Parametric Optimization of Ball-Milled Bimetallic Nanoadsorbents for the Effective Removal of Arsenic Species
Abstract
1. Introduction
2. Nanocomposite Synthesis, Characterization, and Adsorption Studies
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
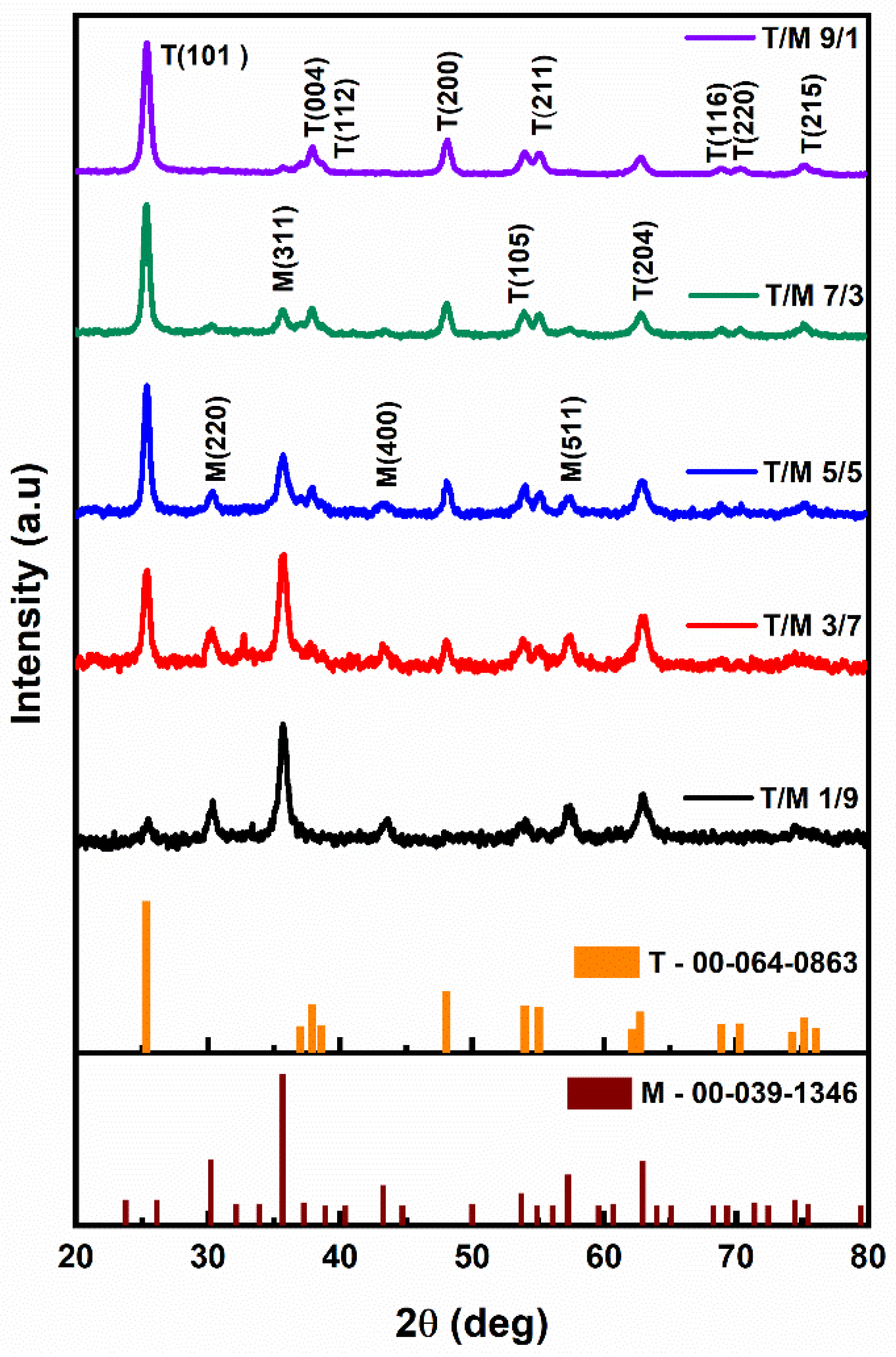
3.1. XRD Diffractograms
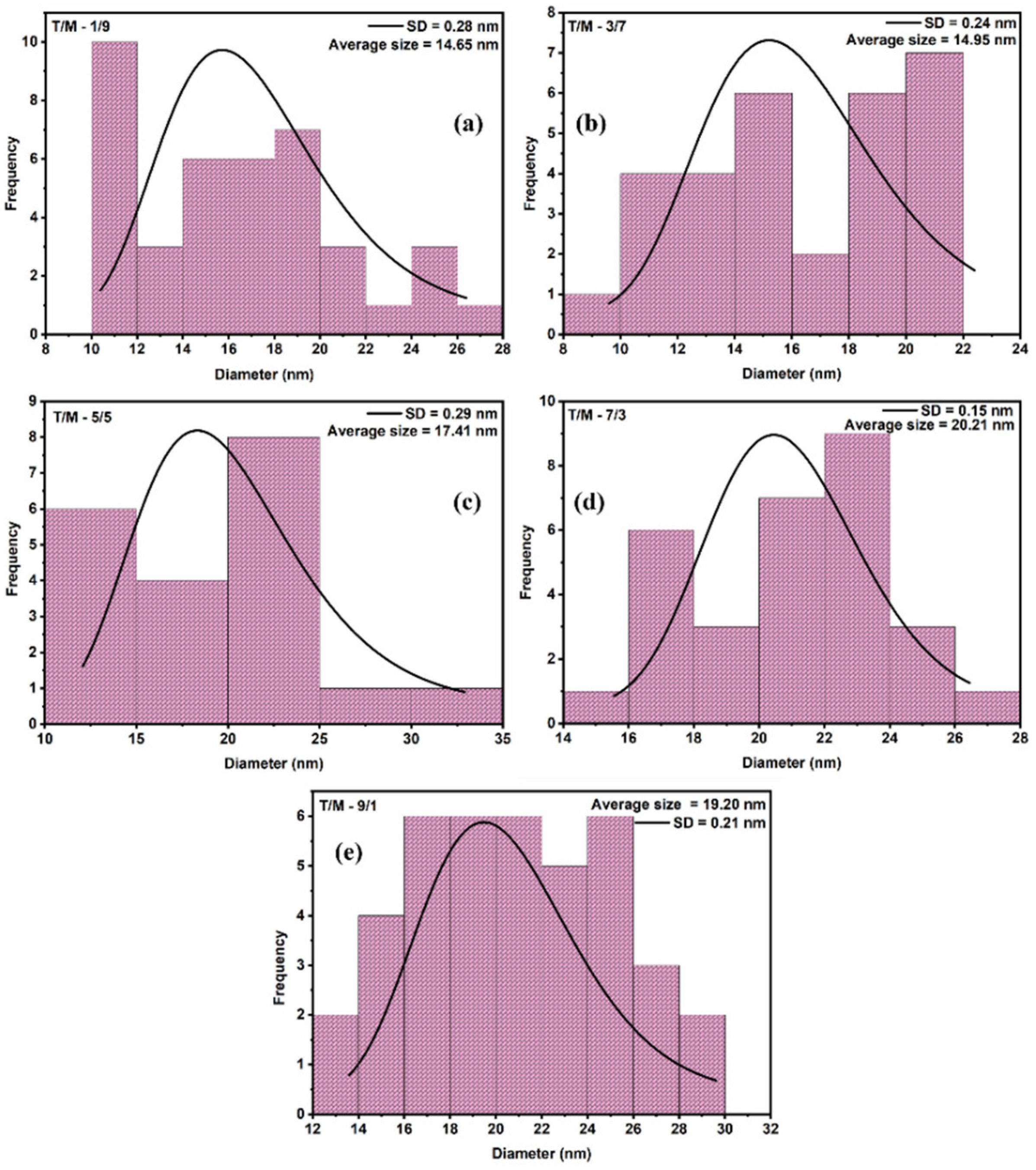
3.2. TEM Analysis
3.3. EDS Analysis
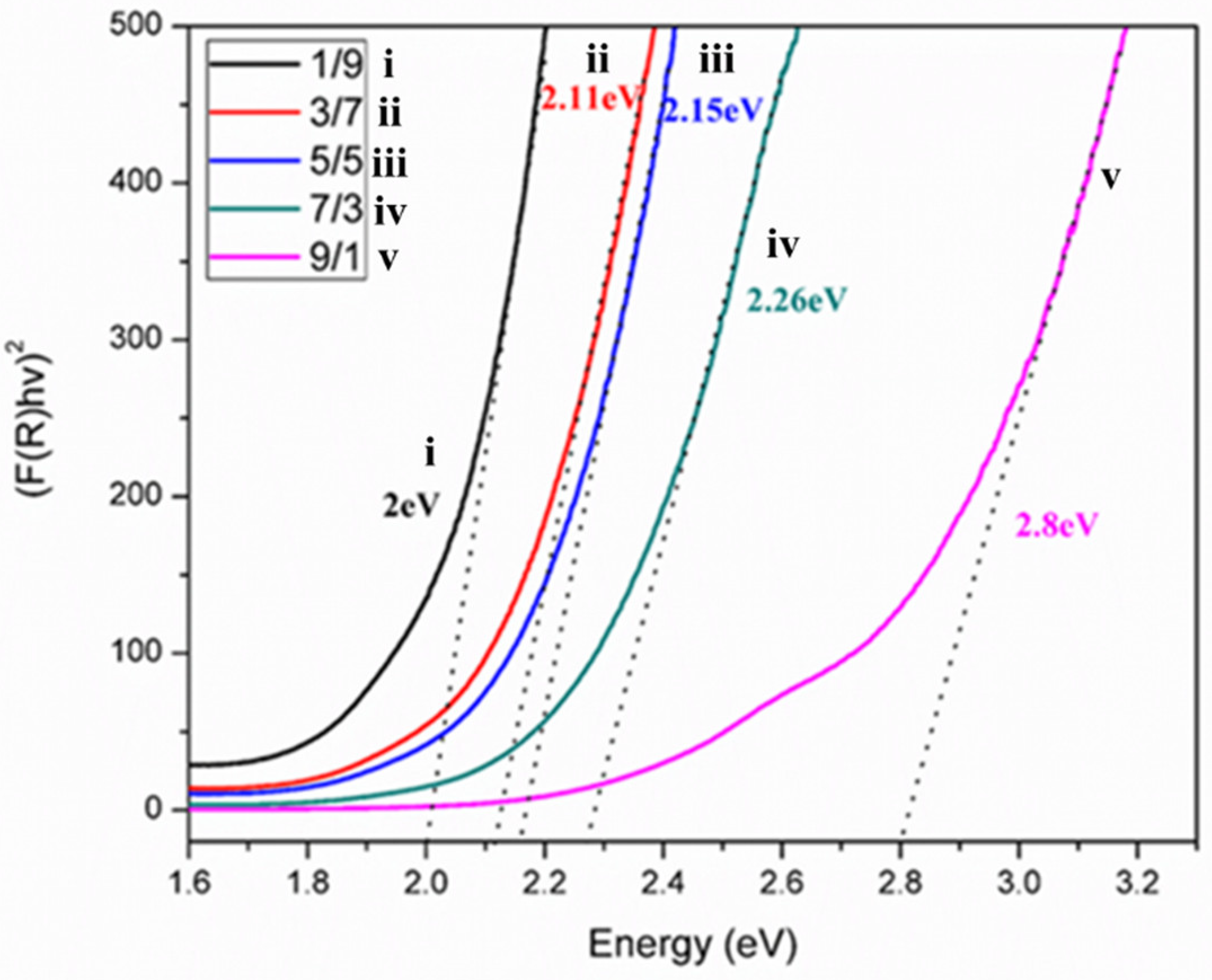
3.4. UV-VIS Diffuse Reflectance Spectrum
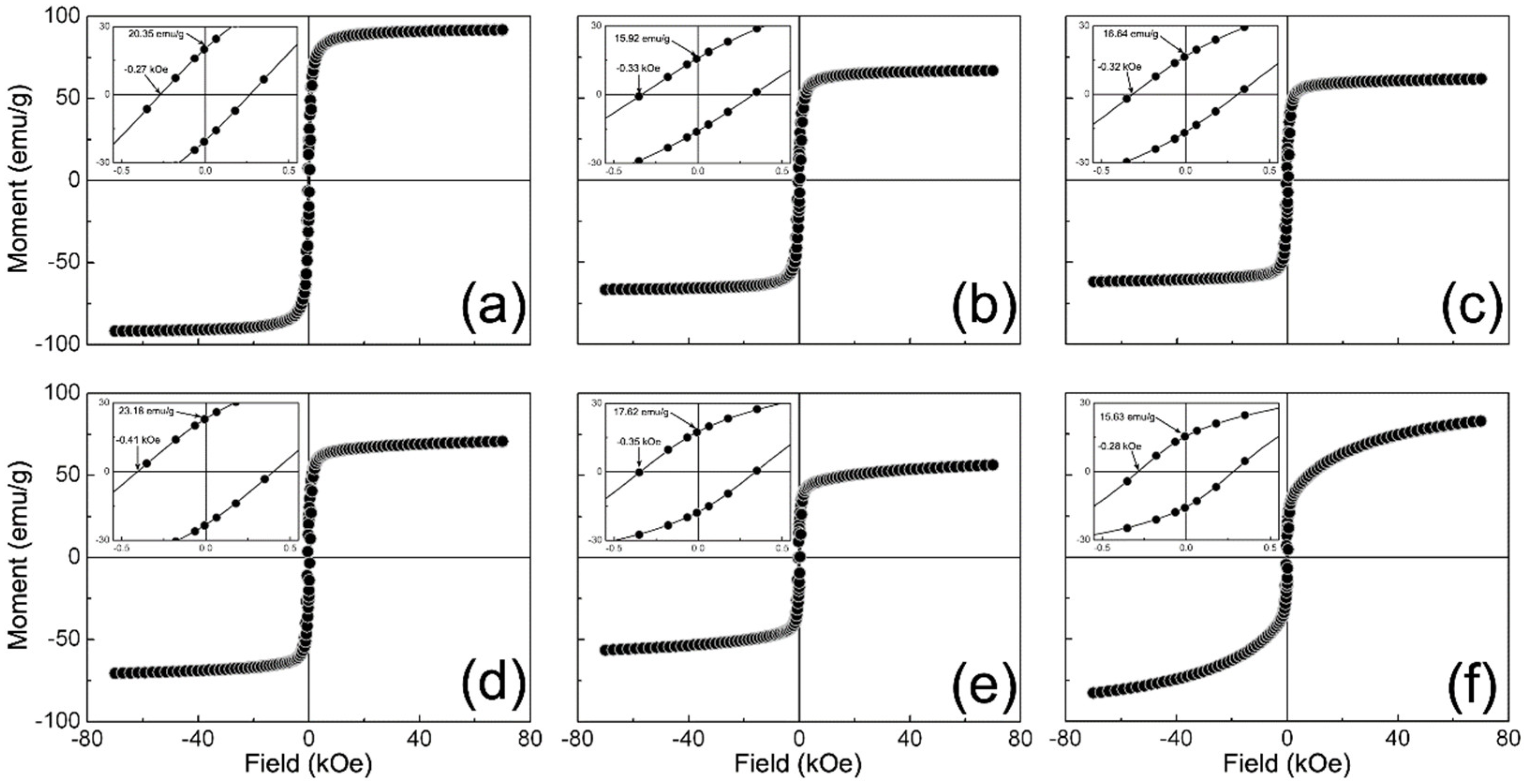
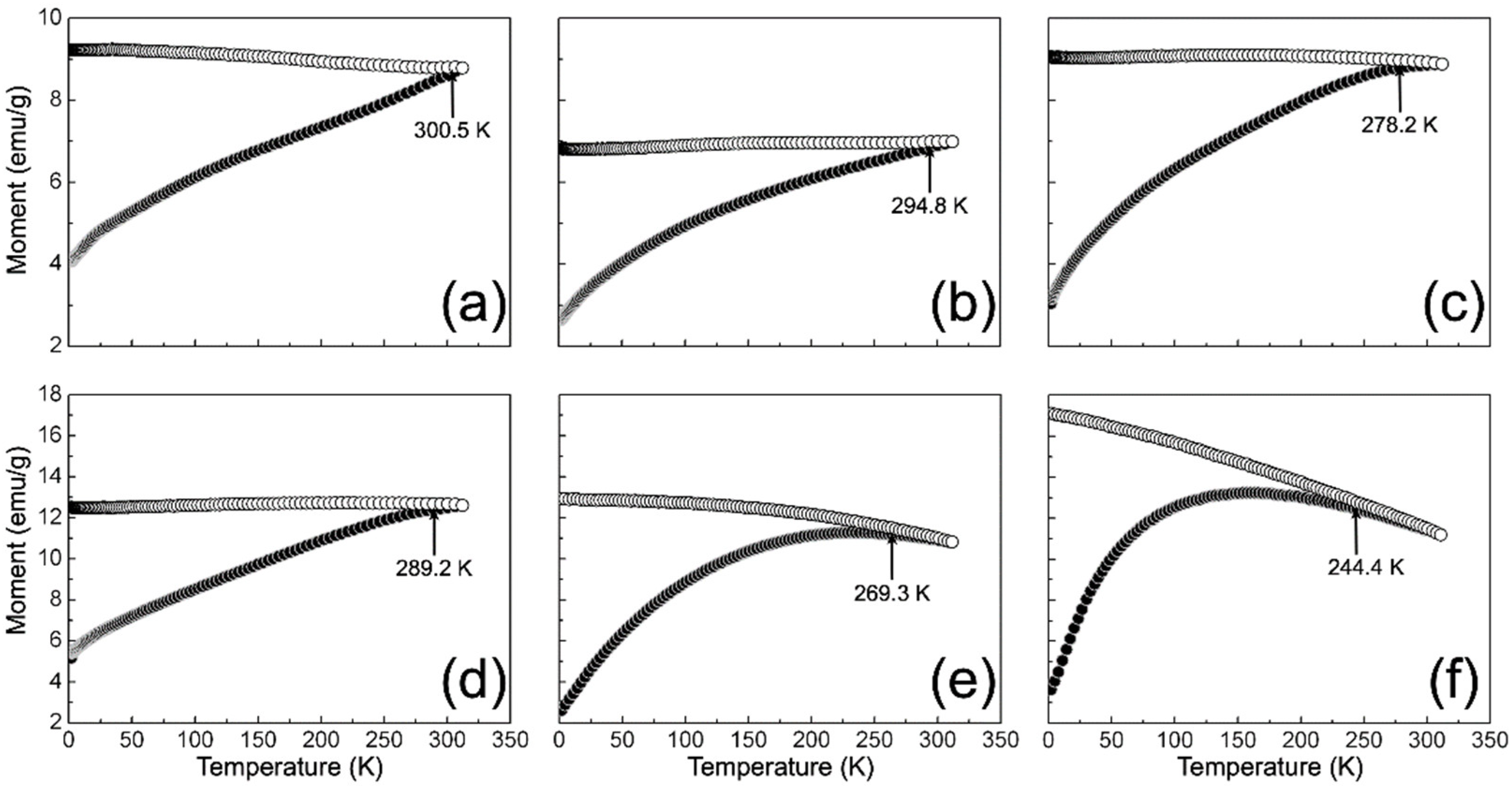
3.5. SQUID Analysis
3.6. Adsorption Studies and Effect of Various Parameters
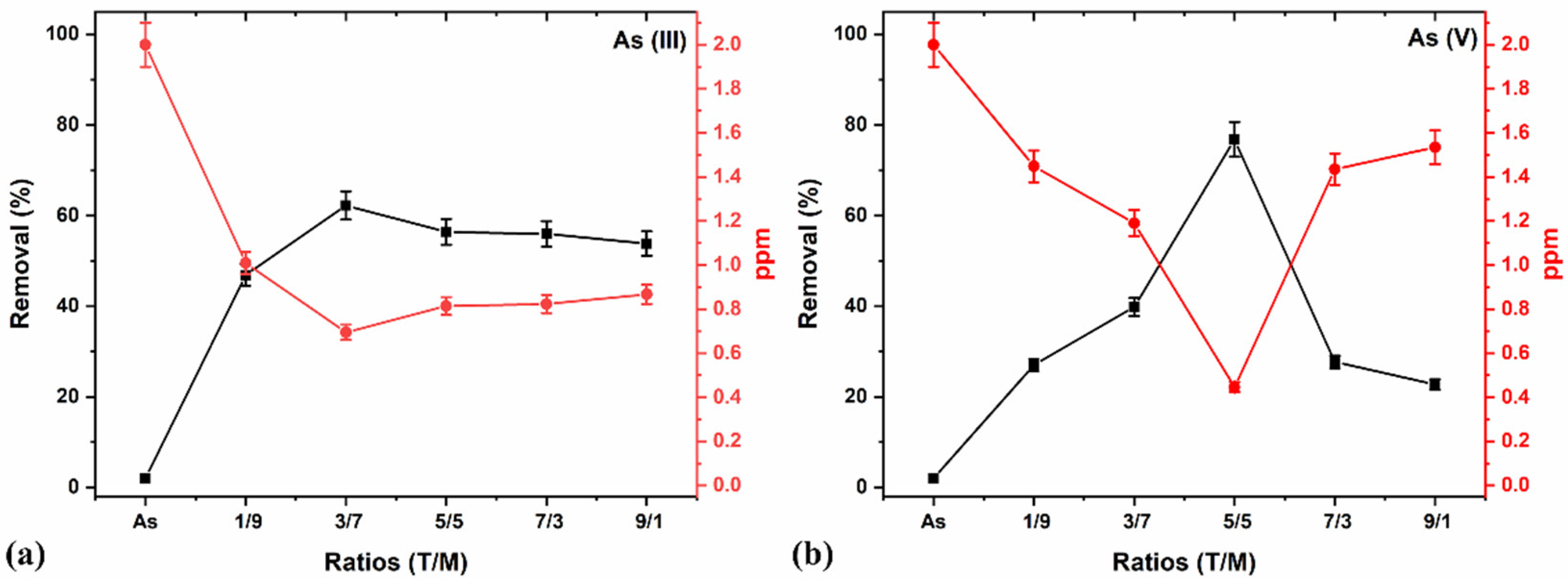
3.6.1. Adsorption Using the Synthesized T/M NC Ratios
3.6.2. Effect of Nano-Adsorbent Dosage
3.6.3. Effect of Contact Time
3.6.4. Effect of As Concentration
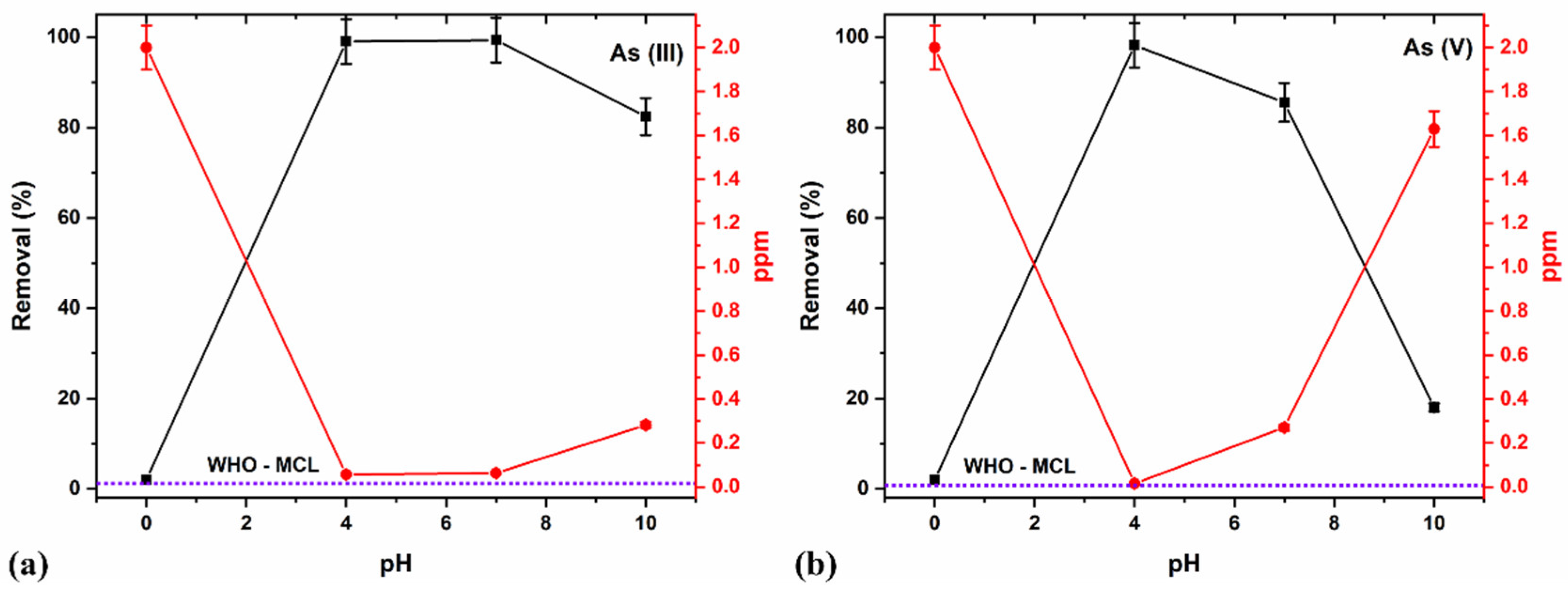
3.6.5. Effect of pH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic Contamination, Consequences and Remediation Techniques: A Review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef]
- Jain, C.K.; Singh, R.D. Technological Options for the Removal of Arsenic with Special Reference to South East Asia. J. Environ. Manag. 2012, 107, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stopelli, E.; Duyen, V.T.; Mai, T.T.; Trang, P.T.K.; Viet, P.H.; Lightfoot, A.; Kipfer, R.; Schneider, M.; Eiche, E.; Kontny, A.; et al. Spatial and Temporal Evolution of Groundwater Arsenic Contamination in the Red River Delta, Vietnam: Interplay of Mobilisation and Retardation Processes. Sci. Total Environ. 2020, 717, 137143. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Mattevada, S.; O’Bryant, S.E. Comparison of the Accuracy of Kriging and IDW Interpolations in Estimating Groundwater Arsenic Concentrations in Texas. Environ. Res. 2014, 130, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in Groundwater of West Bengal, India: A Review of Human Health Risks and Assessment of Possible Intervention Options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef]
- Hashim, M.A.; Kundu, A.; Mukherjee, S.; Ng, Y.S.; Mukhopadhyay, S.; Redzwan, G.; Sen Gupta, B. Arsenic Removal by Adsorption on Activated Carbon in a Rotating Packed Bed. J. Water Process Eng. 2019, 30, 100591. [Google Scholar] [CrossRef]
- Parga, J.R.; Cocke, D.L.; Valenzuela, J.L.; Gomes, J.A.; Kesmez, M.; Irwin, G.; Moreno, H.; Weir, M. Arsenic Removal via Electrocoagulation from Heavy Metal Contaminated Groundwater in La Comarca Lagunera México. J. Hazard. Mater. 2005, 124, 247–254. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Erdogan, H.; Yalçinkaya, Ö.; Türker, A.R. Determination of Inorganic Arsenic Species by Hydride Generation Atomic Absorption Spectrometry in Water Samples after Preconcentration/Separation on Nano ZrO2/B2O3 by Solid Phase Extraction. Desalination 2011, 280, 391–396. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. CHAPTER 1. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. Heavy Met. Water 2014, 1–24. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, Y.; Zhou, J.; Xu, Z.P.; Qian, G.; Lu, G.Q.M. Removal Efficiency of Arsenate and Phosphate from Aqueous Solution Using Layered Double Hydroxide Materials: Intercalation vs. Precipitation. J. Mater. Chem. 2010, 20, 4684–4691. [Google Scholar] [CrossRef]
- Hughes, M.F. Treatment of Arsenic Poisoning: Diagnosis with Biomarkers; Chakrabarty, N., Ed.; CRC Press: Boca Raton, FL, USA, 2015; Volume 7, ISBN 9781482241976. [Google Scholar]
- QA, M.; MS, K. Effect on Human Health Due to Drinking Water Contaminated with Heavy Metals. J. Pollut. Eff. Control 2016, 05, 10–11. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Chowdhury, S. Arsenic Removal Methods for Drinking Water in the Developing Countries: Technological Developments and Research Needs. Environ. Sci. Pollut. Res. 2017, 24, 24102–24120. [Google Scholar] [CrossRef] [PubMed]
- Çermikli, E.; Şen, F.; Altıok, E.; Wolska, J.; Cyganowski, P.; Kabay, N.; Bryjak, M.; Arda, M.; Yüksel, M. Performances of Novel Chelating Ion Exchange Resins for Boron and Arsenic Removal from Saline Geothermal Water Using Adsorption-Membrane Filtration Hybrid Process. Desalination 2020, 491, 114504. [Google Scholar] [CrossRef]
- Pessoa Lopes, M.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. Optimisation of Arsenate Removal from Water by an Integrated Ion-Exchange Membrane Process Coupled with Fe Co-Precipitation. Sep. Purif. Technol. 2020, 246, 116894. [Google Scholar] [CrossRef]
- Abejón, A.; Garea, A.; Irabien, A. Arsenic Removal from Drinking Water by Reverse Osmosis: Minimization of Costs and Energy Consumption. Sep. Purif. Technol. 2015, 144, 46–53. [Google Scholar] [CrossRef]
- Ince, M.; Kaplan İnce, O. An Overview of Adsorption Technique for Heavy Metal Removal from Water/Wastewater: A Critical Review. Int. J. Pure Appl. Sci. 2017, 3, 10–19. [Google Scholar] [CrossRef]
- Gomes, J.A.; Rahman, M.S.; Das, K.; Varma, S.; Cocke, D. A Comparative Electrochemical Study on Arsenic Removal Using Iron, Aluminum, and Copper Electrodes. ECS Trans. 2010, 25, 59–68. [Google Scholar] [CrossRef]
- Harikishore Kumar Reddy, D.; Vijayaraghavan, K.; Kim, J.A.; Yun, Y.S. Valorisation of Post-Sorption Materials: Opportunities, Strategies, and Challenges. Adv. Colloid Interface Sci. 2017, 242, 35–58. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Iron Oxide and Its Modified Forms as an Adsorbent for Arsenic Removal: A Comprehensive Recent Advancement. Process Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen, T.A.; Jones, L.A.; Bhosale, S.V. Graphene-Supported Spinel CuFe2O4 Composites: Novel Adsorbents for Arsenic Removal in Aqueous Media. Sensors 2017, 17, 1292. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- La, D.D.; Patwari, J.M.; Jones, L.A.; Antolasic, F.; Bhosale, S.V. Fabrication of a GNP/Fe-Mg Binary Oxide Composite for Effective Removal of Arsenic from Aqueous Solution. ACS Omega 2017, 2, 218–226. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H.; Pan, K.; Tian, C.; Qu, Y.; Lu, P.; Sun, C.C. Mesoporous TiO2/α-Fe2O3: Bifunctional Composites for Effective Elimination of Arsenite Contamination through Simultaneous Photocatalytic Oxidation and Adsorption. J. Phys. Chem. C 2008, 112, 19584–19589. [Google Scholar] [CrossRef]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Alvarado-Gil, J.J.; Oskam, G.; Rodríguez-Gattorno, G. Influence of Brookite Impurities on the Raman Spectrum of TiO2 Anatase Nanocrystals. J. Phys. Chem. C 2018, 122, 19921–19930. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.K. Review: Efficiently Performing Periodic Elements with Modern Adsorption Technologies for Arsenic Removal. Environ. Sci. Pollut. Res. 2020, 27, 39888–39912. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.T.; Yavuz, C.; Yean, S.; Cong, L.; Shipley, H.; Yu, W.; Falkner, J.; Kan, A.; Tomson, M.; Colvin, V.L. The Effect of Nanocrystalline Magnetite Size on Arsenic Removal. Sci. Technol. Adv. Mater. 2007, 8, 71–75. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-Field Magnetic Separation of Monodisperse Fe3O4 Nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Cumbal, L.; Sengupta, A.K. Arsenic Removal Using Polymer-Supported Hydrated Iron(III) Oxide Nanoparticles: Role of Donnan Membrane Effect. Environ. Sci. Technol. 2005, 39, 6508–6515. [Google Scholar] [CrossRef]
- Bui, T.T.; Le, X.Q.; To, D.P.; Nguyen, V.T. Investigation of Typical Properties of Nanocrystalline Iron Powders Prepared by Ball Milling Techniques. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045003. [Google Scholar] [CrossRef]
- Dar, M.I.; Shivashankar, S.A. Single Crystalline Magnetite, Maghemite, and Hematite Nanoparticles with Rich Coercivity. RSC Adv. 2014, 4, 4105–4113. [Google Scholar] [CrossRef]
- Grau-Crespo, R.; Al-Baitai, A.Y.; Saadoune, I.; De Leeuw, N.H. Vacancy Ordering and Electronic Structure of γ-Fe2O 3 (Maghemite): A Theoretical Investigation. J. Phys. Condens. Matter 2010, 22, 255401. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhang, R.; Zhang, L. Photocatalytic Degradations of Three Dyes with Different Chemical Structures Using Ball-Milled TiO2. Mater. Res. Bull. 2018, 97, 109–114. [Google Scholar] [CrossRef]
- Kong, L.B.; Ma, J.; Huang, H.; Zhang, R.F. Effect of Excess PbO on Microstructure and Electrical Properties of PLZT7/60/40 Ceramics Derived from a High-Energy Ball Milling Process. J. Alloys Compd. 2002, 345, 238–245. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (Ionp) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- Han, Q.; Setchi, R.; Evans, S.L. Synthesis and Characterisation of Advanced Ball-Milled Al-Al2O3 Nanocomposites for Selective Laser Melting. Powder Technol. 2016, 297, 183–192. [Google Scholar] [CrossRef]
- Saravanan, R.; Gupta, V.K.; Prakash, T.; Narayanan, V.; Stephen, A. Synthesis, Characterization and Photocatalytic Activity of Novel Hg Doped ZnO Nanorods Prepared by Thermal Decomposition Method. J. Mol. Liq. 2013, 178, 88–93. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Castrillón Arango, J.A.; Cristóbal, A.A.; Ramos, C.P.; Bercoff, P.G.; Botta, P.M. Mechanochemical Synthesis and Characterization of Nanocrystalline Ni1-XCoxFe2O4 (0 ≤ x ≤ 1) Ferrites. J. Alloys Compd. 2019, 811, 152044. [Google Scholar] [CrossRef]
- Hu, J.; Geng, X.; Duan, Y.; Zhao, W.; Zhu, M.; Ren, S. Effect of Mechanical-Chemical Modification Process on Mercury Removal of Bromine Modified Fly Ash. Energy Fuels 2020, 34, 9829–9839. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J. Mechanically Induced N-Arylation of Amines with Diaryliodonium Salts. ChemistrySelect 2020, 5, 542–548. [Google Scholar] [CrossRef]
- Do, J.L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Babudurai, M.; Nwakanma, O.; Romero-Nuñez, A.; Manisekaran, R.; Subramaniam, V.; Castaneda, H.; Jantrania, A. Mechanical Activation of TiO2/Fe2O3 Nanocomposite for Arsenic Adsorption: Effect of Ball-to-Powder Ratio and Milling Time. J. Nanostruct. Chem. 2021, 11, 619–632. [Google Scholar] [CrossRef]
- Jilani, A.; Melaibari, A.A. MoS2-Cu/CuO@graphene Heterogeneous Photocatalysis for Enhanced Photocatalytic Degradation of MB from Water. Polymers 2022, 14, 3259. [Google Scholar] [CrossRef]
- Karthick, S.; Ríos-Ramírez, J.J.; Chakaravarthy, S.; Velumani, S. Electrical, Optical, and Topographical Properties of RF Magnetron Sputtered Aluminum-Doped Zinc Oxide (AZO) Thin Films Complemented by First-Principles Calculations. J. Mater. Sci. Mater. Electron. 2018, 29, 15383–15395. [Google Scholar] [CrossRef]
- Ohring, M. Mechanical Behavior of Solids. In Engineering Materials Science; Elsevier: Amsterdam, The Netherlands, 1995; p. 299. ISBN 978-0-12-524995-9. [Google Scholar]
- Morán, A.; Nwakanma, O.; Velumani, S.; Castaneda, H. Comparative Study of Optimised Molybdenum Back-Contact Deposition with Different Barriers (Ti, ZnO) on Stainless Steel Substrate for Flexible Solar Cell Application. J. Mater. Sci. Mater. Electron. 2020, 31, 7524–7538. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, G.; Fang, J.; Chen, J. Synthesis, Characterization, and Photocatalysis of Well-Dispersible Phase-Pure Anatase TiO2 Nanoparticles. Int. J. Photoenergy 2013, 2013, 726872. [Google Scholar] [CrossRef]
- Aliahmad, M.; Nasiri Moghaddam, N. Synthesis of Maghemite (γ-Fe2O3) Nanoparticles by Thermal-Decomposition of Magnetite (Fe3O4) Nanoparticles. Mater. Sci. Pol. 2013, 31, 264–268. [Google Scholar] [CrossRef]
- Mercyrani, B.; Hernandez-Maya, R.; Solís-López, M.; Th-Th, C.; Velumani, S. Photocatalytic Degradation of Orange G Using TiO2/Fe3O4 Nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 29, 15436–15444. [Google Scholar] [CrossRef]
- Li, C.Y.; Wang, J.B.; Wang, Y.Q. Microstructure and Photocatalytic Activity of Titanium Dioxide Nanoparticles. Chin. Phys. B 2012, 21, 098102. [Google Scholar] [CrossRef]
- Danish, M.I.; Qazi, I.A.; Zeb, A.; Habib, A.; Awan, M.A.; Khan, Z. Arsenic Removal from Aqueous Solution Using Pure and Metal-Doped Titania Nanoparticles Coated on Glass Beads: Adsorption and Column Studies. J. Nanomater. 2013, 2013, 69. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents-A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Awual, M.R.; Shenashen, M.A.; Yaita, T.; Shiwaku, H.; Jyo, A. Efficient Arsenic(V) Removal from Water by Ligand Exchange Fibrous Adsorbent. Water Res. 2012, 46, 5541–5550. [Google Scholar] [CrossRef]
- Solano, R.A.; Herrera, A.P.; Maestre, D.; Cremades, A. Fe-TiO2 Nanoparticles Synthesized by Green Chemistry for Potential Application in Waste Water Photocatalytic Treatment. J. Nanotechnol. 2019, 2019, 4571848. [Google Scholar] [CrossRef]
- Guirado-López, R.A.; Aguilera-Granja, F. Bimetallic Fe-Ni Cluster Alloys: Stability of Core(Fe)-Shell(Ni) Arrays and Their Role Played in the Structure and Magnetic Behavior. J. Phys. Chem. C 2008, 112, 6729–6739. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and Magnetic Properties of Cobalt Ferrite (CoFe2O4) Nanoparticles Prepared by Wet Chemical Route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Coey, J.M.D. Noncollinear Spin Arrangement in Ultrafine Ferrimagnetic Crystallites. Phys. Rev. Lett. 1971, 27, 1140–1142. [Google Scholar] [CrossRef]
- Peddis, D.; Cannas, C.; Musinu, A.; Ardu, A.; Orruì, F.; Fiorani, D.; Laureti, S.; Rinaldi, D.; Muscas, G.; Concas, G.; et al. Beyond the Effect of Particle Size: Influence of CoFe2O4 Nanoparticle Arrangements on Magnetic Properties. Chem. Mater. 2013, 25, 2005–2013. [Google Scholar] [CrossRef]
- Blanco-Mantecón, M.; O’Grady, K. Interaction and Size Effects in Magnetic Nanoparticles. J. Magn. Magn. Mater. 2006, 296, 124–133. [Google Scholar] [CrossRef]
- Akdogan, O.; Li, W.; Balasubramanian, B.; Sellmyer, D.J.; Hadjipanayis, G.C. Effect of Exchange Interactions on the Coercivity of SmCo5 Nanoparticles Made by Cluster Beam Deposition. Adv. Funct. Mater. 2013, 23, 3262–3267. [Google Scholar] [CrossRef]
- Torres-Martínez, N.E.; Garza-Navarro, M.A.; Lucio-Porto, R.; García-Gutiérrez, D.; Torres-Castro, A.; González-González, V.A. One-Pot Synthesis of Magnetic Hybrid Materials Based on Ovoid-like Carboxymethyl-Cellulose/Cetyltrimethylammonium-Bromide Templates. Mater. Chem. Phys. 2013, 141, 735–743. [Google Scholar] [CrossRef]
- Ray, P.Z.; Shipley, H.J. Inorganic Nano-Adsorbents for the Removal of Heavy Metals and Arsenic: A Review. RSC Adv. 2015, 5, 29885–29907. [Google Scholar] [CrossRef]
- Kütahyali, C.; Şert, S.; Çetinkaya, B.; Inan, S.; Eral, M. Factors Affecting Lanthanum and Cerium Biosorption on Pinus Brutia Leaf Powder. Sep. Sci. Technol. 2010, 45, 1456–1462. [Google Scholar] [CrossRef]
- Esposito, A.; Pagnanelli, F.; Lodi, A.; Solisio, C.; Vegliò, F. Biosorption of Heavy Metals by Sphaerotilus Natans: An Equilibrium Study at Different PH and Biomass Concentrations. Hydrometallurgy 2001, 60, 129–141. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of Rare Earth Metals through Biosorption: An Overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Xie, J.; Lin, Y.; Li, C.; Wu, D.; Kong, H. Removal and Recovery of Phosphate from Water by Activated Aluminum Oxide and Lanthanum Oxide. Powder Technol. 2015, 269, 351–357. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M.; Asadollahzadeh, M.; Hemmati, A.; Khosravi, A. Biosorption of Lanthanum and Cerium from Aqueous Solutions by Grapefruit Peel: Equilibrium, Kinetic and Thermodynamic Studies. Res. Chem. Intermed. 2015, 41, 559–573. [Google Scholar] [CrossRef]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the Factors Affecting the Adsorption of Lanthanum Using Different Adsorbents: A Critical Review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Prasad, P. Health Effects Inflicted by Chronic Low-Level Arsenic Contamination in Groundwater: A Global Public Health Challenge. J. Appl. Toxicol. 2020, 40, 87–131. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Sharma, Y.C.; Sillanpää, M. Green Synthesis of Magnesium Oxide Nanoflower and Its Application for the Removal of Divalent Metallic Species from Synthetic Wastewater. Ceram. Int. 2015, 41, 6702–6709. [Google Scholar] [CrossRef]
- El-Latif, M.M.A.; Ibrahim, A.M.; Showman, M.S.; Hamide, R.R.A. Alumina/Iron Oxide Nano Composite for Cadmium Ions Removal from Aqueous Solutions. Int. J. Nonferrous Metall. 2013, 2, 47–62. [Google Scholar] [CrossRef]
- Mondal, P.; Majumder, C.B.; Mohanty, B. Effects of Adsorbent Dose, Its Particle Size and Initial Arsenic Concentration on the Removal of Arsenic, Iron and Manganese from Simulated Ground Water by Fe3+ Impregnated Activated Carbon. J. Hazard. Mater. 2008, 150, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. Colour Plates. In The Iron Oxides; Wiley: Hoboken, NJ, USA, 2003; ISBN 3527302743. [Google Scholar]
- Ali, S.; Rizwan, M.; Shakoor, M.B.; Jilani, A.; Anjum, R. High Sorption Efficiency for As(III) and As(V) from Aqueous Solutions Using Novel Almond Shell Biochar. Chemosphere 2020, 243, 125330. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.; Muzammal, S.; Shakoor, M.B.; Ahmad, S.R.; Jilani, A.; Iqbal, J.; Al-Sehemi, A.G.; Kalam, A.; Aboushoushah, S.F.O. Synthesis and Application of Egg Shell Biochar for As(V) Removal from Aqueous Solutions. Catalysts 2022, 12, 431. [Google Scholar] [CrossRef]
- Lin, S.; Jin, J.; Sun, S.; Yu, J. Removal of Arsenic Contaminants Using a Novel Porous Nanoadsorbent with Superior Magnetic Recovery. Chem. Eng. Sci. X 2020, 8, 100069. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Ahmed, M.; Siddiqui, S.I.; Ahmed, S. Fe(III)–Sn(IV) Mixed Binary Oxide-Coated Sand Preparation and Its Use for the Removal of As(III) and As(V) from Water: Application of Isotherm, Kinetic and Thermodynamics. J. Mol. Liq. 2016, 224, 431–441. [Google Scholar] [CrossRef]
- Basu, T.; Ghosh, U.C. Influence of Groundwater Occurring Ions on the Kinetics of As(III) Adsorption Reaction with Synthetic Nanostructured Fe(III)-Cr(III) Mixed Oxide. Desalination 2011, 266, 25–32. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, Z.; Zhang, X.; Chen, J. Nanostructured Iron(III)-Copper(II) Binary Oxide: A Novel Adsorbent for Enhanced Arsenic Removal from Aqueous Solutions. Water Res. 2013, 47, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shan, C.; Jin, Y.; Tong, M. Enhanced Removal of Trace Arsenate by Magnetic Nanoparticles Modified with Arginine and Lysine. Chem. Eng. J. 2014, 254, 340–348. [Google Scholar] [CrossRef]
- Beduk, F. Superparamagnetic Nanomaterial Fe3O4-TiO2 for the Removal of As(V) and As(III) from Aqueous Solutions. Environ. Technol. 2016, 37, 1790–1801. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liang, K.; Wu, Y.; Zou, Y.; Zuo, J.; Arriagada, D.C.; Pan, Z.; Hu, G. The Effect of PH on the Adsorption of Arsenic(III) and Arsenic(V) at the TiO2 Anatase [101] Surface. J. Colloid Interface Sci. 2016, 462, 252–259. [Google Scholar] [CrossRef]
- Babu, C.M.; Vinodh, R.; Sundaravel, B.; Abidov, A.; Peng, M.M.; Cha, W.S.; Jang, H.T. Characterization of Reduced Graphene Oxide Supported Mesoporous Fe2O3/TiO2 Nanoparticles and Adsorption of As(III) and As(V) from Potable Water. J. Taiwan Inst. Chem. Eng. 2016, 62, 199–208. [Google Scholar] [CrossRef]
- Monárrez-Cordero, B.E.; Amézaga-Madrid, P.; Leyva-Porras, C.C.; Pizá-Ruiz, P.; Miki-Yoshida, M. Study of the Adsorption of Arsenic (III and V) by Magnetite Nanoparticles Synthetized via AACVD. Mater. Res. 2016, 19, 103–112. [Google Scholar] [CrossRef]
- Hernández-Flores, H.; Pariona, N.; Herrera-Trejo, M.; Hdz-García, H.M.; Mtz-Enriquez, A.I. Concrete/Maghemite Nanocomposites as Novel Adsorbents for Arsenic Removal. J. Mol. Struct. 2018, 1171, 9–16. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Adsorption of Arsenic Using Chitosan Magnetic Graphene Oxide Nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lingamdinne, L.P.; Yang, J.K.; Chang, Y.Y.; Koduru, J.R. Fabrication of Chitosan/Graphene Oxide-Gadolinium Nanorods as a Novel Nanocomposite for Arsenic Removal from Aqueous Solutions. J. Mol. Liq. 2020, 320, 114410. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Qiu, F.; Zhang, T.; Xu, J. Controllable Preparation of FeOOH/CuO@WBC Composite Based on Water Bamboo Cellulose Applied for Enhanced Arsenic Removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
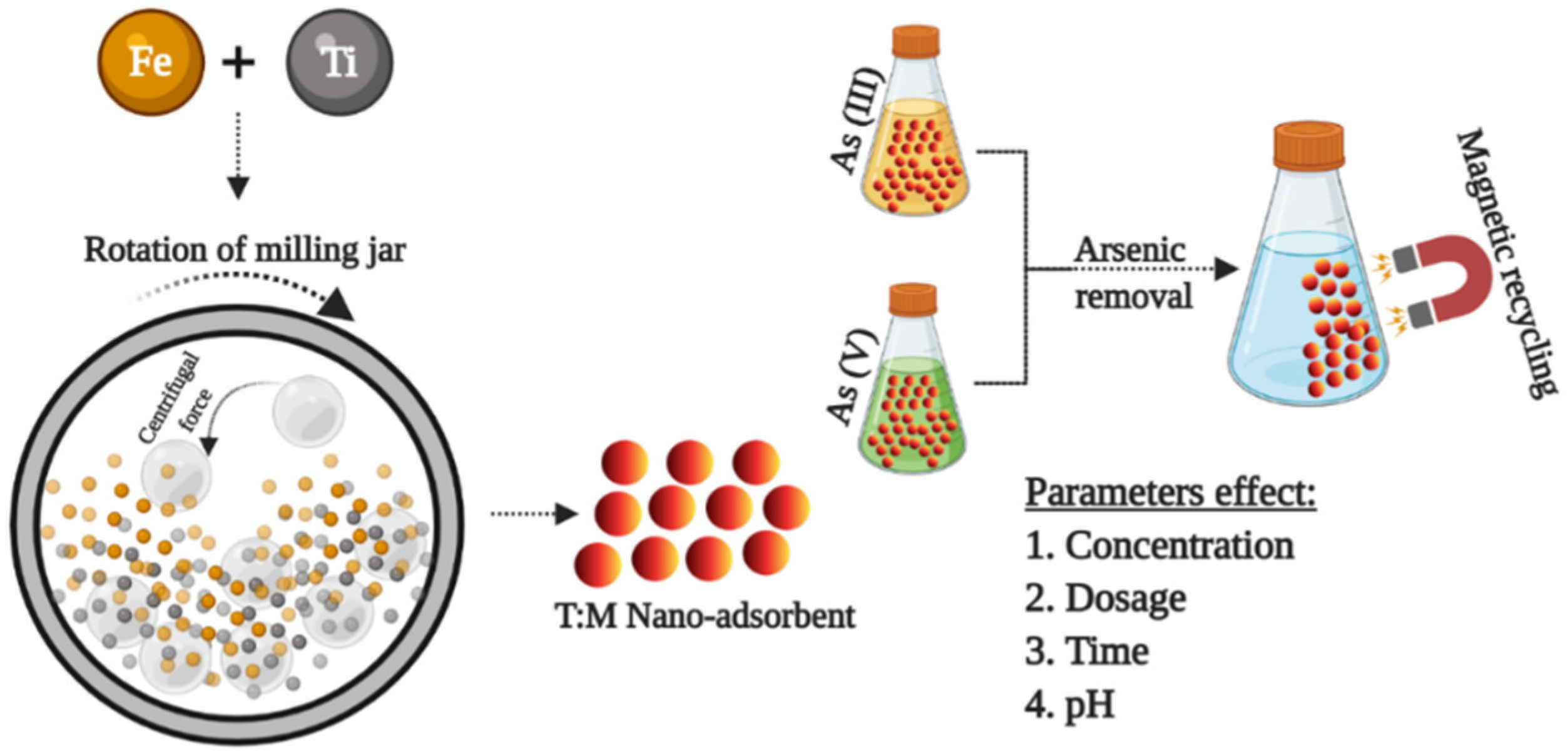





| S.no | Adsorption Parameters | Experimental Conditions | Description |
|---|---|---|---|
| 1 | Initial study (T/M NCs ratios: 1/9, 3/7, 5/5, 7/3, and 9/1) | As (III) and As (V)—2 ppm Dose—0.5 g/L pH—7 Time—5 min | Studies on the optimization of the complete removal of arsenic using the best ratio. |
| 2 | Effect of Dosage | Dose = 0.5, 2, 4, 6, and 8 g/L pH—7 Time—5 min As (III) and As (V)—2 ppm | |
| 3 | Effect of time | Time = 5, 15, 30, and 60 min Dose—8 g/L pH—7 As (III) and As (V)—2 ppm | |
| 4 | Effect of concentration | Concentration = 2, 4, and 6 ppm Dose—8 g/L pH—7 Time—15 min | |
| 5 | Effect of pH | pH = 4, 7, and 10 Dose—8 g/L Time—15 min As (III) and As (V)—2 ppm |
| T/M NC Ratios | 2θ (Degrees) | Average Dp (nm) | I(101)/I(311) | |
|---|---|---|---|---|
| TiO2 | Fe2O3 | |||
| 9/1 | 25.34 | 35.69 | 13 | 6.94 |
| 7/3 | 25.34 | 35.57 | 14 | 2.46 |
| 5/5 | 25.38 | 35.67 | 13 | 1.42 |
| 3/7 | 25.4 | 35.73 | 12 | 0.95 |
| 1/9 | 25.46 | 35.67 | 10 | 0.76 |
| Ratios (T/M NCs) | Ti (%) | Fe (%) |
|---|---|---|
| 9/1 | 90.7 | 9.30 |
| 7/3 | 67.0 | 33.0 |
| 5/5 | 47.2 | 52.8 |
| 3/7 | 29.4 | 70.6 |
| 1/9 | 8.7 | 91.3 |
| Ratios (T/M NCs) | Av. Crystallite Size (nm) | Av. Particle Size (nm) | Bandgap (eV) |
|---|---|---|---|
| 9/1 | 13 | 19 | 2.80 |
| 7/3 | 14 | 20 | 2.26 |
| 5/5 | 13 | 17 | 2.15 |
| 3/7 | 12 | 15 | 2.11 |
| 1/9 | 10 | 15 | 2.00 |
| Sample | Mmax (emu/g) | HC (kOe) | MR (emu/g) | RR = MR/Mmax (Unitless) |
|---|---|---|---|---|
| γ-Fe2O3 | 91.89 | 0.27 | 20.35 | 0.22 |
| 1/9 | 66.72 | 0.33 | 15.92 | 0.24 |
| 3/7 | 61.71 | 0.32 | 16.64 | 0.27 |
| 5/5 | 70.64 | 0.41 | 23.18 | 0.33 |
| 7/3 | 56.36 | 0.35 | 17.62 | 0.31 |
| 9/1 | 82.97 | 0.28 | 15.63 | 0.19 |
| Adsorbents | Synthesis Method | As Removal | Reference |
|---|---|---|---|
| GO-Fe2O3/TiO2 | Sol-gel | As (III) and (V)~92% | [88] |
| GNPs/CuFe2O4 | One-pot hydrothermal | As (V)~98% | [23] |
| TiO2 | Hydrothermal | As (III) and (V)~70% | [87] |
| Fe3O4 | AACVD | As (III)~88% As (V)~100% | [89] |
| GNPs/Fe−Mg | One-pot hydrothermal | As (V)~98% | [25] |
| Concrete/γ-Fe2O3 | Simple mixing | As(V) 10 ppm to 10 ppb | [90] |
| CS magnetic GO | Co-precipitation | As (III)~61% | [91] |
| CS/GO-Gd | Co-precipitation hydrothermal | As (V)~99% | [92] |
| FeOOH/CuO@WBC | Two-step hydrothermal | As (III)~75% | [93] |
| Fe3O4–TiO2 | Co-precipitation | As (III)~93% As (V)~94% | [86] |
| TiO2/Fe2O3 | Ball-milling | As (III) and (V) > 99% | This work * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babudurai, M.; Sekar, K.; Nwakanma, O.M.; Manisekaran, R.; Garza-Navarro, M.A.; Subramaniam, V.; Cuando-Espitia, N.; David, H. Parametric Optimization of Ball-Milled Bimetallic Nanoadsorbents for the Effective Removal of Arsenic Species. Solids 2022, 3, 549-568. https://doi.org/10.3390/solids3030035
Babudurai M, Sekar K, Nwakanma OM, Manisekaran R, Garza-Navarro MA, Subramaniam V, Cuando-Espitia N, David H. Parametric Optimization of Ball-Milled Bimetallic Nanoadsorbents for the Effective Removal of Arsenic Species. Solids. 2022; 3(3):549-568. https://doi.org/10.3390/solids3030035
Chicago/Turabian StyleBabudurai, Mercyrani, Karthick Sekar, Onyekachi Michael Nwakanma, Ravichandran Manisekaran, Marco A. Garza-Navarro, Velumani Subramaniam, Natanael Cuando-Espitia, and Halaney David. 2022. "Parametric Optimization of Ball-Milled Bimetallic Nanoadsorbents for the Effective Removal of Arsenic Species" Solids 3, no. 3: 549-568. https://doi.org/10.3390/solids3030035
APA StyleBabudurai, M., Sekar, K., Nwakanma, O. M., Manisekaran, R., Garza-Navarro, M. A., Subramaniam, V., Cuando-Espitia, N., & David, H. (2022). Parametric Optimization of Ball-Milled Bimetallic Nanoadsorbents for the Effective Removal of Arsenic Species. Solids, 3(3), 549-568. https://doi.org/10.3390/solids3030035








