Two-Dimensional Quantum Dots: From Photoluminescence to Biomedical Applications
Abstract
1. Introduction to 2D Quantum Dots
2. Synthesis of 2D Quantum Dots
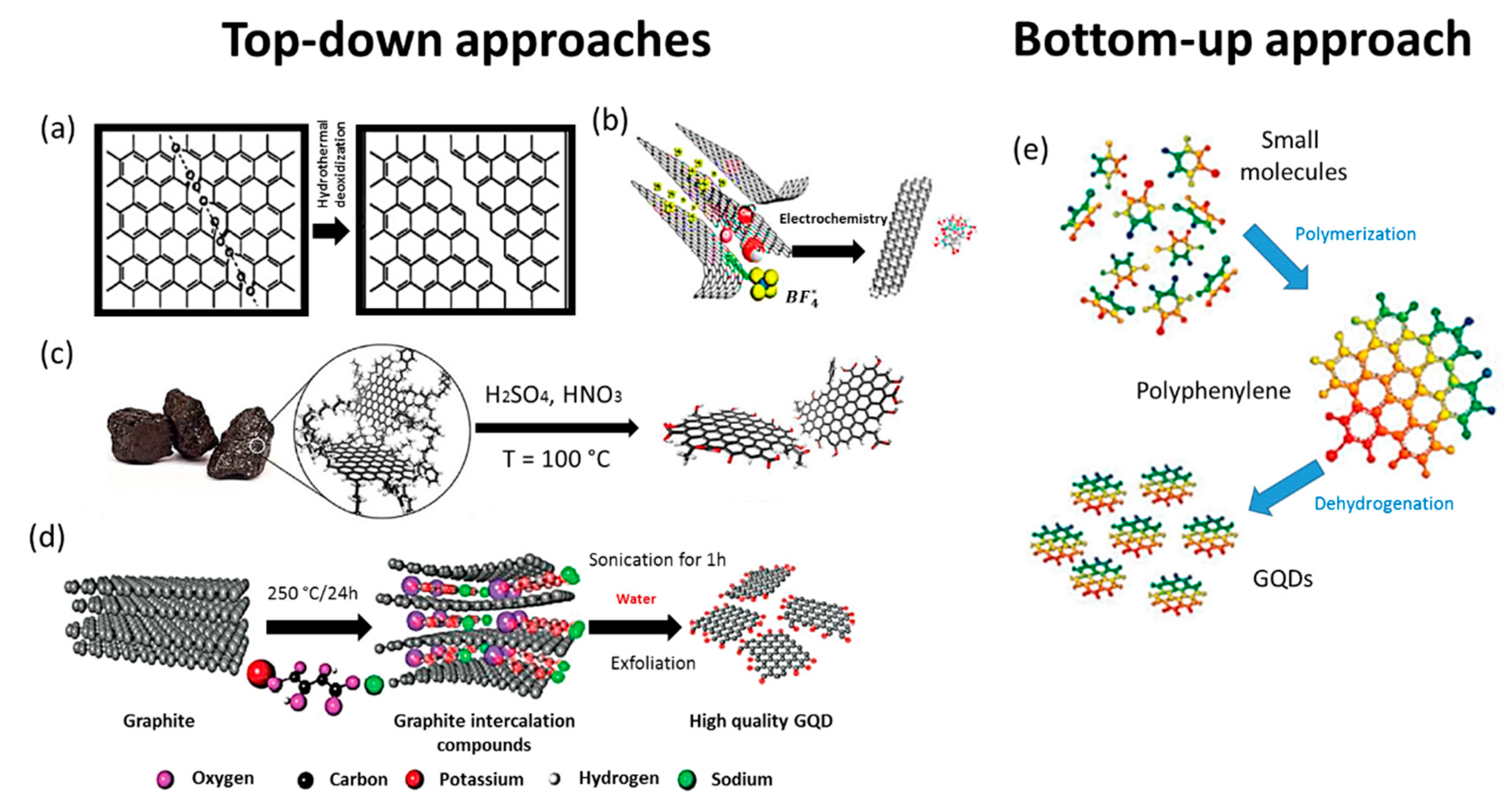
2.1. Top-Down Approaches
2.1.1. Hydrothermal or Solvo-Thermal
2.1.2. Electrochemical Exfoliation with Ion Intercalation
2.1.3. Acid Etching
2.1.4. Ultra-Sonication
2.1.5. Electro-Fenton
2.2. Bottom-Up Approaches
2.2.1. Template Synthesis
2.2.2. Pyrolysis/Carbonization of Organic Precursors
2.2.3. Chemical Vapor Deposition (CVD)
2.2.4. Colloidal Chemical Synthesis
2.2.5. Other Approaches
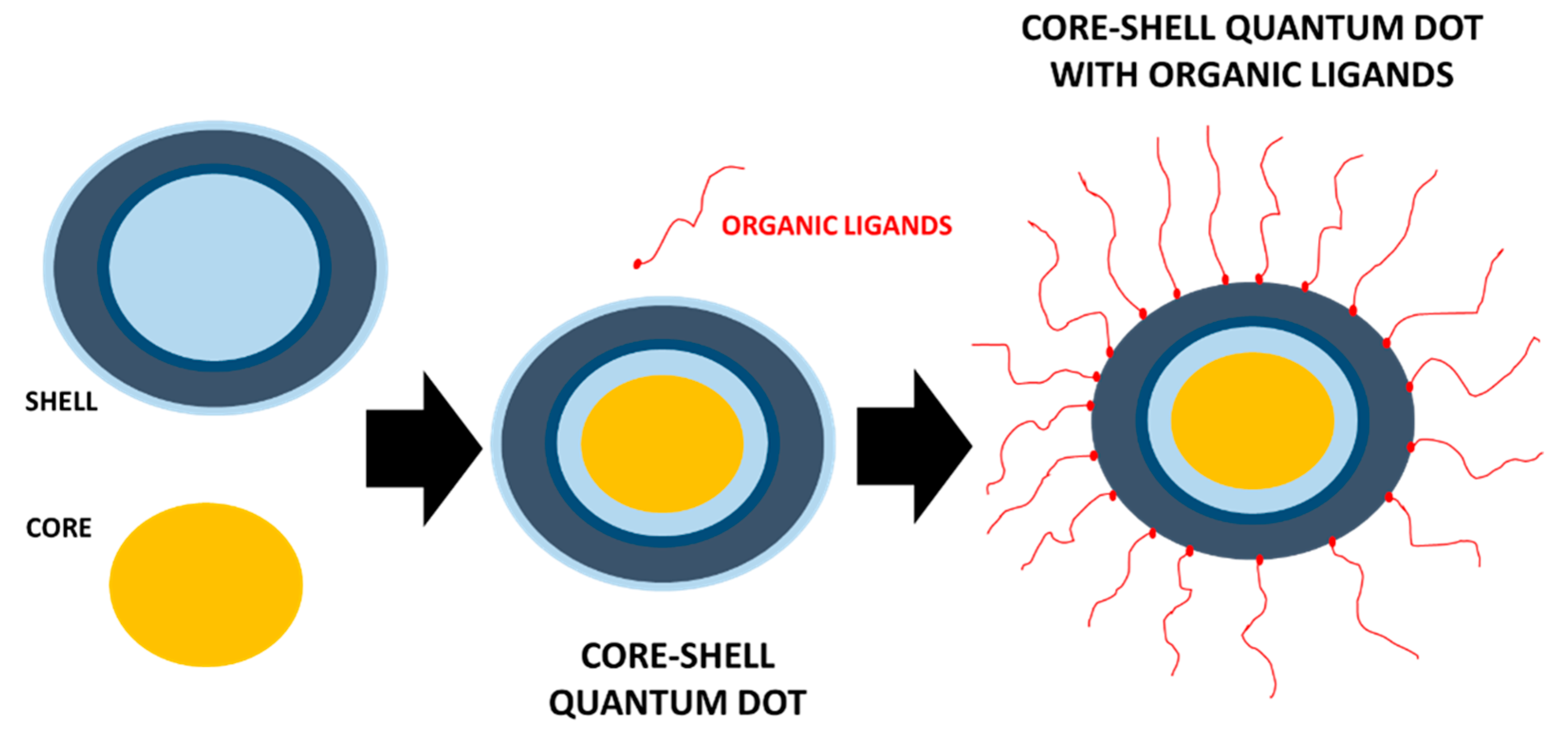
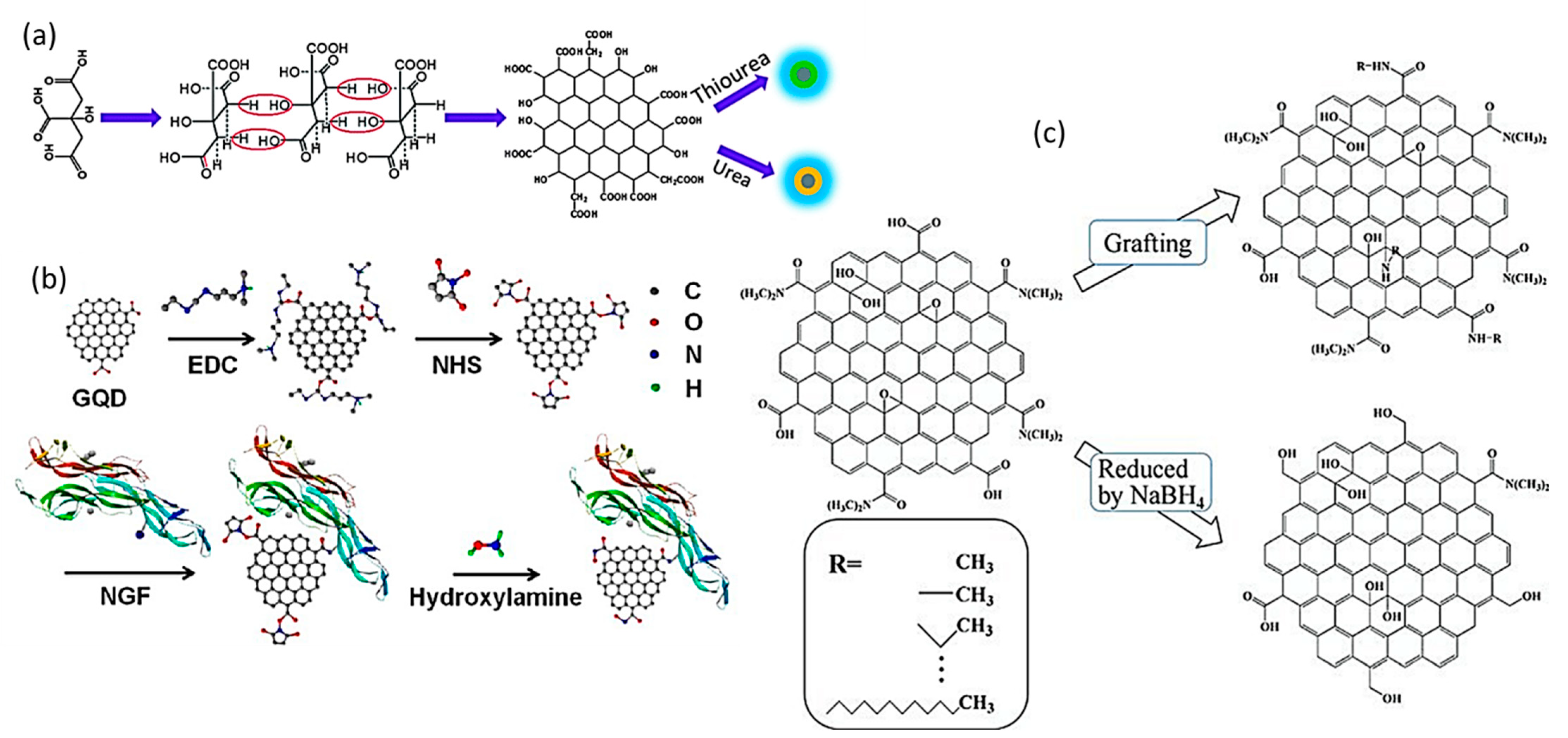
3. Functionalization of 2D Quantum Dots
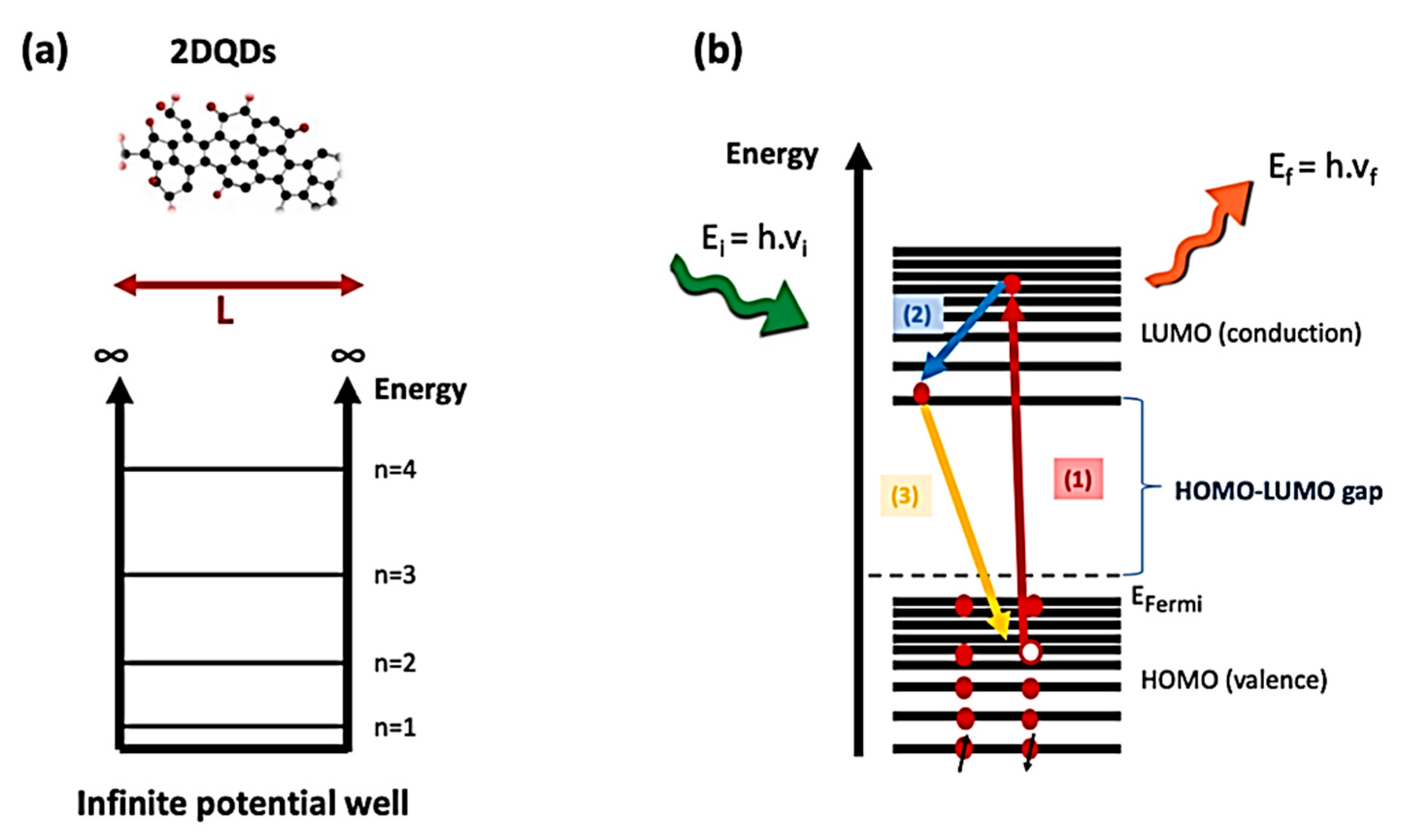
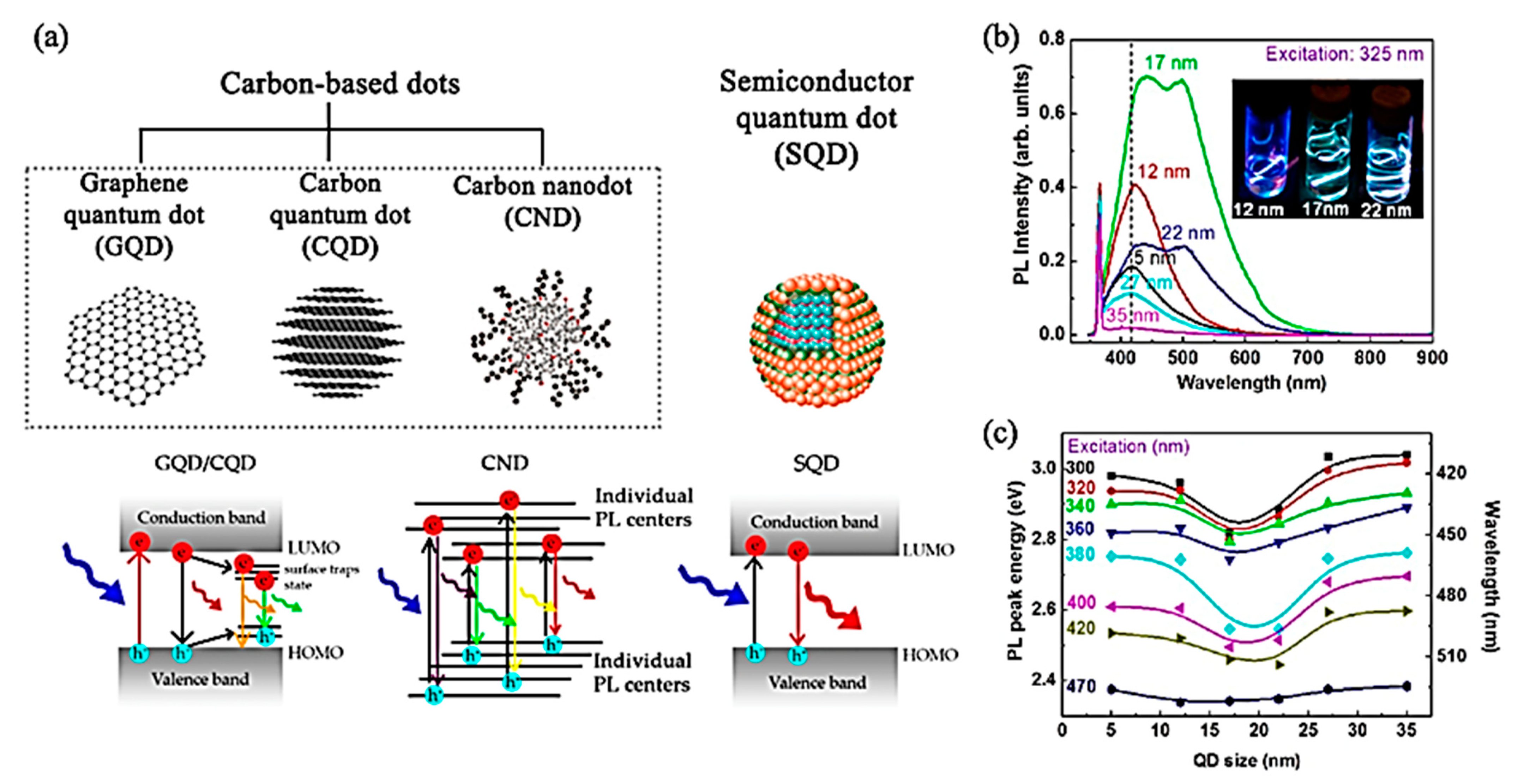
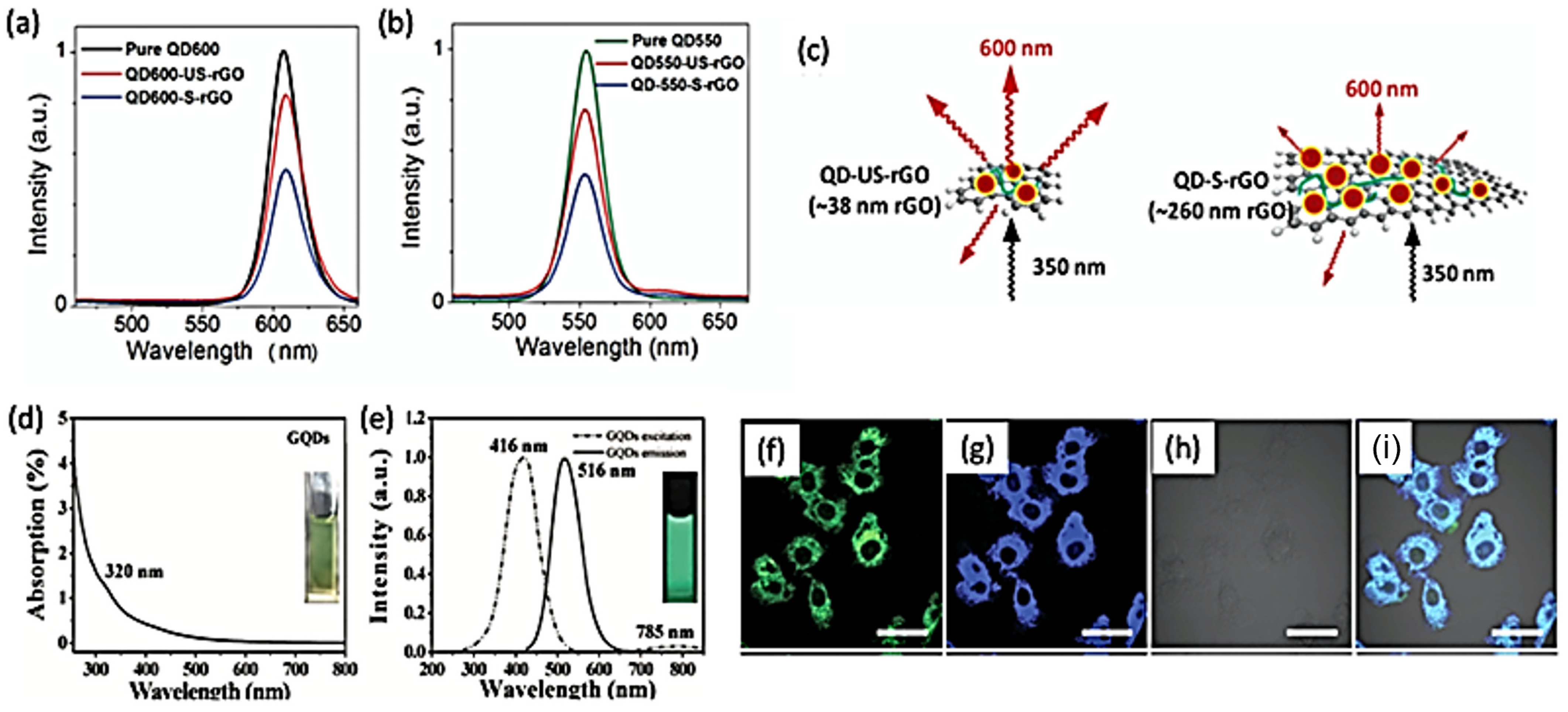
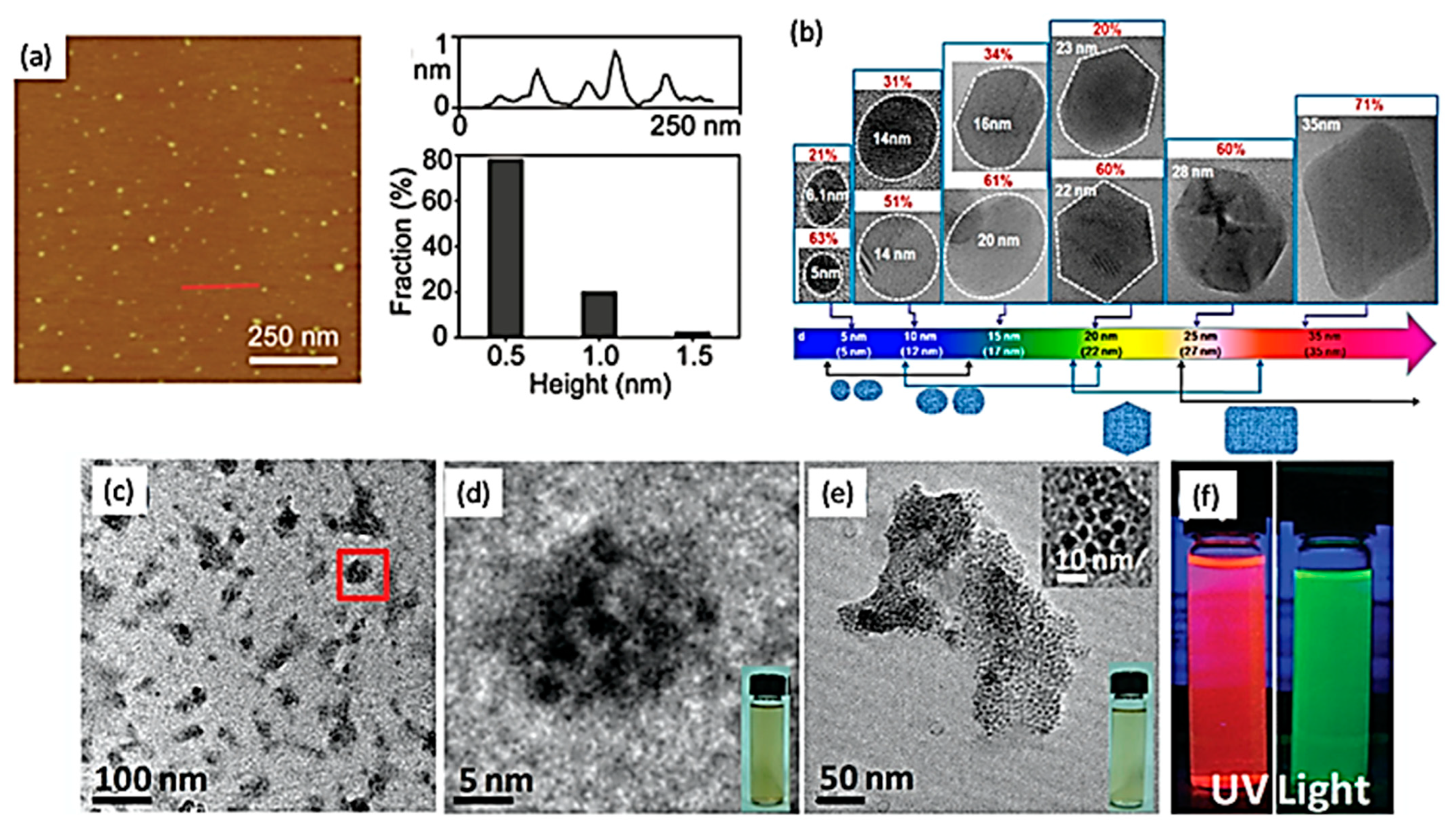
4. Photoluminescence Properties of 2D Quantum Dots
5. Fundamental Characterization Techniques of 2D Quantum Dots
6. Applications of 2D Quantum Dots
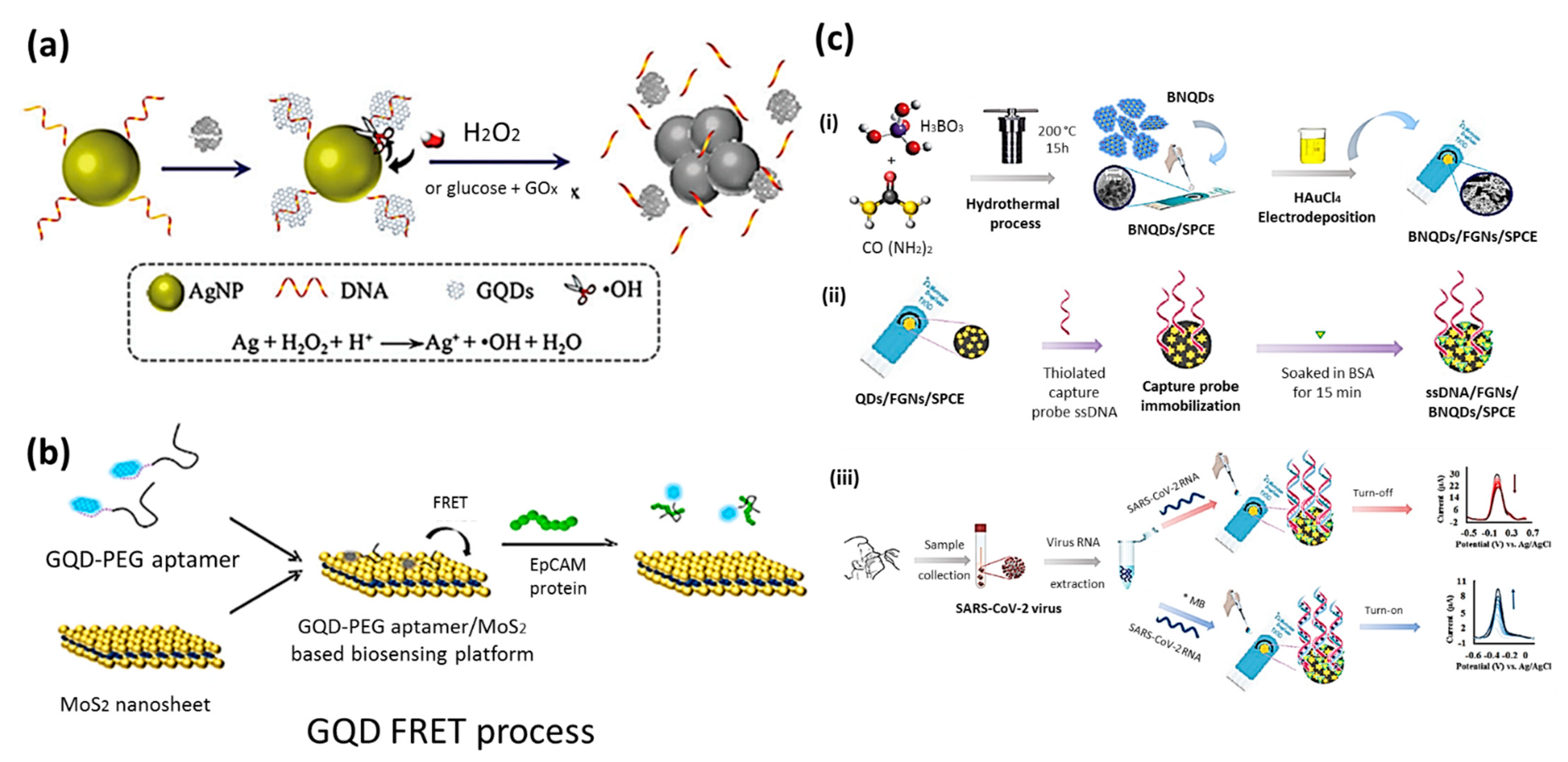
6.1. Biosensing
6.2. Bioimaging
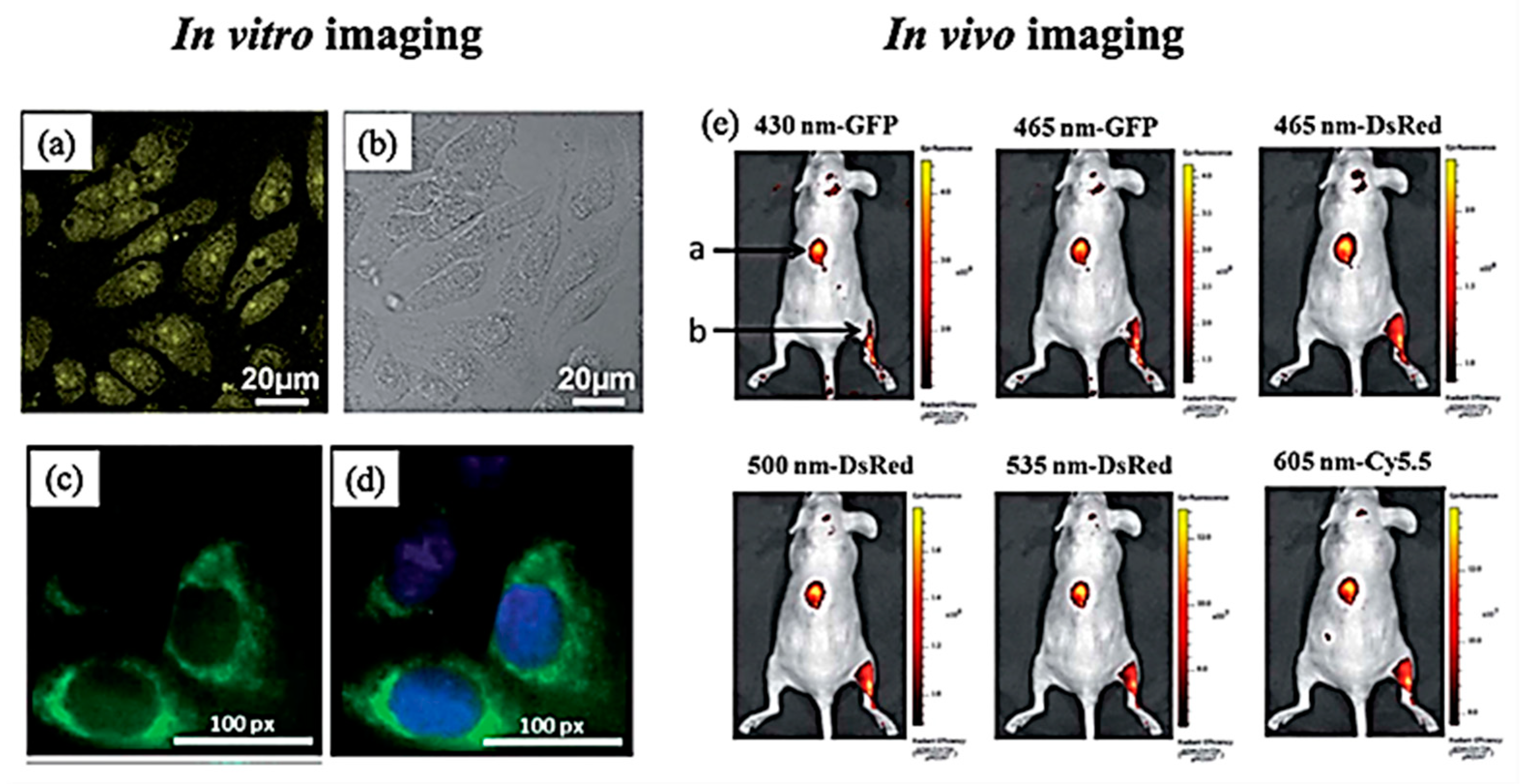
6.2.1. In Vitro Imaging
6.2.2. In Vivo Imaging
6.3. Theranostic Applications of 2DQDs
Phototherapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ISO/TS 80004-13:2017; ISO/TC 229 Nanotechnologies. Nanotechnologies—Vocabulary—Part 13: Graphene and Related Two-dimenszional (2D) Materials. ISO: Geneva, Switzerland, 2017.
- Ekimov, A.I.; Efros, A.L.; Onushchenko, A.A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- Alhassid, Y. The statistical theory of quantum dots. Rev. Mod. Phys. 2000, 72, 895–968. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Jameson, D.M.; James, N.G.; Albanesi, J.P. Fluorescence Fluctuation Spectroscopy Approaches to the Study of Receptors in Live Cells. Methods Enzymol. 2013, 519, 87–113. [Google Scholar] [PubMed]
- Vahala, K.J. Optical microcavities. Nature 2003, 424, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Arul, V.; Edison, T.N.J.I.; Lee, Y.R.; Sethuraman, M.G. Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J. Photochem. Photobiol. B Biol. 2017, 168, 142–148. [Google Scholar] [CrossRef]
- Roy, P.; Periasamy, A.P.; Chuang, C.; Liou, Y.-R.; Chen, Y.-F.; Joly, J.; Liang, C.-T.; Chang, H.-T. Plant leaf-derived graphene quantum dots and applications for white LEDs. New J. Chem. 2014, 38, 4946–4951. [Google Scholar] [CrossRef]
- Coe-Sullivan, S.; Liu, W.; Allen, P.; Steckel, J.S. Quantum Dots for LED Downconversion in Display Applications. ECS J. Solid State Sci. Technol. 2013, 2, R3026–R3030. [Google Scholar] [CrossRef]
- Lim, H.; Liu, Y.; Kim, H.Y.; Son, D.I. Facile synthesis and characterization of carbon quantum dots and photovoltaic applications. Thin Solid Films 2018, 660, 672–677. [Google Scholar] [CrossRef]
- Abolghasemi, R.; Rasuli, R.; Alizadeh, M. Microwave-assisted growth of high-quality CdSe quantum dots and its application as a sensitizer in photovoltaic cells. Mater. Today Commun. 2020, 22, 100827. [Google Scholar] [CrossRef]
- Hetsch, F.; Zhao, N.; Kershaw, S.V.; Rogach, A.L. Quantum dot field effect transistors. Mater. Today 2013, 16, 312–325. [Google Scholar] [CrossRef]
- Choi, B.H.; Hwang, S.W.; Kim, I.G.; Shin, H.C.; Kim, Y.; Kim, E.K. Fabrication and room-temperature characterization of a silicon self-assembled quantum-dot transistor. Appl. Phys. Lett. 1998, 73, 3129–3131. [Google Scholar] [CrossRef]
- Wu, G.Y.; Lue, N.-Y.; Chang, L. Graphene quantum dots for valley-based quantum computing: A feasibility study. Phys. Rev. B 2011, 84, 195463. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W.; et al. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS2 /WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Li, J.; Yao, X.; Li, J. Direct electrochemistry of glucose oxidase and electrochemical biosensing of glucose on quantum dots/carbon nanotubes electrodes. Biosens. Bioelectron. 2007, 22, 3203–3209. [Google Scholar] [CrossRef]
- Freeman, R.; Gill, R.; Shweky, I.; Kotler, M.; Banin, U.; Willner, I. Biosensing and Probing of Intracellular Metabolic Pathways by NADH-Sensitive Quantum Dots. Angew. Chem. Int. Ed. 2009, 48, 309–313. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Dhanabalan, B.; Ponraj, J.S.; Bao, Q.; Zhang, H. 2D–Materials-Based Quantum Dots: Gateway towards Next-Generation Optical Devices. Adv. Opt. Mater. 2017, 5, 1700257. [Google Scholar] [CrossRef]
- LAI, S.; Chen, M.; Khanin, Y.N.; Novoselov, K.S.; Andreeva, D.V. Enhancement of reduced graphene oxide bolometric photoresponse via addition of graphene quantum dots. Surf. Rev. Lett. 2021, 28, 2140011. [Google Scholar] [CrossRef]
- Xu, Q.; Cai, W.; Li, W.; Sreeprasad, T.S.; He, Z.; Ong, W.-J.; Li, N. Two-dimensional quantum dots: Fundamentals, photoluminescence mechanism and their energy and environmental applications. Mater. Today Energy 2018, 10, 222–240. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-471-41526-8. [Google Scholar]
- Chung, I.; Shimizu, K.T.; Bawendi, M.G. Room temperature measurements of the 3D orientation of single CdSe quantum dots using polarization microscopy. Proc. Natl. Acad. Sci. USA 2003, 100, 405–408. [Google Scholar] [CrossRef]
- Cusack, M.A.; Briddon, P.R.; Jaros, M. Electronic structure of InAs/GaAs self-assembled quantum dots. Phys. Rev. B 1996, 54, R2300–R2303. [Google Scholar] [CrossRef]
- Meric, I.; Han, M.Y.; Young, A.F.; Ozyilmaz, B.; Kim, P.; Shepard, K.L. Current saturation in zero-bandgap, top-gated graphene field-effect transistors. Nat. Nanotechnol. 2008, 3, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Schwierz, F. Graphene Transistors: Status, Prospects, and Problems. Proc. IEEE 2013, 101, 1567–1584. [Google Scholar] [CrossRef]
- Ezawa, M. Peculiar band gap structure of graphene nanoribbons. Phys. Status Solidi 2007, 4, 489–492. [Google Scholar] [CrossRef]
- Manikandan, A.; Chen, Y.-Z.; Shen, C.-C.; Sher, C.-W.; Kuo, H.-C.; Chueh, Y.-L. A critical review on two-dimensional quantum dots (2D QDs): From synthesis toward applications in energy and optoelectronics. Prog. Quantum Electron. 2019, 68, 100226. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Li, N.; Chen, P. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016, 45, 2239–2262. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ni, N.; Govindarajan, S.; Ding, X.; Leong, D.T. Phototherapy with layered materials derived quantum dots. Nanoscale 2020, 12, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhu, M.; Lee, X.; Zhang, R.; Wang, K.; Wei, J.; Zhong, M.; Wu, D.; Zhu, H. Direct Synthesis of Graphene Quantum Dots by Chemical Vapor Deposition. Part. Part. Syst. Charact. 2013, 30, 764–769. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, Y.; Li, M.; Sun, L.; Hu, G.; Tang, T.; Wen, J.; Li, X. Tuning the Photoluminescence of Graphene Quantum Dots by Fluorination. J. Nanomater. 2017, 2017, 9682846. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.W.; Kim, M.K.; Shin, D.Y.; Shin, D.H.; Kim, C.O.; Yang, S.B.; Park, J.H.; Hwang, E.; Choi, S.H.; et al. Anomalous behaviors of visible luminescence from graphene quantum dots: Interplay between size and shape. ACS Nano 2012, 6, 8203–8208. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jang, M.H.; Ha, H.D.; Kim, J.H.; Cho, Y.H.; Seo, T.S. Facile synthetic method for pristine graphene quantum dots and graphene oxide quantum dots: Origin of blue and green luminescence. Adv. Mater. 2013, 25, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, L.; Li, X.; Xu, H. Fabrication of transition metal dichalcogenides quantum dots based on femtosecond laser ablation. Sci. Rep. 2019, 9, 2931. [Google Scholar] [CrossRef]
- Lin, L.; Xu, Y.; Zhang, S.; Ross, I.M.; Ong, A.C.M.; Allwood, D.A. Fabrication and luminescence of monolayered boron nitride quantum dots. Small 2014, 10, 60–65. [Google Scholar] [CrossRef]
- Kong, Z.; Hu, W.; Jiao, F.; Zhang, P.; Shen, J.; Cui, B.; Wang, H.; Liang, L. Theoretical Evaluation of DNA Genotoxicity of Graphene Quantum Dots: A Combination of Density Functional Theory and Molecular Dynamics Simulations. J. Phys. Chem. B 2020, 124, 9335–9342. [Google Scholar] [CrossRef]
- Yong, Y.; Cheng, X.; Bao, T.; Zu, M.; Yan, L.; Yin, W.; Ge, C.; Wang, D.; Gu, Z.; Zhao, Y. Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for In Vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy. ACS Nano 2015, 9, 12451–12463. [Google Scholar] [CrossRef]
- Shi, M.; Dong, L.; Zheng, S.; Hou, P.; Cai, L.; Zhao, M.; Zhang, X.; Wang, Q.; Li, J.; Xu, K. “Bottom-up” preparation of MoS2 quantum dots for tumor imaging and their in vivo behavior study. Biochem. Biophys. Res. Commun. 2019, 516, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Periasamy, A.P.; Lin, C.-Y.; Her, G.-M.; Chiu, W.-J.; Li, C.-L.; Shu, C.-L.; Huang, C.-C.; Liang, C.-T.; Chang, H.-T. Photoluminescent graphene quantum dots for in vivo imaging of apoptotic cells. Nanoscale 2015, 7, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, J.F.; Dai, L. Recent advances in graphene quantum dots for fluorescence bioimaging from cells through tissues to animals. Part. Part. Syst. Charact. 2015, 32, 515–523. [Google Scholar] [CrossRef]
- Yadav, V.; Roy, S.; Singh, P.; Khan, Z.; Jaiswal, A. 2D MoS2-Based Nanomaterials for Therapeutic, Bioimaging, and Biosensing Applications. Small 2019, 15, 1803706. [Google Scholar] [CrossRef]
- Hu, S.H.; Chen, Y.W.; Hung, W.T.; Chen, I.W.; Chen, S.Y. Quantum-dot-tagged reduced graphene oxide nanocomposites for bright fluorescence bioimaging and photothermal therapy monitored in situ. Adv. Mater. 2012, 24, 1748–1754. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S. Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Amel Jamehdar, S.; Rizi, K.S.; Mojarrad, M.; Meshkat, Z.; Choobin, H.; Soleimanpour, S.; Boroushaki, M.T. Sensitive and specific clinically diagnosis of SARS-CoV-2 employing a novel biosensor based on boron nitride quantum dots/flower-like gold nanostructures signal amplification. Biosens. Bioelectron. 2022, 207, 114209. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Bakandritsos, A.; Pykal, M.; Zbořil, R.; Otyepka, M. Chemistry, properties, and applications of fluorographene. Appl. Mater. Today 2017, 9, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Fernandes, J.O.; Bernardino, C.A.R.; Braz, B.F.; Mahler, C.F.; Santelli, R.E.; Cincotto, F.H. (Bio)Sensing Materials: Quantum Dots. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Da Costa, M.C.F.; Ribeiro, H.B.; Kessler, F.; de Souza, E.A.T.; Fechine, G.J.M. Micromechanical exfoliation of two-dimensional materials by a polymeric stamp. Mater. Res. Express 2016, 3, 025303. [Google Scholar] [CrossRef]
- Qiu, X.; Bouchiat, V.; Colombet, D.; Ayela, F. Liquid-phase exfoliation of graphite into graphene nanosheets in a hydrocavitating ‘lab-on-a-chip’. RSC Adv. 2019, 9, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, H.; Fan, G.; Li, Y.; Li, L.; Quan, X. Electrolytic exfoliation synthesis of boron doped graphene quantum dots: A new luminescent material for electrochemiluminescence detection of oncogene microRNA-20a. Electrochim. Acta 2016, 190, 1150–1158. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. Appl. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Najafi, L.; Bellani, S.; Martín-García, B.; Oropesa-Nuñez, R.; Del Rio Castillo, A.E.; Prato, M.; Moreels, I.; Bonaccorso, F. Solution-Processed Hybrid Graphene Flake/2H-MoS 2 Quantum Dot Heterostructures for Efficient Electrochemical Hydrogen Evolution. Chem. Mater. 2017, 29, 5782–5786. [Google Scholar] [CrossRef]
- Lin, D.; Su, Z.; Wei, G. Three-dimensional porous reduced graphene oxide decorated with MoS2 quantum dots for electrochemical determination of hydrogen peroxide. Mater. Today Chem. 2018, 7, 76–83. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Rhyner, M.N.; Ruan, G.; Nie, S. A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots. Phys. Chem. Chem. Phys. 2006, 8, 3895. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS 2 Quantum Dot-Interspersed Exfoliated MoS 2 Nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef]
- Li, B.L.; Chen, L.X.; Zou, H.L.; Lei, J.L.; Luo, H.Q.; Li, N.B. Electrochemically induced Fenton reaction of few-layer MoS 2 nanosheets: Preparation of luminescent quantum dots via a transition of nanoporous morphology. Nanoscale 2014, 6, 9831–9838. [Google Scholar] [CrossRef]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-Crystalline Tungsten Oxide Quantum Dots for Fast Pseudocapacitor and Electrochromic Applications. Adv. Mater. 2014, 26, 4260–4267. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Li, L. Synthesis Strategies about 2D Materials. In Two-Dimensional Materials—Synthesis, Characterization and Potential Applications; InTech: Vienna, Austria, 2016. [Google Scholar]
- Li, R.; Liu, Y.; Li, Z.; Shen, J.; Yang, Y.; Cui, X.; Yang, G. Bottom-Up Fabrication of Single-Layered Nitrogen-Doped Graphene Quantum Dots through Intermolecular Carbonization Arrayed in a 2D Plane. Chem. A Eur. J. 2016, 22, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, P.; Tian, F.; Li, W.; Li, F.; Liu, W. One-step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging. J. Mater. Chem. 2011, 21, 13163. [Google Scholar] [CrossRef]
- Liu, J.-J.; Zhang, X.-L.; Cong, Z.-X.; Chen, Z.-T.; Yang, H.-H.; Chen, G.-N. Glutathione-functionalized graphene quantum dots as selective fluorescent probes for phosphate-containing metabolites. Nanoscale 2013, 5, 1810. [Google Scholar] [CrossRef]
- Kagan, C.R.; Bassett, L.C.; Murray, C.B.; Thompson, S.M. Colloidal Quantum Dots as Platforms for Quantum Information Science. Chem. Rev. 2021, 121, 3186–3233. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Wu, J.; Xu, Z.; Huang, Y.; Zhang, C. Colloidal synthesis of MoS 2 quantum dots: Size-dependent tunable photoluminescence and bioimaging. New J. Chem. 2015, 39, 8492–8497. [Google Scholar] [CrossRef]
- Jung, W.; Lee, S.; Yoo, D.; Jeong, S.; Miró, P.; Kuc, A.; Heine, T.; Cheon, J. Colloidal Synthesis of Single-Layer MSe 2 (M = Mo, W) Nanosheets via Anisotropic Solution-Phase Growth Approach. J. Am. Chem. Soc. 2015, 137, 7266–7269. [Google Scholar] [CrossRef]
- Musselman, K.P.; Ibrahim, K.H.; Yavuz, M. Research Update: Beyond graphene—Synthesis of functionalized quantum dots of 2D materials and their applications. APL Mater. 2018, 6, 120701. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Wang, Y.; Chen, X.; Ji, X.; Niu, F.; Song, Z.; Liu, J. Boron Nitride Quantum Dots with Solvent-Regulated Blue/Green Photoluminescence and Electrochemiluminescent Behavior for Versatile Applications. Adv. Opt. Mater. 2017, 5, 1600661. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chen, J.; Wang, A.-J.; Bao, N.; Feng, J.-J.; Wang, W.; Shao, L. Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury(II) detection and bioimaging. J. Mater. Chem. C 2015, 3, 73–78. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Wang, L.; Du, M.; Bai, Y.; Li, W.; Liu, Y.; Chen, S.; Braunstein, P.; Xu, Q.; Pang, H. MIL-96-Al for Li–S Batteries: Shape or Size? Adv. Mater. 2022, 34, 2107836. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, X.; Geng, P.; Du, M.; Jing, Q.; Chen, X.; Zhang, G.; Li, H.; Xu, Q.; Braunstein, P.; et al. Rational Design and General Synthesis of Multimetallic Metal–Organic Framework Nano-Octahedra for Enhanced Li–S Battery. Adv. Mater. 2021, 33, 2105163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Than, A.; Ananthanaraya, A.; Kim, D.-H.; Chen, P. Graphene Quantum Dots as Universal Fluorophores and Their Use in Revealing Regulated Trafficking of Insulin Receptors in Adipocytes. ACS Nano 2013, 7, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Cross, D.; Burmester, J.K. Gene Therapy for Cancer Treatment: Past, Present and Future. Clin. Med. Res. 2006, 4, 218–227. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Yang, Z.; Garate, J.A.; Gu, Z.; Weber, J.K.; Liu, J.; Zhou, R. Reduced Cytotoxicity of Graphene Nanosheets Mediated by Blood-Protein Coating. ACS Nano 2015, 9, 5713–5724. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Emamy, H.; Akhavan, F. Genotoxicity of graphene nanoribbons in human mesenchymal stem cells. Carbon N. Y. 2013, 54, 419–431. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, Y.; Liang, L.; Wei, M.; Hu, W.; Li, X.; Huang, Q. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale 2012, 4, 3861. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Li, J.; Tao, L.; Wei, Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62–68. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, L.; Chen, J.-F.; Dai, L. Can graphene quantum dots cause DNA damage in cells? Nanoscale 2015, 7, 9894–9901. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.-B.; Huang, H.-Y.; Hsieh, M.-L.; Chen, P.-W.; Chiang, S.-E.; Chang, S.H.; Shen, J.-L.; Liu, W.-R.; Yuan, C.-T. Two-Dimensional Self-Assembly of Boric Acid-Functionalized Graphene Quantum Dots: Tunable and Superior Optical Properties for Efficient Eco-Friendly Luminescent Solar Concentrators. ACS Nano 2022, 16, 3994–4003. [Google Scholar] [CrossRef] [PubMed]
- Shayeganfar, F.; Rahimi Tabar, M.R.; Simchi, A.; Beheshtian, J. Effects of functionalization and side defects on single-photon emission in boron nitride quantum dots. Phys. Rev. B 2017, 96, 165307. [Google Scholar] [CrossRef]
- Scotognella, F.; Kriegel, I.; Sassolini, S. Covalent functionalized black phosphorus quantum dots. Opt. Mater. 2018, 75, 521–524. [Google Scholar] [CrossRef]
- Raheman AR, S.; Wilson, H.M.; Momin, B.M.; Annapure, U.S.; Jha, N. CdSe quantum dots modified thiol functionalized g-C3N4: Intimate interfacial charge transfer between 0D/2D nanostructure for visible light H2 evolution. Renew. Energy 2020, 158, 431–443. [Google Scholar] [CrossRef]
- Stokes, G.G. On the change of refrangibility of light. No. II. Abstr. Pap. Commun. R. Soc. Lond. 1854, 6, 333–335. [Google Scholar] [CrossRef]
- Crosby, G.A.; Demas, J.N. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Wen, J.; Xu, Y.; Li, H.; Lu, A.; Sun, S. Recent applications of carbon nanomaterials in fluorescence biosensing and bioimaging. Chem. Commun. 2015, 51, 11346–11358. [Google Scholar] [CrossRef]
- Fan, Z.; Li, S.; Yuan, F.; Fan, L. Fluorescent graphene quantum dots for biosensing and bioimaging. RSC Adv. 2015, 5, 19773–19789. [Google Scholar] [CrossRef]
- Biju, V.; Ishikawa, M. Photoluminescence of CdSe Quantum Dots: Shifting, Enhancement and Blinking. In Molecular Nano Dynamics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 293–314. [Google Scholar]
- ISO 13095:2014; Surface Chemical Analysis—Atomic Force Microscopy—Procedure for in Situ Characterization on AFM Probe Shank Profile Used for Nanostructure Measurement. Standards: Etobicoke, ON, Canada, 2014.
- Kumar, S.; Ojha, A.K.; Ahmed, B.; Kumar, A.; Das, J.; Materny, A. Tunable (violet to green) emission by high-yield graphene quantum dots and exploiting its unique properties towards sun-light-driven photocatalysis and supercapacitor electrode materials. Mater. Today Commun. 2017, 11, 76–86. [Google Scholar] [CrossRef]
- Sarkar, S.; Gandla, D.; Venkatesh, Y.; Bangal, P.R.; Ghosh, S.; Yang, Y.; Misra, S. Graphene quantum dots from graphite by liquid exfoliation showing excitation-independent emission, fluorescence upconversion and delayed fluorescence. Phys. Chem. Chem. Phys. 2016, 18, 21278–21287. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.F.; Marangoni, V.S.; Ng, P.R.; Nguyen, H.T.L.; Carvalho, A.; Castro Neto, A.H. Accelerated Synthesis of Graphene Oxide from Graphene. Nanomaterials 2021, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Costa, M.C.F.; Marangoni, V.S.; Ng, P.R.; Nguyen, T.L.H.; Castro Neto, A.H. The Degree of Oxidation of Graphene Oxide. Nanomaterials 2021, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Fornara, A.; Toprak, M.S.; Bhattacharya, K. Keeping it real: The importance of material characterization in nanotoxicology. Biochem. Biophys. Res. Commun. 2015, 468, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. New insights in establishing the structure-property relations of novel plasmonic nanostructures for clean energy applications. EnergyChem 2022, 4, 100070. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 2006, 102, 29–45. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q. Health and Ecosystem Risks of Graphene. Chem. Rev. 2013, 113, 3815–3835. [Google Scholar] [CrossRef]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Xia, Z.; Wei, W. As-prepared MoS 2 quantum dot as a facile fluorescent probe for long-term tracing of live cells. Nanotechnology 2016, 27, 275101. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Developmental toxicity of CdTe QDs in zebrafish embryos and larvae. J. Nanopart. Res. 2013, 15, 1700. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, K.; Miao, Y.; Dong, Q.; Huang, C.; Wang, H.; Guo, M.; Cui, X. Toxicity assessment of zebrafish following exposure to CdTe QDs. J. Hazard. Mater. 2012, 213–214, 413–420. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zhou, R.; Jiang, D.; Song, J.E.; Xu, Q.; Si, J.; Chen, Y.P.; Zhou, X.; Gan, L.; Li, J.Z.; et al. Toxicity of graphene quantum dots in zebrafish embryo. Biomed. Environ. Sci. 2015, 28, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Appel, J.H.; Li, D.O.; Podlevsky, J.D.; Debnath, A.; Green, A.A.; Wang, Q.H.; Chae, J. Low Cytotoxicity and Genotoxicity of Two-Dimensional MoS 2 and WS 2. ACS Biomater. Sci. Eng. 2016, 2, 361–367. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Kawamura, A.; Miyata, T. Biosensors. Biomater. Nanoarchitect. 2016, 157–176, Chapter 4.2. [Google Scholar] [CrossRef]
- Michelmore, A. Thin Film Growth on Biomaterial Surfaces; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9781782424536. [Google Scholar]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Ma, J.J.; Chen, J.R.; Feng, H. A universal fluorescence sensing strategy based on biocompatible graphene quantum dots and graphene oxide for the detection of DNA. Nanoscale 2014, 6, 5671–5674. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Zhang, R.; He, S.; Chen, W. Nitrogen-doped graphene quantum dots-based fluorescent probe for the sensitive turn-on detection of glutathione and its cellular imaging. RSC Adv. 2014, 4, 52583–52589. [Google Scholar] [CrossRef]
- Wu, S.; Kong, X.J.; Cen, Y.; Yuan, J.; Yu, R.Q.; Chu, X. Fabrication of a LRET-based upconverting hybrid nanocomposite for turn-on sensing of H2O2 and glucose. Nanoscale 2016, 8, 8939–8946. [Google Scholar] [CrossRef]
- Shi, J.; Lyu, J.; Tian, F.; Yang, M. A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens. Bioelectron. 2017, 93, 182–188. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Shen, Y.; Yan, J.; Hou, Z.; Mao, C.; Zhao, W. MoS 2 quantum dots featured fluorescent biosensor for multiple detection of cancer. RSC Adv. 2017, 7, 54638–54643. [Google Scholar] [CrossRef]
- Sreejith, S.; Joshi, H.; Zhao, Y. Graphene-Based Materials in Biosensing, Bioimaging, and Therapeutics. In Graphene-Based Materials in Health and Environment; Springer: Cham, Switzerland, 2016; pp. 35–61. [Google Scholar]
- Dong, H.; Tang, S.; Hao, Y.; Yu, H.; Dai, W.; Zhao, G.; Cao, Y.; Lu, H.; Zhang, X.; Ju, H. Fluorescent MoS 2 Quantum Dots: Ultrasonic Preparation, Up-Conversion and Down-Conversion Bioimaging, and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3107–3114. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Wang, Z.; Pei, Z.; Huang, Y.; Huang, Y.; Song, X.; Zeng, H.; Zhi, C. Hydrothermal synthesis of blue-fluorescent monolayer BN and BCNO quantum dots for bio-imaging probes. RSC Adv. 2016, 6, 79090–79094. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Hou, Y.; Yang, G.; Fei, X.; Zhao, H.; Guo, Y.; Su, C.; Wang, Z.; Zhong, H.; et al. Multifunctional Nanoplatform Based on Black Phosphorus Quantum Dots for Bioimaging and Photodynamic/Photothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 25098–25106. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, Y.; Li, X.; Fan, L.; Zhou, S.; Fang, D.; Yang, S. Surrounding media sensitive photoluminescence of boron-doped graphene quantum dots for highly fluorescent dyed crystals, chemical sensing and bioimaging. Carbon N. Y. 2014, 70, 149–156. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, D.; Li, L.; Liu, T.; Tan, L.; Wu, X.; Tang, F. Multifunctional Gold Nanoshells on Silica Nanorattles: A Platform for the Combination of Photothermal Therapy and Chemotherapy with Low Systemic Toxicity. Angew. Chem. Int. Ed. 2011, 50, 891–895. [Google Scholar] [CrossRef]
- Ding, X.; Peng, F.; Zhou, J.; Gong, W.; Slaven, G.; Loh, K.P.; Lim, C.T.; Leong, D.T. Defect engineered bioactive transition metals dichalcogenides quantum dots. Nat. Commun. 2019, 10, 41. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Shao, Y.-T.; Huang, K.-S.; Chou, T.-M.; Yang, C.-H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef] [PubMed]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef]
- Kholikov, K.; Ilhom, S.; Sajjad, M.; Smith, M.E.; Monroe, J.D.; San, O.; Er, A.O. Improved singlet oxygen generation and antimicrobial activity of sulphur-doped graphene quantum dots coupled with methylene blue for photodynamic therapy applications. Photodiagn. Photodyn. Ther. 2018, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wu, Y.; Lin, Y.; Xu, X.; Lian, H.; Huang, G.; Liu, J.-Z.; Wu, X.; Yang, H.-H. Black Phosphorus Quantum Dots with Renal Clearance Property for Efficient Photodynamic Therapy. Small 2018, 14, 1702815. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Miró, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed]
- Guzman, D.M.; Alyahyaei, H.M.; Jishi, R.A. Superconductivity in graphene-lithium. 2D Mater. 2014, 1, 021005. [Google Scholar] [CrossRef]
- Thiel, L.; Wang, Z.; Tschudin, M.A.; Rohner, D.; Gutiérrez-Lezama, I.; Ubrig, N.; Gibertini, M.; Giannini, E.; Morpurgo, A.F.; Maletinsky, P. Probing magnetism in 2D materials at the nanoscale with single-spin microscopy. Science 2019, 364, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Sethulakshmi, N.; Mishra, A.; Ajayan, P.M.; Kawazoe, Y.; Roy, A.K.; Singh, A.K.; Tiwary, C.S. Magnetism in two-dimensional materials beyond graphene. Mater. Today 2019, 27, 107–122. [Google Scholar] [CrossRef]
| Categories | 2DQDs | Approach | Synthesis | Φ (%) |
|---|---|---|---|---|
| Single-element 2DQD | Carbon dots (CDs) | Bottom-up | Hydrothermal method | 80 |
| Bottom-up | Solvothermal method | 11.4 | ||
| Bottom-up | Microwave radiation | 11.7–22.9 | ||
| Graphene quantum dots (GQDs) | Top-down | Chemical etching from coal precursor | 0.6 | |
| Phosphorene quantum dots (PQDs) | Top-down or bottom-up | Sonication and solvothermal | 8.4 | |
| Top-down | Sonication | N/A | ||
| Double-element 2DQD | TMDs (MoS2, WS2) quantum dots | Top-down | Chemical etching by acid | >95 |
| Top-down | Ultrasonication | N/A | ||
| Top-down | Lithium intercalation | N/A | ||
| Multi-element 2DQD | MXene-type quantum dots | Top-down or bottom-up | Hydrothermal method | 10 |
| Technique | Acronym | Applied for Analyzing: |
|---|---|---|
| Transmission electron microscopy | TEM | Particle size distribution, crystalline organization |
| High-resolution transmission electron microscopy | HRTEM | Crystallinity, d-spacing, planes |
| Energy dispersive X-ray spectroscopy | EDX | Detection of elements |
| X-ray photoelectron spectroscopy | XPS | Understanding chemical states and compositions |
| Atomic force microscopy | AFM | Morphology and thickness |
| X-ray diffraction | XRD | Crystal structure, unit cell dimensions, crystal spacing. |
| Raman spectroscopy | - | Measuring the rotational, vibrational, and other low-frequency modes, and other defect states. |
| UV-Vis spectroscopy (or spectrophotometry) | UV-Vis | Optical properties (light absorption and transmission), qualitative information (size and concentration). |
| Photoluminescence spectroscopy | PL spectroscopy | Electronic transitions, estimation of quantum yield. |
| 2D Quantum Dots | Toxicity Test | Outcome | Applications | Ref. |
|---|---|---|---|---|
| GQDs | In vitro | Photoluminescent GQDs with low toxicity to MC3TW cells obtained by tuning surface chemistry routes. | Strong tool in biomedical field, for up-conversion imaging. | [15] |
| GQDs | In vivo | Fluorescence agents showing efficiency for treatment of cancer cells and tumours | Fluorescence contrast agents for bioimaging | [93,114] |
| GQDs | In vivo | Concentration dependence on the potential toxicity of GQDs to zebrafish embryos | Biological and medical, such as bioimaging, biosensing, and drug delivery. | [108,109,110] |
| GQDs/polyethylene glycol(PEG)/MoS2- | In vivo/in vitro | Fluorescent biosensor for epithelial cell adhesion molecule (EpCAM) detection | Drug delivery | [115] |
| MoS2-QDs | In vitro | Strongly fluorescent, highly photo-stable QDs with low toxicity | Fluorescent probes for long-term live cell tracing | [107] |
| MoS2-QDs | In vitro | Human cervical cancer cells (HeLa) model showed good biocompatibility with no obvious cytotoxicity when concentration ranges from 15 to 100 μg/mL | up-conversion bioimaging | [116] |
| MoS2/WS2 QDs | In vitro | Low cytotoxicity levels in biocompatibility tests, being deleterious to cellular viability and not inducing genetic defects | Medical devices | [111] |
| BN-QDs and BCNO-QDs | In vitro | Fluorescence detected under 405 nm excitation for labelling HeLa cells | Bioimaging probes | [117] |
| PEGylated-BPQDs | In vitro | Low toxicity when integrated into single component platform with fluorescence approach to image cancer cells | Bioimaging probes | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.C.F.; Echeverrigaray, S.G.; Andreeva, D.V.; Novoselov, K.S.; Neto, A.H.C. Two-Dimensional Quantum Dots: From Photoluminescence to Biomedical Applications. Solids 2022, 3, 578-602. https://doi.org/10.3390/solids3040037
Costa MCF, Echeverrigaray SG, Andreeva DV, Novoselov KS, Neto AHC. Two-Dimensional Quantum Dots: From Photoluminescence to Biomedical Applications. Solids. 2022; 3(4):578-602. https://doi.org/10.3390/solids3040037
Chicago/Turabian StyleCosta, Mariana C. F., Sergio G. Echeverrigaray, Daria V. Andreeva, Kostya S. Novoselov, and Antonio H. Castro Neto. 2022. "Two-Dimensional Quantum Dots: From Photoluminescence to Biomedical Applications" Solids 3, no. 4: 578-602. https://doi.org/10.3390/solids3040037
APA StyleCosta, M. C. F., Echeverrigaray, S. G., Andreeva, D. V., Novoselov, K. S., & Neto, A. H. C. (2022). Two-Dimensional Quantum Dots: From Photoluminescence to Biomedical Applications. Solids, 3(4), 578-602. https://doi.org/10.3390/solids3040037










