Durability Assessment of Binary and Ternary Eco-Friendly Mortars with Low Cement Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Partial Replacement
2.2. Mortar Tests
3. Results and Discussion
3.1. Mortar Tests
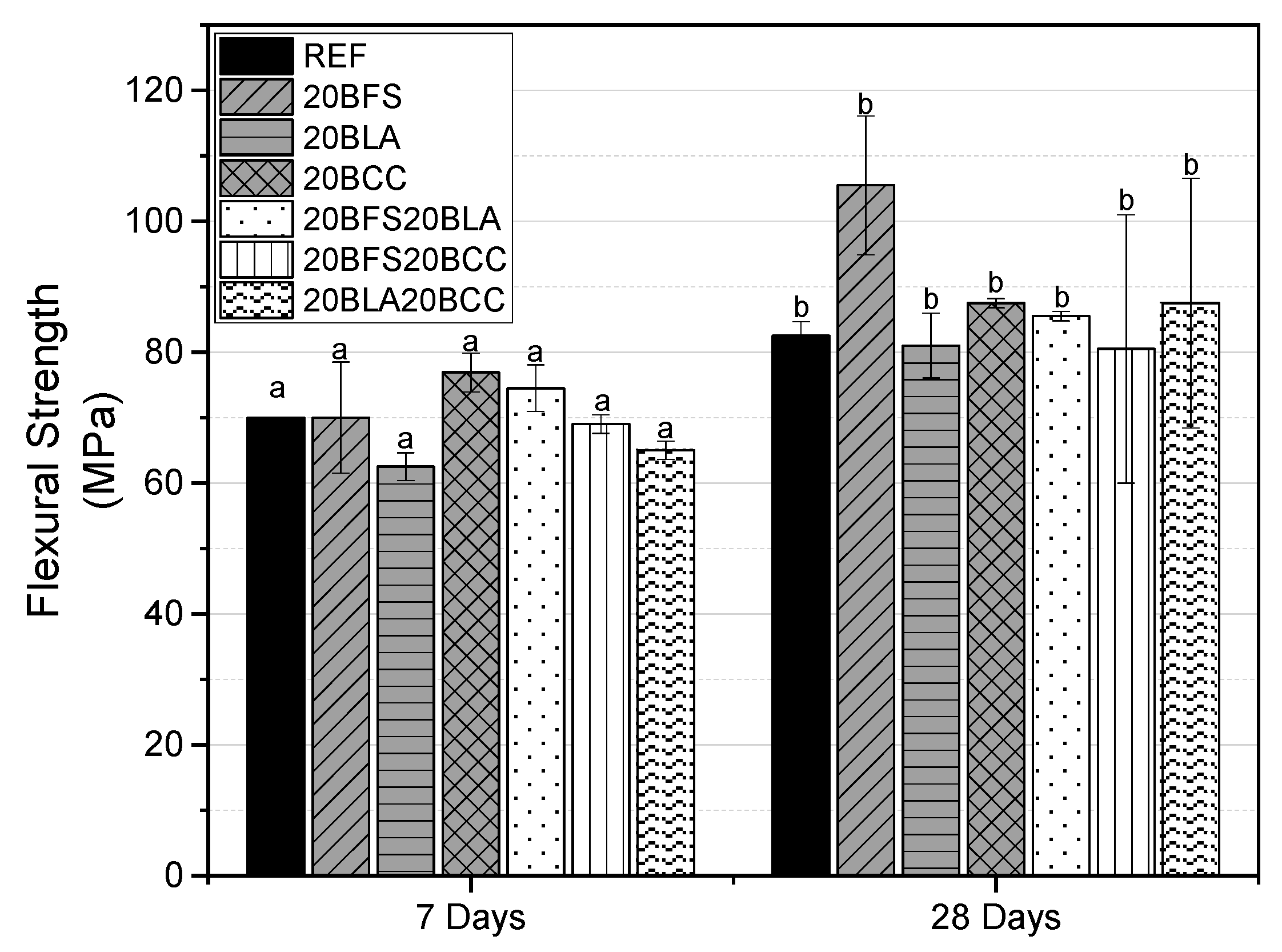
3.1.1. Flexural Strength
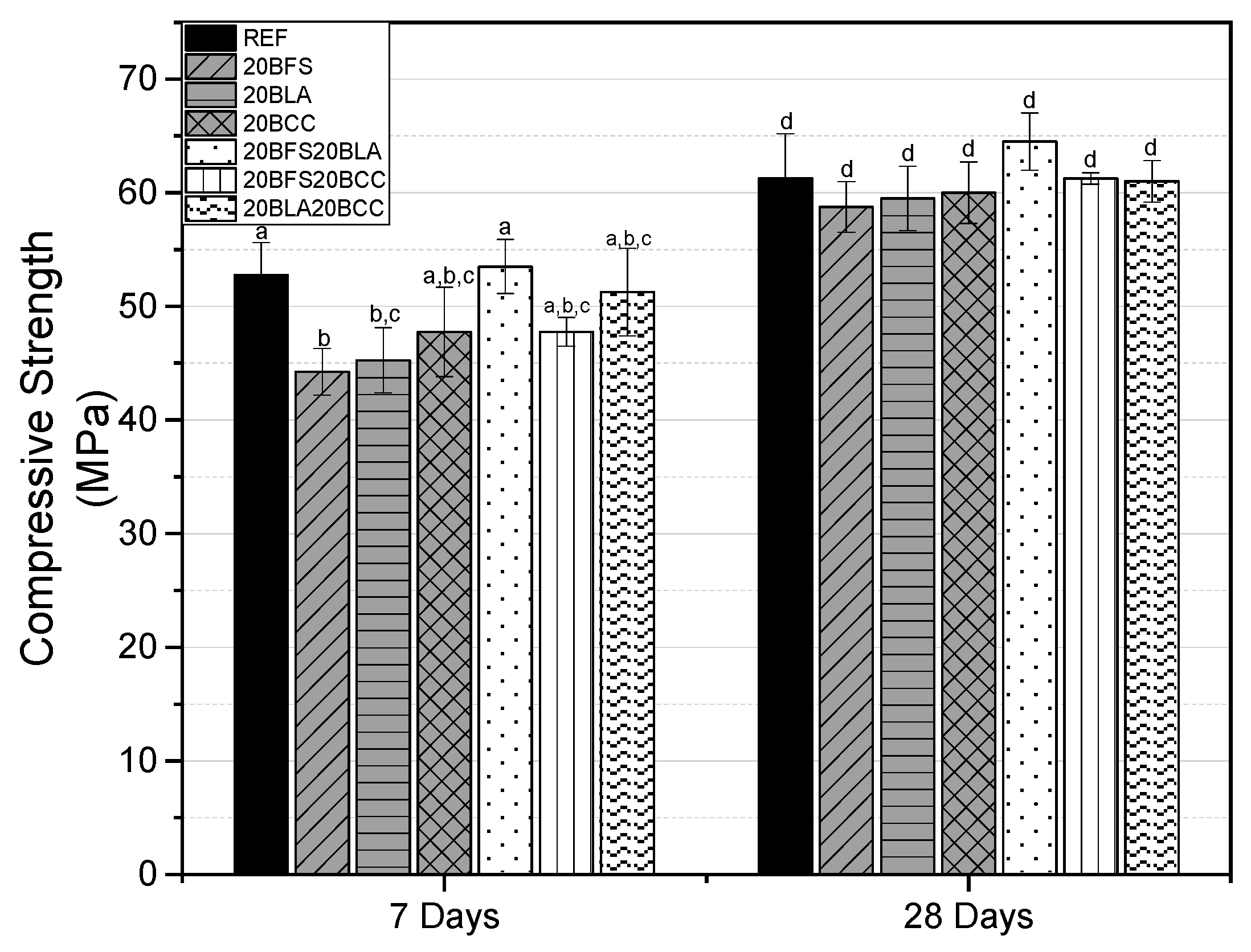
3.1.2. Compressive Strength
3.1.3. Electrical Resistivity of Mortars
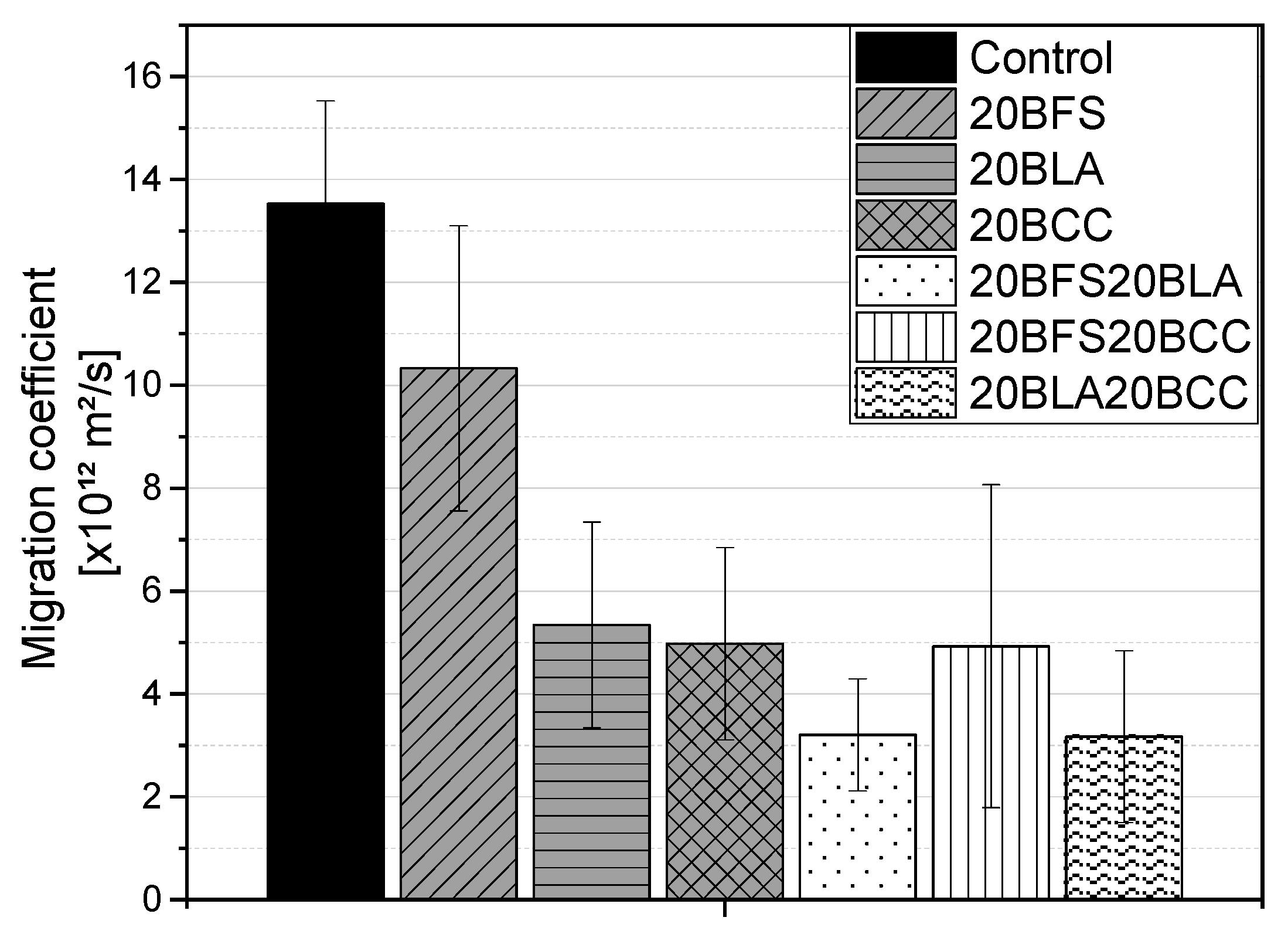
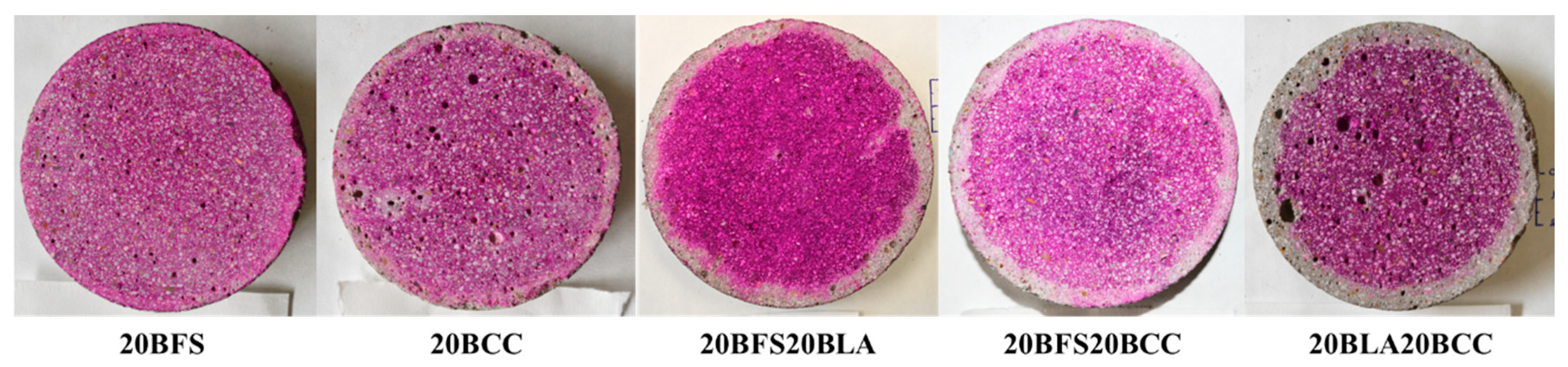
3.1.4. Chloride Migration
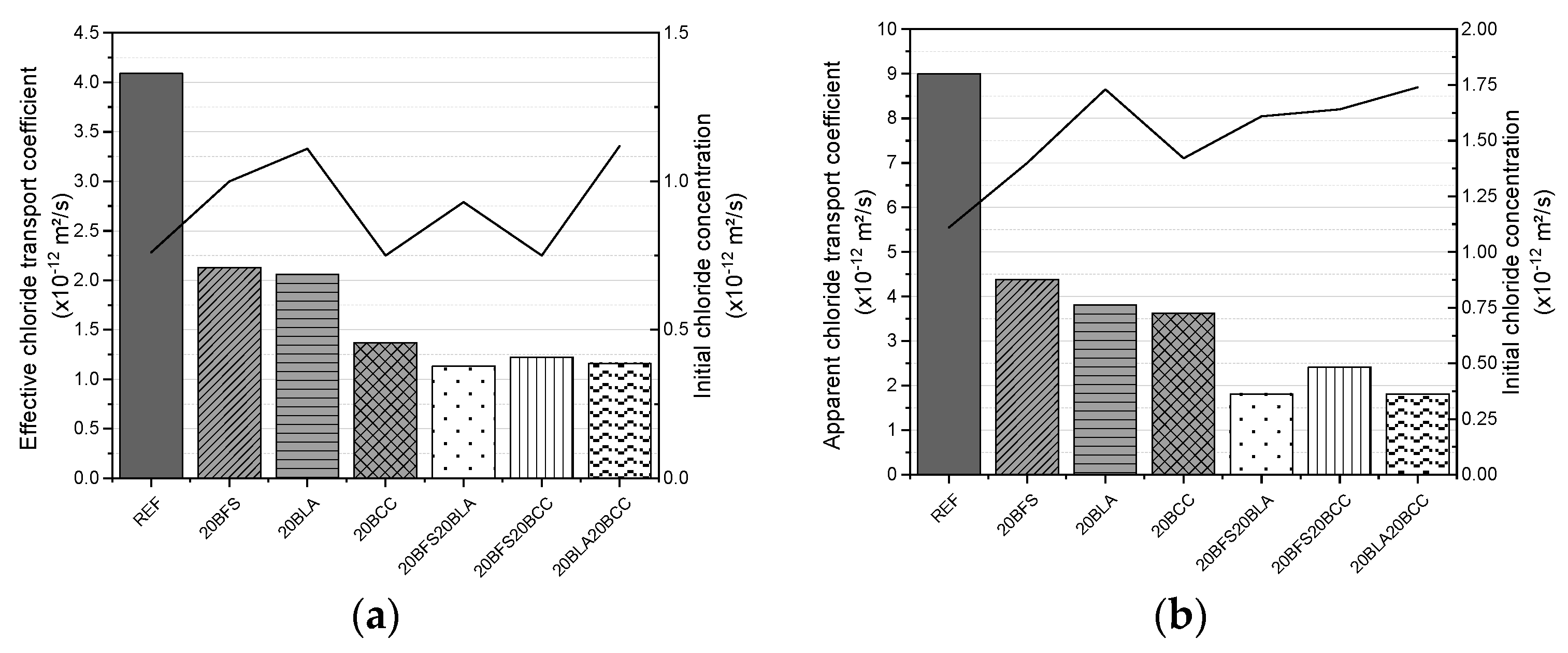
3.1.5. Chloride Diffusion
3.1.6. Carbonatation
4. Conclusions
- Binary and ternary mortars containing 20 to 40 wt.% of waste materials (BFS, BLA, BCC) demonstrate mechanical performance comparable to the reference mix, exceeding the required strength of 42.5 MPa at 28 days.
- All formulations evaluated show significantly higher electrical resistivity compared to the reference mix, particularly the ternary blends, suggesting a reduction in porosity.
- Ternary blends with a 40% replacement rate exhibited the best resistance to chloride ingress, with diffusion and migration coefficients up to four times lower than those of the reference material.
- Binary mixes with a 20% replacement rate exhibited a reduction in diffusion and migration coefficients of approximately half compared to the reference mix.
- BFS and BLA binary mixes displayed superior carbonation resistance, even outperforming the reference material.
- Ternary mixes showed diminished carbonation resistance due to the higher levels of cement replacement and significant pozzolanic activity, particularly from calcined clay, which results in lower portlandite content and reduced alkaline reserve.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| BCC | Brazilian Calcined Clay |
| BFS | Blast Furnace Slag |
| BLA | Bamboo Leaf Ash |
| CAPES | Coordenação de Aperfeiçoamento de Pessoal de Nível Superior |
| CC | Calcined Clay |
| CSH | Calcium Silicate Hydrate |
| IFSP | Federal Institute of Education, Science and Technology |
| OPC | Ordinary Portland Cement |
| PP | Pumice Powder |
| REF | Reference |
| SCM | Supplementary Cementitious Materials |
| sd | Standard Deviation |
| SSA | Specific Surface Area |
| UNB | University of Brasília |
| XRF | X-ray fluorescence |
References
- Jaramillo, A.; González, C.; Lopez, N.; Puga, K.L.N. Estudio experimental en morteros con ceniza de hoja de bambú como material cementante suplementario. Rev. Iniciación Científica 2023, 9, 59–65. [Google Scholar] [CrossRef]
- Rodier, L.; Villar-Cociña, E.; Ballesteros, J.M.; Junior, H.S. Potential use of sugarcane bagasse and bamboo leaf ashes for elaboration of green cementitious materials. J. Clean. Prod. 2019, 231, 54–63. [Google Scholar] [CrossRef]
- Latini, A.O.; Dias, L.D.; Silva, B.R.; Souza, L.M. Advancements in bamboo preservative solutions for sustainable construction in Brazil. Int. J. Environ. Sci. Technol. 2024, 21, 8207–8210. [Google Scholar] [CrossRef]
- Silva, L.H.P.; Nehring, V.; de Paiva, F.F.G.; Tamashiro, J.R.; Galvín, A.P.; López-Uceda, A.; Kinoshita, A. Use of blast furnace slag in cementitious materials for pavements—Systematic literature review and eco-efficiency. Sustain. Chem. Pharm. 2023, 33, 101030. [Google Scholar] [CrossRef]
- Adesanya, D.A.; Raheem, A.A. Development of corn cob ash blended cement. Constr. Build. Mater. 2009, 23, 347–352. [Google Scholar] [CrossRef]
- Oliveira, F.T.; Moreira, C.; da Silva Rêgo, J.H.; Capuzzo, V.M.S. Influence of the Limestone Type on the Compression Strength of LC3 Cements. In Proceedings of the International Conference of Sustainable Production and Use of Cement and Concrete, Villa Clara, Cuba, 23–30 June 2019; pp. 39–45. [Google Scholar] [CrossRef]
- Moreira, C. Efeitos do Teor de Gipsita na Microestrutura das Pastas de Cimento LC. Ph.D. Thesis, Universidade de Brasília, Brasília, Brazil, 2020. [Google Scholar]
- Albuquerque, R.T.O.; de Andrade Lima, N.L. Adições minerais ao concreto: Melhores propriedades, maior economia e mais sustentabilidade. Rev. Interdiscip. Da Univ. Veiga Almeida. 2014, 11, 57–64. [Google Scholar]
- Xuan, M.; Bae, S.C.; Kwon, S.-J.; Wang, X.-Y. Sustainability enhancement of calcined clay and limestone powder hybrid ultra-high-performance concrete using belite-rich Portland cement. Constr. Build. Mater. 2022, 351, 128932. [Google Scholar] [CrossRef]
- Jamhiri, B. Evaluation of Pozzolan-Lime Stabilization on Physical Properties of Fine Sandy Engineering Fills. Konya J. Eng. Sci. 2020, 8, 80–90. [Google Scholar] [CrossRef]
- Maier, M.; Sposito, R.; Beuntner, N.; Thienel, K.-C. Particle characteristics of calcined clays and limestone and their impact on early hydration and sulfate demand of blended cement. Cem. Concr. Res. 2022, 154, 106736. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Nahazanan, H.; Nasir, N.A.M.; Huseien, G.F.; Saad, A.H. Calcium-Based Binders in Concrete or Soil Stabilization: Challenges, Problems, and Calcined Clay as Partial Replacement to Produce Low-Carbon Cement. Materials 2023, 16, 2020. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Xu, Z.; Li, S.; Luo, X.; Chen, G. Long-term hydration and microstructure evolution of blended cement containing ground granulated blast furnace slag and waste clay brick. Cem. Concr. Compos. 2021, 118, 103982. [Google Scholar] [CrossRef]
- Ukpata, J.O.; Basheer, P.A.M.; Black, L. Expansion of CEM I and slag-blended cement mortars exposed to combined chloride-sulphate environments. Cem. Concr. Res. 2019, 123, 105794. [Google Scholar] [CrossRef]
- Aziz, M.A.E.; Aleem, S.A.E.; Heikal, M.; Didamony, H.E. Hydration and durability of sulphate-resisting and slag cement blends in Caron’s Lake water. Cem. Concr. Res. 2005, 35, 1592–1600. [Google Scholar] [CrossRef]
- Silva, L.H.P.; Tamashiro, J.R.; de Paiva, F.F.G.; Santos, L.F.D.; Teixeira, S.R.; Kinoshita, A.; Antunes, P.A. Bamboo leaf ash for use as mineral addition with Portland cement. J. Build. Eng. 2021, 42, 102769. [Google Scholar] [CrossRef]
- Moraes, M.J.B.J.B.; Moraes, J.C.B.C.B.; Tashima, M.M.M.; Akasaki, J.L.L.; Soriano, L.; Borrachero, M.V.V.; Payá, J. Production of bamboo leaf ash by auto-combustion for pozzolanic and sustainable use in cementitious matrices. Constr. Build. Mater. 2019, 208, 369–380. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Frías, M.; Savastano, H.; Rodier, L.; de Rojas, M.I.S.; del Bosque, I.F.S.; Medina, C. Quantitative Comparison of Binary Mix of Agro-Industrial Pozzolanic Additions for Elaborating Ternary Cements: Kinetic Parameters. Materials 2021, 14, 2944. [Google Scholar] [CrossRef]
- UNE-EN 197-1; Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Mohammed, S.; Safiullah, O. Optimization of the SO3 content of an Algerian Portland cement: Study on the effect of various amounts of gypsum on cement properties. Constr. Build. Mater. 2018, 164, 362–370. [Google Scholar] [CrossRef]
- ASTM C989/C989M-18a; Standard Specification for Slag Cement for Use in Concrete and Mortars. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Chen, K.; Wu, D.; Yi, M.; Cai, Q.; Zhang, Z. Mechanical and durability properties of metakaolin blended with slag geopolymer mortars used for pavement repair. Constr. Build. Mater. 2021, 281, 122566. [Google Scholar] [CrossRef]
- Hakeem, I.Y.; Agwa, I.S.; Tayeh, B.A.; Mahmoud, H.A.-E. Effect of using a combination of rice husk and olive waste ashes on high-strength concrete properties. Case Stud. Constr. Mater. 2022, 17, e01486. [Google Scholar] [CrossRef]
- Cociña, E.V.; Savastano, H.; Rodier, L.; Lefran, M.; Frías, M. Pozzolanic Characterization of Cuban Bamboo Leaf Ash: Calcining Temperature and Kinetic Parameters. Waste Biomass Valorization 2016, 9, 691–699. [Google Scholar] [CrossRef]
- Talero, R. Qualitative Analysis of Natural Pozzolanas, Fly Ashes, and Blast Furnace Slags by XRD. J. Mater. Civ. Eng. 1990, 2, 106–115. [Google Scholar] [CrossRef]
- UNE-EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2018.
- Silva, L.H.P.S.; Paiva, F.F.G.d.; Tamashiro, J.R.; Kinoshita, A. Potential of bamboo leaf ash as supplementary binder materials—A systematic literature review. J. Build. Eng. 2023, 71, 106547. [Google Scholar] [CrossRef]
- UNE-EN 934-2; Admixtures for Concrete, Mortar and Grout—Part 2: Concrete Admixtures—Definitions, Requirements, Conformity, Marking and Labelling. European Committee for Standardization: Brussels, Belgium, 2002.
- UNE-EN 83988-1; Concrete Durability. Test Methods. Determination of the Electrical Resistivity. Part 1: Direct Test (Reference Test). European Committee for Standardization: Brussels, Belgium, 2008.
- Garcia, R.; Henao, N.; De la Rubia, M.A.; Moragues, A.; Fernandez, J. Early contributing nanostructured cementitious matrix designs: Benefits in durable features at early ages. Constr. Build. Mater. 2020, 241, 117941. [Google Scholar] [CrossRef]
- NT Build 492; Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments. Nordic Cooperation: Espoo, Finland, 1999.
- Castellote, M.; Andrade, C. RILEM TC 178-TMC: “Testing and modelling chloride penetration in concrete” Round-Robin test on chloride analysis in concrete—Part II: Analysis of water soluble chloride content. Mater. Struct. 2001, 34, 589–598. [Google Scholar] [CrossRef]
- UNE-EN 14629; Productos and Systems for the Protection and Repair of Concrete Strutures. Test Methods. Determination of Cloride Content in Hardened Concrete. European Committee for Standardization: Brussels, Belgium, 2007.
- NT Build 443; Concrete, Hardened: Accelerated Chloride Penetration. Nordic Cooperation: Espoo Finland, 1995.
- UNE-EN 12390-12; Testing Hardened Concrete—Part 12: Determination of the Carbonation Resistance of Concrete—Accelerated Carbonation Method. European Committee for Standardization: Brussels, Belgium, 2020.
- Abebaw, G.; Bewket, B.; Getahun, S. Experimental Investigation on Effect of Partial Replacement of Cement with Bamboo Leaf Ash on Concrete Property. Adv. Civ. Eng. 2021, 2021, 6468444. [Google Scholar] [CrossRef]
- Gyurkó, Z.; Nemes, R. Static Hardness Testing of Cement Mortars Containing Different Types of Recycled Construction Waste Powders. Solids 2021, 2, 331–340. [Google Scholar] [CrossRef]
- Sujjavanich, S.; Suwanvitaya, P.; Chaysuwan, D.; Heness, G. Synergistic effect of metakaolin and fly ash on properties of concrete. Constr. Build. Mater. 2017, 155, 830–837. [Google Scholar] [CrossRef]
- Khatib, J.M.; Hibbert, J.J. Selected engineering properties of concrete incorporating slag and metakaolin. Constr. Build. Mater. 2005, 19, 460–472. [Google Scholar] [CrossRef]
- Adem, H.H.; Cherkos, F.D. Analyzing the Mechanical, Durability, and Microstructural Impact of Partial Cement Replacement with Pumice Powder and Bamboo Leaf Ash in Concrete. Adv. Civ. Eng. 2024, 2024, 5119850. [Google Scholar] [CrossRef]
- García, R.; Reyes, E.; Villanueva, P.; de la Rubia, M.; Fernández, J.; Moragues, A. Service Life and Early Age Durability Enhancement due to Combined Metakaolin and Nanosilica in Mortars for Marine Applications. Materials 2020, 13, 1169. [Google Scholar] [CrossRef]
- McCarter, W.J.; Chrisp, T.M.; Starrs, G.; Adamson, A.; Basheer, P.A.M.; Nanukuttan, S.V.; Srinivasan, S.; Green, C. Characterization of physio-chemical processes and hydration kinetics in concretes containing supplementary cementitious materials using electrical property measurements. Cem. Concr. Res. 2013, 50, 26–33. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Argiz, C.; Gálvez, J.C.; Reyes, E. Combined effect of nano-SiO2 and nano-Fe2O3 on compressive strength, flexural strength, porosity and electrical resistivity in cement mortars. Mater. Construcción 2018, 68, 150. [Google Scholar] [CrossRef]
- Noushini, A.; Nguyen, Q.D.; Castel, A. Assessing alkali-activated concrete performance in chloride environments using NT Build 492. Mater. Struct. 2021, 54, 57. [Google Scholar] [CrossRef]
- Maes, M.; Gruyaert, E.; De Belie, N. Resistance of concrete with blast-furnace slag against chlorides, investigated by comparing chloride profiles after migration and diffusion. Mater. Struct. 2013, 46, 89–103. [Google Scholar] [CrossRef]
- Pack, S.-W.; Jung, M.-S.; Song, H.-W.; Kim, S.-H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structures exposed to a marine environment. Cem. Concr. Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Heikal, M.; Zaki, M.E.A.; Ibrahim, S.M. Preparation, physico-mechanical characteristics and durability of eco-alkali-activated binder from blast-furnace slag, cement kiln-by-pass dust and microsilica ternary system. Constr. Build. Mater. 2020, 260, 119947. [Google Scholar] [CrossRef]
- Wang, D.; Noguchi, T.; Nozaki, T.; Higo, Y. Investigation of the carbonation performance of cement-based materials under high temperatures. Constr. Build. Mater. 2021, 272, 121634. [Google Scholar] [CrossRef]
- Silva, R.V.; Neves, R.; de Brito, J.; Dhir, R.K. Carbonation behaviour of recycled aggregate concrete. Cem. Concr. Compos. 2015, 62, 22–32. [Google Scholar] [CrossRef]
- Pauletti, C.; Possan, E.; Molin, D.C.C.D. Accelerated carbonation: State-of-the art of the research in Brazil. Ambiente Construído 2007, 7, 7–20. [Google Scholar]
- Alonso-Domínguez, D.; Álvarez-Serrano, I.; Reyes, E.; Moragues, A. New mortars fabricated by electrostatic dry deposition of nano and microsilica additions: Enhanced properties. Constr. Build. Mater. 2017, 135, 186–193. [Google Scholar] [CrossRef]
- Cuenca-Moyano, G.M.; Cabrera, M.; López-Alonso, M.; Martínez-Echevarría, M.J.; Agrela, F.; Rosales, J. Design of lightweight concrete with olive biomass bottom ash for use in buildings. J. Build. Eng. 2023, 69, 106289. [Google Scholar] [CrossRef]
- Sáez del Bosque, I.F.; Van den Heede, P.; De Belie, N.; Sánchez de Rojas, M.I.; Medina, C. Carbonation of concrete with construction and demolition waste based recycled aggregates and cement with recycled content. Constr. Build. Mater. 2020, 234, 117336. [Google Scholar] [CrossRef]
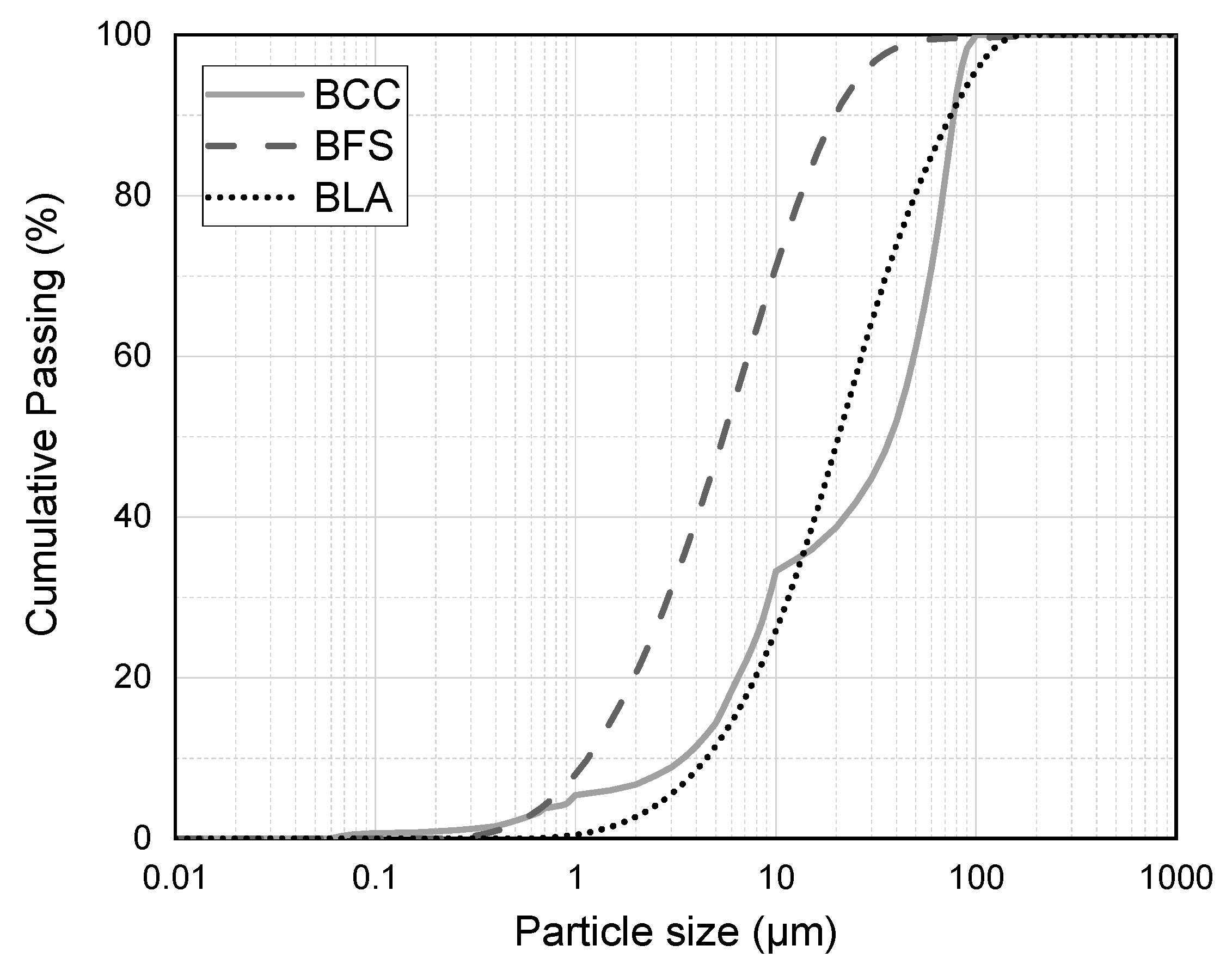
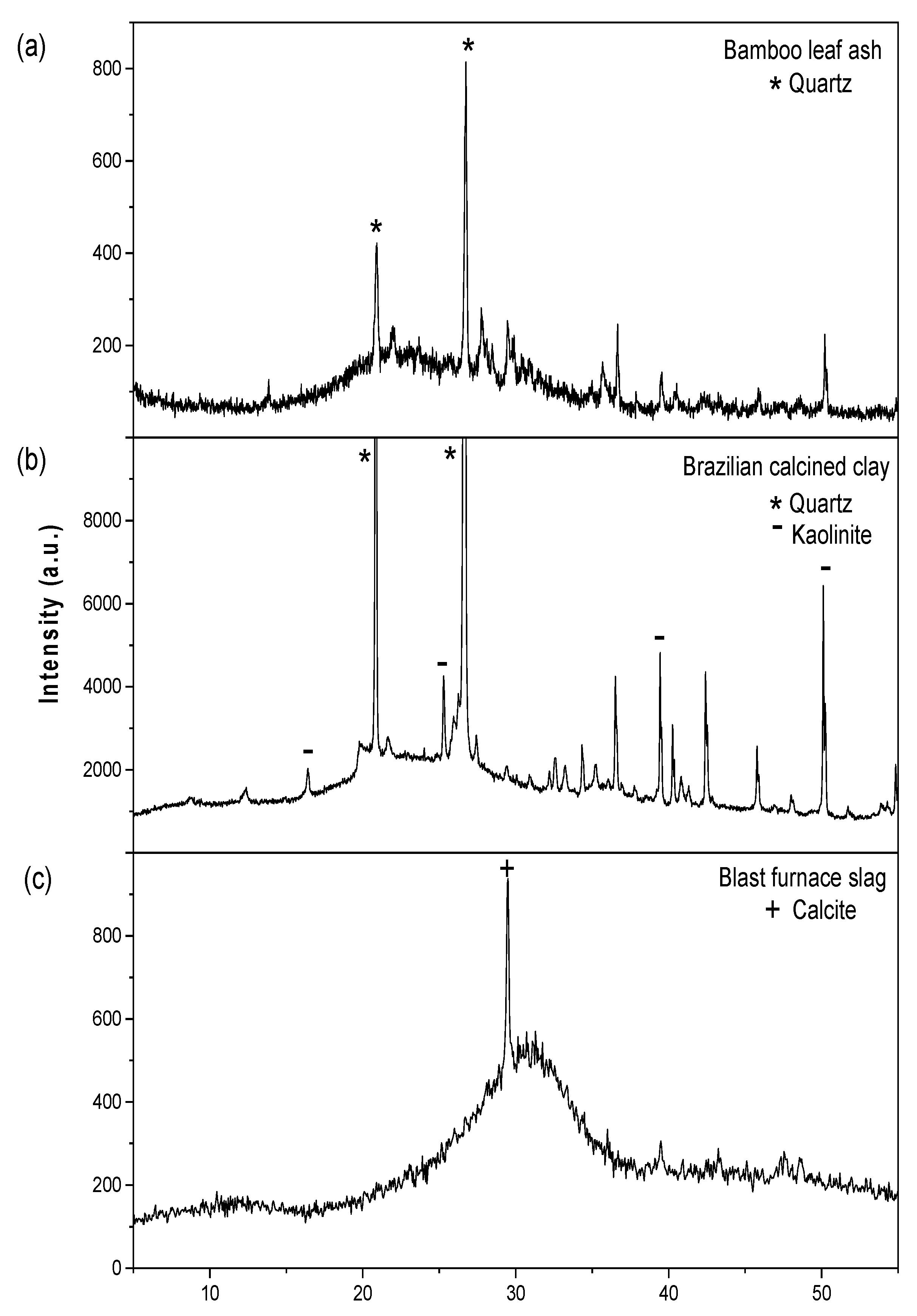




| SiO2 | CaO | K2O | Fe2O3 | MgO | Al2O3 | P2O5 | SO3 | TiO2 | MnO | LOI 1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 20.00 | 63.50 | 0.86 | 2.63 | 1.90 | 4.51 | 0.11 | 3.00 | 0.20 | 0.08 | 3.15 |
| BFS | 34.10 | 42.70 | 0.43 | 0.34 | 7.80 | 10.50 | 0.04 | 2.00 | 0.58 | 0.14 | 0.34 |
| BLA | 70.99 | 8.31 | 5.98 | 4.04 | 2.83 | 2.56 | 2.14 | 2.14 | 0.60 | 0.17 | 8.34 |
| BCC | 59.07 | 2.96 | 0.55 | 3.10 | 0.43 | 29.32 | 1.48 | 0.38 | 2.47 | 0.01 | 2.20 |
| Sieve mesh (mm) | 2 | 1.6 | 1 | 0.5 | 0.16 | 0.08 |
| Cumulative volume (%) | 0 | 7 ± 5 | 33 ± 5 | 67 ± 5 | 87 ± 5 | 99 ± 1 |
| Samples | OPC (g) | BFS (g) | BLA (g) | BCC (g) | Sand (g) | Water (g) |
| REF | 450 | 0 | 0 | 0 | 1450 | 225 |
| 20BFS | 360 | 90 | 0 | 0 | 1450 | 225 |
| 20BLA | 360 | 0 | 90 | 0 | 1450 | 225 |
| 20BCC | 360 | 0 | 0 | 90 | 1450 | 225 |
| 20BFS20BLA | 270 | 90 | 90 | 0 | 1450 | 225 |
| 20BFS20BCC | 270 | 90 | 0 | 90 | 1450 | 225 |
| 20BLA20BCC | 270 | 0 | 90 | 90 | 1450 | 225 |
| Samples | α | R |
|---|---|---|
| REF | 1.16985 | 0.16985 |
| 20BFS | 1.50459 | 0.50459 |
| 20BLA | 1.34138 | 0.34138 |
| 20BCC | 2.28863 | 1.18863 |
| 20BFS20BLA | 1.77748 | 0.77748 |
| 20BFS20BCC | 2.07243 | 1.07243 |
| 20BLA20BCC | 1.70739 | 0.70739 |
| Samples | 7 Days | 28 Days | 70 Days | |||
|---|---|---|---|---|---|---|
| dk (mm) | KAC (mm/d1/2) | dk (mm) | KAC (mm/d1/2) | dk (mm) | KAC (mm/d1/2) | |
| REF | 0.68 | 0.13 | 1.20 | 0.11 | 2.12 | 0.20 |
| 20BFS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20BLA | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20BCC | 0.00 | 0.00 | 0.00 | 0.00 | 4.72 | 0.28 |
| 20BFS20BLA | 0.00 | 0.00 | 2.25 | 0.21 | 4.80 | 0.28 |
| 20BFS20BCC | 0.00 | 0.00 | 0.00 | 0.00 | 5.77 | 0.34 |
| 20BLA20BCC | 0.00 | 0.00 | 0.00 | 0.00 | 7.30 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.H.P.; Tamashiro, J.R.; de Paiva, F.F.G.; da Silva Rego, J.H.; de la Rubia, M.A.; Kinoshita, A.; Terrades, A.M. Durability Assessment of Binary and Ternary Eco-Friendly Mortars with Low Cement Content. Solids 2025, 6, 28. https://doi.org/10.3390/solids6020028
Silva LHP, Tamashiro JR, de Paiva FFG, da Silva Rego JH, de la Rubia MA, Kinoshita A, Terrades AM. Durability Assessment of Binary and Ternary Eco-Friendly Mortars with Low Cement Content. Solids. 2025; 6(2):28. https://doi.org/10.3390/solids6020028
Chicago/Turabian StyleSilva, Lucas Henrique Pereira, Jacqueline Roberta Tamashiro, Fabio Friol Guedes de Paiva, João Henrique da Silva Rego, Miguel Angel de la Rubia, Angela Kinoshita, and Amparo Moragues Terrades. 2025. "Durability Assessment of Binary and Ternary Eco-Friendly Mortars with Low Cement Content" Solids 6, no. 2: 28. https://doi.org/10.3390/solids6020028
APA StyleSilva, L. H. P., Tamashiro, J. R., de Paiva, F. F. G., da Silva Rego, J. H., de la Rubia, M. A., Kinoshita, A., & Terrades, A. M. (2025). Durability Assessment of Binary and Ternary Eco-Friendly Mortars with Low Cement Content. Solids, 6(2), 28. https://doi.org/10.3390/solids6020028







