Advanced Prussian Blue Cathodes for Rechargeable Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
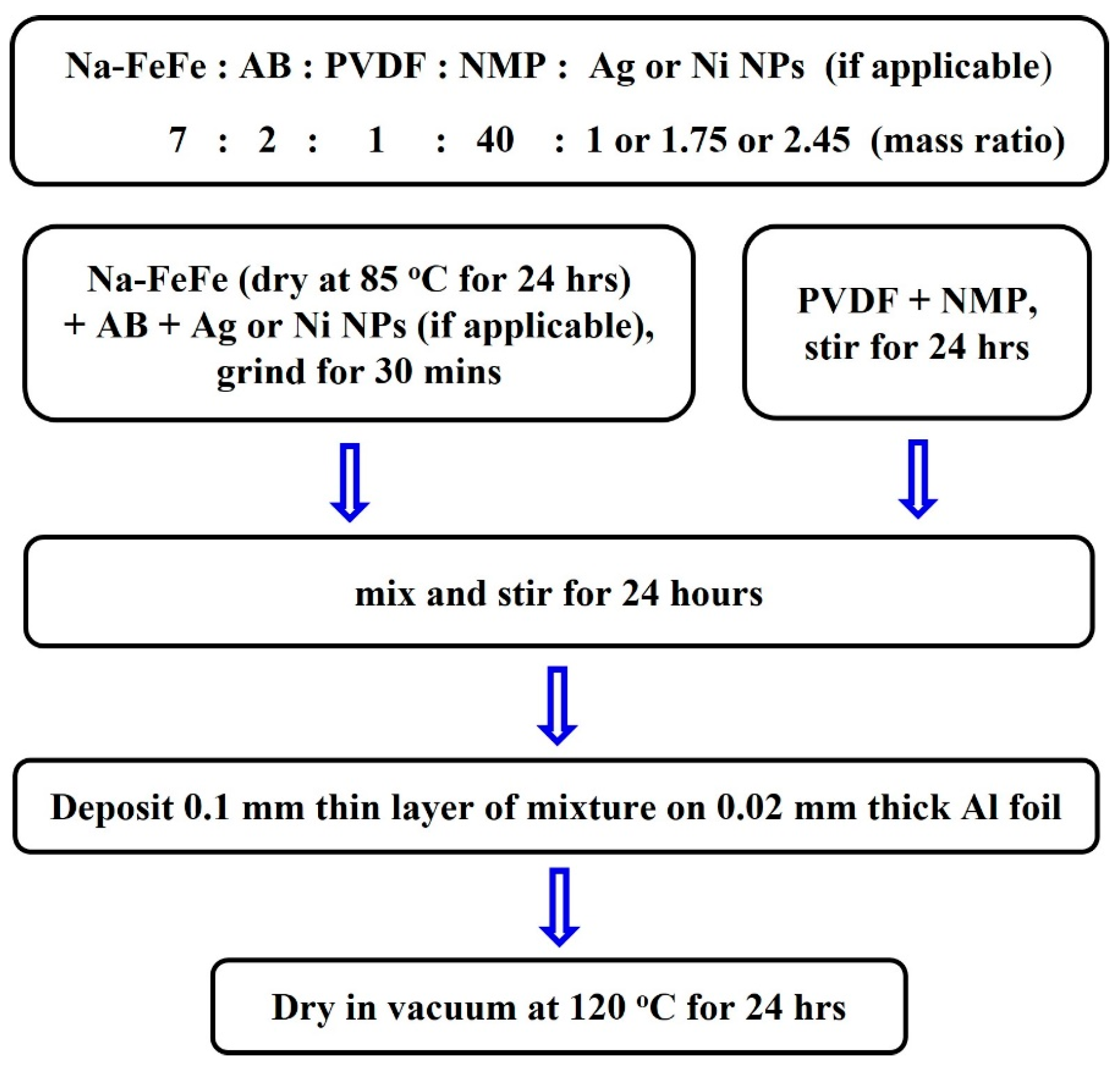
2.1. Synthesis of Prussian Blue Nanoparticles
2.2. Synthesis of Ag and Ni Nanoparticles
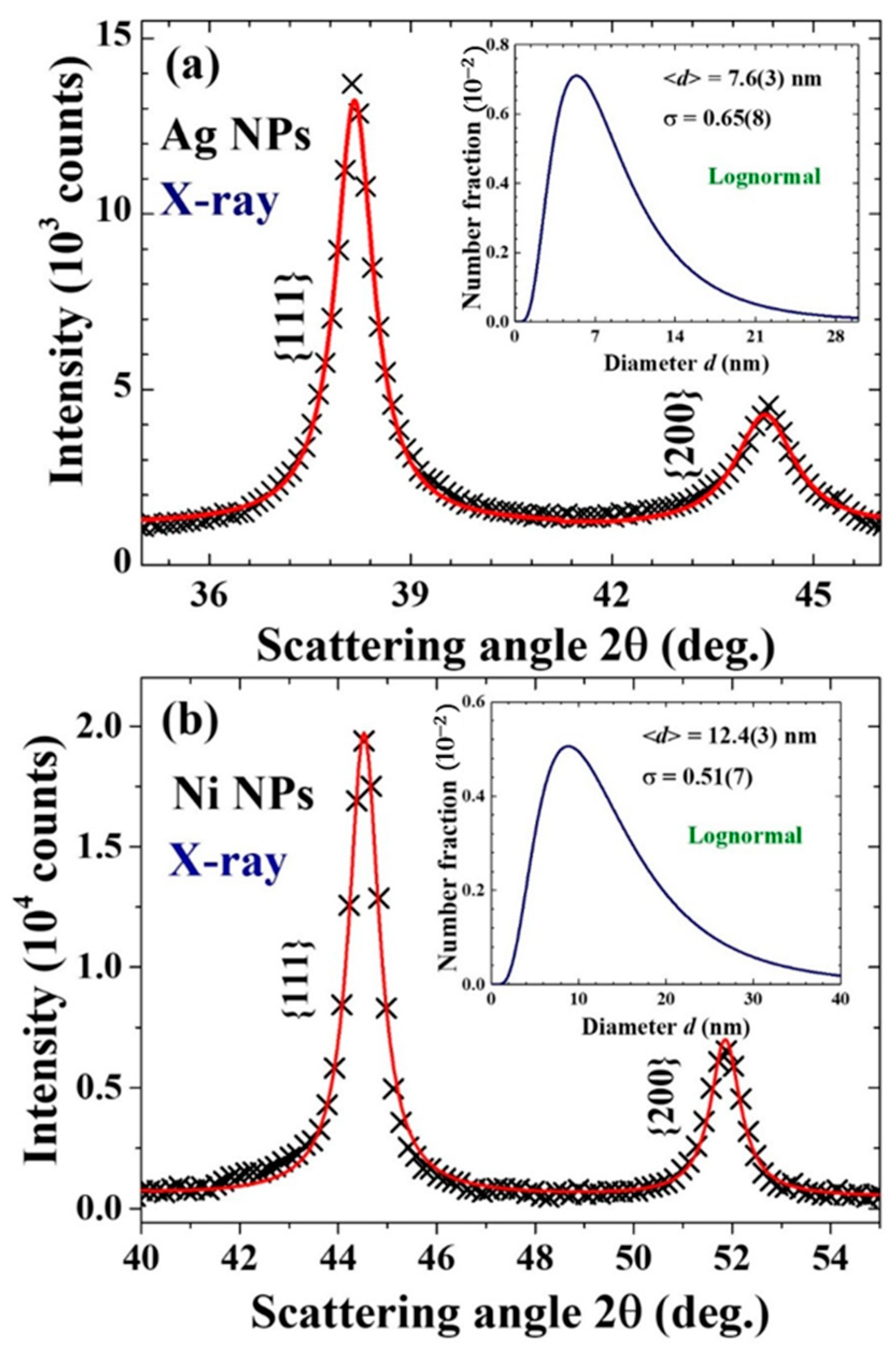
2.3. X-ray Diffraction
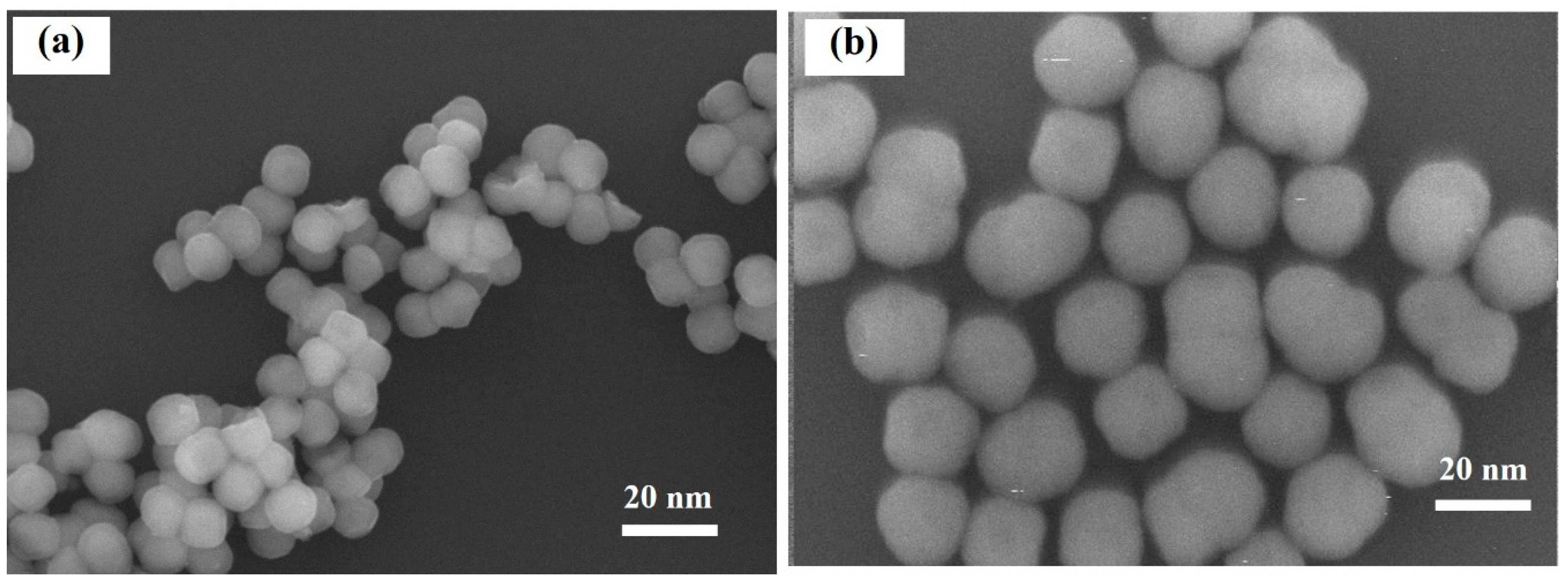
2.4. Transmission Electron Microscopy Images
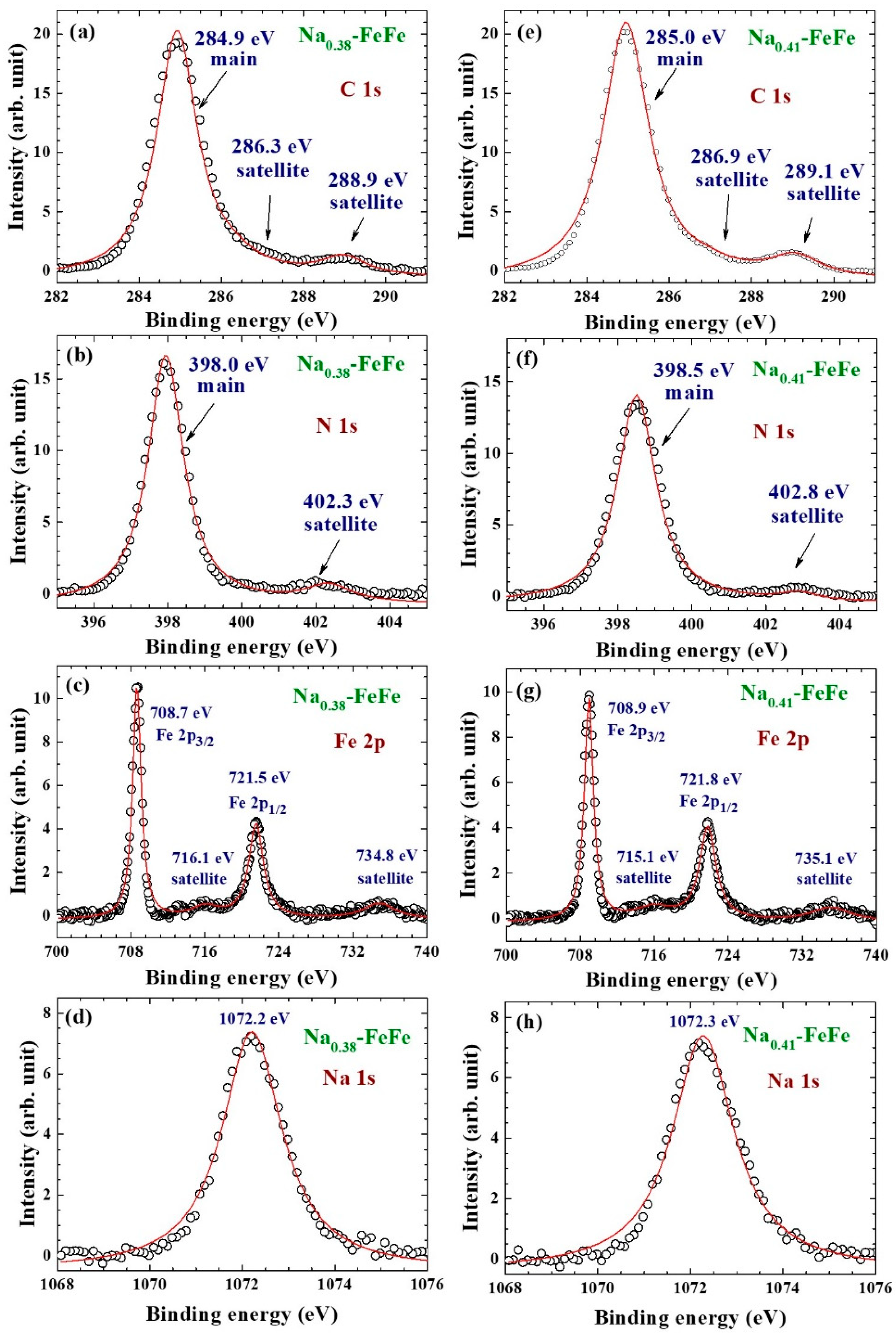
2.5. X-ray Photoemission Spectrum
2.6. Electrochemical Experiments
3. Results and Discussion
3.1. Chemical Composition
3.2. Particle Size
3.3. Ultimate Size for Energy Storage
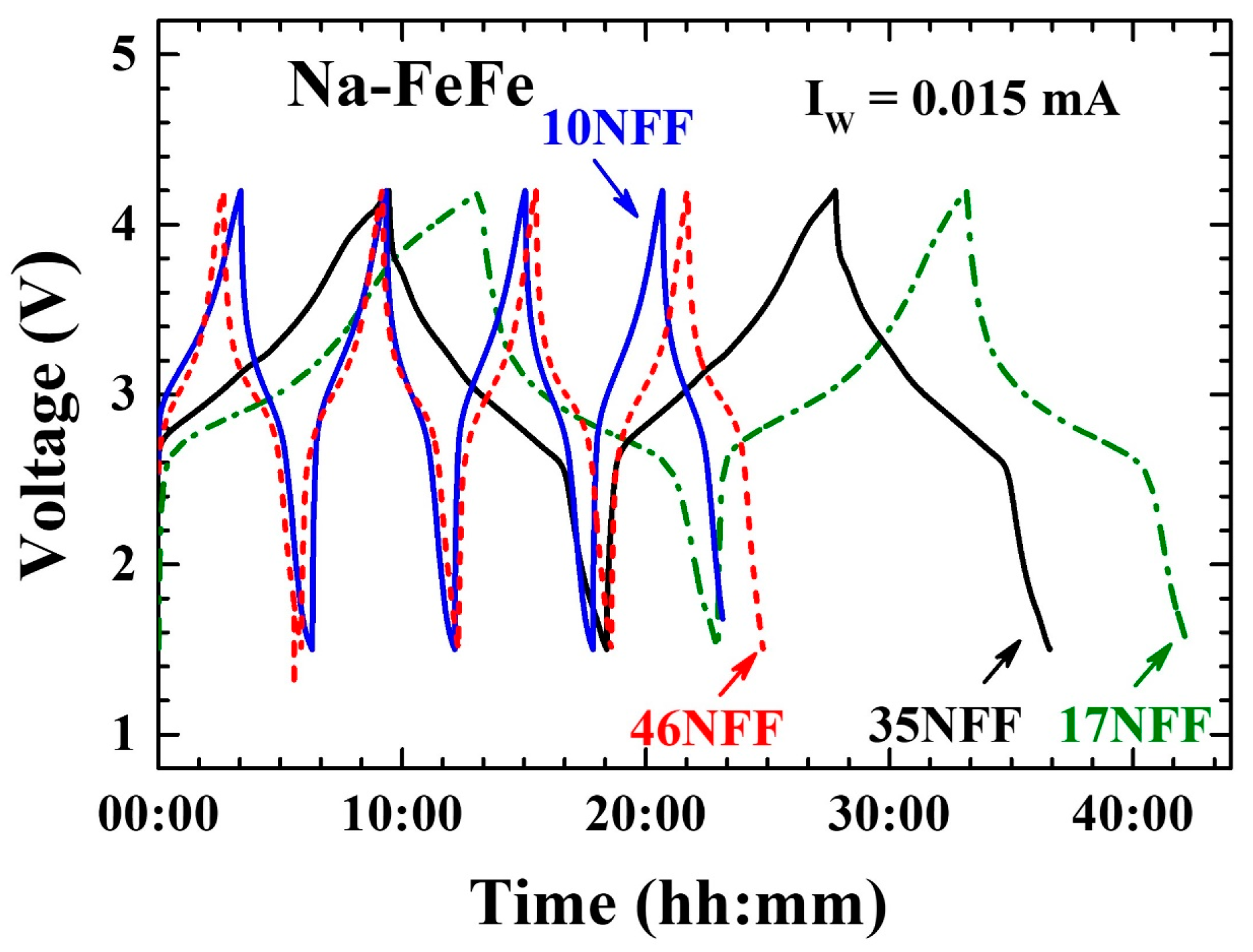
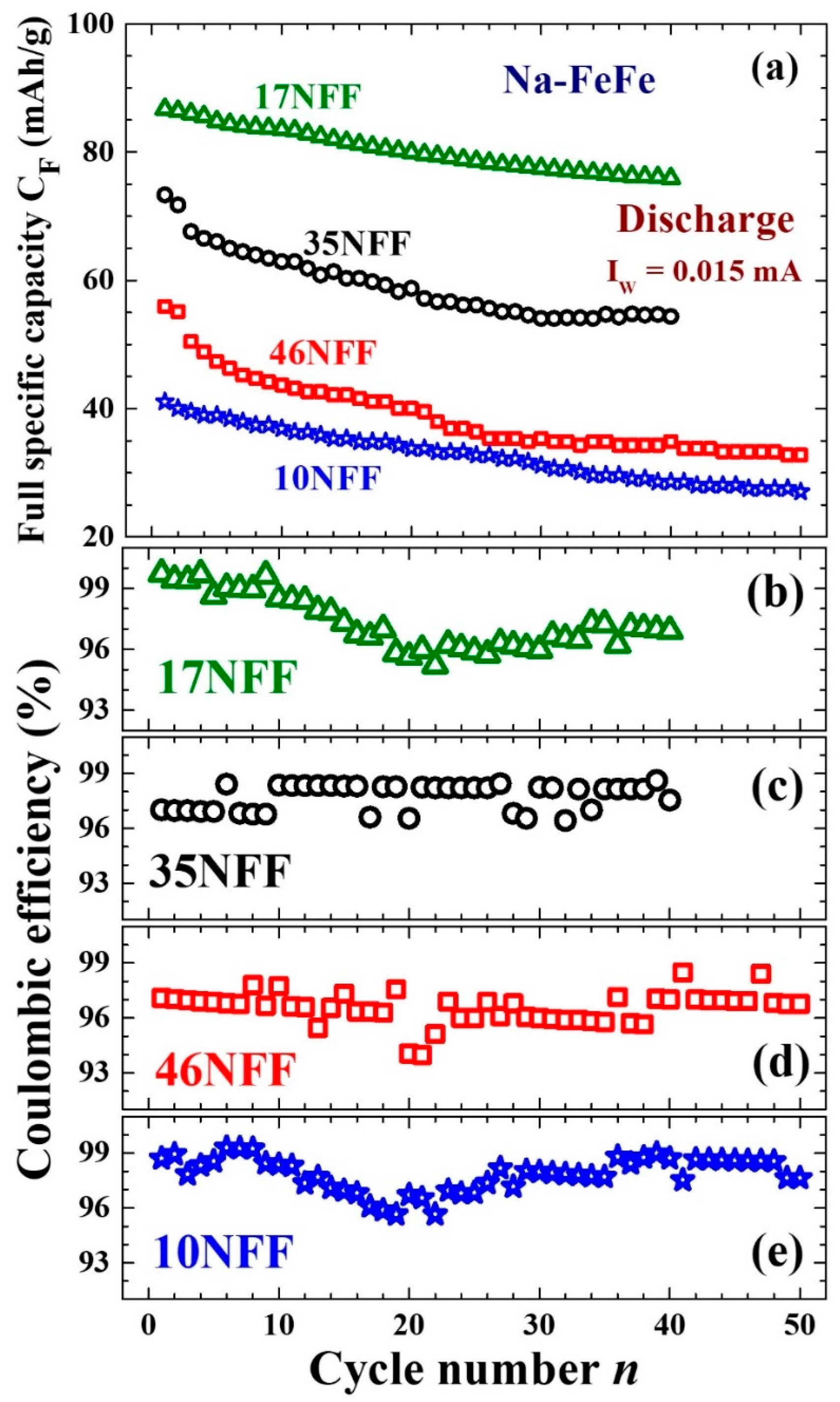
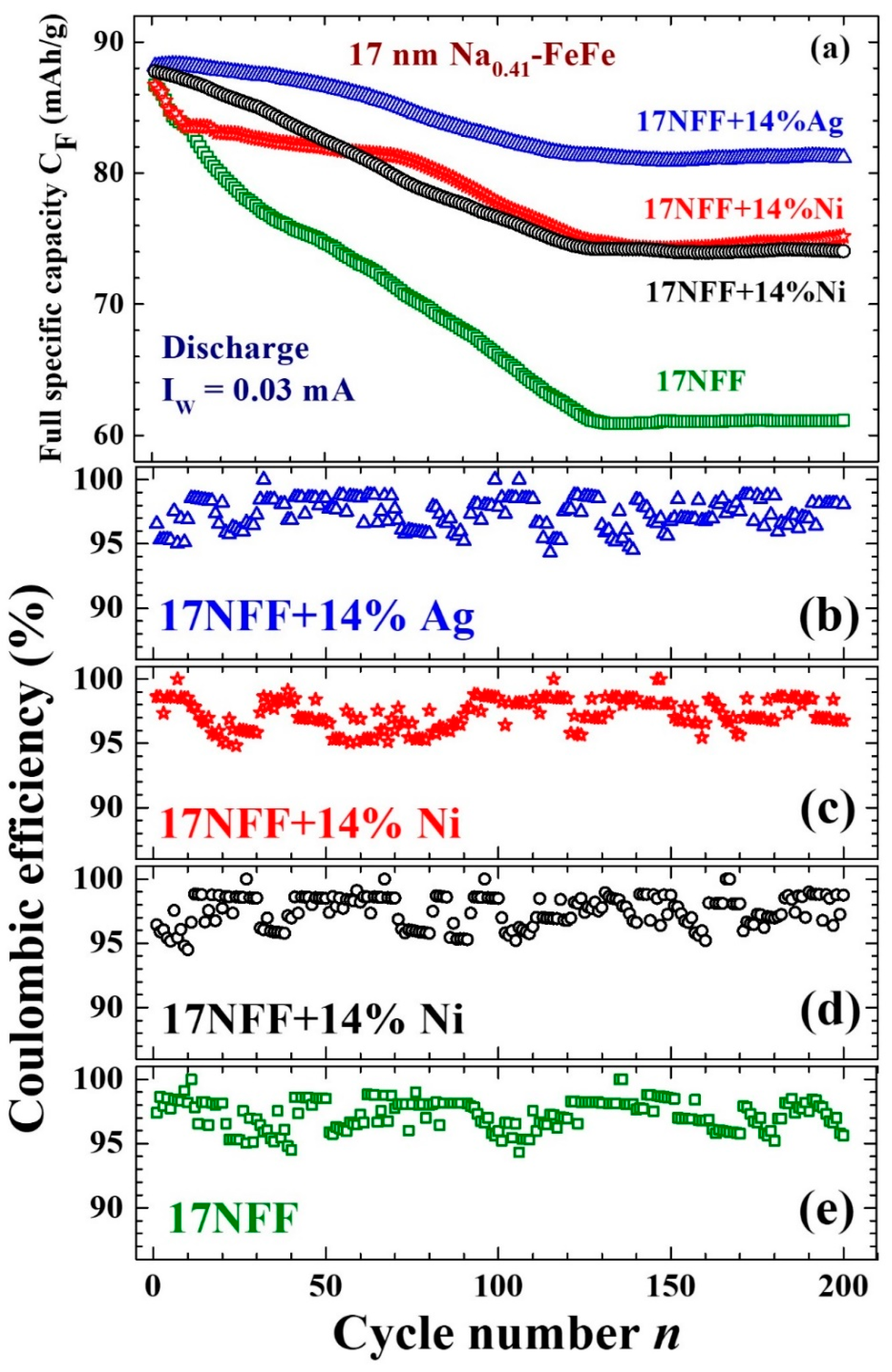
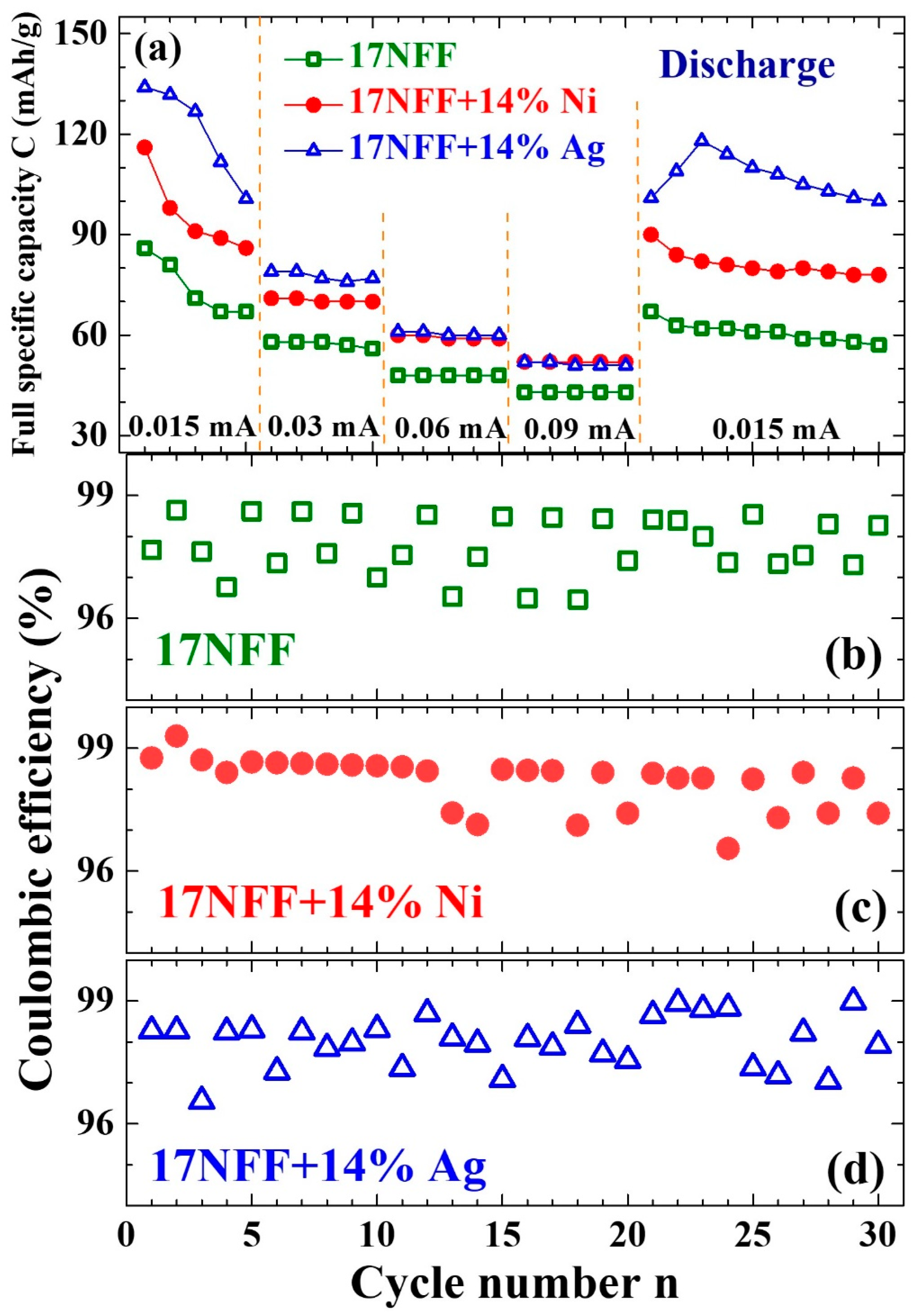
3.4. Enhanced Electrochemical Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Dong, C.; Gao, W.; Jin, B.; Jiang, Q. Advances in cathode materials for high-performance lithium-sulfur batteries. iScience 2018, 6, 151–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, X.; Zhang, S.; Wang, J.; Huang, Q.; Yu, N.; Zhu, Y.; Fu, L.; Wang, F.; Chen, Y.; et al. Three-dimensional ordered porous electrode materials for electrochemical energy storage. NPG Asia Mater. 2019, 11, 12. [Google Scholar] [CrossRef]
- Khan, S.A.; Ali, S.; Saeed, K.; Usman, M.; Khan, I. Advanced cathode materials and efficient electrolytes for rechargeable batteries: Practical challenges and future perspectives. J. Mater. Chem. A 2019, 7, 10159–10173. [Google Scholar] [CrossRef]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A.J.I.C. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3·xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Verdaguer, M.; Girolami, G.S. Magnetic Prussian Blue Analogs. In Magnetism: Molecules to Materials V; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 283–346. [Google Scholar]
- Okubo, M.; Asakura, D.; Mizuno, Y.; Kim, J.D.; Mizokawa, T.; Kudo, T.; Honma, I. Switching redox-active sites by valence tautomerism in Prussian blue analogues AxMny[Fe(CN)6]·nH2O (A: K, Rb): Robust frameworks for reversible Li storage. J. Phys. Chem. Lett. 2010, 1, 2063–2071. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Wang, X.; Bahlawane, N.; Pan, H.; Yan, M.; Jiang, Y. Prussian blue analogs for rechargeable batteries. iScience 2018, 3, 110–133. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian blue analogs as battery materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Yamane, R.; Takeda, Y.; Yamamoto, O.; Sakaebe, H.; Tabuchi, M. Lithium intercalation behavior of iron cyanometallates. J. Power Sources 1999, 81, 530–534. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Scholz, F. Performance characteristics of zinc hexacyanoferrate/Prussian blue and copper hexacyanoferrate/Prussian blue solid state secondary cells. J. Power Sources 2000, 91, 217–233. [Google Scholar] [CrossRef]
- Eftekhari, A. Potassium secondary cell based on Prussian blue cathode. J. Power Sources 2004, 126, 221–228. [Google Scholar] [CrossRef]
- Okubo, M.; Asakura, D.; Mizuno, Y.; Kudo, T.; Zhou, H.; Okazawa, A.; Kojima, N.; Ikedo, K.; Mizokawa, T.; Honma, I. Ion-Induced Transformation of Magnetism in a Bimetallic CuFe Prussian Blue Analogue. Angew. Chem. 2011, 123, 6393–6397. [Google Scholar] [CrossRef]
- Wang, R.Y.; Wessells, C.D.; Huggins, R.A.; Cui, Y. Highly reversible open framework nanoscale electrodes for divalent ion batteries. Nano Lett. 2013, 13, 5748–5752. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A superior low-cost cathode for a Na-ion battery. Angew. Chem. 2013, 52, 2018–2021. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Liu, N.; Nelson, J.; McDowell, M.T.; Huggins, R.A.; Toney, M.F.; Cui, Y. Full open-framework batteries for stationary energy storage. Nat. Commun. 2014, 5, 3007. [Google Scholar] [CrossRef] [PubMed]
- Mullaliu, A.; Asenbauer, J.; Aquilanti, G.; Passerini, S.; Giorgetti, M. Highlighting the Reversible Manganese Electroactivity in Na-Rich Manganese Hexacyanoferrate Material for Li-and Na-Ion Storage. Small Methods 2020, 4, 1900529. [Google Scholar] [CrossRef]
- Brant, W.R.; Mogensen, R.; Colbin, S.; Ojwang, D.O.; Schmid, S.; Häggström, L.; Ericsson, T.; Jaworski, A.; Pell, A.J.; Younesi, R. Selective control of composition in Prussian white for enhanced material properties. Chem. Mater. 2019, 31, 7203–7211. [Google Scholar] [CrossRef]
- Asakura, D.; Li, C.H.; Mizuno, Y.; Okubo, M.; Zhou, H.; Talham, D.R. Bimetallic cyanide-bridged coordination polymers as lithium ion cathode materials: Core@shell nanoparticles with enhanced cyclability. J. Am. Chem. Soc. 2013, 135, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Okubo, M.; Kagesawa, K.; Asakura, D.; Kudo, T.; Zhou, H.; Oh-Ishi, K.; Okazawa, A.; Kojima, N. Precise electrochemical control of ferromagnetism in a cyanide-bridged bimetallic coordination polymer. Inorg. Chem. 2012, 51, 10311–10316. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Nanba, Y.; Asakura, D.; Okubo, M.; Talham, D.R. Li-ion and Na-ion insertion into size-controlled nickel hexacyanoferrate nanoparticles. RSC Adv. 2014, 4, 24955–24961. [Google Scholar] [CrossRef]
- Moo Lee, K.; Tanaka, H.; Ho Kim, K.; Kawamura, M.; Abe, Y.; Kawamoto, T. Improvement of redox reactions by miniaturizing nanoparticles of zinc Prussian blue analog. Appl. Phys. Lett. 2013, 102, 141901. [Google Scholar] [CrossRef]
- Fiore, M.; Wheeler, S.; Hurlbutt, K.; Capone, I.; Fawdon, J.; Ruffo, R.; Pasta, M. Paving the way toward highly efficient, high-energy potassium-ion batteries with ionic liquid electrolytes. Chem. Mater. 2020, 32, 7653–7661. [Google Scholar] [CrossRef]
- Li, C.H.; Peprah, M.K.; Asakura, D.; Meisel, M.W.; Okubo, M.; Talham, D.R. Stepwise Reduction of Electrochemically Lithiated Core–Shell Heterostructures Based on the Prussian Blue Analogue Coordination Polymers K0.1Cu[Fe(CN)6]0.7·3.5H2O and K0.1Ni[Fe(CN)6]0.7·4.4H2O. Chem. Mater. 2015, 27, 1524–1530. [Google Scholar] [CrossRef]
- Fu, H.; Liu, C.; Zhang, C.; Ma, W.; Wang, K.; Li, Z.; Lu, X.; Cao, G. Enhanced storage of sodium ions in Prussian blue cathode material through nickel doping. J. Mater. Chem. A 2017, 5, 9604–9610. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, W.; Xue, L.; Ding, X.; Wang, T.; Liu, X.; Liu, J.; Li, X.; Huang, Y. Polypyrrole-promoted superior cyclability and rate capability of NaxFe[Fe(CN)6] cathodes for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 6036–6041. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L.; Luan, J.; Huang, X.; Tang, Y.; Xie, H.; Wang, H. Surface engineering induced core-shell Prussian blue@ polyaniline nanocubes as a high-rate and long-life sodium-ion battery cathode. J. Power Sources 2018, 395, 305–313. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Tang, Y.; Xu, B.B.; Liang, C.; Yan, M.; Jiang, Y. Manganese hexacyanoferrate reinforced by PEDOT coating towards high-rate and long-life sodium-ion battery cathode. J. Mater. Chem. A 2020, 8, 3222–3227. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.; Wang, B.; Li, Y.; Sun, W.; Lu, Y.; Yan, M.; Song, B.; Dou, S. Prussian blue@C composite as an ultrahigh-rate and long-life sodium-ion battery cathode. Adv. Funct. Mater. 2016, 26, 5315–5321. [Google Scholar] [CrossRef]
- Batsaikhan, E.; Ma, M.-H.; Yang, C.C.; Wu, C.-M.; Li, W.-H. Boosting the electrochemical performance of Prussian-Blue-analogue based Li-ion rechargeable batteries by the addition of Ag or Ni nanoparticles into the cathode. Inorg. Chem. Commun. 2023, 150, 110509. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, C.M.; Batsaikhan, E.; Li, H.C.; Li, C.H.; Peprah, M.K.; Talham, D.R.; Meisel, M.W.; Li, W.H. Complex Magnetic Phases in Nanosized Core@Shell Prussian Blue Analogue Cubes: Rb0.48Co[Fe(CN)6]0.75[(H2O)6]0.25·0.34H2O @K0.36Ni[Cr(CN)6]0.74[(H2O)6]0.26·0.11H2O. J. Phys. Chem. C 2015, 119, 29138–29147. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System, Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994. [Google Scholar]
- Warren, B.E. X-ray Diffraction; Dover Publications: Dover, UK, 1990; Volume 251. [Google Scholar]
- Li, W.H.; Lee, C.H. Spin polarization and small size effect in bare silver nanoparticles. In Complex Magnetic Nanostructures; Springer: Cham, Switzerland, 2017; pp. 195–224. [Google Scholar]

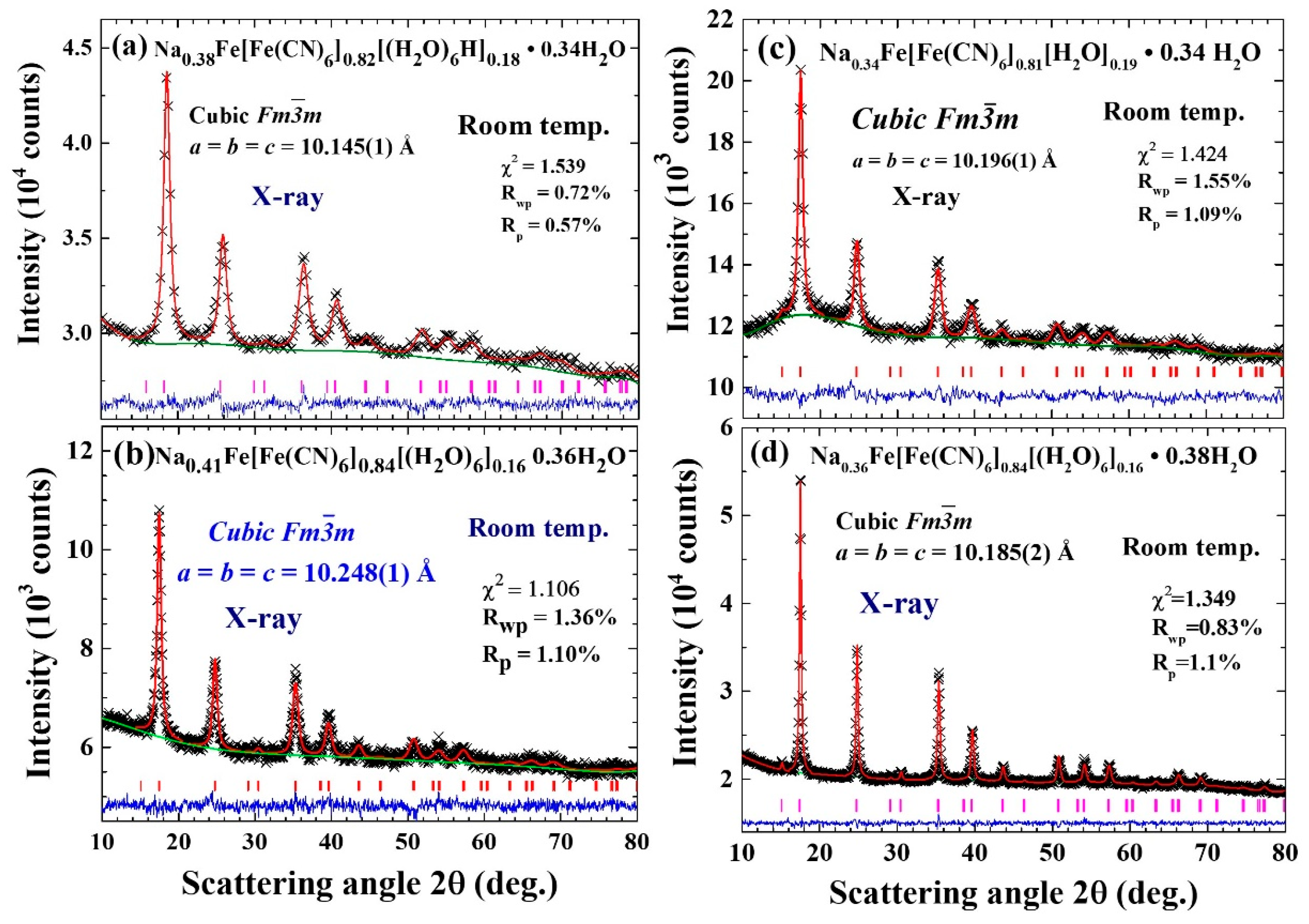
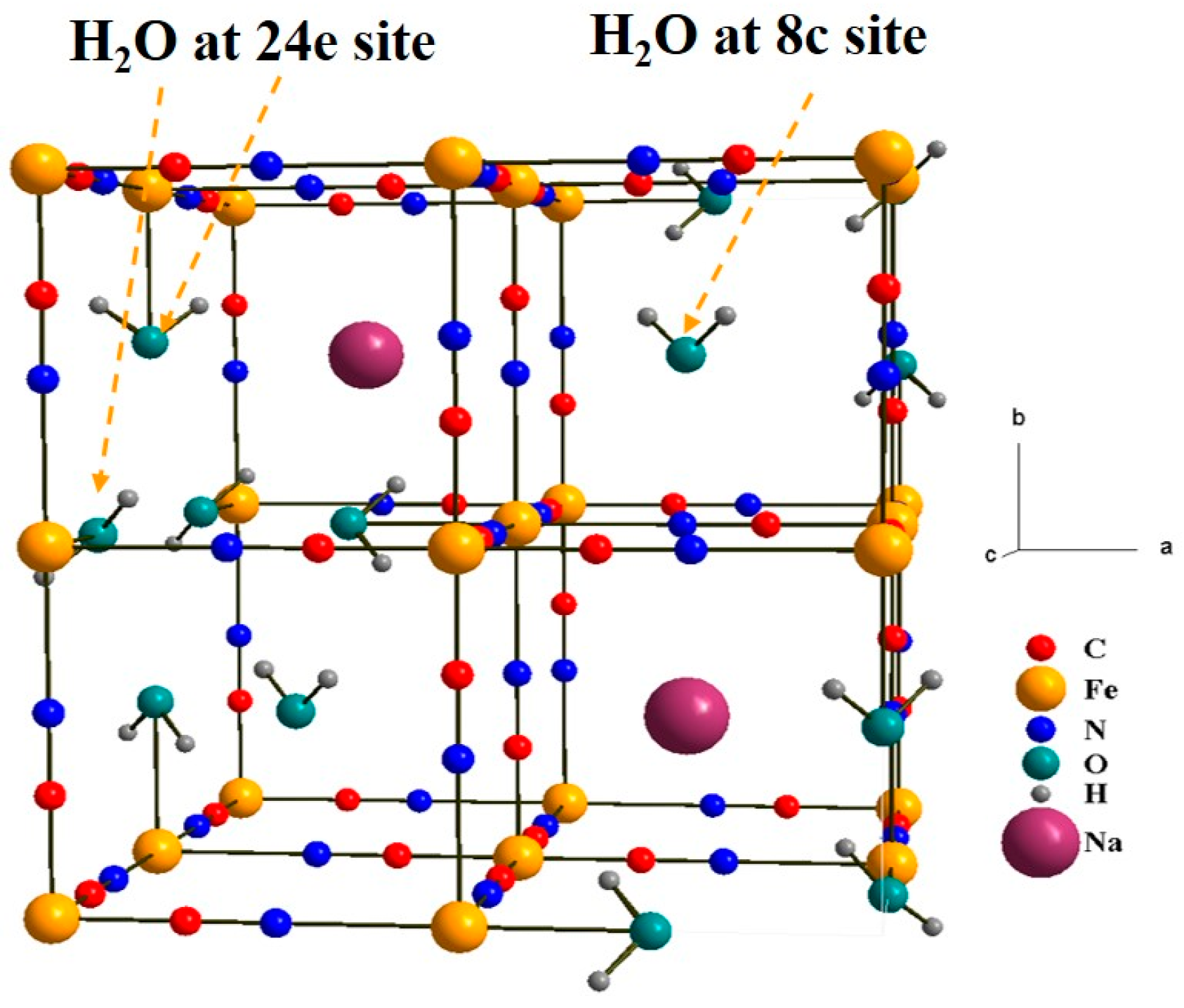

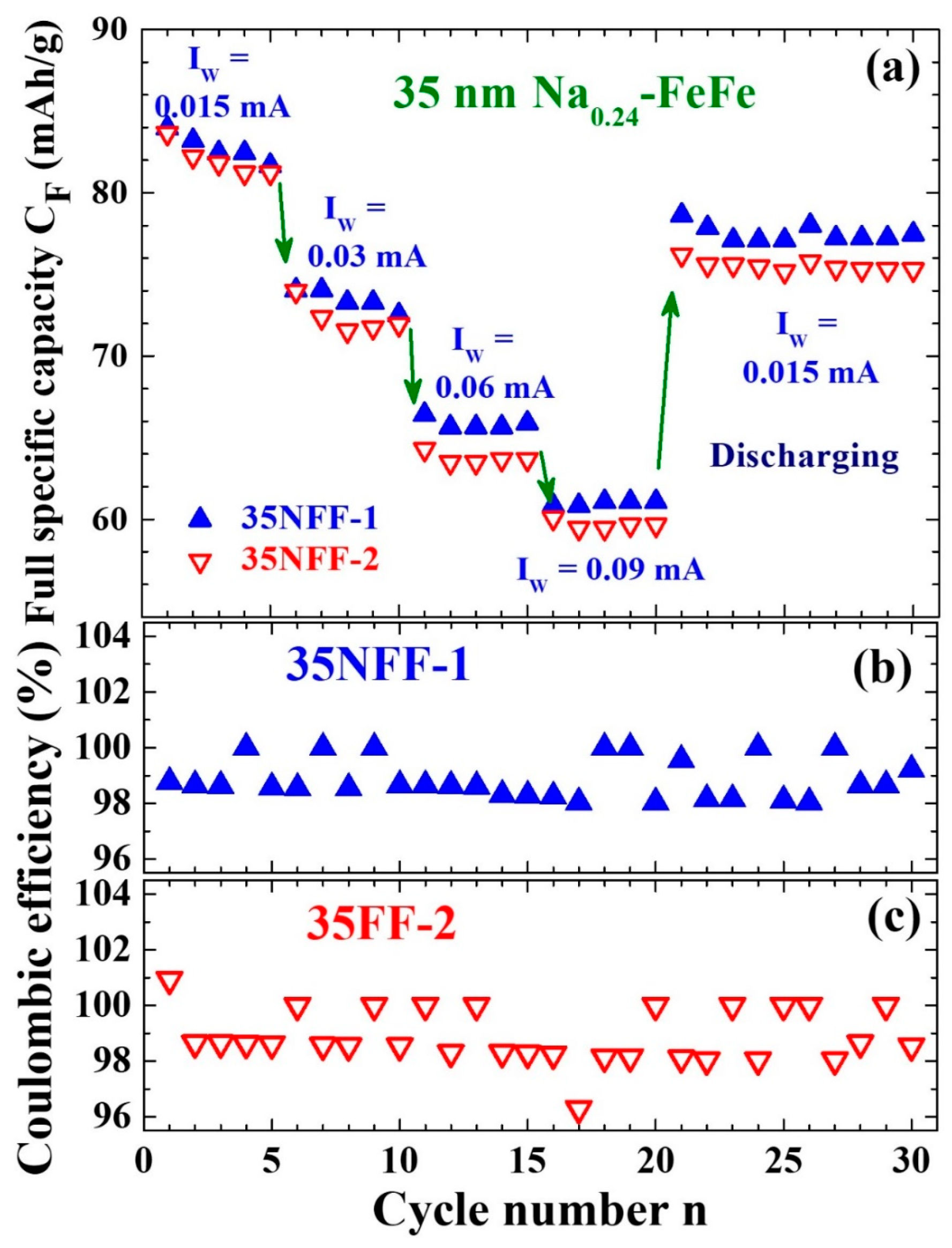
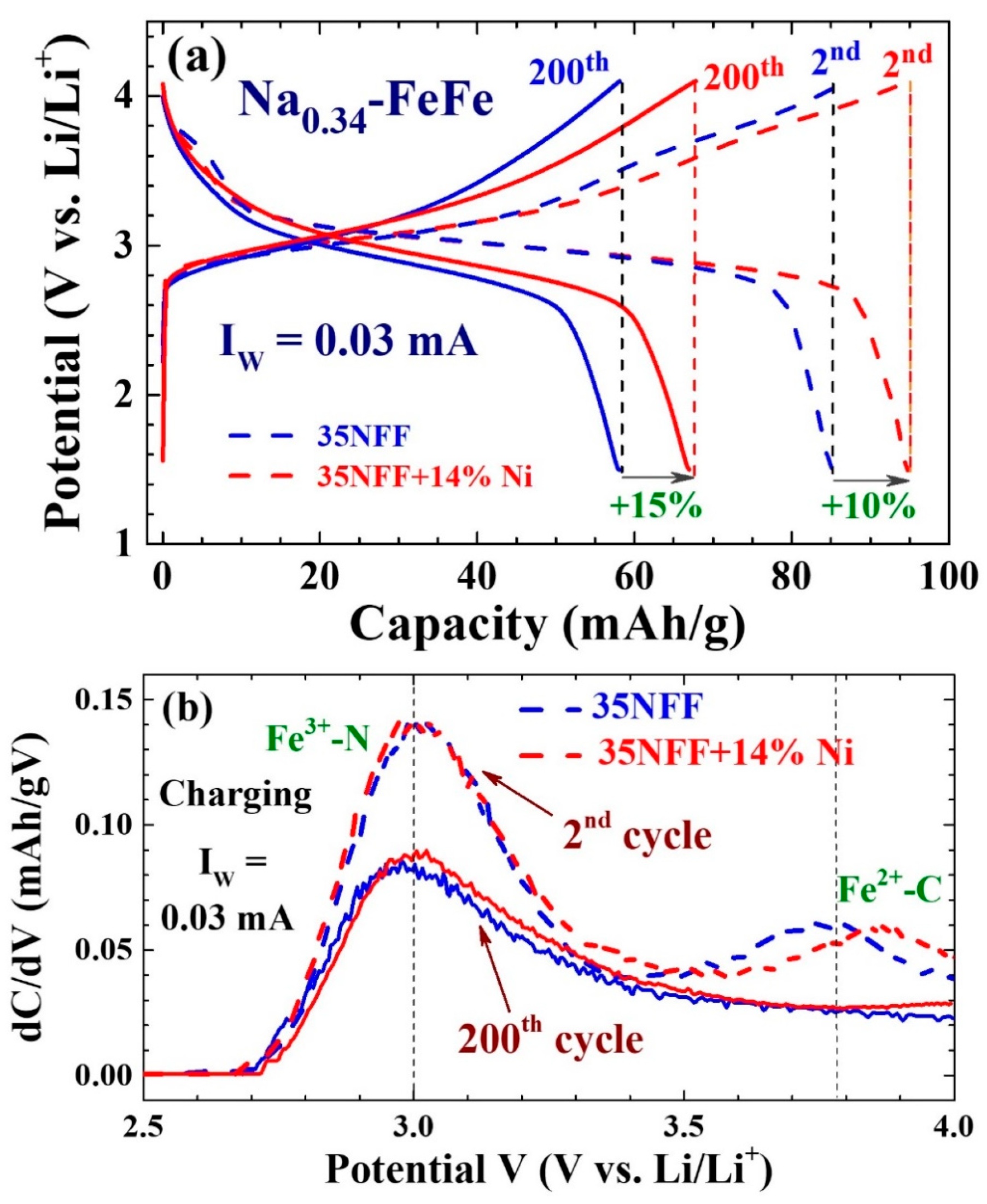
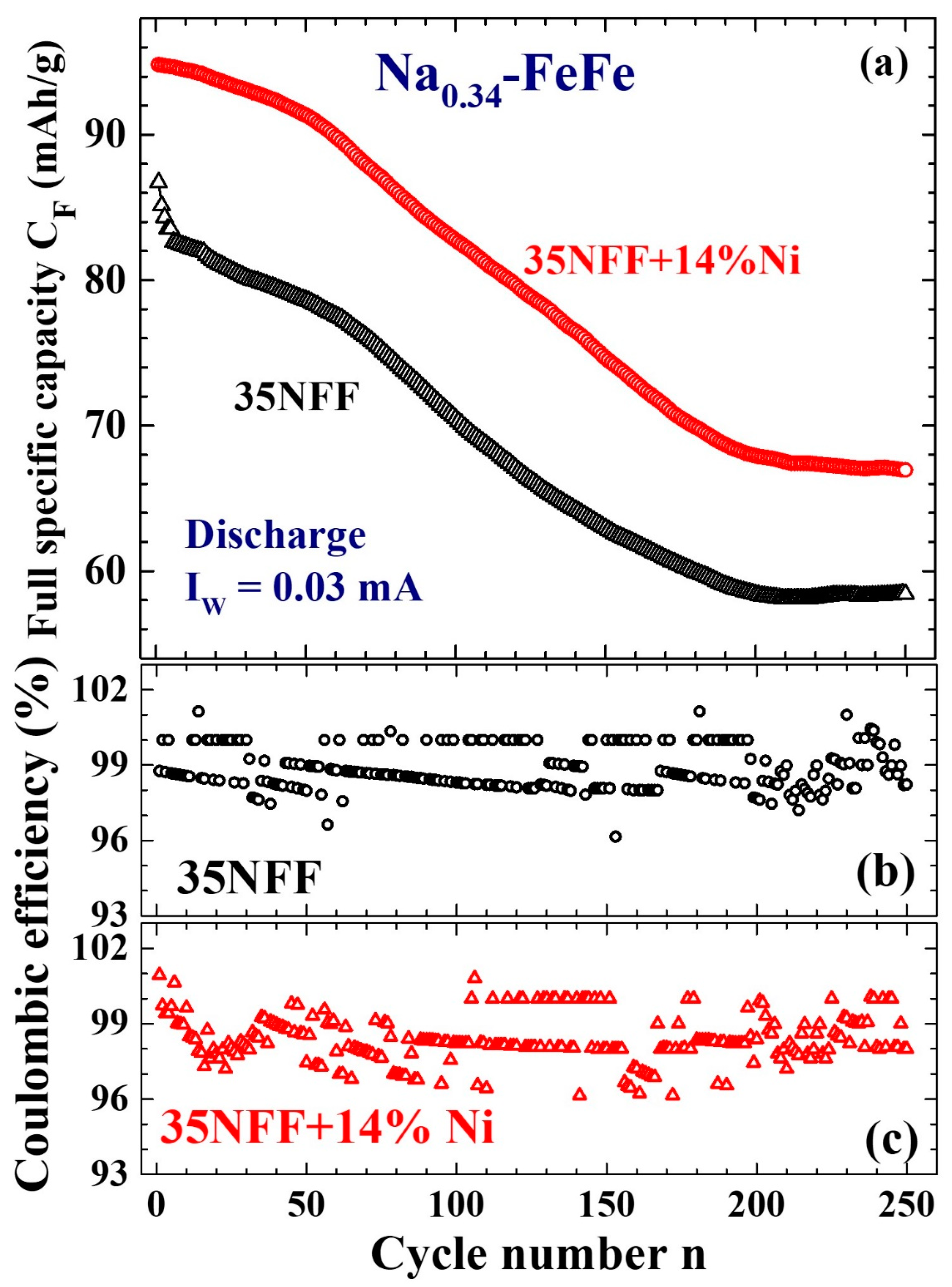
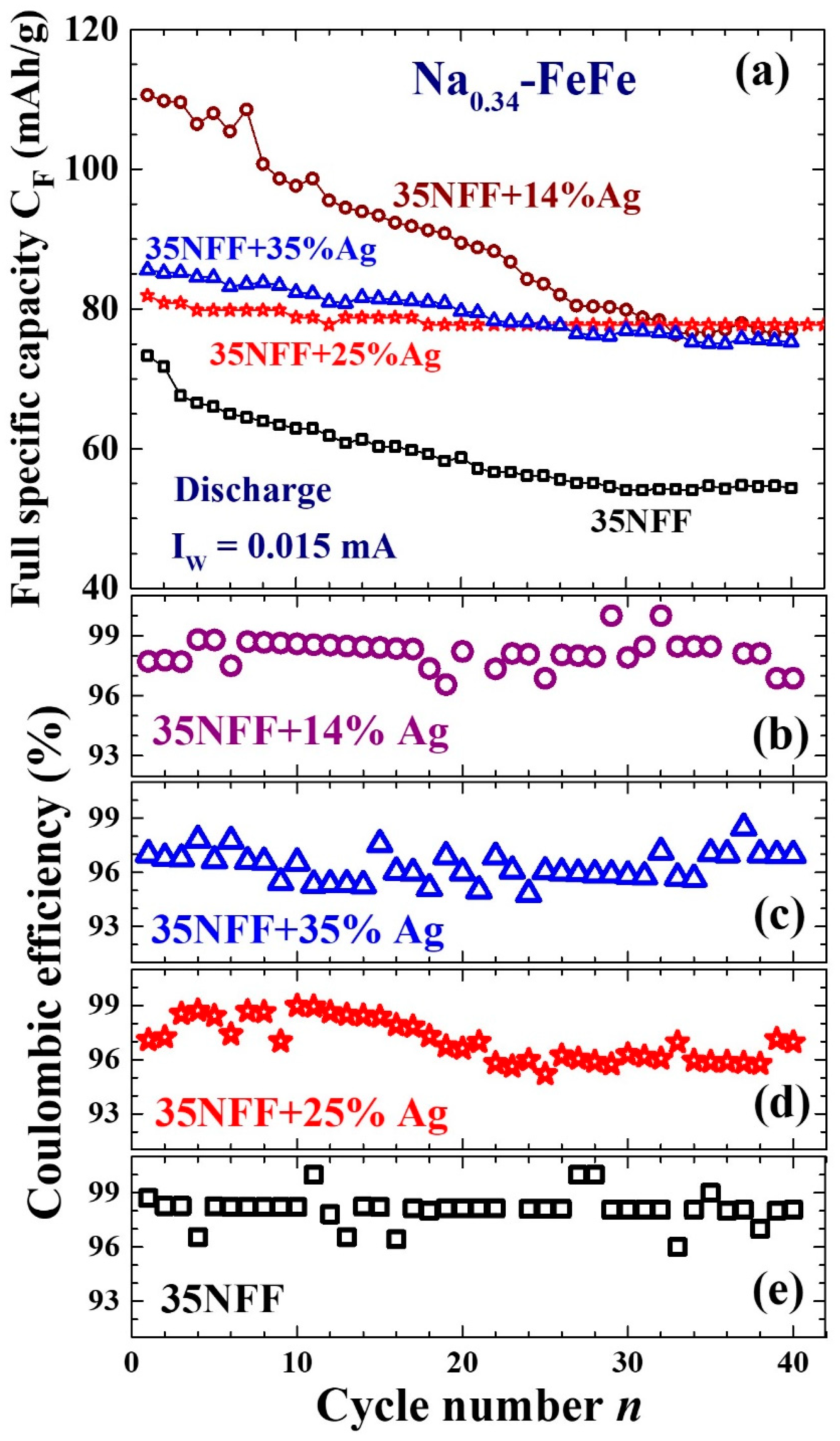
| Label | T (°C) | Chemical Composition | a (Å) | d (nm) | σ |
|---|---|---|---|---|---|
| Na0.38-FeFe | 25 | Na0.38Fe[Fe(CN)6]0.82[(H2O)6]0.18·0.34H2O | 10.145(1) | 10(1) | 0.45(2) |
| Na0.41-FeFe | 50 | Na0.41Fe[Fe(CN)6]0.84[(H2O)6]0.16·0.36H2O | 10.248(1) | 17(2) | 0.65(1) |
| Na0.34-FeFe | 70 | Na0.34Fe[Fe(CN)6]0.81[(H2O)6]0.19·0.34H2O | 10.196(1) | 35(2) | 0.50(2) |
| Na0.36-FeFe | 85 | Na0.36Fe[Fe(CN)6]0.84[(H2O)6]0.16·0.38H2O | 10.185(2) | 46(2) | 0.55(4) |
| Ag | Ag | 4.088(1) | 7.6(3) | 0.65(8) | |
| Ni | Ni | 3.526(3) | 12.4(3) | 0.51(7) |
| Chemical Composition | d (nm) | mAg (%) | mNi (%) | IW (mA) | CFi (mAh/g) | CFs (mAh/g) | n |
|---|---|---|---|---|---|---|---|
| Na0.38Fe[Fe(CN)6]0.82 [(H2O)6]0.18·0.34H2O (this work) | 10 | 0.015 | 42 | 28 | 50 | ||
| Na0.41Fe[Fe(CN)6]0.84 [(H2O)6]0.16·0.36H2O (this work) | 17 | 0.03 | 86 | 61 | 130 | ||
| 14 | 86 | 81 | 130 | ||||
| 14 | 86 | 74 | 120 | ||||
| Na0.34Fe[Fe(CN)6]0.81 [(H2O)6]0.19·0.34H2O (this work) | 35 | 0.015 | 74 | 55 | 35 | ||
| 14 | 110 | 78 | 30 | ||||
| 25 | 82 | 78 | 10 | ||||
| 35 | 86 | 78 | 20 | ||||
| 0.03 | 86 | 59 | 200 | ||||
| 14 | 95 | 67 | 210 | ||||
| Na0.36Fe[Fe(CN)6]0.84 [(H2O)6]0.16·0.38H2O (this work) | 46 | 0.015 | 56 | 35 | 25 | ||
| K0.58Co[Co(CN)6]0.86 [(H2O)6]0.14·0.24H2O (Ref. [36]) | 120 | 0.03 | 300 | 40 | 60 | ||
| 14 | 340 | 130 | 120 | ||||
| 10 | 305 | 40 | 125 | ||||
| 14 | 310 | 125 | 120 | ||||
| Na0.46Co[Fe(CN)6]0.84 [(H2O)6]0.16·0.25H2O (Ref. [36]) | 80 | 0.03 | 110 | 56 | 90 | ||
| 10 | 110 | 60 | 95 | ||||
| 10 | 110 | 70 | 110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-J.; Li, W.-H.; Batsaikhan, E.; Ma, M.-H.; Yang, C.-C. Advanced Prussian Blue Cathodes for Rechargeable Li-Ion Batteries. Solids 2024, 5, 208-226. https://doi.org/10.3390/solids5020014
Wu S-J, Li W-H, Batsaikhan E, Ma M-H, Yang C-C. Advanced Prussian Blue Cathodes for Rechargeable Li-Ion Batteries. Solids. 2024; 5(2):208-226. https://doi.org/10.3390/solids5020014
Chicago/Turabian StyleWu, Shun-Ji, Wen-Hsien Li, Erdembayalag Batsaikhan, Ma-Hsuan Ma, and Chun-Chuen Yang. 2024. "Advanced Prussian Blue Cathodes for Rechargeable Li-Ion Batteries" Solids 5, no. 2: 208-226. https://doi.org/10.3390/solids5020014
APA StyleWu, S.-J., Li, W.-H., Batsaikhan, E., Ma, M.-H., & Yang, C.-C. (2024). Advanced Prussian Blue Cathodes for Rechargeable Li-Ion Batteries. Solids, 5(2), 208-226. https://doi.org/10.3390/solids5020014






