Thermal Expansion Behavior in the A2M3O12 Family of Materials
Abstract
1. Introduction
2. Positive Thermal Expansion
3. Negative Thermal Expansion
3.1. Mechanism of Negative Thermal Expansion in the A2M3O12 Family
3.2. Hygroscopicity in the A2M3O12 Family
3.3. Non-Hygroscopic A2M3O12 Compositions with Corner-Shared Networks
3.3.1. Single Ion Substitution
3.3.2. Aliovalent Substitutions
3.4. Pressure-Induced Phase Transitions in the A2M3O12 Family
3.4.1. High Pressure Behavior of NTE Tungstates
3.4.2. High Pressure Behavior of NTE Molybdates
4. Controllable Thermal Expansion in the A2M3O12 Family
4.1. Heterogeneous Composites
4.2. Solid Solution Formation
4.2.1. Single Ion Substitution at the A/M Site
4.2.2. Aliovalent Ion Substitution at the A/M Site
5. Potential Challenges for Use of NTE Materials
6. Conclusions
Funding
Conflicts of Interest
References
- Chapman, K.W.; Chupas, P.J.; Kepert, C.J. Direct observation of a transverse vibrational mechanism for negative thermal expansion in Zn(CN)2: An atomic pair distribution function analysis. J. Am Chem. Soc. 2005, 127, 15630–15636. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Kepert, C.J. Compositional dependence of negative thermal expansion in the Prussian blue analogues (MPtIV)-PtII(CN)6 (M = Mn, Fe, Co, Ni, Cu, Zn, Cd). J. Am Chem. Soc. 2006, 128, 7009–7014. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.L.; Kepert, C.J. Negative thermal expansion and low-frequency modes in cyanide-bridged framework materials. Phys. Rev. B 2005, 71, 140301(R). [Google Scholar] [CrossRef]
- Margadonna, S.; Prassides, K.; Fitch, A.N. Zero thermal expansion in a Prussian blue analogue. J. Am. Chem. Soc. 2004, 126, 15390–15391. [Google Scholar] [CrossRef]
- Matsuda, T.; Kim, J.E.; Ohoyama, K.; Moritomo, Y. Universal thermal response of the Prussian blue lattice. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef]
- Adak, S.; Daemen, L.L.; Hartl, M.; Williams, D.; Summerhill, J.; Nakotte, H. Thermal expansion in 3d-metal Prussian Blue Analogs-A survey study. J. Solid State Chem. 2011, 184, 2854–2861. [Google Scholar] [CrossRef]
- Greve, B.K.; Martin, K.L.; Lee, P.L.; Chupas, P.J.; Chapman, K.W.; Wilkinson, A.P. Pronounced Negative Thermal Expansion from a Simple Structure: Cubic ScF3. J. Am. Chem. Soc. 2010, 132, 15496–15498. [Google Scholar] [CrossRef]
- Hester, B.R.; Hancock, J.C.; Lapidus, S.H.; Wilkinson, A.P. Composition, Response to Pressure, and Negative Thermal Expansion in MIIBIVF6 (M = Ca, Mg; B = Zr, Nb). Chem. Mater. 2017, 29, 823–831. [Google Scholar] [CrossRef]
- Li, C.W.; Tang, X.; Munoz, J.A.; Keith, J.B.; Tracy, S.J.; Abernathy, D.L.; Fultz, B. Structural Relationship between Negative Thermal Expansion and Quartic Anharmonicity of Cubic ScF3. Phys. Rev. Lett. 2011, 107. [Google Scholar] [CrossRef]
- Ticknor, J.O.; Hester, B.R.; Adkins, J.W.; Xu, W.Q.; Yakovenko, A.A.; Wilkinson, A.P. Zero Thermal Expansion and Abrupt Amorphization on Compression in Anion Excess ReO3-Type Cubic YbZrF7. Chem. Mater. 2018, 30, 3071–3077. [Google Scholar] [CrossRef]
- Baxter, S.J.; Hester, B.R.; Wright, B.R.; Wilkinson, A.P. Controlling the Negative Thermal Expansion and Response to Pressure in ReO3-type Fluorides by the Deliberate Introduction of Excess Fluoride: Mg1−xZr1+xF6+2x, x = 0.15, 0.30, 0.40, and 0.50. Chem. Mater. 2019, 31, 3440–3448. [Google Scholar] [CrossRef]
- Chatterji, T.; Zbiri, M.; Hansen, T.C. Negative thermal expansion in ZnF2. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Han, F.; Hu, L.; Liu, Z.N.; Li, Q.; Wang, T.; Ren, Y.; Deng, J.X.; Chen, J.; Xing, X.R. Local structure and controllable thermal expansion in the solid solution (Mn1−xNix)ZrF6. Inorg. CHem. Front. 2017, 4, 343–347. [Google Scholar] [CrossRef]
- Hancock, J.C.; Chapman, K.W.; Halder, G.J.; Morelock, C.R.; Karlan, B.S.; Gallington, L.C.; Bongiorno, A.; Han, C.; Zhou, S.; Wilkinson, A.P. Large Negative Thermal Expansion and Anomalous Behavior on Compression in Cubic ReO3-Type AIIBIVF6: CaZrF6 and CaHfF6. Chem. Mater. 2015, 27, 3912–3918. [Google Scholar] [CrossRef]
- Morelock, C.R.; Gallington, L.C.; Wilkinson, A.P. Evolution of Negative Thermal Expansion and Phase Transitions in Sc1−xTixF3. Chem. Mater. 2014, 26, 1936–1940. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.G.; Bai, J.M.; Qu, B.Y.; Tong, P.; Wang, M.; Lin, J.C.; Zhang, R.R.; Tong, H.Y.; Wu, Y.; et al. Crossover of thermal expansion from positive to negative by removing the excess fluorines in cubic ReO3-type TiZrF7−x. J. Mater. Chem. C 2018, 6, 5148–5152. [Google Scholar] [CrossRef]
- Takenaka, K.; Takagi, H. Giant negative thermal expansion in Ge-doped anti-perovskite manganese nitrides. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Wen, Y.C.; Chu, L.H.; Pan, H.; Niez, M. Negative Thermal Expansion and Magnetic Transition in Anti-Perovskite Structured Mn3Zn1−xSnxN Compounds. J. Am. Ceram. Soc. 2010, 93, 2178–2181. [Google Scholar] [CrossRef]
- Hamada, T.; Takenaka, K. Giant negative thermal expansion in antiperovskite manganese nitrides. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Takenaka, K.; Ichigo, M.; Hamada, T.; Ozawa, A.; Shibayama, T.; Inagaki, T.; Asano, K. Magnetovolume effects in manganese nitrides with antiperovskite structure. Sci. Technol. Adv. Mater. 2014, 15, 015009. [Google Scholar] [CrossRef]
- Iikubo, S.; Kodama, K.; Takenaka, K.; Takagi, H.; Shamoto, S. Magnetovolume effect in Mn3Cu1−xGexN related to the magnetic structure: Neutron powder diffraction measurements. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef]
- Huang, R.J.; Li, L.F.; Cai, F.S.; Xu, X.D.; Qian, L.H. Low-temperature negative thermal expansion of the antiperovskite manganese nitride Mn3CuN codoped with Ge and Si. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Iikubo, S.; Kodama, K.; Takenaka, K.; Takagi, H.; Takigawa, M.; Shamoto, S. Local Lattice Distortion in the Giant Negative Thermal Expansion Material Mn3Cu1−xGexN. Phys. Rev. Lett. 2008, 101. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Liu, Y.Y.; Fan, W.; Tan, J.; Xiao, F.R.; Qian, L.H.; Li, L.F. Giant Negative Thermal Expansion in NaZn13-Type La(Fe, Si, Co)13 Compounds. J. Am Chem. Soc. 2013, 135, 11469–11472. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Huang, R.J.; Zhao, Y.Q.; Li, W.; Wang, W.; Huang, C.J.; Gong, P.F.; Lin, Z.S.; Li, L.F. Broad Negative Thermal Expansion Operation-Temperature Window Achieved by Adjusting Fe-Fe Magnetic Exchange Coupling in La(Fe,Si)13 Compounds. Inorg. Chem. 2015, 54, 7868–7872. [Google Scholar] [CrossRef]
- Li, S.P.; Huang, R.J.; Zhao, Y.Q.; Wang, W.; Han, Y.M.; Li, L.F. Zero Thermal Expansion Achieved by an Electrolytic Hydriding Method in La(Fe, Si)13 Compounds. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Song, Y.Z.; Huang, R.J.; Liu, Y.; Zhang, Z.H.; Huang, Q.Z.; Jiang, Y.; Wang, S.G.; Li, L.F.; Xing, X.R.; Chen, J. Magnetic-Field-Induced Strong Negative Thermal Expansion in La(Fe,Al)13. Chem. Mater. 2020, 32, 7535–7541. [Google Scholar] [CrossRef]
- Sun, W.T.; Zhang, H.; Li, W.; Huang, R.J.; Zhao, Y.Q.; Wang, W.; Li, L.F. Controllable negative thermal expansion in NaZn13-type La(Fe, Co, Al)13 compounds. AIP Adv. 2020, 10. [Google Scholar] [CrossRef]
- Hu, J.Y.; Lin, K.; Cao, Y.L.; Yu, C.Y.; Li, W.J.; Huang, R.J.; Fischer, H.E.; Kato, K.; Song, Y.Z.; Chen, J.; et al. Adjustable Magnetic Phase Transition Inducing Unusual Zero Thermal Expansion in Cubic RCo2-Based Intermetallic Compounds (R = Rare Earth). Inorg. Chem. 2019, 58, 5401–5405. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.R.; Gu, F.; Hogan, T.; Kanatzidis, M.G. Zero thermal expansion in YbGaGe due to an electronic valence transition. Nature 2003, 425, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Gruner, M.E.; Keune, W.; Roldan Cuenya, B.; Weis, C.; Landers, J.; Makarov, S.I.; Klar, D.; Hu, M.Y.; Alp, E.E.; Zhao, J.; et al. Element-Resolved Thermodynamics of Magnetocaloric LaFe13−xSix. Phys. Rev. Lett. 2015, 114. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Hu, Z.; Jorgensen, J.D.; Argyriou, D.N.; Short, S.; Sleight, A.W. Compressibility, Phase Transitions, and Oxygen Migration in Zirconium Tungstate, ZrW2O8. Science 1997, 275, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S.O.; Mary, T.A.; Vogt, T.; Subramanian, M.A.; Sleight, A.W. Negative Thermal Expansion in ZrW2O8 and HfW2O8. Chem. Mater. 1996, 8, 2809–2823. [Google Scholar] [CrossRef]
- Mary, T.A.; Evans, J.S.O.; Vogt, T.; Sleight, A.W. Negative Thermal Expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 1996, 272, 90–92. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P.; Hu, Z.B.; Short, S.; Jorgensen, J.D. Synthesis and Properties of the Negative Thermal Expansion Material Cubic ZrMo2O8. Chem. Mater. 1998, 10, 2335–2337. [Google Scholar] [CrossRef]
- Perottoni, C.A.; da Jornada, J.A.H. Pressure-induced amorphization and negative thermal expansion in ZrW2O8. Science 1998, 280, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Pryde, A.K.A.; Hammonds, K.D.; Dove, M.T.; Heine, V.; Gale, J.D.; Warren, M.C. Rigid Unit Modes and the Negative Thermal Expansion in ZrW2O8. Phase Transit. 1997, 61, 141–153. [Google Scholar] [CrossRef]
- Ramirez, A.P.; Kowach, G.R. Large Low Temperature Specific Heat in the Negative Thermal Expansion Compound ZrW2O8. Phys. Rev. Lett. 1998, 80, 4903–4906. [Google Scholar] [CrossRef]
- Korthuis, V.; Khosrovani, N.; Sleight, A.W.; Roberts, N.; Dupree, R.; Warren, W.W. Negative thermal expansion and phase transitions in the ZrV2−xPxO7 series. Chem. Mater. 1995, 7, 412–417. [Google Scholar] [CrossRef]
- Khosrovani, K.; Sleight, A.W.; Vogt, T. Structure of ZrV2O7 from −263 to 470 °C. J. Solid State Chem. 1997, 132, 355–360. [Google Scholar] [CrossRef]
- Carlson, S.; Andersen, A.M.K. High-pressure properties of TiP2O7, ZrP2O7 and ZrV2O7. J. Appl. Crystallogr. 2001, 34, 7–12. [Google Scholar] [CrossRef]
- Xing, X.R.; Zhu, Z.Q.; Qiu, X.P.; Liu, G.R. Zero-thermal expansion and heat capacity of zirconium pyrovanadate doped with zirconia and vanadium (V) oxide. Rare Metals 2001, 20, 1–4. [Google Scholar]
- Beccara, S.A.; Dalba, G.; Fornasini, P.; Grisenti, R.; Sanson, A. Local thermal expansion in a cuprite structure: The case of Ag2O. Phys. Rev. Lett. 2002, 89, 025503. [Google Scholar] [CrossRef] [PubMed]
- Tiano, W.; Dapiaggi, M.; Artioli, G. Thermal expansion in cuprite-type structures from 10 K to decomposition temperature: Cu2O and Ag2O. J. Appl. Crystallogr. 2003, 36, 1461–1463. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Kubota, Y.; Kato, K. Negative thermal expansion and phase transition behaviour in Ag2O. Solid State Commun. 2005, 136, 177–180. [Google Scholar] [CrossRef]
- Fornasini, P.; Dalba, G.; Grisenti, R.; Purans, J.; Vaccari, M.; Rocca, F.; Sanson, A. Local behaviour of negative thermal expansion materials. Nucl. Inst. Methods Phys. Res. B 2006, 246, 180–183. [Google Scholar] [CrossRef]
- Sanson, A.; Rocca, F.; Dalba, G.; Fornasini, P.; Grisenti, R.; Dapiaggi, M.; Artioli, G. Negative thermal expansion and local dynamics in Cu2O and Ag2O. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Negative Thermal Expansion Materials. Phys. B 1998, 241–243, 311–316. [Google Scholar] [CrossRef]
- Forster, P.M.; Sleight, A.W. Negative Thermal Expansion in Y2W3O12. Int. J. Inorg. Mater. 1999, 1, 123–127. [Google Scholar] [CrossRef]
- Mary, T.A.; Sleight, A.W. Bulk thermal expansion for tungstate and molybdates of the type A2M3O12. J. Mater. Res. 1999, 14, 912–915. [Google Scholar] [CrossRef]
- Nassau, K.; Levinstein, H.J.; Loiacono, G.M. A comprehensive study of trivalent tungstates and molybdates of the type L2(MO4)3. Phys. Chem. Solids 1965, 26, 1815–1816. [Google Scholar] [CrossRef]
- Cao, W.G.; Li, Q.; Lin, K.; Liu, Z.N.; Deng, J.X.; Chen, J.; Xing, X.R. Phase transition and negative thermal expansion in orthorhombic Dy2W3O12. RSC Adv. 2016, 6, 96275–96280. [Google Scholar] [CrossRef]
- Roy, M.; Choudhary, R.N.P.; Acharya, H.N. X-ray and thermal studies of ferroelectric Dy2(MoO4)3. J. Therm. Anal. 1989, 35, 1471–1476. [Google Scholar] [CrossRef]
- Jeitschko, W. Comprehensive X-ray study of the ferroelectric-ferroelastic and paraelectric-paraelastic phases of gadolinium molybdate. Acta Crystallogr. B 1972, 28, 60–76. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Negative thermal expansion in Sc2(WO4)3. J. Solid State Chem. 1998, 137, 148–160. [Google Scholar] [CrossRef]
- Liu, H.F.; Yang, L.; Zhang, Z.P.; Pan, K.M.; Zhang, F.; Cheng, H.H.; Zeng, X.H.; Chen, X.B. Preparation and optical, nanomechanical, negative thermal expansion properties of Sc2W3O12 thin film grown by pulsed laser deposition. Ceram. Int. 2016, 42, 8809–8814. [Google Scholar] [CrossRef]
- Forster, P.M.; Yokochi, A.; Sleight, A.W. Enhanced Negative Thermal Expansion in Lu2W3O12. J. Solid State Chem. 1998, 140, 157–158. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Negative Thermal Expansion in a Large Molybdate and Tungstate Family. J. Solid State Chem. 1997, 133, 580–583. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A. Structural phase transitions and negative thermal expansion in Sc2(MoO4)3. Int. J. Inorg. Mater. 2000, 2, 143–151. [Google Scholar] [CrossRef]
- Pernicone, N.; Fagherazzi, G. A new iron tungstate: Fe2W3O12. J. Inorg. Nucl. Chem. 1969, 31, 3323–3324. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, Q.; Du, H.L.; Jia, Q.L. Preparation and thermal expansion of Fe2−xYxW3O12 powder by citrate sol-gel process. Chem. Eng. Commun. 2008, 195, 243–255. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Chowdhry, U.; Machiels, C.J.; Sleight, A.W.; Cheetham, A.K. Preparation of ferric tungstate and its catalytic behavior toward methanol. J. Solid State Chem. 1985, 60, 101–106. [Google Scholar] [CrossRef]
- Kendrick, E.; Swiatek, A.; Barker, J. Synthesis and characterisation of iron tungstate anode materials. J. Power Sources 2009, 189, 611–615. [Google Scholar] [CrossRef]
- Sriraman, A.K.; Tyagi, A.K. A new method of Fe2(WO4)3 preparation and its thermal stability. Thermochim. Acta 2003, 406, 29–33. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Li, L.-C.; Feng, M. Negative thermal expansion property of Cr2(WO4)3 and Cr2(MoO4)3. Chin. J. Inorg. Chem. 2007, 23, 382–386. [Google Scholar]
- Yang, G.; Liu, X.S.; Sun, X.W.; Liang, E.J.; Zhang, W.F. Synthesis process control of low-thermal-expansion Fe2W3O12 by suppressing the intermediate phase Fe2WO6. Ceram. Int. 2018, 44, 22032–22035. [Google Scholar] [CrossRef]
- Gates, S.D.; Colin, J.A.; Lind, C. Non-hydrolytic sol-gel synthesis, properties, and high-pressure behavior of gallium molybdate. J. Mater. Chem. 2006, 16, 4214–4219. [Google Scholar] [CrossRef]
- Sumithra, S.; Umarji, A.M. Negative thermal expansion in rare earth molybdates. Solid State Sci. 2006, 8, 1453–1458. [Google Scholar] [CrossRef]
- Woodcock, D.A.; Lightfoot, P.; Ritter, C. Negative thermal expansion in Y2(WO4)3. J. Solid State Chem. 2000, 149, 92–98. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Yang, L.; Liu, H.F.; Pan, K.M.; Wang, W.; Zeng, X.H.; Chen, X.B. Preparation and negative thermal expansion properties of Y2W3O12 thin films grown by pulsed laser deposition. Ceram. Int. 2016, 42, 18902–18906. [Google Scholar] [CrossRef]
- Xiao, X.L.; Cheng, Y.Z.; Peng, J.; Wu, M.M.; Chen, D.F.; Hu, Z.B.; Kiyanagi, R.; Fieramosca, J.S.; Short, S.; Jorgensen, J. Thermal expansion properties of A2(MO4)3 (A = Ho and Tm; M = W and Mo). Solid State Sci. 2008, 10, 321–325. [Google Scholar] [CrossRef]
- Sumithra, S.; Tyagi, A.K.; Umarji, A.M. Negative thermal expansion in Er2W3O12 and Yb2W3O12 by high temperature X-ray diffraction. Mater. Sci. Eng. B 2005, 116, 14–18. [Google Scholar] [CrossRef]
- Dove, M.T.; Fang, H. Negative thermal expansion and associated anomalous physical properties: Review of the lattice dynamics theoretical foundation. Rep. Prog. Phys. 2016, 79, 066503. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Gupta, M.K.; Chaplot, S.L. Phonons and anomalous thermal expansion behaviour in crystalline solids. Prog. Mater. Sci. 2018, 92, 360–445. [Google Scholar] [CrossRef]
- Barrera, G.D.; Bruno, J.A.O.; Barron, T.H.K.; Allan, N.L. Negative thermal expansion. J. Phys. Condens. Matter 2005, 17, R217–R252. [Google Scholar] [CrossRef]
- Dove, M.T.; Trachenko, K.O.; Tucker, M.G.; Keen, D.A. Rigid unit modes in framework structures: Theory, experiment and applications. Transf. Processes Miner. 2000, 39, 1–33. [Google Scholar]
- Pryde, A.K.A.; Hammonds, K.D.; Dove, M.T.; Heine, V.; Gale, J.D.; Warren, M.C. Origin of the Negative Thermal Expansion in ZrW2O8 and ZrV2O7. J. Phys. Condens. Matter 1996, 8, 10973–10982. [Google Scholar] [CrossRef]
- Tao, J.Z.; Sleight, A.W. The role of rigid unit modes in negative thermal expansion. J. Solid State Chem. 2003, 173, 442–448. [Google Scholar] [CrossRef]
- Ernst, G.; Broholm, C.; Kowach, G.R.; Ramirez, A.P. Phonon density of states and negative thermal expansion in ZrW2O8. Nature 1998, 396, 147–149. [Google Scholar] [CrossRef]
- Rimmer, L.H.N.; Dove, M.T. Simulation study of negative thermal expansion in yttrium tungstate Y2W3O12. J. Phys. Condens. Matter 2015, 27, 185401. [Google Scholar] [CrossRef]
- Romao, C.P.; Donegan, S.P.; Zwanziger, J.W.; White, M.A. Relationships between elastic anisotropy and thermal expansion in A2Mo3O12 materials. Chem. Phys. Phys. Chem. 2016, 18, 30652–30661. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Jardim, P.M.; de Avillez, R.R.; Rizzo, F. Negative thermal expansion in Y2Mo3O12. Solid State Sci. 2005, 7, 1377–1383. [Google Scholar] [CrossRef]
- Cao, W.G.; Zhu, H.; Liu, Z.N.; Deng, J.X.; Chen, J.; Xing, X.R. Phase transition and thermal expansion of Ho2W3O12. Inorg. Chem. Front. 2016, 73, 111–114. [Google Scholar] [CrossRef]
- Sumithra, S.; Umarji, A.M. Hygroscopicity and bulk thermal expansion Y2W3O12. Mater. Res. Bull. 2005, 40, 167–176. [Google Scholar] [CrossRef]
- Kol’tsova, T.N. X-ray Diffraction Study of Y2W3O12 • 3H2O. Inorg. Mater. 2001, 37, 1175–1177. [Google Scholar] [CrossRef]
- Liang, E.; Huo, H.; Wang, J.; Chao, M. Effect of water species on the phonon modes in orthorhombic Y2(MoO4)3 revealed by Raman spectroscopy. J. Phys. Chem. C 2008, 112, 6577–6581. [Google Scholar] [CrossRef]
- Li, Z.Y.; Song, W.B.; Liang, E.J. Structures, Phase Transition, and Crystal Water of Fe2−xYxMo3O12. J. Phys. Chem. C 2011, 115, 17806–17811. [Google Scholar] [CrossRef]
- Li, Q.J.; Yuan, B.H.; Song, W.B.; Liang, E.J.; Yuan, B. The phase transition, hygroscopicity, and thermal expansion properties of Yb2−xAlxMo3O12. Chin. Phys. B 2012, 21, 046501. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Yu, Z.Q.; Che, G.F.; Yao, J.L.; Sun, X.J.; Cheng, X.N.; Yang, J. Synthesis and tunable thermal expansion properties of Sc2−xYxW3O12 solid solutions. Ceram. Int. 2014, 40, 8195–8199. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Wu, M.M.; Peng, J.; Xiao, X.L.; Li, Z.X.; Hu, Z.B.; Kiyanagi, R.; Fieramosca, J.S.; Short, S.; Jorgensen, J. Structures, thermal expansion properties and phase transitions of ErxFe2−x(MoO4)3 (0.0 ≤ x ≤ 2.0). Solid State Sci. 2007, 9, 693–698. [Google Scholar] [CrossRef]
- Wu, M.M.; Peng, J.; Zu, Y.; Liu, R.D.; Hu, Z.B.; Liu, Y.T.; Chen, D.F. Thermal expansion properties of Lu2−xFexMo3O12. Chin. Phys. B 2012, 21, 116102. [Google Scholar] [CrossRef]
- Wu, M.M.; Xiao, X.L.; Hu, Z.B.; Liu, Y.T.; Chen, D.F. Controllable thermal expansion and phase transition in Yb2−xCrxMo3O12. Solid State Sci. 2009, 11, 325–329. [Google Scholar] [CrossRef]
- Wu, M.M.; Hu, Z.B.; Liu, Y.T.; Chen, D.F. Thermal expansion properties of Ln2−xCrxMo3O12 (Ln = Er and Y). Mater. Res. Bull. 2009, 44, 1943–1947. [Google Scholar] [CrossRef]
- Liu, H.F.; Wang, X.C.; Zhang, Z.P.; Chen, X.B. Synthesis and thermal expansion properties of Y2−xLaxMo3O12 (x = 0, 0.5, 2). Ceram. Int. 2012, 38, 6349–6352. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhang, W.; Zhang, Z.P.; Chen, X.B. Synthesis and negative thermal expansion properties of solid solutions Yb2−xLaxW3O12 (0 ≤ x ≤ 2). Ceram. Int. 2012, 38, 2951–2956. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhang, Z.P.; Zhang, W.; Zeng, X.H.; Chen, X.B. Synthesis and negative thermal expansion property of Y2−xLaxW3O12 (0 ≤ x ≤ 2). Ceram. Int. 2013, 39, 2781–2786. [Google Scholar] [CrossRef]
- Liu, H.F.; Sun, W.K.; Zhang, Z.P.; Zhou, M.; Meng, X.D.; Zeng, X.H. Tailorable thermal expansion and hygroscopic properties of cerium-substituted Y2W3O12 ceramics. J. Alloys Compd. 2018, 751, 49–55. [Google Scholar] [CrossRef]
- Cheng, Y.G.; Liu, X.S.; Song, W.B.; Yuan, B.H.; Wang, X.L.; Chao, M.J.; Liang, E.J. Relationship between hygroscopicity reduction and morphology evolution of Y2Mo3O12 doped with (LiMg)3+. Mater. Res. Bull. 2015, 65, 273–278. [Google Scholar] [CrossRef]
- Liu, X.S.; Yuan, B.H.; Cheng, Y.G.; Ge, X.H.; Liang, E.J.; Zhang, W.F. Avoiding the invasion of H2O into Y2Mo3O12 by coating with C3N4 to improve negative thermal expansion properties. Phys. Chem. Chem. Phys. 2017, 19, 13443–13448. [Google Scholar] [CrossRef]
- Ari, M.; Jardim, P.M.; Marinkovic, B.A.; Rizzo, F.; Ferreira, F.F. Thermal expansion of Cr2xFe2-2xMo3O12, Al2xFe2-2xMo3O12 and Al2xCr2-2xMo3O12 solid solutions. J. Solid State Chem. 2008, 181, 1472–1479. [Google Scholar] [CrossRef]
- Sleight, A.W.; Brixner, L.H. A New Ferroelastic Transition in Some A2(MO4)3 Molybdates and Tungstates. J. Solid State Chem. 1973, 7, 172–174. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Ari, M.; Jardim, P.M.; de Avillez, R.R.; Rizzo, F.; Ferreira, F.F. In2Mo3O12: A low negative thermal expansion compound. Thermochim. Acta 2010, 499, 48–53. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhang, Z.P.; Ma, J.; Jun, Z.; Zeng, X.H. Effect of isovalent substitution on phase transition and negative thermal expansion of In2−xScxW3O12 ceramics. Ceram. Int. 2015, 41, 9873–9877. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Achary, S.N.; Mathews, M.D. Phase transition and negative thermal expansion in A2(MoO4)3 system (A=Fe3+, Cr3+ and Al3+). J. Alloys Compd. 2002, 339, 207–210. [Google Scholar] [CrossRef]
- Truitt, R.; Hermes, I.; Leright, A.; Sendecki, A.; Lind, C. Non-hydrolytic sol-gel synthesis of homogeneous AlScMo3O12. Materials 2015, 8, 700–716. [Google Scholar] [CrossRef]
- Shen, R.; Wang, T.M. The synthesis and thermal expansion of Al2Mo3−xWxO12. Rare Metal Mater. Eng. 2004, 33, 91–95. [Google Scholar]
- Liu, X.Z.; Hao, L.J.; Wu, M.M.; Ma, X.B.; Chen, D.F.; Liu, Y.T. The structure, thermal expansion and phase transition properties of Ho2Mo3−xWxO12 (x=0, 1.0, 2.0) solid solutions. Mater. Res. Bull. 2015, 70, 640–644. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Sun, W.K.; Zheng, Q.; Liu, H.F.; Zhou, M.; Wang, W.; Chen, X.B. Tuning the phase transition temperature of Cr2(MoO4)3 by A-site substitution of scandium. Ceram. Int. 2018, 44, 22165–22171. [Google Scholar] [CrossRef]
- Wu, M.M.; Peng, J.; Han, S.B.; Hu, Z.B.; Liu, Y.T.; Chen, D.F. Phase transition and negative thermal expansion properties of Sc2−xCrxMo3O12. Ceram. Int. 2012, 38, 6525–6529. [Google Scholar] [CrossRef]
- Gates, S.D. Cation Influence of Negative Thermal Expansion in the A2M3O12 Family. Ph.D. Thesis, The University of Toledo, Toledo, OH, USA, 2008. [Google Scholar]
- Wu, M.; Peng, J.; Cheng, Y.; Xiao, X.; Chen, D.; Hu, Z. Structural and controllable thermal expansion properties of Sc2−xAlxMo3O12. J. Alloys Compd. 2013, 577, 295–298. [Google Scholar] [CrossRef]
- Sun, W.K.; Zhang, Z.P.; Liu, H.F.; Wang, W.; Zeng, X.H. Tailored phase transition temperature and negative thermal expansion of Sc-substituted Fe2Mo3O12 synthesized by the co-precipitation method. J. Alloys Compd. 2019, 794, 1–7. [Google Scholar] [CrossRef]
- Wu, M.M.; Liu, X.Z.; Chen, D.F.; Huang, Q.Z.; Wu, H.; Liu, Y.T. Structure, Phase Transition, and Controllable Thermal Expansion Behaviors of Sc2−xFexMo3O12. Inorg. Chem. 2014, 53, 9206–9212. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Wang, Y.N.; Sun, W.K.; Zhang, X.Y.; Liu, H.F.; Chen, X.B.; Zeng, X.H. Phase transition temperature and negative thermal expansion of Sc-substituted In2(MoO4)3 ceramics. J. Mater. Sci. 2020, 55, 5730–5740. [Google Scholar] [CrossRef]
- Sugimoto, T.; Aoki, Y.; Niwa, E.; Hashimoto, T.; Morito, Y. Thermal expansion and phase transition behavior of Al2−xMx(WO4)3 (M = Y, Ga and Sc) ceramics. J. Ceram. Soc. Jpn. 2007, 115, 176–181. [Google Scholar] [CrossRef]
- Koseva, I.; Yordanova, A.; Tzvetkov, P.; Nikolov, V.; Nihtianova, D. Nanosized pure and Cr doped Al2−xInx(WO4)3 solid solutions. Mater. Chem. Phys. 2012, 132, 808–814. [Google Scholar] [CrossRef]
- Zhang, F.; Lei, Y.; Zhang, Z.P.; Liu, H.F.; Zhang, X.Y.; Chen, X.B.; Zeng, X.H. Negative thermal expansion coefficient of Sc-doped indium tungstate ceramics synthesized by co-precipitation. Ceram. Int. 2020, 46, 7259–7267. [Google Scholar] [CrossRef]
- Dasgupta, N.; Sorge, E.; Butler, B.; Wen, T.C.; Shetty, D.K.; Cambrea, L.R.; Harris, D.C. Synthesis and characterization of Al2−xScx(WO4)3 ceramics for low-expansion infrared-transmitting windows. J. Mater. Sci. 2012, 47, 6286–6296. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Ari, M.; de Avillez, R.R.; Rizzo, F.; Ferreira, F.F.; Miller, K.J.; Johnson, M.B.; White, M.A. Correlation between AO6 Polyhedral Distortion and Negative Thermal Expansion on Orthorhombic Y2Mo3O12 and Related Materials. Chem. Mater. 2009, 21, 2886–2894. [Google Scholar] [CrossRef]
- Li, S.L.; Ge, X.H.; Yuan, H.L.; Chen, D.X.; Guo, J.; Shen, R.F.; Chao, M.J.; Liang, E.J. Near-Zero Thermal Expansion and Phase Transitions in HfMg1−xZnxMo3O12. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ge, X.H.; Liu, X.S.; Cheng, Y.G.; Liu, Y.M.; Yuan, H.L.; Li, S.L.; Liu, Y.Y.; Guo, J.; Sun, Q.; et al. Enhanced negative thermal expansion by solid solution of HfMgMo1.5W1.5O12. Mater. Express 2016, 6, 515–520. [Google Scholar] [CrossRef]
- Shen, R.F.; Yuan, B.H.; Li, S.L.; Ge, X.H.; Guo, J.; Liang, E.J. Near-zero thermal expansion of ZrxHf1−xMgMo3O12 in a larger temperature range. Optik 2018, 165, 1–6. [Google Scholar] [CrossRef]
- Song, W.-B.; Wang, J.-Q.; Li, Z.-Y.; Liu, X.-S.; Yuan, B.-H.; Liang, E.-J. Phase transition and thermal expansion property of Cr2−xZr0.5xMg0.5xMo3O12 solid solution. Chin. Phys. B 2014, 23, 066501. [Google Scholar] [CrossRef]
- Suzuki, T.; Omote, A. Negative Thermal Expansion in HfMg(WO4)3. J. Am. Ceram. Soc. 2004, 87, 1365–1367. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yuan, B.H.; Cheng, Y.G.; Liang, E.J.; Ge, X.H.; Yuan, H.L.; Zhang, Y.; Guo, J.; Chao, M.J. Phase transition and negative thermal expansion of HfMnMo3O12. Mater. Res. Bull. 2018, 99, 255–259. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Jardim, P.M.; Ari, M.; de Avillez, R.R.; Rizzo, F.; Ferreira, F.F. Low positive thermal expansion in HfMgMo3O12. Phys. Status Solidi B 2008, 245, 2514–2519. [Google Scholar] [CrossRef]
- Miller, K.J.; Johnson, M.B.; White, M.A.; Marinkovic, B.A. Low-temperature investigations of the open-framework material HfMgMo3O12. Solid State Commun. 2012, 152, 1748–1752. [Google Scholar] [CrossRef]
- Ge, X.H.; Mao, Y.C.; Li, L.; Li, L.P.; Yuan, N.; Cheng, Y.G.; Guo, J.; Chao, M.J.; Liang, E.J. Phase Transition and Negative Thermal Expansion Property of ZrMnMo3O12. Chin. Phys. Lett. 2016, 33, 046503. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.S.; Cheng, Y.G.; Ge, X.H.; Zhang, M.D.; Lian, H.; Zhang, Y.; Liang, E.J.; Li, Y.X. Zero and controllable thermal expansion in HfMgMo3−xWxO12. Chin. Phys. B 2017, 26, 016501. [Google Scholar] [CrossRef]
- Chen, D.X.; Yuan, B.H.; Cheng, Y.G.; Ge, X.H.; Jia, Y.; Liang, E.J.; Chao, M.J. Phase transition and near-zero thermal expansion in ZrFeMo2VO12. Phys. Lett. A 2016, 380, 4070–4074. [Google Scholar] [CrossRef]
- Cheng, Y.G.; Liang, Y.; Mao, Y.C.; Ge, X.H.; Yuan, B.H.; Guo, J.; Chao, M.J.; Liang, E. A novel material of HfScW2PO12 with negative thermal expansion from 140 K to 1469 K and intense blue photoluminescence. Mater. Res. Bull. 2017, 85, 176–180. [Google Scholar] [CrossRef]
- Ge, X.H.; Liu, X.S.; Cheng, Y.G.; Yuan, B.H.; Chen, D.X.; Chao, M.J.; Guo, J.; Wang, J.Q.; Liang, E.J. Negative thermal expansion and photoluminescence properties in a novel material ZrScW2PO12. J. Appl. Phys. 2016, 120. [Google Scholar] [CrossRef]
- Ge, X.H.; Mao, Y.C.; Liu, X.S.; Cheng, Y.G.; Yuan, B.H.; Chao, M.J.; Liang, E.J. Negative thermal expansion and broad band photoluminescence in a novel material of ZrScMo2VO12. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Structure of Zr2(WO4)(PO4)2 from powder X-ray data-cation ordering with no superstructure. J. Solid State Chem. 1995, 120, 101–104. [Google Scholar] [CrossRef]
- Cetinkol, M.; Wilkinson, A.P. Pressure dependence of negative thermal expansion in Zr2(WO4)(PO4)2. Solid State Commun. 2009, 149, 421–424. [Google Scholar] [CrossRef]
- Cetinkol, M.; Wilkinson, A.P.; Lee, P.L. Structural changes accompanying negative thermal expansion in Zr2(MoO4)(PO4)2. J. Solid State Chem. 2009, 182, 1304–1311. [Google Scholar] [CrossRef]
- Baiz, T.I.; Gindhart, A.M.; Kraemer, S.K.; Lind, C. Synthesis of MgHf(WO4)3 and MgZr(WO4)3 using a non-hydrolytic sol-gel method. J. Sol-Gel Sci. Technol. 2008, 47, 128–130. [Google Scholar] [CrossRef]
- Romao, C.P.; Perras, F.A.; Werner-Zwanziger, U.; Lussier, J.A.; Miller, K.J.; Calahoo, C.M.; Zwanziger, J.W.; Bieringer, M.; Marinkovic, B.A.; Bryce, D.L.; et al. Zero Thermal Expansion in ZrMgMo3O12: NMR Crystallography Reveals Origins of Thermoelastic Properties. Chem. Mater. 2015, 27, 2633–2646. [Google Scholar] [CrossRef]
- Gindhart, A.M.; Lind, C.; Green, M. Polymorphism in the negative thermal expansion material magnesium hafnium tungstate. J. Mater. Res. 2008, 23, 210–213. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.S.; Song, W.B.; Yuan, B.H.; Cheng, Y.G.; Yuan, H.L.; Cheng, F.X.; Chao, M.J.; Liang, E.J. Phase transition, crystal water and low thermal expansion behavior of Al2-2x(ZrMg)xW3O12•n(H2O). J. Solid State Chem. 2014, 218, 15–22. [Google Scholar] [CrossRef]
- Zeng, G.J.; Yuan, H.L.; Guo, J.; Sun, Q.; Gao, Q.L.; Chao, M.J.; Ren, X.; Liang, E.J. Hydrate formation and its effects on the thermal expansion properties of HfMgW3O12. Phys. Chem. Chem. Phys. 2020, 22, 12605–12612. [Google Scholar] [CrossRef] [PubMed]
- Omote, A.; Yotsuhashi, S.; Zenitani, Y.; Yamada, Y. High Ion Conductivity in MgHf(WO4)3 Solids with Ordered Structure: 1-D Alignments of Mg2+ and Hf4+ Ions. J. Am. Ceram. Soc. 2011, 94, 2285–2288. [Google Scholar] [CrossRef]
- Madrid, A.; Ponton, P.I.; Garcia, F.; Johnson, M.B.; White, M.A.; Marinkovic, B.A. Solubility limit of Zn2+ in low thermal expansion ZrMgMo3O12 and its influence on phase transition temperature. Ceram. Int. 2020, 46, 3979–3983. [Google Scholar] [CrossRef]
- Prisco, L.P.; Ponton, P.I.; Paraguassu, W.; Romao, C.P.; White, M.A.; Marinkovic, B.A. Near-zero thermal expansion and phase transition in In0.5(ZrMg)0.75Mo3O12. J. Mater. Res. 2016, 31, 3240–3248. [Google Scholar] [CrossRef]
- Suzuki, T.; Omote, A. Zero thermal expansion in (Al2x(HfMg)1−x)(WO4)3. J. Am. Ceram. Soc. 2006, 89, 691–693. [Google Scholar] [CrossRef]
- Song, W.; Yuan, B.; Liu, X.; Li, Z.; Wang, J.; Liang, E. Tuning the monoclinic-to-orthorhombic phase transition temperature of Fe2Mo3O12 by substitutional co-incorporation of Zr4+ and Mg2+. J. Mater. Res. 2014, 29, 849–855. [Google Scholar] [CrossRef]
- Miller, K.J.; Romao, C.P.; Bieringer, M.; Marinkovic, B.A.; Prisco, L.; White, M.A. Near-Zero Thermal Expansion in In(HfMg)0.5Mo3O12. J. Am. Ceram. Soc. 2013, 96, 561–566. [Google Scholar] [CrossRef]
- Cheng, Y.G.; Mao, Y.C.; Liu, X.S.; Yuan, B.H.; Chao, M.J.; Liang, E.J. Near-zero thermal expansion of In2(1−x)(HfMg)xMo3O12 with tailored phase transition. Chin. Phys. B 2016, 25, 086501. [Google Scholar] [CrossRef]
- Cheng, Y.G.; Liang, Y.; Ge, X.H.; Liu, X.S.; Yuan, B.H.; Guo, J.; Chao, M.J.; Liang, E. A novel material of HfScMo2VO12 with negative thermal expansion and intense white-light emission. RSC Adv. 2016, 6, 53657–53661. [Google Scholar] [CrossRef]
- Liang, Y.; Cheng, Y.G.; Ge, X.H.; Yuan, B.H.; Guo, J.; Sun, Q.; Liang, E.J. Negative thermal expansion and photoluminescence in solid solution (HfSc)0.83W2.25P0.83O12-δ. Chin. Phys. B 2017, 26, 106501. [Google Scholar] [CrossRef]
- Yuan, H.L.; Wang, C.Y.; Gao, Q.L.; Ge, X.H.; Sun, H.; Lapidus, S.H.; Guo, J.; Chao, M.J.; Jia, Y.; Liang, E.J. Structure and Negative Thermal Expansion in Zr0.3Sc1.7Mo2.7V0.3O12. Inorg. Chem. 2020, 59, 4090–4095. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, W.J.; Chao, M.J.; Mao, Y.C.; Guo, J.; Li, Y.X.; Feng, D.S.; Liang, E.J. Negative thermal expansion, optical and electrical properties of HfMnMo2PO12−δ. Ceram. Int. 2015, 41, 15170–15175. [Google Scholar] [CrossRef]
- Chen, D.X.; Yuan, B.H.; Yuan, H.L.; Ge, X.H.; Guo, J.; Liang, E.J.; Chao, M.J. Phase transition and thermal expansion properties of Cr1.5−xScxZr0.5Mo2.5V0.5O12. Ceram. Int. 2018, 44, 9609–9615. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, L.L.; Qi, H.; Xu, Q.; Yuan, B.H.; Liu, X.S.; Xu, L. Substitutions of Zr4+/V5+ for Y3+/Mo6+ in Y2Mo3O12 for Less Hygroscopicity and Low Thermal Expansion Properties. Materials 2019, 12, 3945. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Wilkinson, A.P.; Lind, C.; Bassett, W.A.; Zha, C.-S. High pressure synchrotron X-ray powder diffraction study of Sc2Mo3O12 and Al2W3O12. J. Phys. Condens. Matter 2005, 17, 4271–4283. [Google Scholar] [CrossRef]
- Varga, T.; Wilkinson, A.P.; Jorgensen, J.D.; Short, S. Neutron powder diffraction study of the orthorhombic to monoclinic transition in Sc2W3O12 on compression. Solid State Sci. 2006, 8, 289–295. [Google Scholar] [CrossRef]
- Torres Dias, A.C.; Luz Lima, C.; Paraguassu, W.; Pereira da Silva, K.; Freire, P.T.C.; Mendes Filho, J.; Marinkovic, B.A.; Miller, K.J.; White, M.A.; Souza Filho, A.G. Pressure-Induced Crystal-Amorphous Transformation In Y2Mo3O12. Vib. Spectrosc. 2013, 68, 251–256. [Google Scholar] [CrossRef]
- Baiz, T.I.; Heinrich, C.P.; Banek, N.A.; Vivekens, B.L.; Lind, C. In-situ non-ambient X-ray diffraction studies of indium tungstate. J. Solid State Chem. 2012, 187, 195–199. [Google Scholar] [CrossRef]
- Liu, H.; Secco, R.A.; Imanaka, N.; Adachi, G. X-ray diffraction study of pressure-induced amorphization in Lu2(WO4)3. Solid State Commun. 2002, 121, 177–180. [Google Scholar] [CrossRef]
- Garg, N.; Murli, C.; Tyagi, A.K.; Sharma, S.M. Phase transitions in Sc2(WO4)3 under high pressure. Phys. Rev. B 2005, 72, 064106. [Google Scholar] [CrossRef]
- Cetinkol, M.; Wilkinson, A.P.; Lind, C. In situ high-pressure synchrotron X-ray diffraction study of Zr2(WO4)(PO4)2 up to 16 GPa. Phys. Rev. B 2009, 79, 224118. [Google Scholar] [CrossRef]
- Young, L.; Gadient, J.; Lind, C. High Pressure Behavior of Chromium and Yttrium Molybdate (Cr2Mo3O12, Y2Mo3O12). Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Secco, R.A.; Liu, H.; Imanaka, N.; Adachi, G.; Rutter, M.D. Electrical conductivity and amorphization of Sc2(WO4)3 at high pressures and temperatures. J. Phys. Chem. Solids 2002, 63, 425–431. [Google Scholar] [CrossRef]
- Secco, R.A.; Liu, H.; Imanaka, N.; Adachi, G. Anomalous ionic conductivity of Sc2(WO4)3 mediated by structural changes at high pressures and temperatures. J. Phys. Condens. Matter 2002, 14, 11285–11289. [Google Scholar] [CrossRef]
- Mukherjee, G.D.; Vijaykumar, V.; Achary, S.N.; Tyagi, A.K.; Godwal, B.K. Phase transitions in Al2(WO4)3: High pressure investigations of low frequency dielectric constant and crystal structure. J. Phys. Condens. Matter 2004, 16, 7321–7330. [Google Scholar] [CrossRef]
- Karmakar, S.; Deb, S.K.; Tyagi, A.K.; Sharma, S.M. Pressure-induced amorphization in Y2(WO4)3: In situ X-ray diffraction and Raman studies. J. Solid State Chem. 2004, 177, 4087–4092. [Google Scholar] [CrossRef]
- Young, L.; Gadient, J.; Xiaodong, G.; Lind, C. High pressure studies of A2Mo3O12 negative thermal expansion materials (A2 = Al2, Fe2, FeAl, AlGa). J. Solid State Chem. 2016, 237, 121–128. [Google Scholar] [CrossRef]
- Tran, K.D.; Groshens, T.J.; Nelson, J.G. Fabrication of near-zero thermal expansion (FexSc1−x)2Mo3O12-MoO3 ceramic composite using the reaction sintering process. Mater. Sci. Eng. A 2001, 303, 234–240. [Google Scholar] [CrossRef]
- Yanase, I.; Miyagi, M.; Kobayashi, H. Fabrication of zero-thermal-expansion ZrSiO4/Y2W3O12 sintered body. J. Eur. Ceram. Soc. 2009, 29, 3129–3134. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, W.J.; Liu, X.S.; Chao, M.J.; Li, Y.C.; Zhang, N.; Liu, Y.M.; Li, Y.X.; Feng, D.S.; Liang, E.J. Electrical properties of Al-ZrMgMo3O12 with controllable thermal expansion. Ceram. Int. 2015, 41, 2361–2366. [Google Scholar] [CrossRef]
- Liu, X.S.; Cheng, F.X.; Wang, J.Q.; Song, W.B.; Yuan, B.H.; Liang, E.J. The control of thermal expansion and impedance of Al-Zr2(WO4)(PO4)2 nano-cermets for near-zero-strain Al alloy and fine electrical components. J. Alloys Compd. 2013, 553, 1–7. [Google Scholar] [CrossRef]
- Yanase, I.; Sakai, H.; Kobayashi, H. Fabrication of Zr2WP2O12/ZrV0.6P1.4O7 composite with a nearly zero-thermal-expansion property. Mater. Lett. 2017, 207, 221–224. [Google Scholar] [CrossRef][Green Version]
- Liu, H.F.; Sun, W.K.; Xie, X.; Yang, L.; Zhang, Z.P.; Zhou, M.; Zeng, X.H.; Chen, X.B. Adjustable Thermal Expansion Properties in Zr2MoP2O12/ZrO2 Ceramic Composites. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Sun, W.K.; Liu, H.F.; Xie, G.; Chen, X.B.; Zeng, X.H. Synthesis of Zr2WP2O12/ZrO2 Composites with Adjustable Thermal Expansion. Front. Chem. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Fan, C.Y.; Wu, G.D.; Zhao, Y.H.; Sun, X.J.; Cheng, X.N.; Shen, J.T.; Hu, Y.M. In-situ synthesis of Sc2W3O12/YSZ ceramic composites with controllable thermal expansion. Ceram. Int. 2015, 41, 8267–8271. [Google Scholar] [CrossRef]
- Wu, M.M.; Zu, Y.; Peng, J.; Liu, R.D.; Hu, Z.B.; Liu, Y.T.; Chen, D.F. Controllable thermal expansion properties of In2−xCrxMo3O12. Cryst. Res. Technol. 2012, 47, 793–798. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Sun, X.Y.; Xiao, X.L.; Liu, X.F.; Xue, L.; Hu, Z.B. Effects of doping Fe cations on crystal structure and thermal expansion property of Yb2Mo3O12. Chin. Chem. Lett. 2017, 28, 1600–1606. [Google Scholar] [CrossRef]
- Cheng, Y.G.; Mao, Y.C.; Yuan, B.H.; Ge, X.H.; Guo, J.A.; Chao, M.J.; Liang, E.J. Enhanced negative thermal expansion and optical absorption of In0.6(HfMg)0.7Mo3O12 with oxygen vacancies. Phys. Lett. A 2017, 381, 2195–2199. [Google Scholar] [CrossRef]
- Liu, X.S.; Ge, X.H.; Liang, E.J.; Zhang, W.F. Effects of Al particles and thin layer on thermal expansion and conductivity of Al-Y2Mo3O12 cermets. Chin. Phys. B 2017, 26, 118101. [Google Scholar] [CrossRef]
- Yang, J.R.; Wang, L.; Tan, X.R.; Zhi, Q.; Yang, R.B.; Zhang, G.P.; Liu, Z.X.; Ge, X.H.; Liang, E.J. Effect of sintering temperature on the thermal expansion behavior of ZrMgMo3O12p/2024Al composite. Ceram. Int. 2018, 44, 10744–10752. [Google Scholar] [CrossRef]
- Kohler, J.; Imanaka, N.; Adachi, G.Y. New cation conducting solid electrolytes with the Sc2(WO4)3 type structure. J. Mater. Chem. 1999, 9, 1357–1362. [Google Scholar] [CrossRef]
- Imanaka, N. Novel multivalent cation conducting ceramics and their application. J. Ceram. Soc. Jpn. 2005, 113, 387–393. [Google Scholar] [CrossRef]
- Zhou, Y.K.; Adams, S.; Rao, R.P.; Edwards, D.D.; Neiman, A.; Pestereva, N. Charge Transport by Polyatomic Anion Diffusion in SC2(WO4)3. Chem. Mater. 2008, 20, 6335–6345. [Google Scholar] [CrossRef]
- Arfaoui, A.; Mhamdi, A.; Jlidi, D.; Belgacem, S. Physical and ethanol sensing properties of sprayed Fe2(MoO4)3 thin films. J. Alloys Compd. 2017, 719, 392–400. [Google Scholar] [CrossRef]
- Grissa, R.; Martinez, H.; Pele, V.; Cotte, S.; Pecquenard, B.; Le Cras, F. An X-ray photoelectron spectroscopy study of the electrochemical behaviour of iron molybdate thin films in lithium and sodium cells. J. Power Sources 2017, 342, 796–807. [Google Scholar] [CrossRef]
- Heo, J.W.; Hyoung, J.; Hong, S.T. Unveiling the Intercalation Mechanism in Fe2(MoO4)3 as an Electrode Material for Na-Ion Batteries by Structural Determination. Inorg. Chem. 2018, 57, 11901–11908. [Google Scholar] [CrossRef] [PubMed]
- Huu, H.T.; Im, W.B. Facile Green Synthesis of Pseudocapacitance-Contributed Ultrahigh Capacity Fe2(MoO4)3 as an Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 35152–35163. [Google Scholar] [CrossRef]
- Lin, Z.D.; Xu, M.Y.; Fu, P.; Deng, Q.R. Crystal plane control of 3D iron molybdate and the facet effect on gas sensing performances. Sens. Actuators B Chem. 2018, 254, 755–762. [Google Scholar] [CrossRef]
- Nguyen, V.; Liu, Y.L.; Yang, X.; Chenz, W. Fe2(MoO4)3/Nanosilver Composite as a Cathode for Sodium-Ion Batteries. ECS Electrochem. Lett. 2015, 4, A29–A32. [Google Scholar] [CrossRef]
- Niu, Y.B.; Xu, M.W. Reduced graphene oxide and Fe2(MoO4)3 composite for sodium-ion batteries cathode with improved performance. J. Alloys Compd. 2016, 674, 392–398. [Google Scholar] [CrossRef]
- Zhang, F.H.; Wang, Y.C.; Wang, L.; Liu, J.; Ge, H.L.; Wang, B.; Huang, X.Y.; Wang, X.D.; Chi, Z.T.; Xie, W.F. High performance In2(MoO4)3@In2O3 nanocomposites gas sensor with long-term stability. J. Alloys Compd. 2019, 805, 180–188. [Google Scholar] [CrossRef]
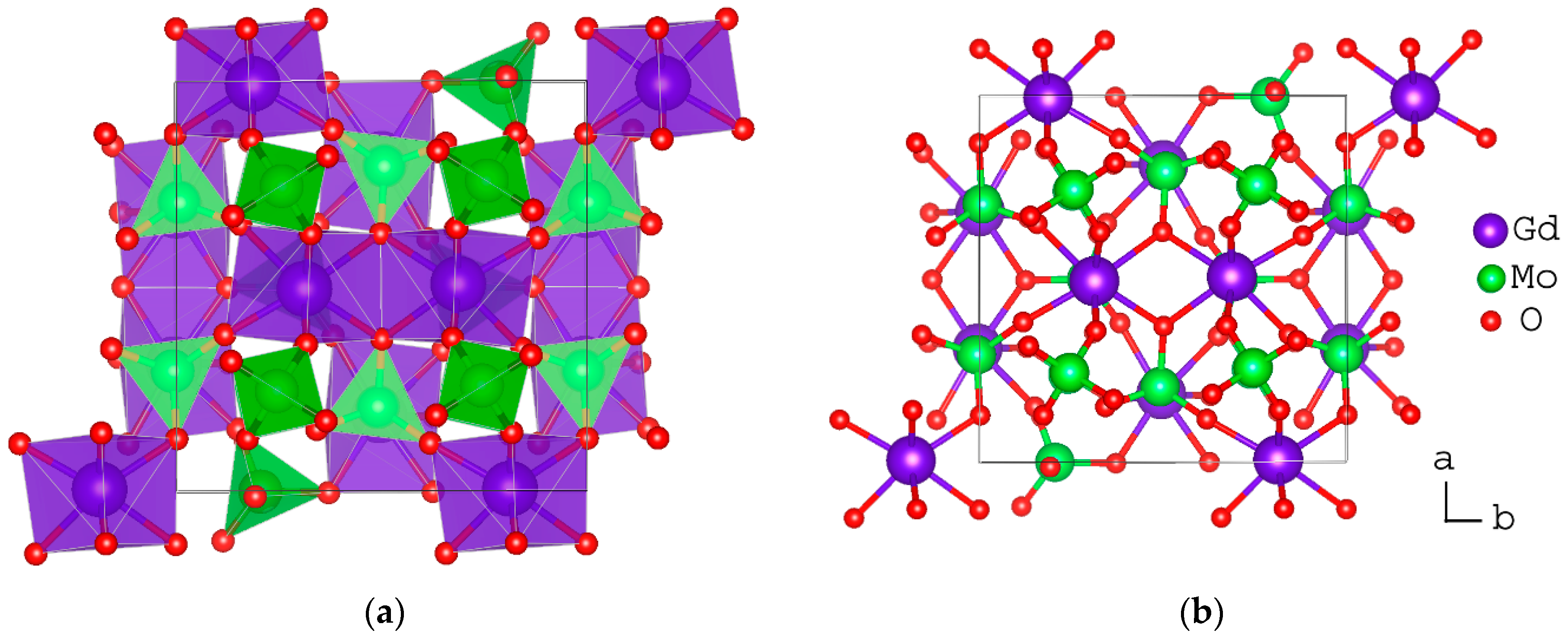
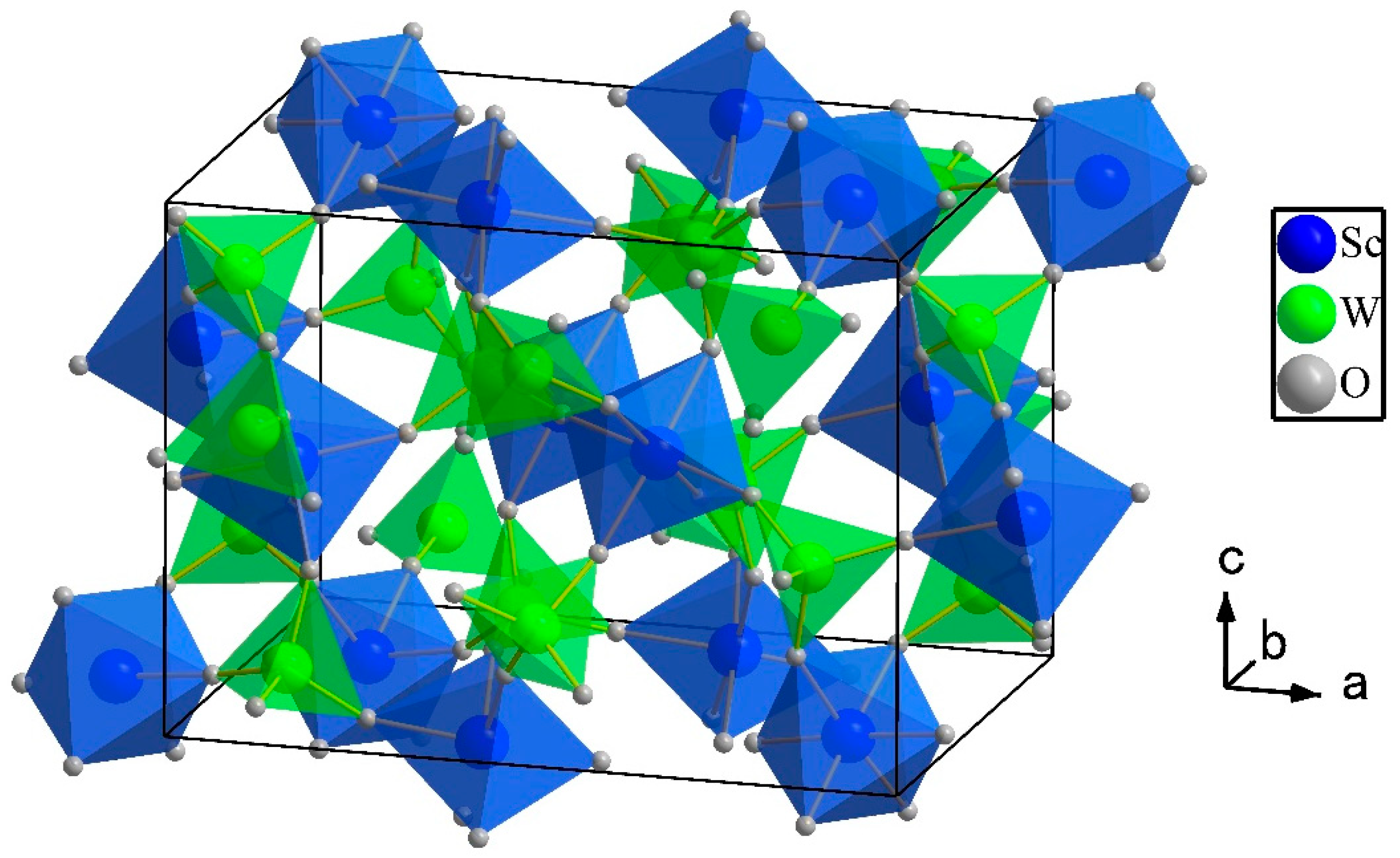

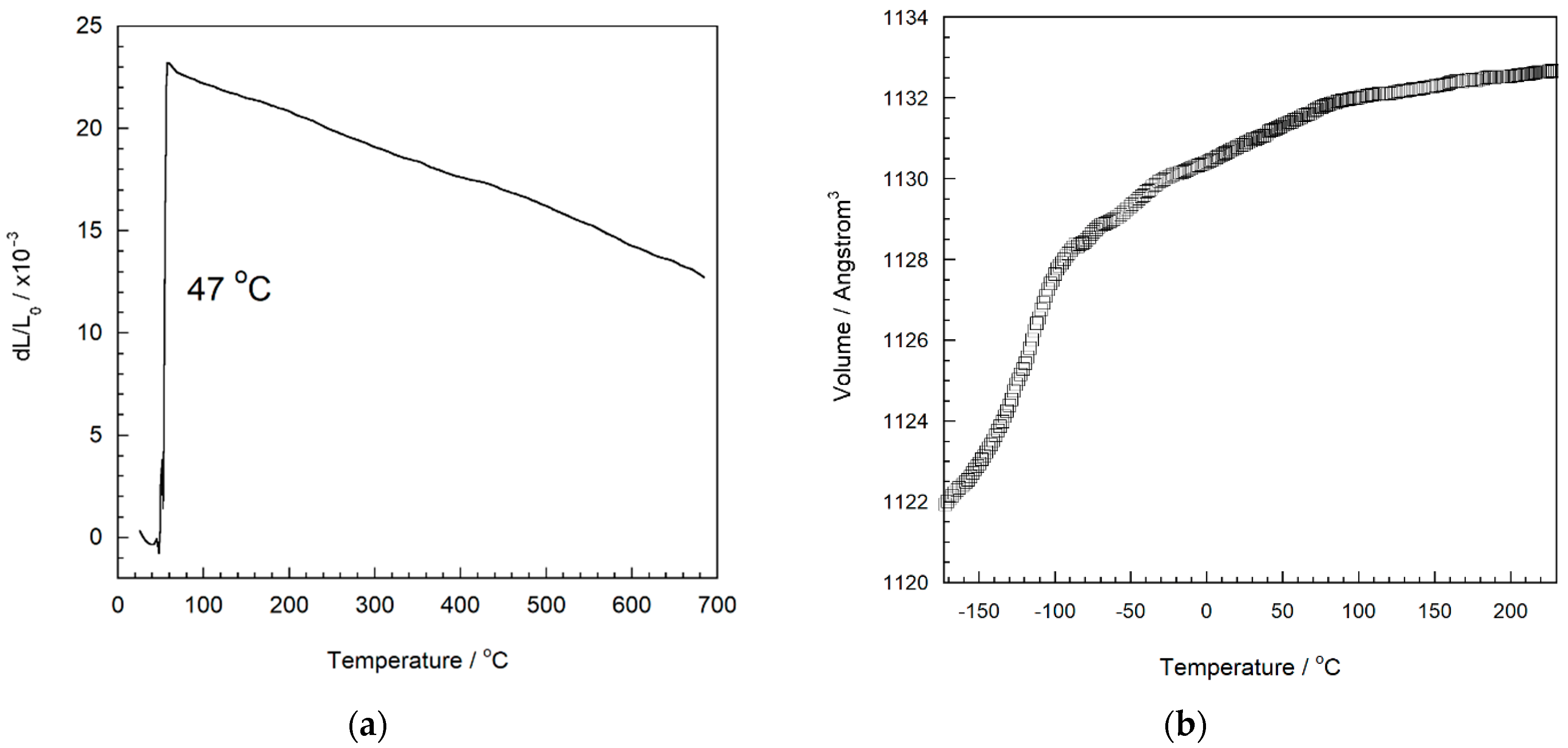
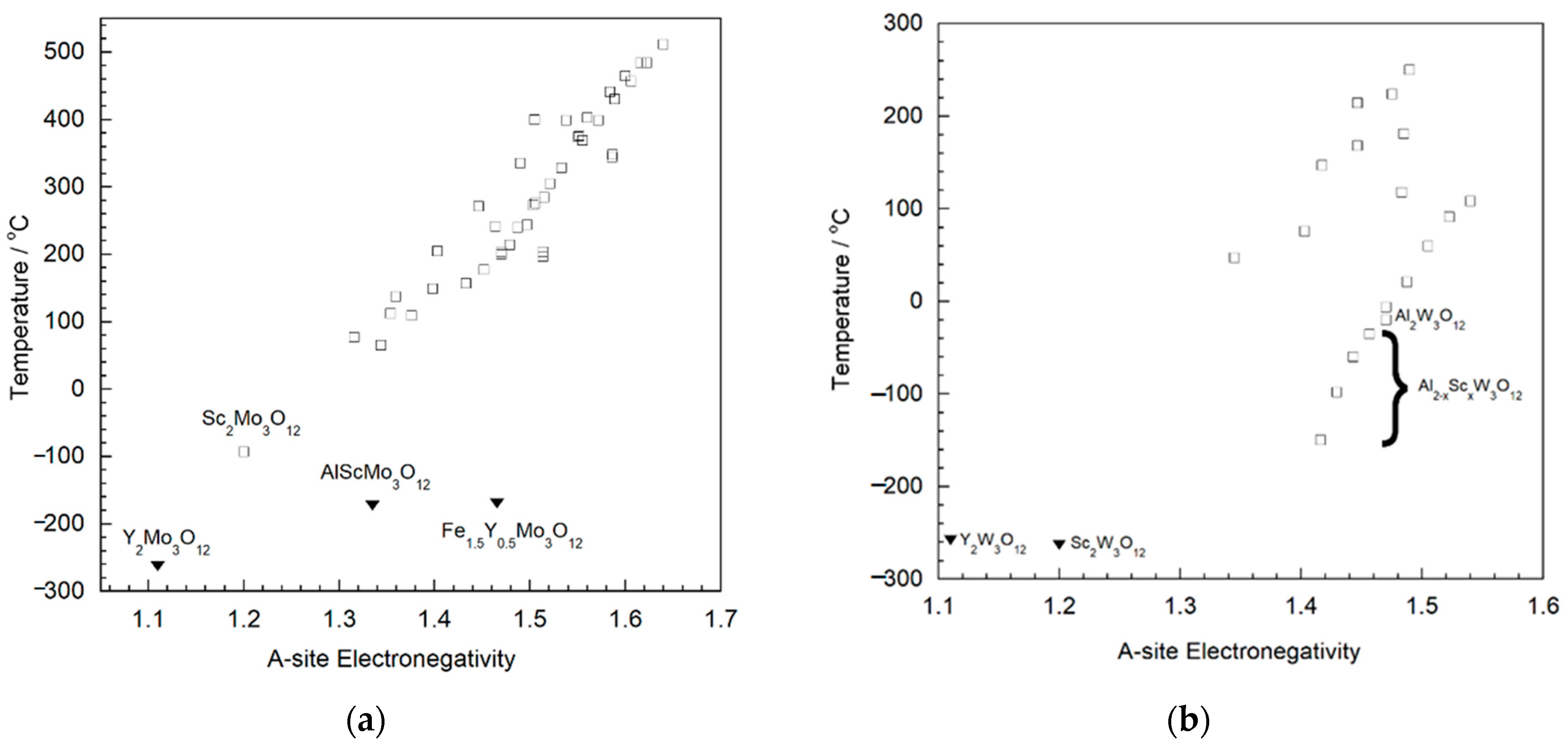
| Compound | αl (×10−6 °C−1) | T Range (°C) | Ref. |
|---|---|---|---|
| Y2Mo3O12 | −9.36 1 | 25–800 | [68] |
| Y2W3O12 | −7.34 1 | 200–800 | [72] |
| Dy2W3O12 | −8.60 | 150–500 | [52] |
| Ho2Mo3O12 | −11.56 | 200–700 | [71] |
| Ho2W3O12 | −6.97 | 200–600 | [83] |
| Er2Mo3O12 | −7.56 1 | 25–800 | [68] |
| Er2W3O12 | −6.74 1 | 200–800 | [72] |
| Tm2Mo3O12 | −4.04 | 200–800 | [71] |
| Tm2W3O12 | −3.99 | 200–800 | [71] |
| Lu2Mo3O12 | −6.02 1 | 25–800 | [68] |
| Lu2W3O12 | −6.18 1 | 200–800 | [72] |
| Yb2Mo3O12 | −6.04 1 | 25–800 | [68] |
| Yb2W3O12 | −6.38 1 | 200–800 | [72] |
| Compound | TPT (°C) | αl (×10−6 °C−1) | T Range | Ref. |
|---|---|---|---|---|
| Al2Mo3O12 | 200 | 2.32 | 250–650 | [100] |
| Al2W3O12 | −6 | 1.51 | 20–800 | [69,101] |
| Sc2Mo3O12 | −93 | −2.11 | −73–27 | [59] |
| Sc2W3O12 | NR | −2.20 | −263–177 | [55] |
| Cr2Mo3O12 | 403 | 0.67 | 420–740 | [100] |
| Fe2Mo3O12 | 512 | 1.72 | 550–740 | [100] |
| Fe2W3O12 | 414–445 | 1.35 1 | 445–600 | [66] |
| Ga2W3O12 | NR | −5 1 | NR | [58] |
| ln2Mo3O12 | 335 | −1.85 | 370–760 | [102] |
| In2W3O12 | 250 | −3.00 1 | 277–700 | [103] |
| Compound | TPT (°C) | αl (×10−6 °C−1) | Ref. | Compound | TPT (°C) | αl (×10−6 °C−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Al2Mo3O12 | 200 | 2.32 | [100] | Cr1.8Er0.2Mo3O12 | 197 | 0.47 | [93] |
| Al1.8Cr0.2Mo3O12 | 214 | NR | [100] | Cr0.2Er1.8Mo3O12 | <RT | −4.20 | [93] |
| Al1.4Cr0.6Mo3O12 | 244 | 2.37 | [100] | Cr1.4Fe0.6Mo3O12 | 441 | 0.97 | [100] |
| AlCrMo3O12 | 284 | 1.85 | [100] | CrFeMo3O12 | 465 | 1.21 | [100] |
| Al0.6Cr1.4Mo3O12 | 328 | 1.38 | [100] | Cr0.6Fe1.4Mo3O12 | 484 | 1.40 | [100] |
| Al0.2Cr1.8Mo3O12 | 374 | NR | [100] | Cr1.7Sc0.3Mo3O12 | 276 | −4.34 1 | [108] |
| Al1.8Fe0.2Mo3O12 | 240 | NR | [100] | Cr1.5Sc0.5Mo3O12 | 204 | 0.99 | [109] |
| Al1.6Fe0.4Mo3O12 | 273 | NR | [100] | Cr1.4Sc0.6Mo3O12 | 177 | −2.81 1 | [108] |
| Al1.4Fe0.6Mo3O12 | 305 | 3.40 | [100] | Cr1.1Sc0.9Mo3O12 | 149 | −5.87 1 | [108] |
| Al1.2Fe0.8Mo3O12 | 399 | NR | [100] | Cr0.8Sc1.2Mo3O12 | 65 | −4.57 1 | [108] |
| AlFeMo3O12 | 369 | 3.29 | [100] | Cr0.7Sc1.3Mo3O12 | <RT | −0.47 | [109] |
| Al0.8Fe1.2Mo3O12 | 399 | NR | [100] | Cr0.6Sc1.4Mo3O12 | <RT | −11.17 1 | [108] |
| Al0.6Fe1.4Mo3O12 | 430 | NR | [100] | Cr0.5Sc1.5Mo3O12 | <RT | −0.51 | [109] |
| Al0.4Fe1.6Mo3O12 | 457 | NR | [100] | Cr1.8Yb0.2Mo3O12 | 204 | 1.13 | [92] |
| Al0.2Fe1.8Mo3O12 | 484 | NR | [100] | Cr0.4Yb1.6Mo3O12 | <RT | −1.69 | [92] |
| Al1.5Ga0.5Mo3O12 | 400 | 4.5 | [110] | Cr0.2Yb1.8Mo3O12 | <RT | −4.73 | [92] |
| AlGaMo3O12 | 300 | 1.9 | [110] | Fe2Mo3O12 | 512 | 1.72 | [100] |
| Al0.5Ga1.5Mo3O12 | 250 | 0.1 | [110] | Er0.2Fe1.8Mo3O12 | 344 | NR | [90] |
| Al1.3Sc0.7Mo3O12 | <RT | 3.06 | [111] | Fe1.5Lu0.5Mo3O12 | 400 | 2.31 | [91] |
| AlScMo3O12 | <−173 | 3.60 | [105] | FeLuMo3O12 | <RT | 0.99 | [91] |
| Al0.3Sc1.7Mo3O12 | <RT | −0.73 | [111] | Fe0.3Lu1.7Mo3O12 | <RT | −3.13 | [91] |
| Al1.8Yb0.2Mo3O12 | 157 | 9.5 1 | [88] | Fe1.6Sc0.4Mo3O12 | 376 | −6.25 1 | [112] |
| Al1.6Yb0.4Mo3O12 | <RT | 5.74 1 | [88] | Fe1.2Sc0.8Mo3O12 | 241 | 1.17 | [113] |
| Al0.4Yb1.6Mo3O12 | <RT | −5.5 1 | [88] | Fe0.8Sc1.2Mo3O12 | 109 | −4.18 1 | [112] |
| Al0.2Yb1.8Mo3O12 | <RT | −9.1 1 | [88] | Fe0.7Sc1.3Mo3O12 | 112 | 0.09 | [113] |
| Al2Mo2.5W0.5O12 | 127 | 4.85 1 | [106] | Fe0.4Sc1.6Mo3O12 | <RT | −0.83 | [113] |
| Al2Mo2.5W0.5O12 | 118 | 5.20 1 | [106] | Fe1.8Y0.2Mo3O12 | 348 | NR | [87] |
| Al2Mo2.5W0.5O12 | 101 | 4.00 1 | [106] | ln2Mo3O12 | 335 | −1.85 | [102] |
| Al2Mo2.5W0.5O12 | 71 | 0.80 1 | [106] | In1.7Sc0.3Mo3O12 | 271 | −8.41 1 | [114] |
| Al2Mo2.5W0.5O12 | NR | 0.05 1 | [106] | In1.4Sc0.6Mo3O12 | 205 | −6.32 1 | [114] |
| Al2W3O12 | −6 | 1.51 | [69] | In1.1Sc0.9Mo3O12 | 137 | −5.83 1 | [114] |
| Al1.9Ga0.1W3O12 | 21 | NR | [115] | In0.8Sc1.2Mo3O12 | 77 | −11.27 1 | [114] |
| Al1.8Ga0.2W3O12 | 60 | NR | [115] | In0.5Sc1.5Mo3O12 | <RT | −5.08 1 | [114] |
| Al1.7Ga0.3W3O12 | 91 | NR | [115] | In2W3O12 | 250 | −3.00 1 | [103] |
| Al1.6Ga0.4W3O12 | 108 | NR | [115] | ErInW3O12 | 135 | NR | [50] |
| Al0.7In1.3W3O12 | 118 | NR | [116] | In1.9Sc0.1W3O12 | 224 | −5.29 1 | [103] |
| Al0.5In1.5W3O12 | 181 | NR | [116] | In1.7Sc0.3W3O12 | 168 | NR | [117] |
| Al1.9Sc0.1W3O12 | −35 | ~0.2 1 | [115] | In1.5Sc0.5W3O12 | 147 | −1.28 1 | [103] |
| Al1.8Sc0.2W3O12 | −60 | ~0.6 1 | [115] | In1.4Sc0.6W3O12 | 76 | NR | [117] |
| Al1.7Sc0.3W3O12 | −98 | ~1.4 1 | [115] | In1.1Sc0.9W3O12 | <RT | −5.35 1 | [117] |
| Al1.6Sc0.4W3O12 | <−150 | ~1.4 1 | [115] | In0.8Sc1.2W3O12 | <RT | NR | [117] |
| Al0.8Sc1.2W3O12 | <RT | 1.21 | [118] | Sc2Mo3O12 | −93 | −2.11 | [59] |
| Al0.5Sc1.5W3O12 | <RT | −0.32 | [118] | Sc2W3O12 | <−263 | −2.20 | [55] |
| Al0.3Sc1.7W3O12 | <RT | −0.93 | [118] | Y2Mo3O12 | <−263 | −9.02 | [119] |
| Cr2Mo3O12 | 403 | 0.67 | [100] | Y2W3O12 | <−258 | −7.0 | [49] |
| Compound | TPT (°C) | αl (×10−6 °C−1) | T Range | Ref. |
|---|---|---|---|---|
| Zr2MoP2O12 | <−264 | −4.46 | −151–123 | [136] |
| Hf2MoP2O12 | NR | −4 1 | NR | [58] |
| Zr2WP2O12 | <−213 | −4.70 | −213–27 | [135] |
| Hf2WP2O12 | NR | −5 1 | NR | [58] |
| MgZrMo3O12 | −126 | 0.13 | 25–450 | [138] |
| MgHfMo3O12 | −98 | 1.02 | 25–740 | [126,127] |
| MgZrW3O12 | NR | −1.15 1 | 167–698 | [140] |
| MgHfW3O12 | <−193 | 1.18 | −193–300 | [141] |
| MnZrMo3O12 | 90 | −2.8 | 100–500 | [128] |
| MnHfMo3O12 | 75 | −2.46 | 200–300 | [125] |
| Mg0.65Zn0.35ZrMo3O12 | −20 | NR | NA | [143] |
| Mg0.5Zn0.5HfMo3O12 | 50 | −0.11 1 | 100–400 | [120] |
| Al1.8(MgZr)0.1W3O12 | −43 | 1.61 1 | 21–770 | [140] |
| Al1.6(MgZr)0.2W3O12 | −70 | NR | NA | [140] |
| Al1.4(MgZr)0.3W3O12 | <−160 | 2.34 1 | 21−774 | [140] |
| Cr1.5(MgZr)0.75Mo3O12 | 250 | NR | NA | [123] |
| Cr0.3(MgZr)0.85Mo3O12 | <−170 | 0.62 | 127–727 | [123] |
| Fe0.6(MgZr)0.7Mo3O12 | 67 | NR | NA | [146] |
| Fe0.4(MgZr)0.8Mo3O12 | <−169 | 2.02 | 127–727 | [146] |
| In0.5(MgZr)0.75Mo3O12 | 82 | −0.16 | 100–500 | [144] |
| In(MgHf)0.5Mo3O12 | 152 | −0.4 | 225–650 | [147] |
| ZrFeMo2VO12 | ~32 | 0.68 | 150–300 | [130] |
| ZrScMo2VO12 | ~−190 | −2.19 | 25–500 | [133] |
| ZrScW2PO12 | −199 | −1.75 | 25–600 | [132] |
| HfScMo2VO12 | <−123 | −2.11 | 25–557 | [149] |
| HfScW2PO12 | NR | −1.27 | 25–1000 | [131] |
| Zr0.3Sc1.7Mo2.7V0.3O12 | −140 | −1.53 | 25–485 | [151] |
| CrSc0.5Zr0.5Mo2.5V0.5O12 | ~−55 | 1.29 | −41–299 | [153] |
| Cr0.5ScZr0.5Mo2.5V0.5O12 | <−192 | 0.95 | −192–299 | [153] |
| Compositions | dL/L0 (×10−6 °C−1) | T Range (°C) | Ref. |
|---|---|---|---|
| Fe0.4Sc1.6Mo3O12/MoO3 | 0.2 | 25–500 | [168] |
| ZrSiO4/Y2W3O12 | −0.08 | 25–1000 | [169] |
| Al/ZrMgMo3O12 | 0.77 | 25–400 | [170] |
| Al/Zr2WP2O12 | −0.0021 | 20–600 | [171] |
| Sc2W3O12/(ZrO2+Y2O3) | 1.04 | 25–500 | [175] |
| Zr2WP2O12/ZrV0.6P1.4O7 | −0.029 | 25–500 | [172] |
| Zr2MoP2O12/ZrO2 | −0.0065 | 25–700 | [173] |
| Zr2WP2O12/ZrO2 | −0.09 | 25–700 | [174] |
| Compound | αl (×10−6 °C−1) | T Range (°C) | Ref. |
|---|---|---|---|
| Al0.3Sc1.7Mo3O12 | −0.73 | 25–450 | [111] |
| Al2Mo0.5W2.5O12 | 0.05 1 | 25–800 | [106] |
| Al0.5Sc1.5W3O12 | −0.32 | 25–600 | [118] |
| Fe0.4Sc1.6Mo3O12 | −0.83 | 25–800 | [113] |
| Fe0.7Sc1.3Mo3O12 | 0.09 | 250–800 | [113] |
| ErFeMo3O12 | −0.60 | 180–400 | [90] |
| FeLuMo3O12 | 0.99 | 200–800 | [91] |
| In1.3Cr0.7Mo3O12 | −0.76 | 400–750 | [176] |
| InCrMo3O12 | 0.94 | 400–750 | [176] |
| Sc1.5Cr0.5Mo3O12 | −0.51 | 25–800 | [109] |
| Sc1.3Cr0.7Mo3O12 | −0.47 | 25–800 | [109] |
| Y0.25Ce1.75W3O12 | −0.82 1 | 182–700 | [97] |
| Yb0.6Fe1.4Mo3O12 | 0.55 | 300–500 | [177] |
| Compound | αl (×10−6 °C−1) | T Range (°C) | Ref. |
|---|---|---|---|
| MgZrMo3O12 | 0.13 | 25–450 | [138] |
| MgHfMo3O12 | 1.02 | 25–740 | [126] |
| HfMgMo2.5W0.5O12 | −0.08 | 25–400 | [129] |
| Cr0.3(MgZr)0.85Mo3O12 | 0.62 | 127–727 | [123] |
| In0.5(MgZr)0.75Mo3O12 | −0.16 | 100–500 | [144] |
| In(MgHf)0.5Mo3O12 | −0.40 | 225–650 | [147] |
| ZrFeMo2VO12 | 0.68 | 150–300 | [130] |
| (MgHf)0.83ScW2.25P0.83O12-δ | −1.03 | −18–300 | [150] |
| Cr0.5ScZr0.5Mo2.5V0.5O12 | 0.95 | −192–300 | [153] |
| Cr0.8Sc0.7Zr0.5Mo2.5V0.5O12 | 0.84 | −94–300 | [153] |
| Sc1.5Zr0.5Mo2.5V0.5O12 | −0.07 | −192–300 | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Sun, W.; Zhang, Z.; Lovings, L.; Lind, C. Thermal Expansion Behavior in the A2M3O12 Family of Materials. Solids 2021, 2, 87-107. https://doi.org/10.3390/solids2010005
Liu H, Sun W, Zhang Z, Lovings L, Lind C. Thermal Expansion Behavior in the A2M3O12 Family of Materials. Solids. 2021; 2(1):87-107. https://doi.org/10.3390/solids2010005
Chicago/Turabian StyleLiu, Hongfei, Weikang Sun, Zhiping Zhang, La’Nese Lovings, and Cora Lind. 2021. "Thermal Expansion Behavior in the A2M3O12 Family of Materials" Solids 2, no. 1: 87-107. https://doi.org/10.3390/solids2010005
APA StyleLiu, H., Sun, W., Zhang, Z., Lovings, L., & Lind, C. (2021). Thermal Expansion Behavior in the A2M3O12 Family of Materials. Solids, 2(1), 87-107. https://doi.org/10.3390/solids2010005






