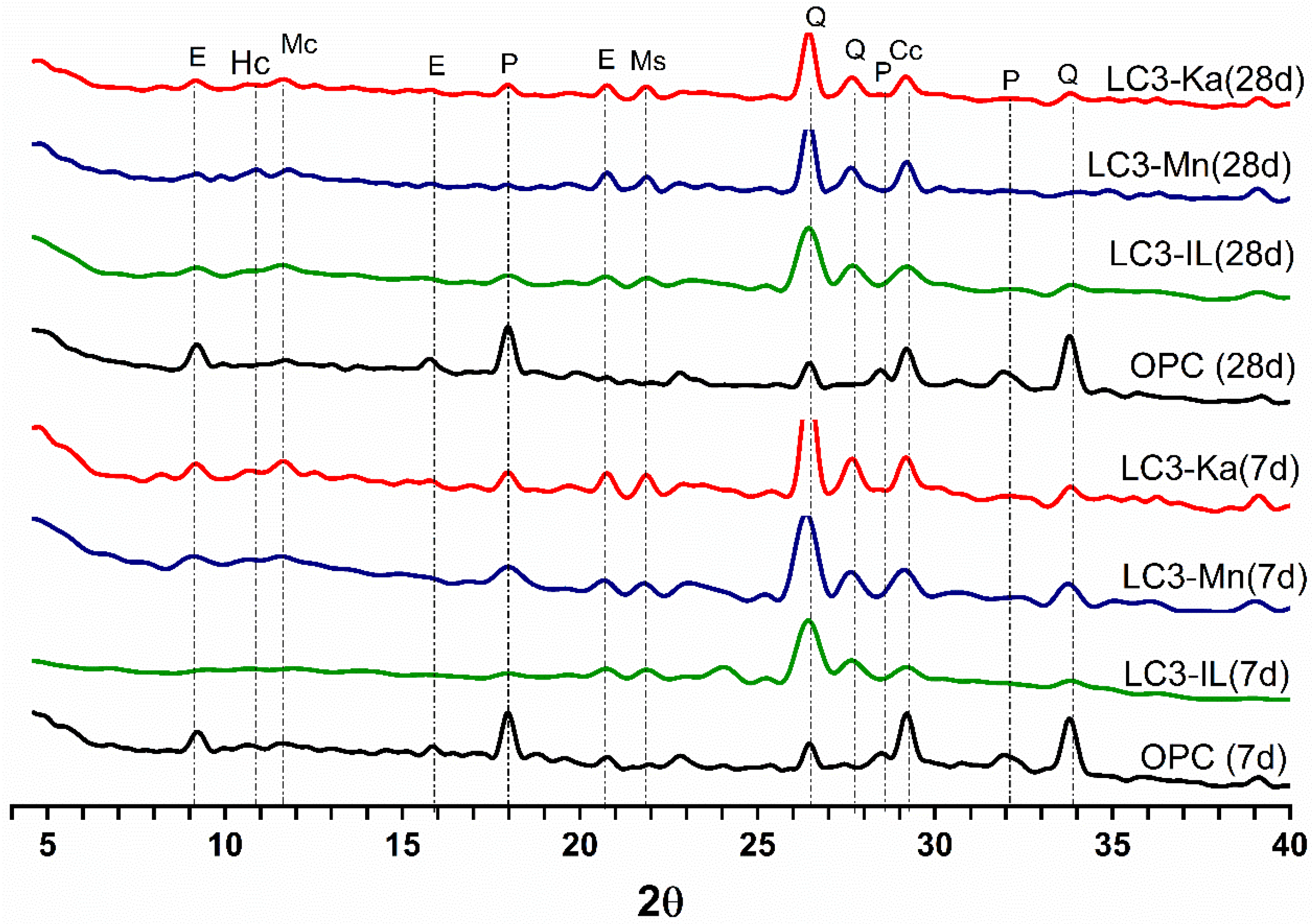
3.3.1. X-Ray Diffraction (XRD)
3.3.2. Thermogravimetric Analysis (TGA)
3.3.3. Scanning Electron Microscopy (SEM)
3.4. Setting Time and Consistency of Blends
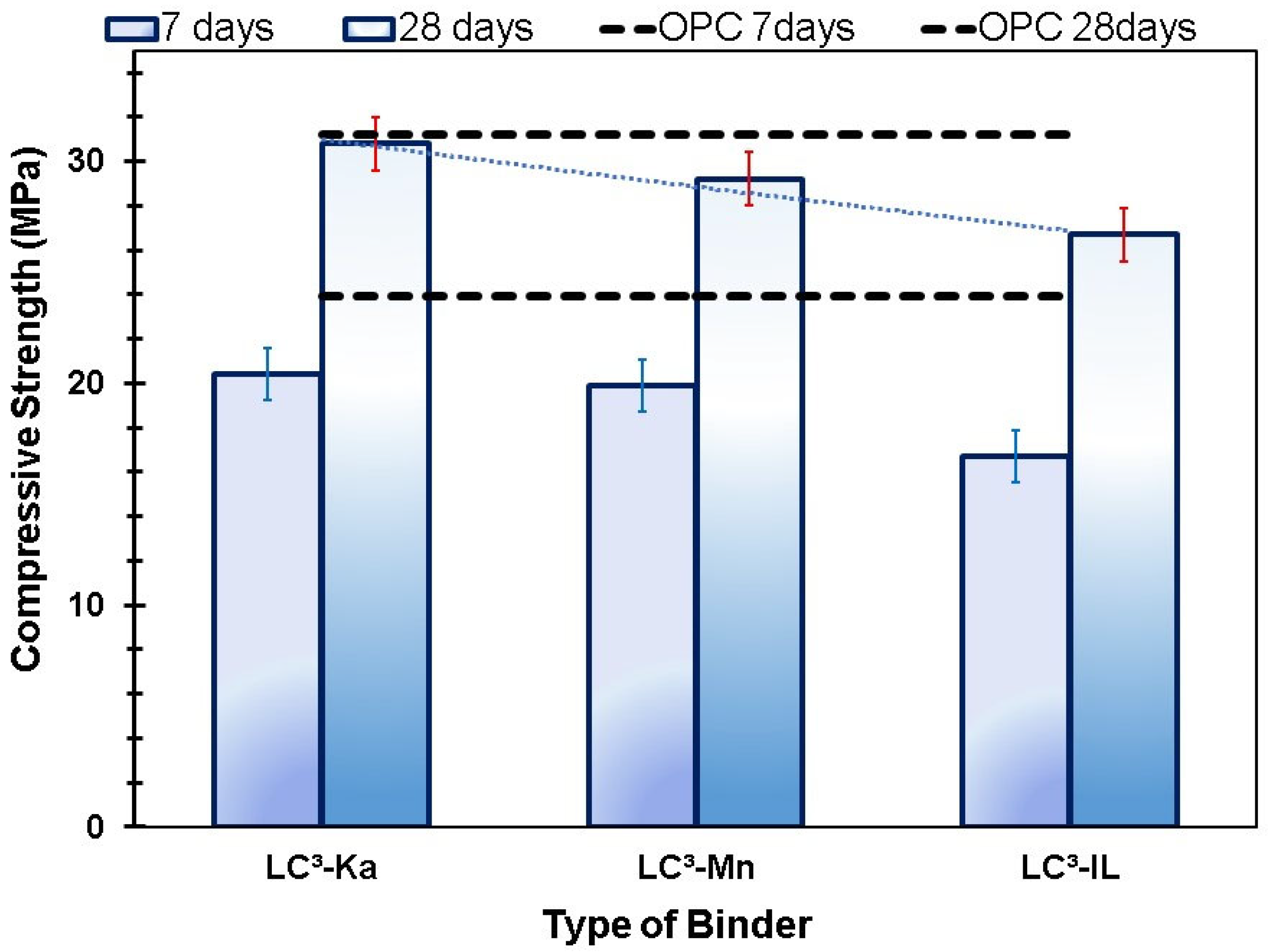
3.5. Compressive Strength
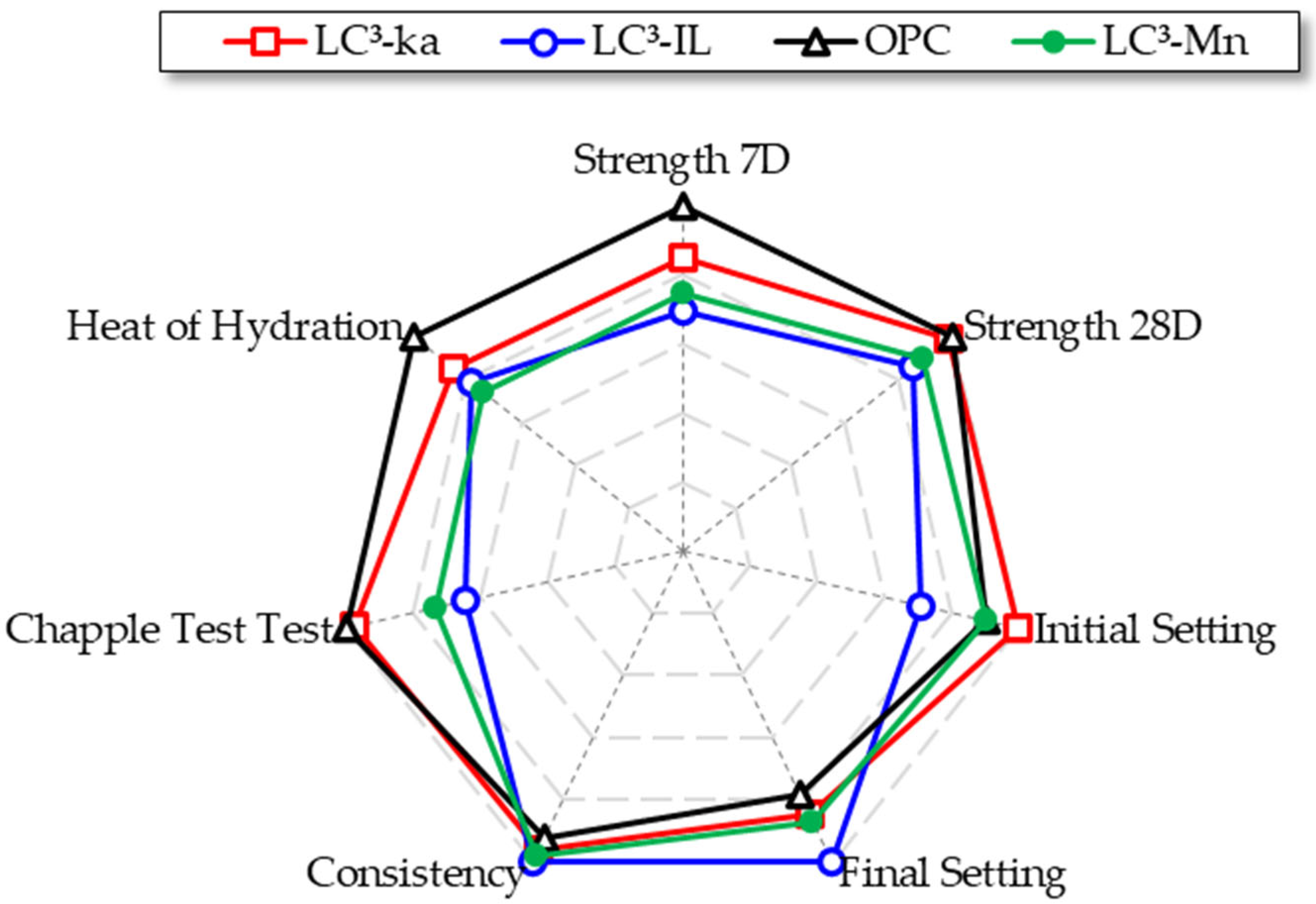
3.6. Overall Performance Summary
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC3 | Limestone calcined clay cement (LC3) |
| OPC | Ordinary Portland Cement (OPC) |
| IL | Illite |
References
- Py, L.G.; Neto, J.S.A.; Longhi, M.A.; Kirchheim, A.P. Evaluation of ultrafine calcined clays on LC3 cements on the sulfate requirement, water demand and strength development. Mater. Struct. 2024, 57, 13. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting factors and properties of limestone calcined clay cements (LC3). Green Mater. 2019, 7, 3–14. [Google Scholar] [CrossRef]
- Sun, J.; Zunino, F.; Scrivener, K. Hydration and phase assemblage of limestone calcined clay cements (LC3) with clinker content below 50%. Cem. Concr. Res. 2024, 177, 107417. [Google Scholar] [CrossRef]
- Kafodya, I.; Basuroy, D.; Marangu, J.M.; Kululanga, G.; Maddalena, R.; Novelli, V.I. Mechanical performance and physico-chemical properties of limestone calcined clay cement (LC3) in Malawi. Buildings 2023, 13, 740. [Google Scholar] [CrossRef]
- Sui, H.; Hou, P.; Liu, Y.; Sagoe-Crentsil, K.; de Souza, F.B.; Duan, W. Limestone calcined clay cement: Mechanical properties, crystallography, and microstructure development. J. Sustain. Cem. Mater. 2023, 12, 427–440. [Google Scholar] [CrossRef]
- Yu, J.; Mishra, D.K.; Hu, C.; Leung, C.K.; Shah, S.P. Mechanical, environmental and economic performance of sustainable Grade 45 concrete with ultrahigh-volume Limestone-Calcined Clay (LCC). Resour. Conserv. Recycl. 2021, 175, 105846. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. structure and mineralogy of clay minerals. In Developments in Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 21–81. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A com-parison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Li, K.J.; Kurniawan, A.; Christidis, G.E.; He, J.Y.; Zhou, C.H. Interfacial interactions controlling adsorption of metal cations on montmorillonite. Am. Miner. 2024, 109, 633–655. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. Clay Clay Min. 2021, 24, 1–29. [Google Scholar]
- Ghosh, B.; Chakraborty, D. An overview of the clay minerals: Composition, classification, internal structure and properties. In Clay Minerals: Their Antimicrobial and Antitoxic Applications; Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 1–24. [Google Scholar]
- Hay, R.; Celik, K. Performance enhancement and characterization of limestone calcined clay cement (LC3) produced with low-reactivity kaolinitic clay. Constr. Build. Mater. 2023, 392, 131831. [Google Scholar] [CrossRef]
- Hay, R.; Celik, K. Comparative performance of OPC and LC3-based composites under combined action of carbonation and chloride exposure. Constr. Build. Mater. 2024, 429, 136428. [Google Scholar] [CrossRef]
- Her, S.; Im, S.; Liu, J.; Suh, H.; Kim, G.; Sim, S.; Wi, K.; Park, D.; Bae, S. Exploring the potential of pulverized oyster shell as a limestone substitute in limestone calcined clay cement (LC3) and its implications for performance. Constr. Build. Mater. 2024, 425, 135918. [Google Scholar] [CrossRef]
- Han, F.; Li, Y.; Zhu, Z.; Zhang, Z. Synergistic utilization of Al-rich and carbonate-rich mineral admixtures toward sustainable low-carbon cementitious materials: A review. J. Build. Eng. 2025, 105, 112523. [Google Scholar] [CrossRef]
- Diaz, A.A.; Reyes, R.S.A.; Hanein, T.; Irassar, E.F.; Juenger, M.; Kanavaris, F.; Maier, M.; Marsh, A.T.; Sui, T.; Thienel, K.-C.; et al. Properties and occurrence of clay resources for use as supplementary cementitious materials: A paper of RILEM TC 282-CCL. Mater. Struct. 2022, 55, 139. [Google Scholar] [CrossRef]
- Blouch, N.; Rashid, K.; Zafar, I.; Ltifi, M.; Ju, M. Prioritization of low-grade kaolinite and mixed clays for performance evaluation of Limestone Calcined Clay Cement (LC3): Multi-criteria assessment. Appl. Clay Sci. 2023, 243, 107080. [Google Scholar] [CrossRef]
- Krishnan, S.; Rao, D.G.; Bishnoi, S. Why Low-Grade Calcined Clays Are the Ideal for the Production of Limestone Calcined Clay Cement (LC3). In Calcined Clays for Sustainable Concrete: Proceedings of the 3rd International Conference on Calcined Clays for Sustainable Concrete, New Delhi, India, 15–17 October 2019; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 25, p. 125. [Google Scholar]
- Zolfagharnasab, A.; Ramezanianpour, A.A.; Bahman-Zadeh, F. Investigating the potential of low-grade calcined clays to produce durable LC3 binders against chloride ions attack. Constr. Build. Mater. 2021, 303, 124541. [Google Scholar] [CrossRef]
- De Maio, U.; Gaetano, D.; Greco, F.; Lonetti, P.; Pranno, A. An adaptive cohesive interface model for fracture propagation analysis in heterogeneous media. Eng. Fract. Mech. 2025, 325, 111330. [Google Scholar] [CrossRef]
- Tijssens, M.; Sluys, L.; van der Giessen, E. Simulation of fracture of cementitious composites with explicit modeling of microstructural features. Eng. Fract. Mech. 2001, 68, 1245–1263. [Google Scholar] [CrossRef]
- Werling, N.; Kaltenbach, J.; Weidler, P.G.; Schuhmann, R.; Dehn, F.; Emmerich, K. Solubility of Calcined Kaolinite, Montmorillonite, and Illite in High Molar NaOH and Suitability as Precursors for Geopolymers. Clays Clay Miner. 2022, 70, 270–289. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Kassa, A.E.; Shibeshi, N.T.; Tizazu, B.Z. Kinetic analysis of dehydroxylation of Ethiopian kaolinite during calcination. J. Therm. Anal. Calorim. 2022, 147, 12837–12853. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Castellano, C.C.; Trezza, M.A.; Rahhal, V.F.; Cordoba, G.; Lemma, R. Calcined illite-chlorite shale as supplementary cementing material: Thermal treatment, grinding, color and pozzolanic activity. Appl. Clay Sci. 2019, 179, 105143. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.; Lund, J.; Herfort, D. Influence of fineness of raw clay and calcination temperature on the performance of calcined clay-limestone blended cements. Appl. Clay Sci. 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Waheed, I.F.; Awsaj, M.M.; Dahham, O.S.; Jabbar, M.Q.; Al-Abady, F.M.; Al-Samarrai, M.A.F. Synthesis of CoMnFe2O4 hollow microstructure decorated GO for photocatalytic degradation of organic dyes. Arab. J. Chem. 2024, 17, 105934. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Guo, Z.; Zhang, S.; Wu, P.; Sun, X. Activation the hydration properties of illite-containing tailings to prepare a binder for cemented paste backfill. Constr. Build. Mater. 2022, 318, 125989. [Google Scholar] [CrossRef]
- Marsh, A.T.; Krishnan, S.; Bernal, S.A. Structural features of thermally or mechano-chemically treated montmorillonite clays as precursors for alkali-activated cements production. Cem. Concr. Res. 2024, 181, 107546. [Google Scholar] [CrossRef]
- Rakhimova, N. Montmorillonite clays in Portland clinker-reduced, non-clinker cements, and cement composites: A review. Constr. Build. Mater. 2024, 411, 134678. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.; Allen, C.; Gu, Y.; Xi, Y. Thermal behaviors of clay minerals as key components and additives for fired brick properties: A review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Reactivity of kaolinitic clays calcined in the 650 °C–1050 °C temperature range: Towards a robust assessment of overcalcination. Cem. Concr. Compos. 2024, 146, 105380. [Google Scholar] [CrossRef]
- Luzu, B.; Duc, M.; Djerbi, A.; Gautron, L. High performance illitic clay-based geopolymer: Influence of thermal/mechanical activation on strength development. Appl. Clay Sci. 2024, 258, 107445. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- ASTM C 191-92; Standard Test Method for Time of Setting of Hydraulic Cement by Vicat Needle, Annual Book of ASTM. ASTM International: West Conshohocken, PA, USA, 1995; Volume 4.
- ASTM C 191-08; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2008.
- Chen, C.; Zhang, Y.; Li, X.; He, J.; Gao, F.; Chen, Z. Investigations into methane hydrate formation, accumulation, and distribution in sediments with different contents of illite clay. Appl. Energy 2024, 359, 122661. [Google Scholar] [CrossRef]
- Yaman, İ.Ö. Effects of Clay Type on the Properties of Limestone Calcined Clay Cement; Middle East Technical University: Ankara, Turkey, 2024. [Google Scholar]
- Allegretta, I.; Pinto, D.; Eramo, G. Effects of grain size on the reactivity of limestone temper in a kaolinitic clay. Appl. Clay Sci. 2016, 126, 223–234. [Google Scholar] [CrossRef]
- Fatima, M.; Rashid, K.; Blouch, N. Thermo-Mechano-Chemo Dynamics of Clay’s Reactivity and its Correlation with Limestone Calcined Clay Cement (LC3) Performance. J. Adv. Concr. Technol. 2024, 22, 689–705. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties, compatibility, environmental benefits and future directions of limestone calcined clay cement (LC3) concrete: A review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, K.; Ye, H. Understanding reactivity of calcined marine clay as a supplementary cementitious material through structural transformation of clay minerals. Cem. Concr. Compos. 2025, 160, 106066. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Blouch, N.; Rashid, K.; Ju, M. Exploring low-grade clay minerals diving into limestone calcined clay cement (LC3): Characterization—Hydration—Performance. J. Clean. Prod. 2023, 426, 139065. [Google Scholar] [CrossRef]
- Boakye, K.; Khorami, M. Performance of Calcined Impure Kaolinitic Clay as a Partial Substitute for Portland Cement Concrete: A Review. J. Compos. Sci. 2025, 9, 145. [Google Scholar] [CrossRef]
- Adem, J.K.; Seo, J.; Park, S.; Kim, G. Effects of low-lime calcium silicate cement addition on the hydration, carbonation, and microstructural characteristics of OPC pastes by carbonation curing. J. Build. Eng. 2025, 107, 112683. [Google Scholar] [CrossRef]
- Jayathilakage, R.; Gunasekara, C.; Law, D.; Setunge, S. A combination technique to improve natural low-grade illite as supplementary cementitious material for concrete. Constr. Build. Mater. 2025, 477, 141334. [Google Scholar] [CrossRef]
- Bansal, T.; Talakokula, V. Comparative analysis of very-early age hydration process: LC3 vs. conventional and blended cement pastes using embedded piezo sensors. Measurement 2024, 229, 114433. [Google Scholar] [CrossRef]
- Shao, Z.; Cao, M. Hydration mechanism of limestone calcined clay cement containing calcined coal gangue. Constr. Build. Mater. 2024, 438, 136906. [Google Scholar] [CrossRef]
- Shen, X.; He, H.; He, C.; Li, B.; Luo, W.; Ren, P. Low-carbon blended cement containing wet carbonated municipal solid waste incineration fly ash and mechanically activated coal fly ash. Case Stud. Constr. Mater. 2024, 21, e03671. [Google Scholar] [CrossRef]
- Ponomar, V.; Yliniemi, J.; Kilpimaa, K. Influence of calcium oxide and hydroxide on non-ferrous metallurgical slag hydration in alkaline environments. Cem. Concr. Compos. 2024, 152, 105673. [Google Scholar] [CrossRef]
- Ren, S.; Huang, G.; Zhang, W.; Sha, X.; Liu, G.; Lin, R.-S.; Chen, L. Enhancing cement-based materials hydration and carbonation efficiency with pre-carbonated lime mud. J. CO2 Util. 2024, 88, 102928. [Google Scholar] [CrossRef]
- Mañosa, J.; Calderón, A.; Salgado-Pizarro, R.; Maldonado-Alameda, A.; Chimenos, J.M. Research evolution of limestone calcined clay cement (LC3), a promising low-carbon binder—A comprehensive overview. Heliyon 2024, 10, e25117. [Google Scholar] [CrossRef]
- Wu, H.; He, M.; Cheng, J.; Wang, T.; Che, Y.; Du, Y. Study on the synergistic effects of OPC and silica fume on the mechanical and microstructural properties of geopolymer mortar. Constr. Build. Mater. 2024, 434, 136740. [Google Scholar] [CrossRef]
- Georget, F.; Wilson, W.; Scrivener, K.L. Edxia: Microstructure characterisation from quantified SEM-EDS hypermaps. Cem. Concr. Res. 2021, 141, 106327. [Google Scholar] [CrossRef]
- Atasever, M.; Erdoğan, S.T. Effects of clay type and component fineness on the hydration and properties of limestone calcined clay cement. Mater. Struct. 2024, 57, 183. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, L.; Yan, Y.; Geng, G. Performance of limestone calcined clay cement (LC3) incorporating low-grade marine clay. Case Stud. Constr. Mater. 2024, 20, e03283. [Google Scholar] [CrossRef]
- Rehman, M.U.; MacLeod, A.J.; Gates, W.P. Phase development and mechanical strength of limestone calcined clay cement utilising Australian bentonite and plasterboard waste. Constr. Build. Mater. 2024, 445, 137937. [Google Scholar] [CrossRef]
- Ferreiro, S.; Herfort, D.; Damtoft, J. Effect of raw clay type, fineness, water-to-cement ratio and fly ash addition on workability and strength performance of calcined clay—Limestone Portland cements. Cem. Concr. Res. 2017, 101, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Q.; Zhang, X. Exploring the influence of calcined clay grade on the rheological dynamics of LC3 mortar. Constr. Build. Mater. 2024, 444, 137852. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y. A Review on Hydration Process and Setting Time of Limestone Calcined Clay Cement (LC3). Solids 2023, 4, 24–38. [Google Scholar] [CrossRef]
- Gu, H.; Meng, Z.; Wang, Y.; Gao, X.; Wang, R.; Wang, D.; Sheng, J.; Wang, J. Investigations into Replacing Calcined Clay with Sewage Sludge Ash in Limestone Calcined Clay Cement (LC3). Materials 2025, 18, 782. [Google Scholar] [CrossRef]
- da Silva, M.R.C.; Andrade Neto, J.d.S.; Walkley, B.; Kirchheim, A.P. Effects of kaolinite and montmorillonite calcined clays on the sulfate balance, early hydration, and artificial pore solution of limestone calcined clay cements (LC3). Mater. Struct. 2024, 57, 187. [Google Scholar] [CrossRef]
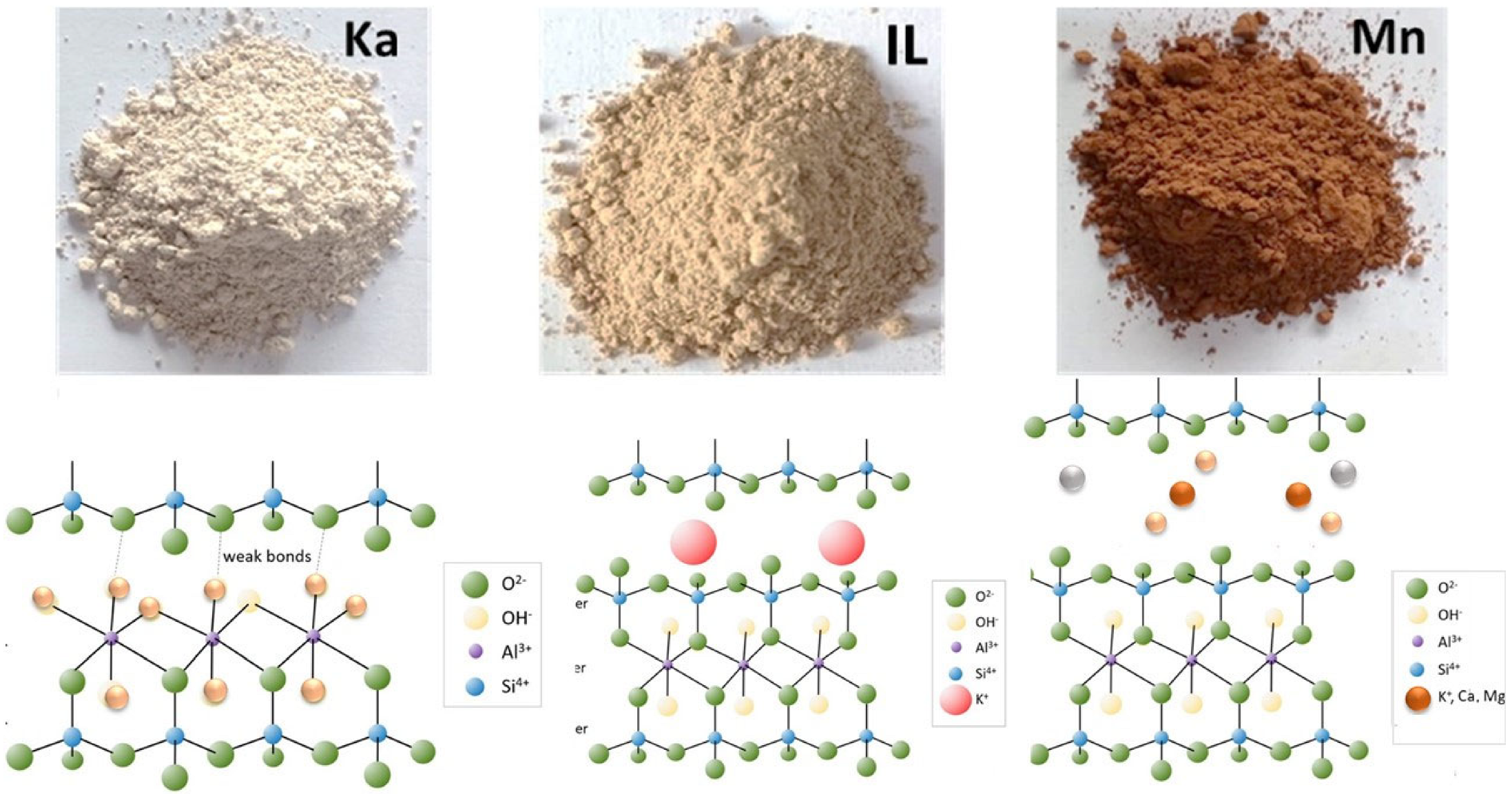
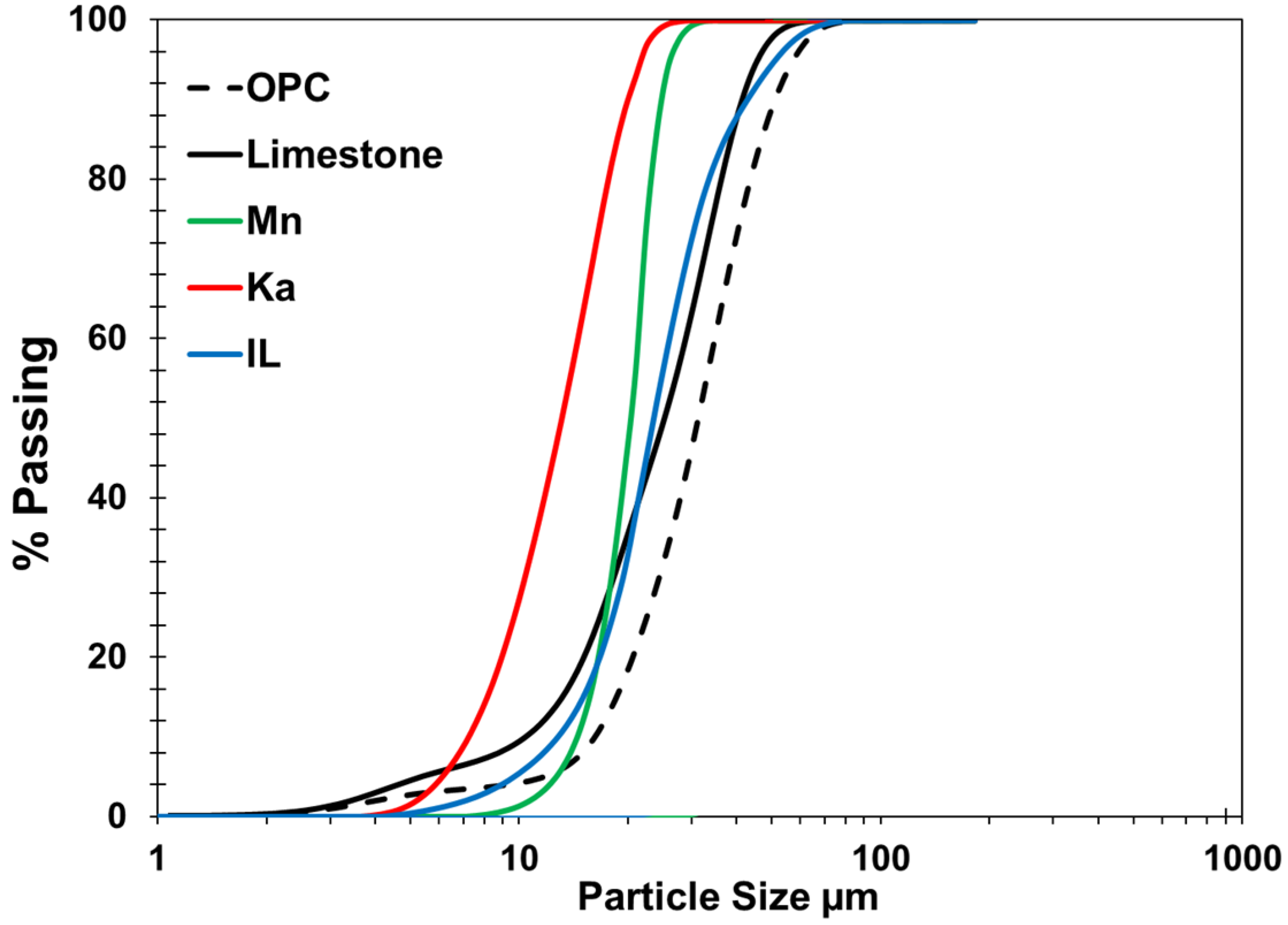
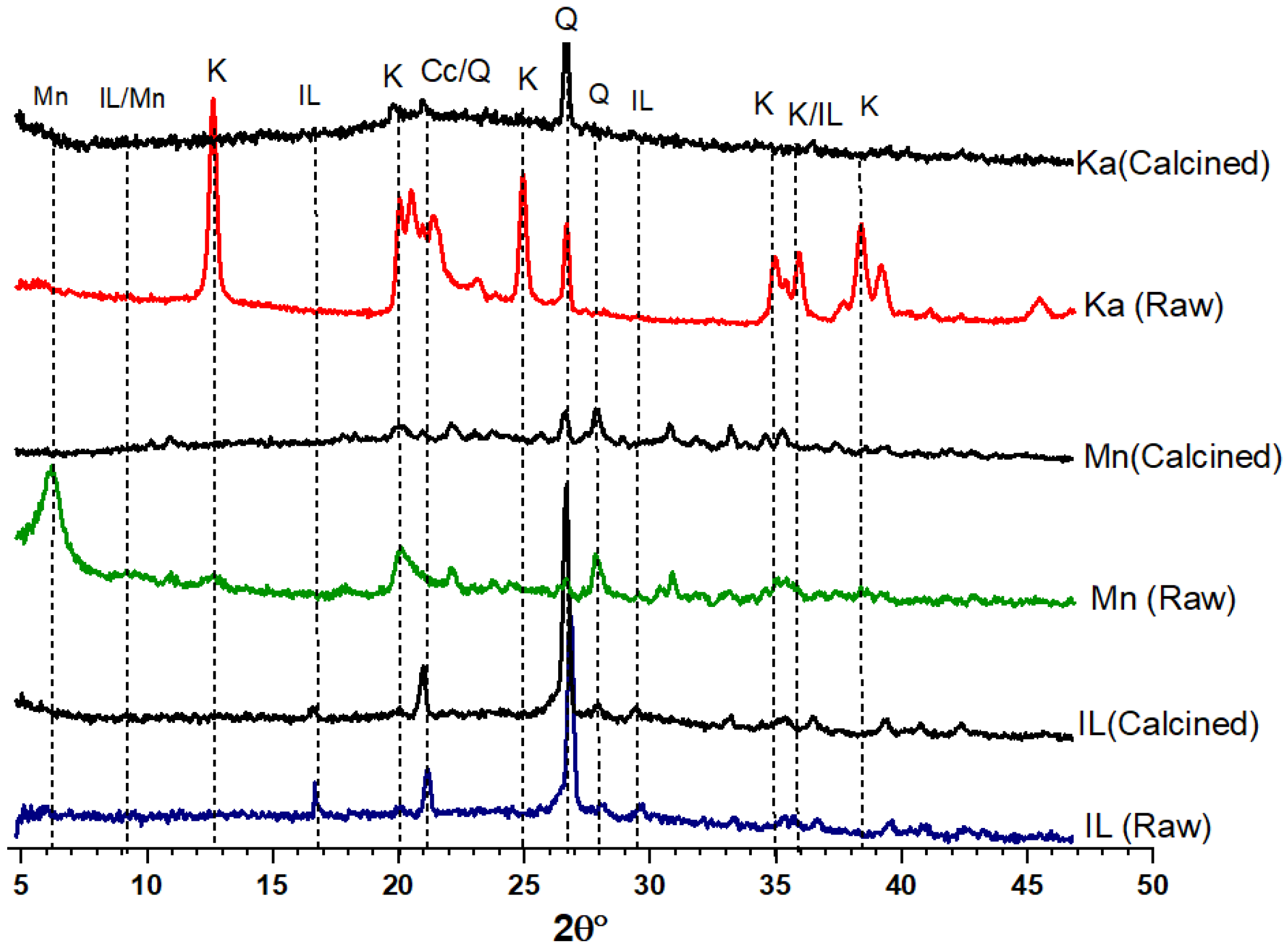
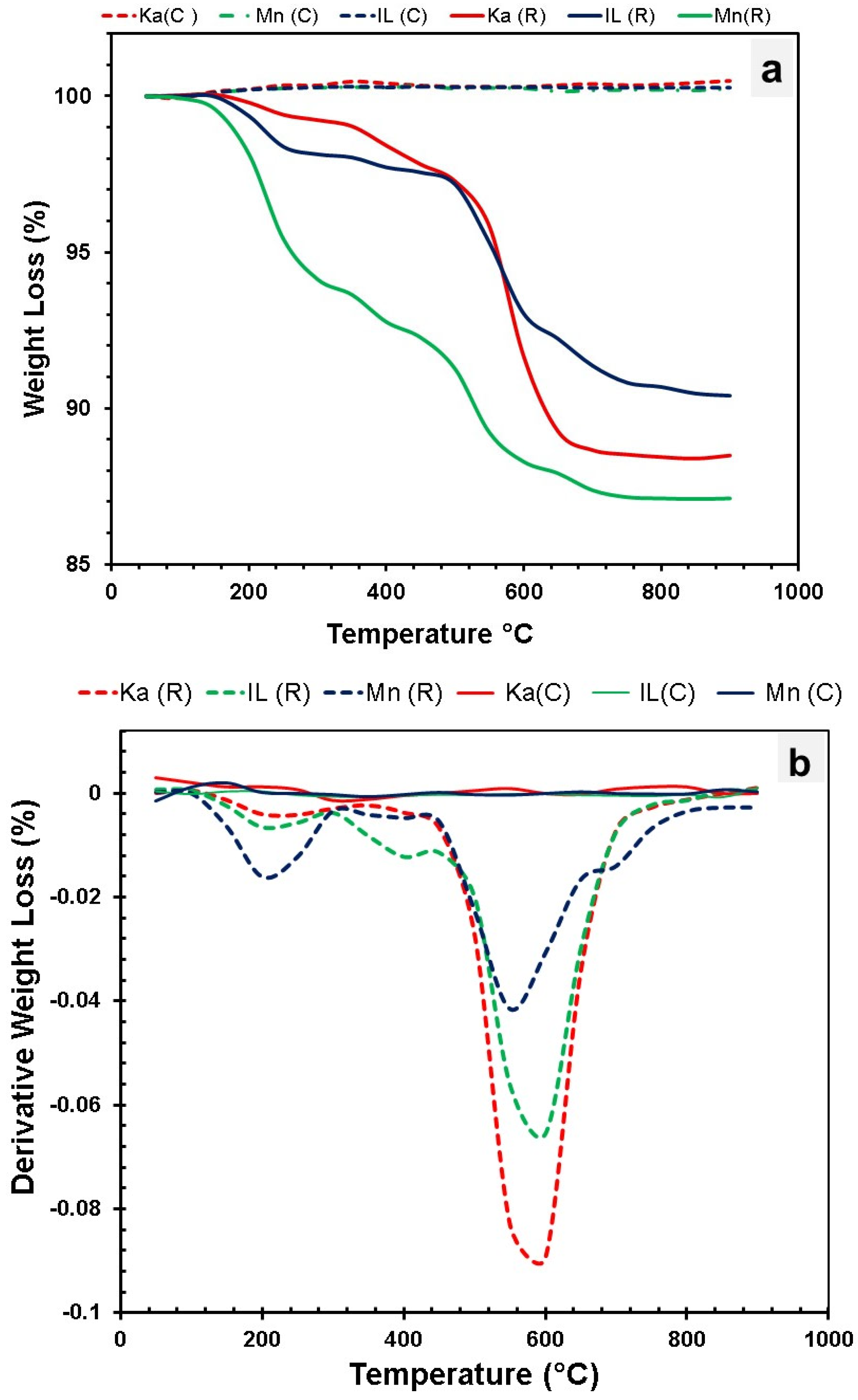







| Clay Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | Cl | Blain Finess (cm2/g) | LO1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ka (R) | 58.7 | 28.8 | 3.5 | 2.3 | 0.2 | 0.3 | 0.1 | 0.2 | 0.0 | 6552 | 12.15 |
| Ka (C) | 53.16 | 34.28 | 3.36 | 2.67 | 0.04 | 0.01 | 0.02 | 0.06 | 0.00 | 10,930 | 2.97 |
| Mn (R) | 41.45 | 29.23 | 2.32 | 9.60 | 0.68 | 2.55 | 0.65 | 0.03 | - | 4545 | 10.34 |
| Mn (C) | 41.45 | 27.13 | 2.02 | 8.76 | 0.60 | 2.55 | 0.65 | 0.03 | - | 10,000 | 0.69 |
| IL (R) | 56.40 | 19.57 | 6.98 | 4.73 | 1.71 | 1.49 | 1.39 | 0.1 | 0.72 | 4650 | 10.29 |
| IL (C) | 54.06 | 18.64 | 7.13 | 4.64 | 1.80 | 1.57 | 1.59 | 0.08 | 0.17 | 8765 | 0.48 |
| Limestone | 19.28 | 5.44 | 2.09 | 42.5 | 0.96 | 0.78 | 0.37 | 0.15 | 0.00 | 11,535 | 43.93 |
| OPC | 19.4 | 4.81 | 3.54 | 60.9 | 2.58 | 2.65 | 0.25 | 0.77 | 0 | 3200 | 2.5 |
| OPC | Clay (kg/m3) | Limestone | Sand | Water | |||
|---|---|---|---|---|---|---|---|
| kg/m3 | Ka | Mn | IL | kg/m3 | kg/m3 | kg/m3 | |
| LC3-Ka | 170 | 212 | --- | ---- | 98 | 1039 | 115 |
| LC3-Mn | 170 | ---- | 212 | --- | 98 | 1039 | 115 |
| LC3-IL | 170 | --- | --- | 212 | 98 | 1039 | 115 |
| Control Mix | 569 | --- | --- | --- | --- | 1039 | 115 |
| Clay Type | Clay Structure | PSD | XRD | TGA | Blain Fineness | Weight Loss (%) | Oxide Content (%) | MCT | Reactivity of the Clay | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D50 µm | Crystallinity | Dehydroxylation | cm2/g | LOI | Al2O3 Content | SiO2 Content | K2O Content | CaO Content | Fe2O3 Content | Bound Portlandite Content | |||
| Ka (R) | 1:1 | 4.5 | Highly crystalline | 700–750 °C | 6552 | 12.15 | 37.91 | 48.08 | 0.04 | 3.15 | 1.32 | -- | Excellent |
| Ka (C) | Disorder | 8.5 | Very low crystalline with a sharp hump at 20–40° 2θ | --- | 10,930 | 2.97 | 39.50 | 50.22 | 0.06 | 1.89 | 1.55 | 1164 | |
| Mn (R) | 2:1 | 4.9 | Medium crystalline | 700–750 °C | 4545 | 29.23 | 41.45 | 2.55 | 9.60 | 2.32 | --- | Good | |
| Mn (C) | Disorder | 6.1 | Low crystalline | 10,000 | 27.13 | 41.45 | 2.55 | 8.76 | 2.02 | 881 | |||
| IL (R) | 2:1 | 11 | Low crystals | 800–850 °C | 4650 | 10.29 | 19.57 | 66.40 | 1.49 | 4.73 | 6.98 | ---- | Average |
| IL (C) | Partially disorder | 16.8 | Resembles raw clay structure | 8765 | 0.89 | 18.64 | 64.06 | 1.57 | 4.64 | 7.13 | 783 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blouch, N.; Kazmi, S.N.H.; Akram, N.; Saleem, M.J.; Khan, I.A.; Javed, K.; Ahmad, S.; Khan, A. Performance Evaluation of Low-Grade Clay Minerals in LC3-Based Cementitious Composites. Solids 2025, 6, 35. https://doi.org/10.3390/solids6030035
Blouch N, Kazmi SNH, Akram N, Saleem MJ, Khan IA, Javed K, Ahmad S, Khan A. Performance Evaluation of Low-Grade Clay Minerals in LC3-Based Cementitious Composites. Solids. 2025; 6(3):35. https://doi.org/10.3390/solids6030035
Chicago/Turabian StyleBlouch, Nosheen, Syed Noman Hussain Kazmi, Nijah Akram, Muhammad Junaid Saleem, Imran Ahmad Khan, Kashif Javed, Sajjad Ahmad, and Asfandyar Khan. 2025. "Performance Evaluation of Low-Grade Clay Minerals in LC3-Based Cementitious Composites" Solids 6, no. 3: 35. https://doi.org/10.3390/solids6030035
APA StyleBlouch, N., Kazmi, S. N. H., Akram, N., Saleem, M. J., Khan, I. A., Javed, K., Ahmad, S., & Khan, A. (2025). Performance Evaluation of Low-Grade Clay Minerals in LC3-Based Cementitious Composites. Solids, 6(3), 35. https://doi.org/10.3390/solids6030035







