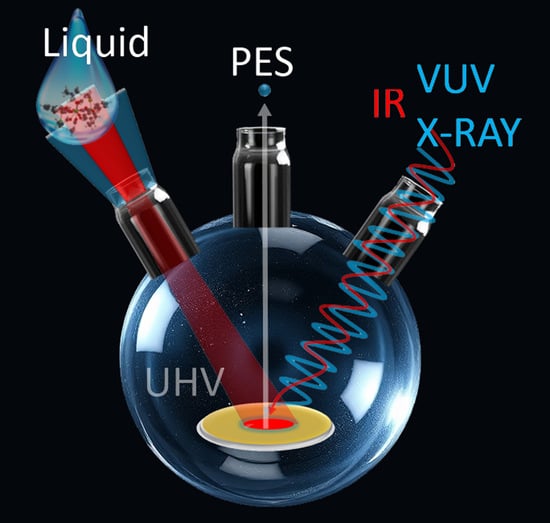
Liquid Phase Preparation of Organic Thin Films Consisting of Complex Molecules—The Example of the Metallacrown CuCu4
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
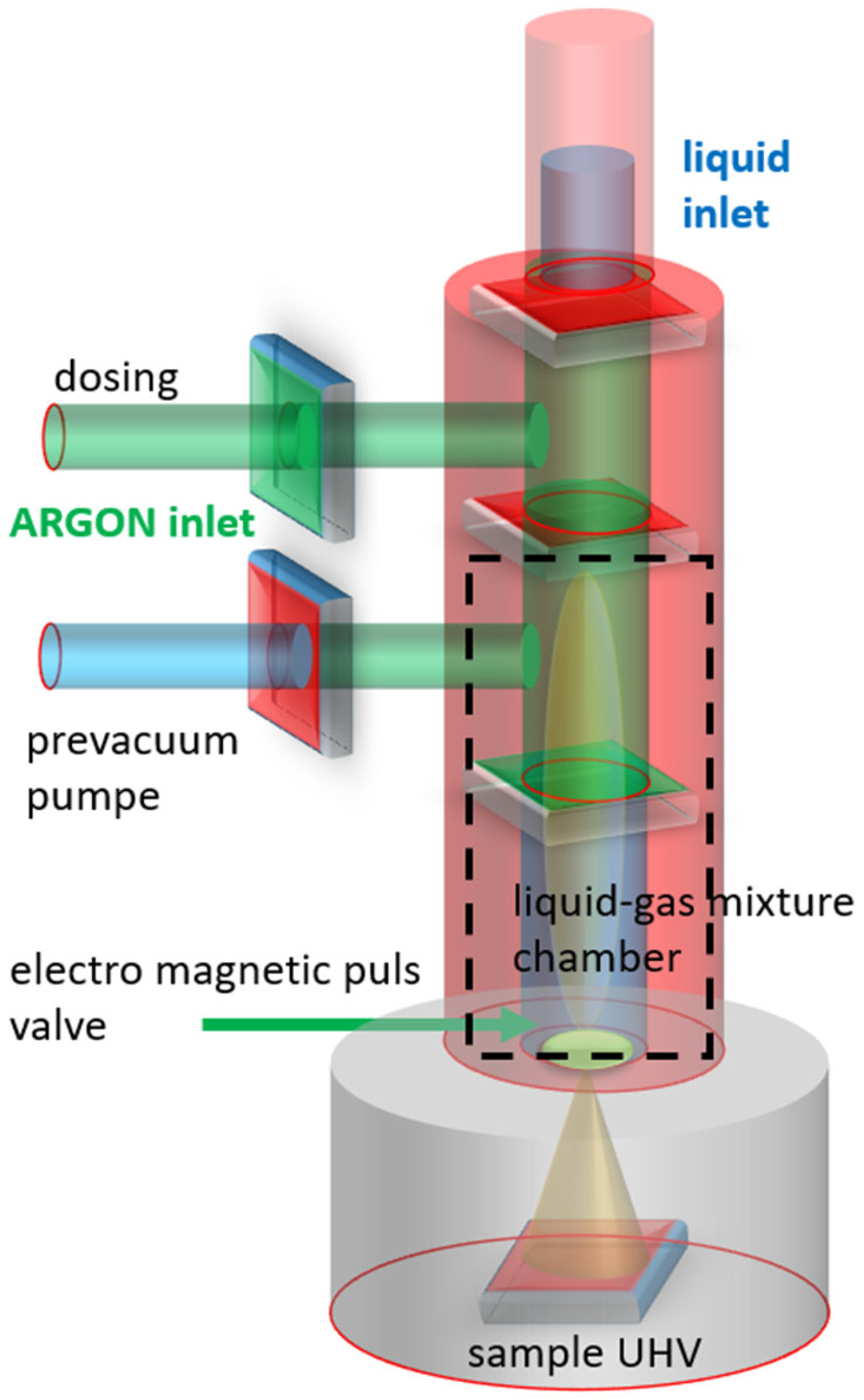
2.2.1. Liquid Injection (LI)
2.2.2. Electrospray Injection (ESI)
2.2.3. In Situ Preparation
2.2.4. Ex Situ Preparation
2.2.5. Scanning Tunneling Microscopy
2.2.6. Infrared Spectroscopy
2.2.7. Photoemission Spectroscopy
3. Results and Discussion
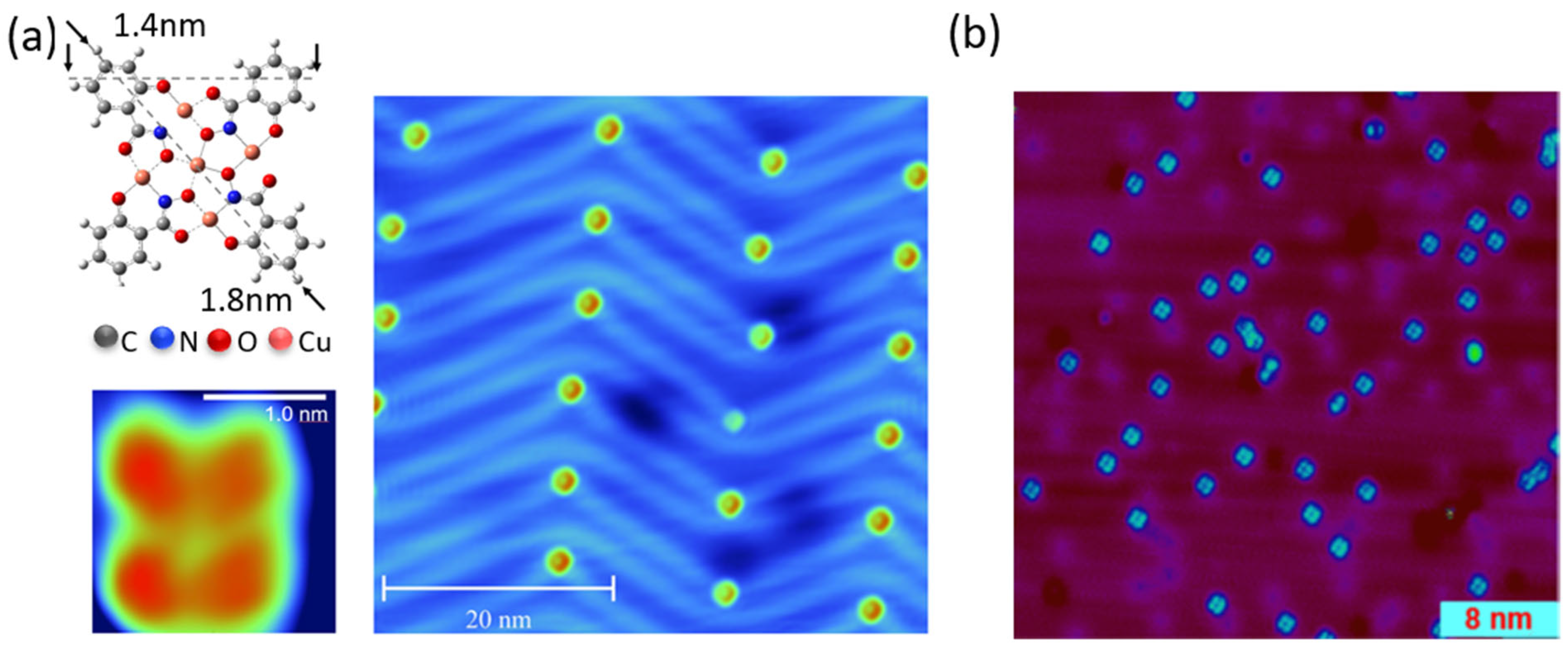
3.1. STM
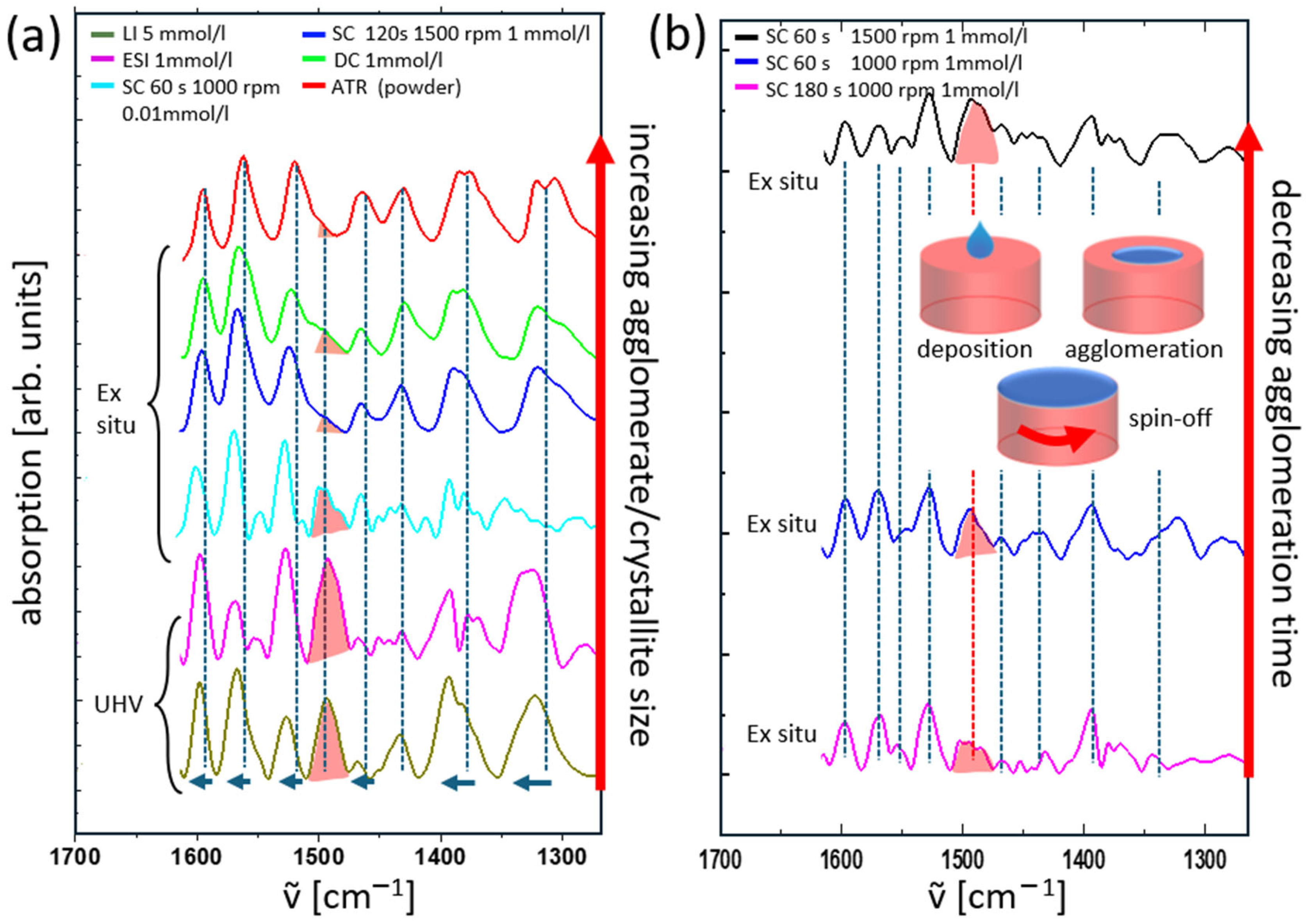
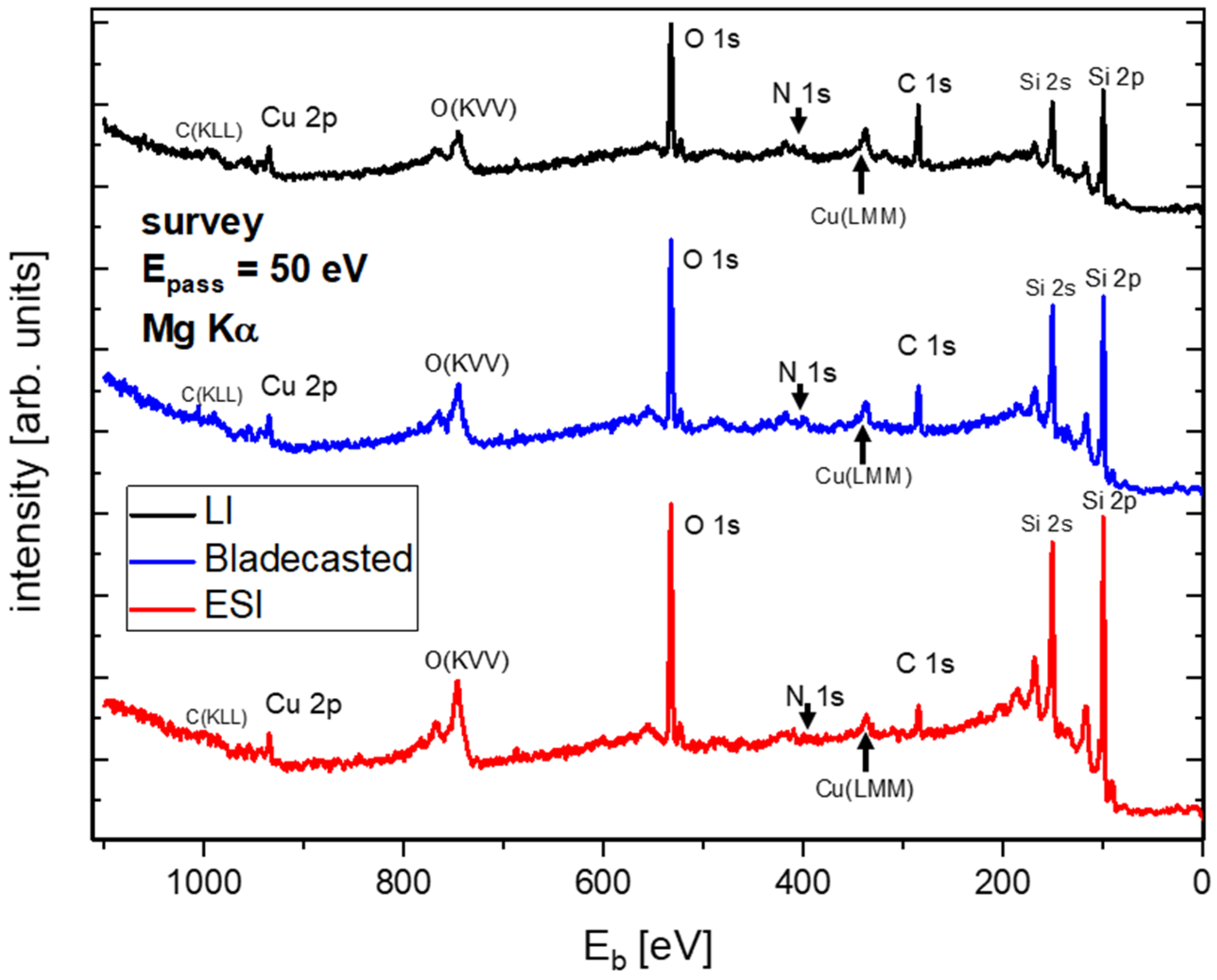
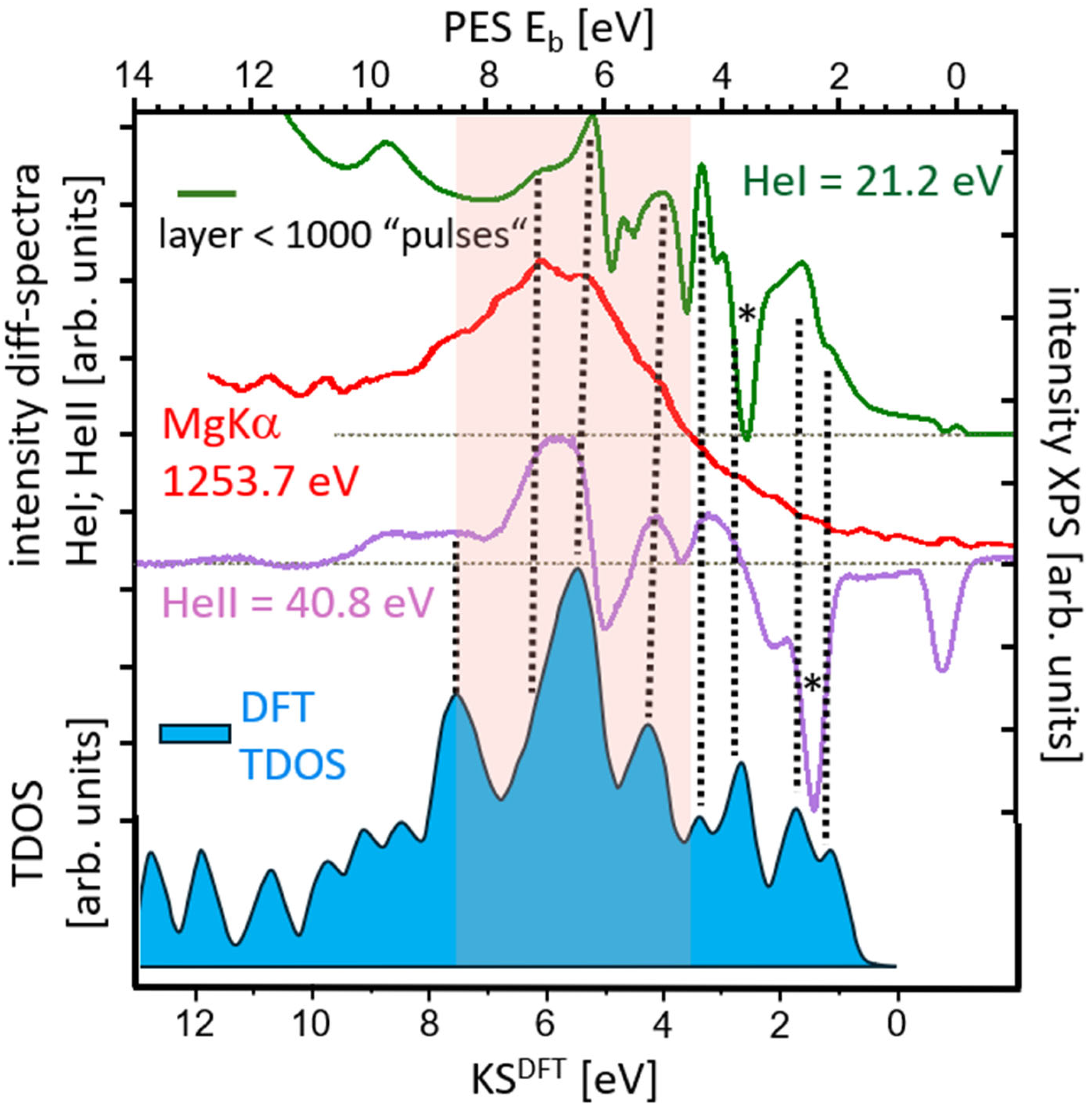
3.2. Comparison of Ex Situ and In Situ Prepared CuCu4 Films
3.3. Improvements of the LI System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atodiresei, N.; Brede, J.; Lazić, P.; Caciuc, V.; Hoffmann, G.; Wiesendanger, R.; Blügel, S. Design of the Local Spin Polarization at the Organic-Ferromagnetic Interface. Phys. Rev. Lett. 2010, 105, 066601. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, S. Molecular spintronics: The rise of spinterface science. Nat. Phys. 2010, 6, 562–564. [Google Scholar] [CrossRef]
- Schmaus, S.; Bagrets, A.; Nahas, Y.; Yamada, T.K.; Bork, A.; Bowen, M.; Beaurepaire, E.; Evers, F.; Wulfhekel, W. Giant magnetoresistance through a single molecule. Nat. Nanotech. 2011, 6, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Cinchetti, M.; Dediu, V.A.; Hueso, L.E. Activating the molecular spinterface. Nat. Mater. 2017, 16, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Droghetti, A.; Thielen, P.; Rungger, I.; Haag, N.; Groszmann, N.; Stockl, J.; Stadtmuller, B.; Aeschlimann, M.; Sanvito, S.; Cinchetti, M. Dynamic spin filtering at the Co/Alq3 interface mediated by weakly coupled second layer molecules. Nat. Commun. 2016, 7, 12668. [Google Scholar] [CrossRef]
- Dediu, V.A.; Hueso, L.E.; Bergenti, I.; Taliani, C. Spin routes in organic semiconductors. Nat. Mater. 2009, 8, 707–716. [Google Scholar] [CrossRef]
- Pardo, E.; Ruiz-García, R.; Cano, J.; Ottenwaelder, X.; Lescouëzec, R.; Journaux, Y.; Lloret, F.; Julve, M. Ligand design for multidimensional magnetic materials: A metallosupramolecular perspective. Dalton Trans. 2008, 21, 2780–2805. [Google Scholar] [CrossRef]
- Homberg, J.; Weismann, A.; Berndt, R.; Gruber, M. Inducing and Controlling Molecular Magnetism through Supramolecular Manipulation. ACS Nano 2020, 14, 17387–17395. [Google Scholar] [CrossRef]
- Kipgen, L.; Bernien, M.; Tuczek, F.; Kuch, W. Spin-Crossover Molecules on Surfaces: From Isolated Molecules to Ultrathin Films. Adv. Mater. 2021, 33, 2008141. [Google Scholar] [CrossRef]
- Viswanatha, C.B.; Stöckl, J.; Arnoldi, B.; Becker, S.; Aeschlimann, M.; Stadtmüller, B. Vectorial Electron Spin Filtering by an All-Chiral Metal–Molecule Heterostructure. J. Phys. Chem. Lett. 2022, 13, 6244–6249. [Google Scholar] [CrossRef]
- Kahn, A.; Koch, N.; Gao, W. Electronic structure and electrical properties of interfaces between metals and π-conjugated molecular films. J. Polym. Sci. B 2003, 41, 2529–2548. [Google Scholar] [CrossRef]
- Koch, N. Organic electronic devices and their functional interfaces. ChemPhysChem 2007, 8, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-level alignment at organic/metal and organic/organic interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Fahlman, M.; Fabiano, S.; Simon, V.G.D.; Berggren, M.; Crispin, X. Interfaces in organic electronics. Nat. Rev. Mater. 2019, 4, 627–650. [Google Scholar] [CrossRef]
- Zhan, Y.Q.; Fahlman, M. The study of organic semiconductor/ferromagnet interfaces in organic spintronics: A short review of recent progress. J. Polym. Sci. B 2012, 50, 1453–1462. [Google Scholar] [CrossRef]
- Panish, M.; Holtkamp, D.; Lange, W.; Jirikowsky, M.; Benninghoven, A. UHV preparation of organic overlayers by a molecular beam technique. Appl. Surf. Sci. 1984, 17, 296–308. [Google Scholar]
- Forrest, S.R. Ultrathin Organic Films Grown by Organic Molecular Beam Deposition and Related Techniques. Chem. Rev. 1997, 97, 1793–1896. [Google Scholar] [CrossRef]
- Kowarik, S.; Gerlach, A.; Schreiber, F. Organic molecular beam deposition: Fundamentals, growth dynamics, and in situ studies. J. Phys. Condens. Matter 2008, 20, 184005–184017. [Google Scholar] [CrossRef]
- Mustafa, H.A.; Jameel, D.A. Modeling and the main stages of spin coating process: A review. J. Appl. Sci. Technol. Trends 2021, 2, 119–123. [Google Scholar] [CrossRef]
- Tyona, M.D. A theoretical study on spin coating technique. Adv. Mater. Res. 2013, 2, 195–208. [Google Scholar] [CrossRef]
- Ramos-Hernández, R.; Calvo, F.D.; Pérez-Gutiérrez, E.; Percino, M.J. Large area small-molecule thin films deposited by the doctor blade technique implemented with computer numerical control machine. Thin Solid Films 2023, 771, 139787–139796. [Google Scholar] [CrossRef]
- Kumar, A.K.S.; Zhang, Y.; Li, D.; Compton, R.G. A mini-review: How reliable is the drop casting technique? Electrochem. Comm. 2020, 121, 106867–106877. [Google Scholar] [CrossRef]
- Rasool, S.; Kim, J.Y. Prospects of glove-box versus air-processed organic solar cells. Phys. Chem. Chem. Phys. 2023, 25, 19337–19357. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B. Electrospray Wings for Molecular Elephants (Nobel Lecture). Angew. Chem. Int. Ed. 2003, 42, 3871–3894. [Google Scholar] [CrossRef]
- Kawai, T.; Tanaka, H.; Nakagawa, T. Low dimensional self-organization of DNA-base molecules on Cu(111) surfaces. Surf. Sci. 1997, 386, 124–136. [Google Scholar] [CrossRef]
- Yamashita, M.; Fenn, J.B. Electrospray Ion-Source−Another Variation on the Free-Jet Theme. J. Phys. Chem. 1984, 88, 4451–4459. [Google Scholar] [CrossRef]
- Rauschenbach, S.; Vogelgesang, R.; Malinowski, N.; Gerlach, J.W.; Benyouce, M.; Costantini, G.; Deng, Z.; Thontasen, N.; Kern, K. Electrospray Ion Beam Deposition: Soft-Landing and Fragmentation of Functional Molecules at Solid Surfaces. Nano 2009, 3, 2901–2910. [Google Scholar] [CrossRef]
- Grill, L.; Stass, I.; Rieder, K.H.; Moresco, F. Preparation of self-ordered molecular layers by pulse injection. Surf. Sci. 2006, 600, L143–L147. [Google Scholar] [CrossRef]
- Zambelli, T.; Boutayeb, Y.; Gayral, F.; Lagoute, J.; Girdhar, N.K.; Gourdon, A.; Gauthier, S.; Blanco, M.-J.; Chambron, J.-C.; Heitz, V.; et al. Deposition of large organic molecules in ultra-high vacuum: A comparison between thermal sublimation and pulse-injection. Int. J. Nanosci. 2004, 3, 12. [Google Scholar] [CrossRef]
- Happ, P.; Rentschler, E. Enforcement of a High-Spin Ground State for the First 3d Heterometallic 12-Metallacrown-4 Complex. Dalton Trans. 2014, 41, 15308–15312. [Google Scholar] [CrossRef]
- Ranecki, R.; Lach, S.; Lüpke, A.; Rentschler, E.; Ziegler, C. Spin-Flip Inelastic Electron Tunneling Spectroscopy on a CuCu4 Metallacrown Complex on Au(111). J. Phys. Chem. C 2023, 127, 13186–13195. [Google Scholar] [CrossRef]
- Sobrado, J.M.; Martin-Gago, J.A. Controlled injection of a liquid into ultra-high vacuum: Submonolayers of adenosine triphosphate deposited on Cu(110). J. Appl. Phys. 2016, 120, 145307. [Google Scholar] [CrossRef]
- Pütz, F. Präparation Organischer Schichten Aus Der Flüssigphase. Master’s Thesis, TU Kaiserslautern, Kaiserslautern, Germany, 2022. [Google Scholar]
- Necas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys 2012, 10, 181–188. [Google Scholar]
- Ranecki, R.; Lach, S.; Lüpke, A.; Athanasopoulou, A.; Rentschler, E.; Ziegler, C. Competing Intramolecular Superexchange Interactions in the CuFe4 Metallacrown on Au(111)—An Inelastic Tunneling Spectroscopy Study. J. Phys. Chem. C 2022, 126, 15907–15914. [Google Scholar]
- Kurek, Y. Infrarotspektroskopische Untersuchungen von In-Situ und Ex-Situ Präparierten CuCu4-Metallakronen-Komplexen. Ph.D. Thesis, TU Kaiserslautern, Kaiserslautern, Germany, 2022. [Google Scholar]
- Dendrinou-Samara, C.; Psomas, G.; Iordanidis, L.; Tangoulis, V.; Kessissoglou, D.P. Host–Guest Interaction of 12-MC-4, 15-MC-5, and Fused 12-MC-4 Metallacrowns with Mononuclear and Binuclear Carboxylato Complexes: Structure and Magnetic Behavior. Chem. Eur. J. 2001, 7, 5041–5051. [Google Scholar] [CrossRef]
- Kessissoglou, D.P.; Bodwin, J.J.; Kampf, J.; Dendrinou-Samara, C.; Pecoraro, V.L. Pseudohalide complexation by manganese 12-metallacrowns-4 complexes. Inorg. Chimica Acta 2002, 331, 73–80. [Google Scholar] [CrossRef]
- Dendrinou-Samara, C.; Alevizopoulou, L.; Iordanidis, L.; Samaras, E.; Kessissoglou, D.P. 15-MC-5 manganese metallacrowns hosting herbicide complexes. Structure and bioactivity. J. Inorg. Biochem. 2002, 89, 89–96. [Google Scholar] [CrossRef]
- Alexiou, M.; Tsivikas, I.; Dendrinou-Samara, C.; Pantazaki, A.A.; Trikalitis, P.; Lalioti, N.; Kyriakidis, D.A.; Kessissoglou, D.P. High nuclearity nickel compounds with three, four or five metal atoms showing antibacterial activity. J. Inorg. Biochem. 2003, 93, 256–264. [Google Scholar] [CrossRef]
- Dendrinou-Samara, C.; Papadopoulos, A.N.; Malamatari, D.A.; Tarushi, A.; Raptopoulou, C.P.; Terzis, A.; Samaras, E.; Kessissoglou, D.P. Inter-conversion of 15-MC-5 to 12-MC-4 manganese metallacrowns: Structure and bioactivity of metallacrowns hosting carboxylato complexes. J. Inorg. Biochem. 2005, 99, 864. [Google Scholar] [CrossRef]
- Blättner, R. Vergleich von Methoden Zur Präparation von Metallakronen-Komplexen. Bachelor Thesis, TU Kaiserslautern, Kaiserslautern, Germany, 2022. [Google Scholar]
- Biesinger, M.C.; Laua, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Torres-Ochoa, J.A.; Cabrera-German, D.; Cortazar-Martinez, O.; Bravo-Sanchez, M.; Gomez-Sosa, G.; Herrera-Gomez, A. Peak-fitting of Cu 2p photoemission spectra in Cu0, Cu1+, and Cu2+ oxides: A method for discriminating Cu0 from Cu1+. Appl. Surf. Sci. 2023, 622, 156960–156971. [Google Scholar] [CrossRef]
- Parmigiani, F.; Depero, L.E.; Minerva, T.; Torrance, J.J.B. The fine structure of the Cu2p32 X-ray photoelectron spectra of copper oxide based compounds. J. Electron Spectrosc. Relat. Phenom. 1992, 58, 315–323. [Google Scholar] [CrossRef]
- Yeh, J.J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. At. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Pavlyukh, Y.; Rentschler, E.; Elmers, H.-J.; Hübner, W.; Lefkidis, G. Broken symmetry states of metallacrowns: Distribution of spins and the g-tensor. Phys. Rev. B 2019, 99, 144418. [Google Scholar] [CrossRef]
- Tavakoli, A. (RPTU Kaiserslautern, Kaiserslautern, Germany); Puschnik, P. (University of Graz, Graz, Austria); Schneider, H.-C. (RPTU Kaiserslautern, Kaiserslautern, Germany). Private communication, 2024.
- Toader, T.; Gavrila, G.; Braun, W.; Ivanco, J.; Zahn, D.T. Valence band fine structure of copper phtahlocyanine thin films: Effect of molecular orientation. Phys. Status Solidi 2009, 7, 1510–1518. [Google Scholar] [CrossRef]
- Evangelista, F.; Carravetta, V.; Stefani, G.; Jansik, B.; Alagia, M.; Stranges, S.; Ruocco, A. Elektronic Structure of copper phthalocyanine: An experimental and theoretical study of occupied an unoccupied levels. J. Chem. Phys. 2007, 126, 124709. [Google Scholar] [CrossRef]
- Lach, S.; Altenhof, A.; Tarafder, K.; Schmidt, F.; Ali, E.; Vogel, M.; Sauther, J.; Oppeneer, P.M.; Ziegler, C. Metal–organic hybrid interface states of a ferromagnet/organic semiconductor hybrid junction as basis for engineering spin injection in organic spintronics. Adv. Funct. Mater. 2012, 22, 989–997. [Google Scholar] [CrossRef]
- Faubel, M.; Steiner, B.; Toennies, J.P. Measurement of He I photoelectron spectra of liquid water, formamide and ethylene glycol in fast-flowing microjets. J. Electron Spectrosc. Relat. Phenom. 1998, 95, 159–169. [Google Scholar] [CrossRef]
- Salim, M.; Hurst, J.; Montgomery, M.; Tolman, N.; Liu, H. Airborne contamination of graphite as analyzed by ultra-violet photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2019, 235, 8–15. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C121448&Type=IR-SPEC&Index=0 (accessed on 7 January 2025).
- Hamid, Y.; Fathi, M.R. A Novel Cationic Surfactant-Assisted Switchable Solvent-Based Dispersive Liquid–Liquid Microextraction for Determination for Orange II in Food Samples. Food Analyt. Methods 2018, 11, 2131–2140. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Happ, P.; Sapozhnik, A.; Klanke, J.; Czaja, P.; Chernenkaya, A.; Medanik, K.; Schuppler, S.; Nagle, P.; Merz, M.; Rentschler, E.; et al. Analyzing the enforcement of a high-spin ground state for a metallacrown single-molecule magnet. Phys. Rev. B 2016, 93, 174404. [Google Scholar] [CrossRef]
- Marom, N.; Kronik, L. Density functional theory of transition metal phthalocyanines II: Electronic structure of MnPc and FePc—symmetry and symmetry breaking. Appl. Phys. A 2009, 95, 165–172. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pütz, F.; Blättner, R.; Kurek, Y.; Bolz, L.; Ehnert, S.; Wendels, R.; Stephan, D.; Schreyer, P.; Ranecki, R.; Brennfleck, E.; et al. Liquid Phase Preparation of Organic Thin Films Consisting of Complex Molecules—The Example of the Metallacrown CuCu4. Solids 2025, 6, 13. https://doi.org/10.3390/solids6010013
Pütz F, Blättner R, Kurek Y, Bolz L, Ehnert S, Wendels R, Stephan D, Schreyer P, Ranecki R, Brennfleck E, et al. Liquid Phase Preparation of Organic Thin Films Consisting of Complex Molecules—The Example of the Metallacrown CuCu4. Solids. 2025; 6(1):13. https://doi.org/10.3390/solids6010013
Chicago/Turabian StylePütz, Frederik, Richard Blättner, Yves Kurek, Lukas Bolz, Swen Ehnert, Robert Wendels, Dominic Stephan, Philip Schreyer, Robert Ranecki, Ellen Brennfleck, and et al. 2025. "Liquid Phase Preparation of Organic Thin Films Consisting of Complex Molecules—The Example of the Metallacrown CuCu4" Solids 6, no. 1: 13. https://doi.org/10.3390/solids6010013
APA StylePütz, F., Blättner, R., Kurek, Y., Bolz, L., Ehnert, S., Wendels, R., Stephan, D., Schreyer, P., Ranecki, R., Brennfleck, E., Lüpke, A., Laible, D., Baumann, B., Lach, S., Rentschler, E., & Ziegler, C. (2025). Liquid Phase Preparation of Organic Thin Films Consisting of Complex Molecules—The Example of the Metallacrown CuCu4. Solids, 6(1), 13. https://doi.org/10.3390/solids6010013