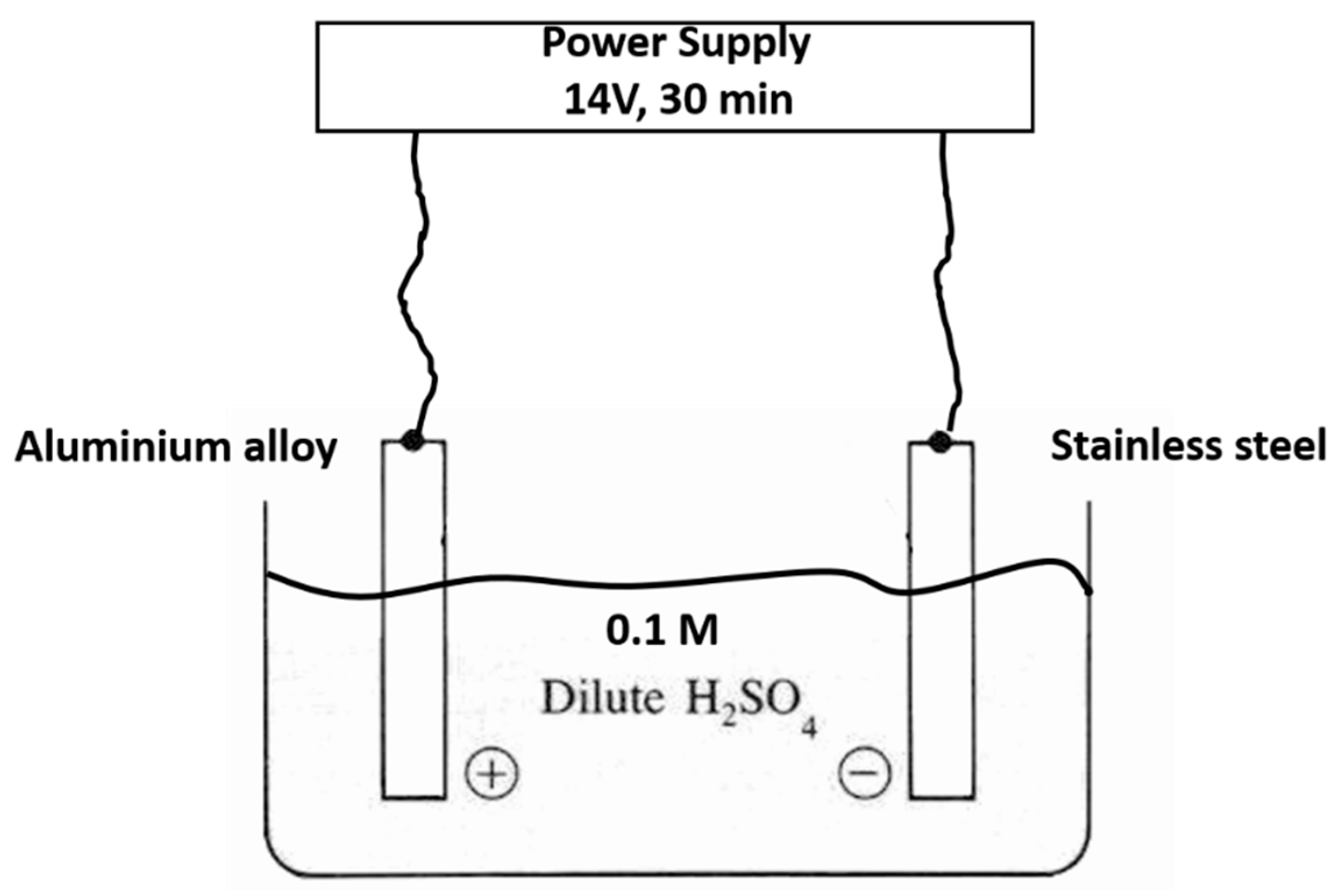
1. Introduction
Aluminum and its alloys are extensively used in a variety of applications due to their good formability, light weight, and high thermal–electrical conductivity properties. However, aggressive solutions are found to disrupt the protective aluminum oxide layer, which causes an initiation of the corrosion reaction and limits their usage in the application ranges [
1]. Layered double hydroxides (LDHs) have received considerable attention due to their unique characteristics, like ion-exchange properties, tunable interior architecture, porosity, interlayer distance, and flexible composition range, which make them suitable in numerous fields like catalyst [
2], fertilization [
3], environmental science [
4], adsorption [
5], and energy systems [
6].
In recent years, layered double hydroxide (LDH)-based coating systems have been thoroughly investigated to substitute the traditional chromate coating systems in order to protect aluminum alloys [
7,
8]. LDHs are a class of synthetic anionic clays composed of positively charged metal hydroxide layers and interlayer anions. A wide range of divalent metal cations was used to develop M-Al-LDH (M = Li, Ca, Mg, Zn, Ni, etc.) on the aluminum alloys, and the corrosion resistance properties were investigated for LiAl-LDHs [
9], MgAl-LDHs [
10], ZnAl-LDHs [
11], CaAl-LDHs [
12], NiAl-LDHs [
13], where LDHs have shown the following key features: (a) Active protection due to LDH anion-exchange capabilities; (b) self-healing characteristics; and (c) barrier effect, which inhibits the diffusion of corrosive species to the underlying metal. To further improve the barrier properties, numerous works are reported to develop layered double hydroxide directly on the anodized aluminum surface, where the anodic surface provides the additional protective layer and the source of Al
3+ for the dense growth of LDH [
14]. In recent works, double doped layered double hydroxide has shown long term corrosion resistance properties, where the synergic effect of corrosion inhibitors demonstrated the self-healing characteristics and multifunctional properties, along with high corrosion resistance properties [
15,
16]. The current work builds on our previous work [
17,
18], where we reported the introduction of cerium (III) and perfluorodecyl trichlorosilane species inside the LDH framework to develop double doped LDHs directly on the anodic aluminum source, and a synergetic corrosion protection mechanism was proposed based on the long-term monitoring of EIS and XRD results. However, the stearate-based dopants are further known to improve individual efficiency as corrosion inhibitors in the LDH structure to protect the aluminum surface [
19]. The current work is focused on studying the cerium-based LDHs intercalated with stearate groups on the anodic AA6082 alloy, where the stearate endows superhydrophobicity and the cerium act as a corrosion inhibitor, both of which play a significant role in improving the corrosion resistance of LDH. There is no study on such coating modifications, while long-term corrosion resistance investigation will facilitate understanding the combined effect of cerium and stearate addition on LDH stability and performance. The monitoring of long-term corrosion resistance properties and knowledge of superhydrophobicity against the corrosive solution is of valuable significance for a detailed understanding of coatings for practical applications. We focused on the surface superhydrophobicity behavior against UV radiation and different household species for indoor and outdoor coating applications. The relationship between microstructure of superhydrophobic film and anticorrosion performance against corrosive solution is discussed.
4. Results and Discussion
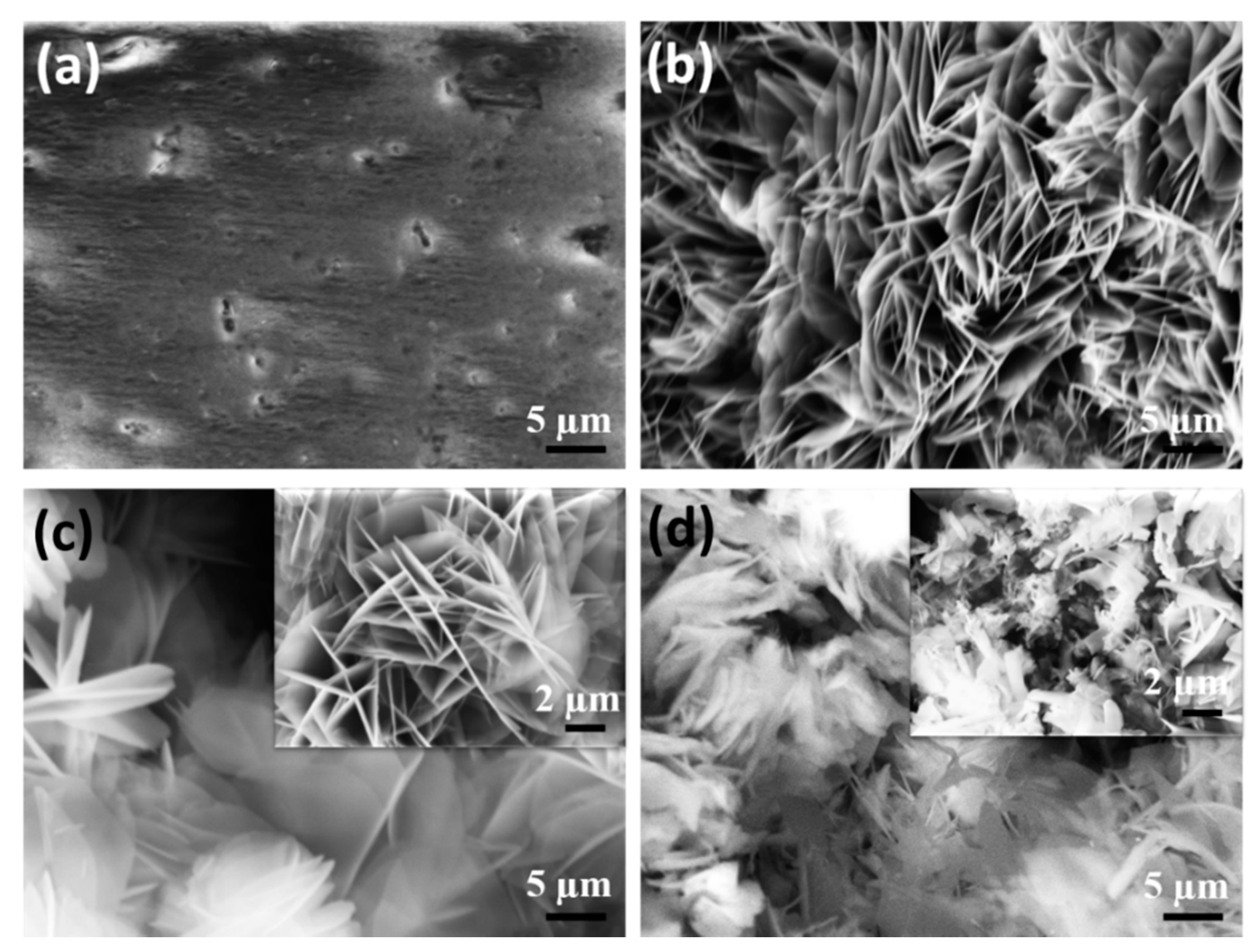
The surface morphology of the developed LDH specimens before and after modification with cerium and stearate group is characterized by SEM analysis, as shown in
Figure 2a–d. MgAl-LDHs and Ce-LDHs showed curved hexagonal platelet structures grown perpendicular to the substrate and distributed randomly on the surface (
Figure 2b,c). The high nucleation density is possibly due to the high concentration of Al(OH)
4 that comes from the anodic aluminum surface, where the sealing treatment of the porous anodic structure by LDH precursors turned the alumina into hydrated alumina. The platelet structure in the case of Ce-LDHs remains unchanged except that the thickness may slightly increase the platelet LDHs. The Ce-LDH precursors also provide a sealing effect for the anodized porous surface. However, after intercalation with stearate groups, the surface morphology changed considerably; the disordered packed platelet structure can be seen in
Figure 2d. The intercalation of stearate groups induced considerable stress inside the LDH framework and caused the formation of a disordered packed arrangement. The EDS analysis demonstrated that the mass and atomic percentage ratio of Mg/(Al+Ce) is equal to (31.81/3.56 + 4.44 = 3.97) and (22.7/2.55 + 1.12 = 6.18), respectively. The anodic film thickness is around 26.5±1.8 µm, while after the development of Ce-LDHs and S-LDHs on the anodic film, the total film thickness became around 30.6 ± 1.9 and 34.2 ± 1.7 µm, respectively.
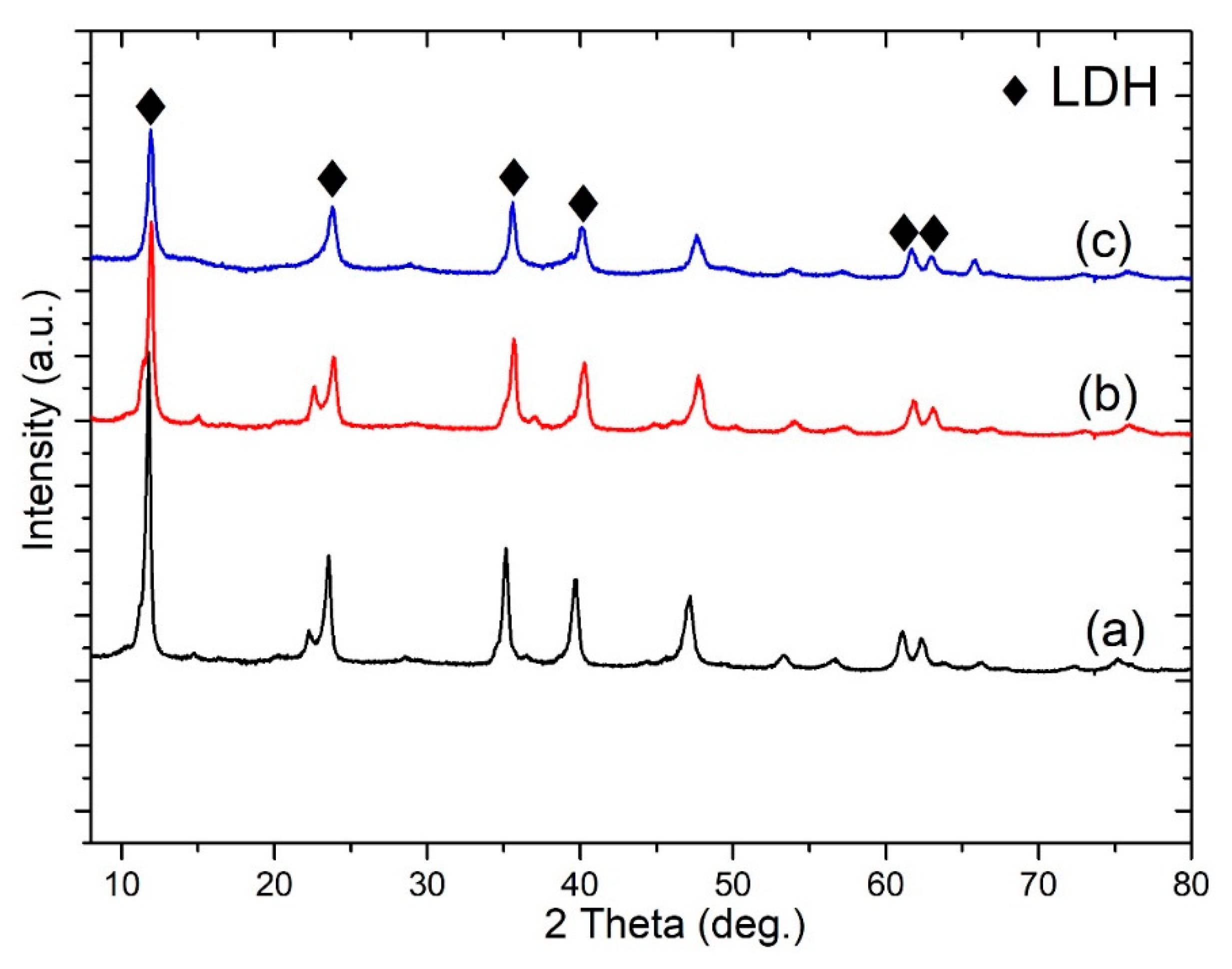
Figure 3 shows the XRD patterns of MgAl-LDH, Ce-LDH, and S-LDH developed on an anodized aluminum surface. MgAl-LDH reflects the characteristics peak of the LDH structure at 11.8°, 23°, 35°, 61.5°, and 63°, corresponding to (003), (006), (012), (110), and (113) diffraction planes, while on the addition of cerium no distinct difference was observed in reflection peaks. The interlayer thickness (d
003, 0.76 nm) and (d
006, 0.38 nm) based on Bragg’s law were very consistent with the MgAl-CO
3-LDHs [
20]. The calculated value of 0.76 nm for d
003 is characteristic of carbonate intercalated layered double hydroxide and corresponds to x = 0.33 with flat-lying anion orientation in the interlayers [
21]. The sharp diffraction peak of the (003) crystal plane showed a good preferred orientation and crystal quality concerning LDHs. S-LDH diffraction peaks overlap with that of MgAl-LDHs and Ce-LDHs. No change on the diffraction angle of (003) was observed after modification with stearate groups, except for the decrement in the intensity of corresponding diffraction peaks. This explains the slight weakening of crystal quality after stearate modification and did not make any impact on the crystal structure and interlayer thickness [
22]. The intense reflection peaks can further be used to evaluate cell parameters “c” and “a”, which can be evaluated by the correlations, c = (3d
003 + 6d
006)/2 and a = 2d
110. The cell parameter “c” remains around 2.34 nm; however, in the case of modified MgAl-LDHs, a very slight shift is found for the parameter “a”, where 0.302 nm for MgAl-LDHs shifted to 0.300 nm for Ce-LDHs and S-LDHs. The synthesis experiments were performed without a controlled environment and in such circumstances contamination with atmospheric CO
2 cannot be avoided. It is evident that in the case of higher pH value, and without a controlled environment, the potential of formation of carbonate-based LDHs is much higher compared to nitrate-based LDHs. A similar concept has already been explained well in the work of Li, KunWei, et al. [
23], where higher pH results in the formation of carbonate-based LDHs, despite the synthesis of LDHs in the nitrate-based solution. It may be assumed that stearate would cause the specific low intensity diffraction peak at ~2.3° of 2θ due to diffuse scattering arising [
24]; that would be very consistent with the densely packed structure SEM image of the film (
Figure 2d).
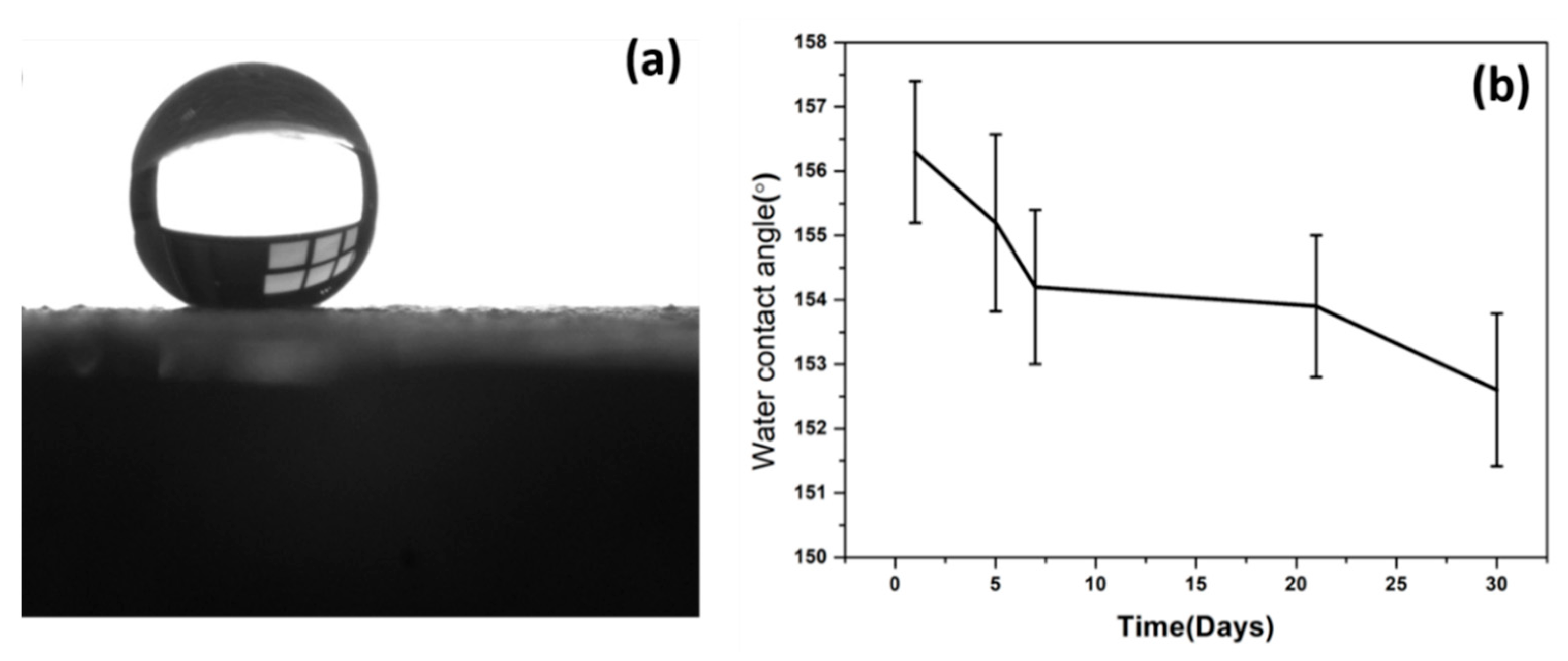
The S-LDHs showed superhydrophobic properties with water contact angles (WCAs) of around ~156° (
Figure 4a). Superhydrophobicity is commonly related to the synergic effect of surface roughness and lower surface energy. Due to the high surface roughness of the LDH structure, the air is trapped within the textured surface and on spikes, resulting in the upper layer of air that modeled Cassie–Baxter regimes, where the liquid–air–solid interaction is superimposed, rather than the liquid–solid model. This causes an increase in superhydrophobic characteristics with substantial-high WCA contact angle hysteresis. It is of great importance to understand the film’s durability against UV radiation and its effect on the superhydrophobicity of the LDHs to evaluate outdoor applications. As can be seen, WCAs exhibited a decline of around 3° after 30 days of exposure with UV irradiation (
Figure 4b), indicating strong resistance to UV irradiation, which is well correlated with the literature work [
18]. This may be due to the UV-blocking properties of LDH, which resist the UV aging where LDH particle size distribution and layered composition have shown an influential effect on the UV aging properties [
25].
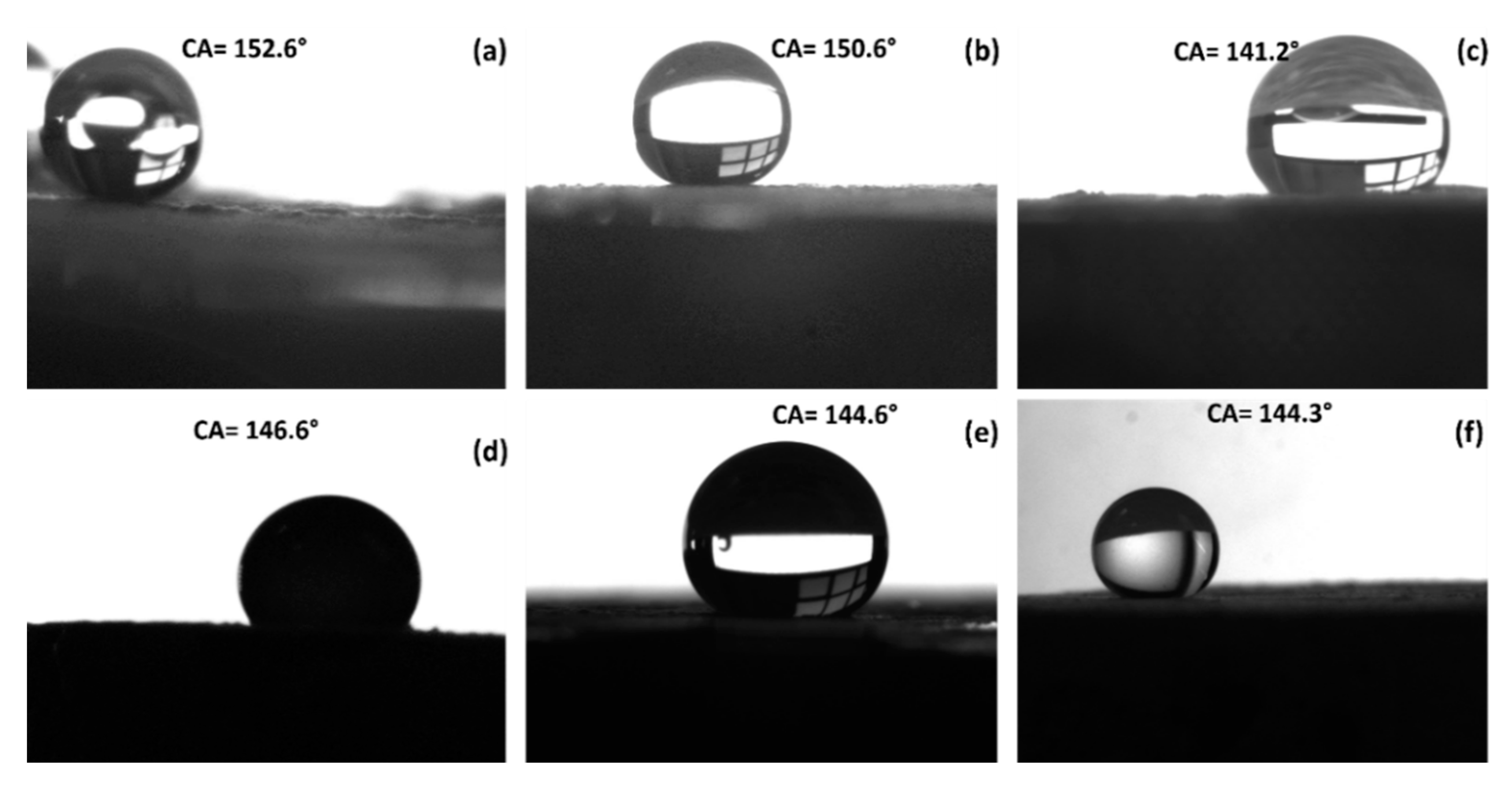
As a common indoor coating material, the coating films may be exposed to common household liquids and thus it is important to analyze the domestic liquid-repellence of the LDHs. The digital images of the contact angle measurements against six common household liquid items (tea, acidic drops, basic liquid, corrosive liquids, coffee, and cola) are shown in
Figure 5. Liquid droplets in all cases stood on the thin film with a sphere shape and showed good repellency; however, in the case of acidic media, degradation of the thin film was also observed.
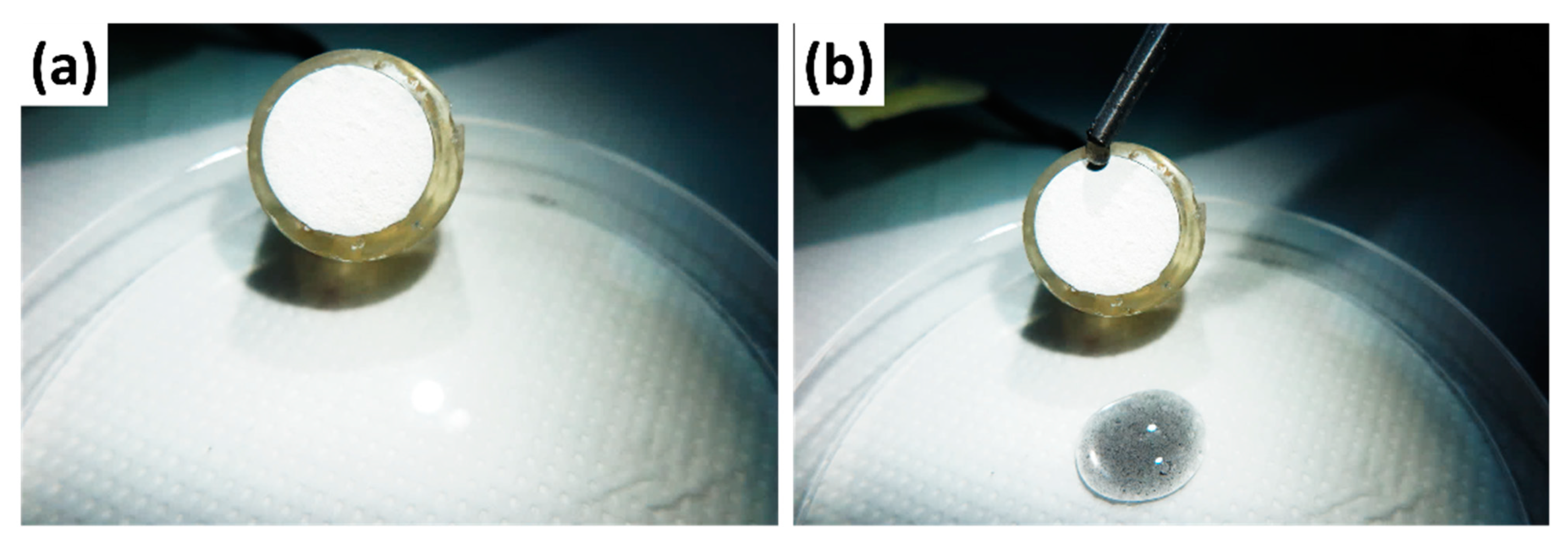
Dust contaminations are another parameter to investigate the understanding of self-cleaning properties, where the dust particles cannot be avoided in daily life. To analyze the self-cleaning properties, the graphene-suspended water is used by mixing the graphene in water to evaluate the liquid contamination effect on the film. The graphene suspension was deliberately put on the film surface (
Figure 6). It is interesting to observe that the graphene suspension readily rolled away from the S-LDHs without traces on the surface, indicating the well-suited self-cleaning characteristics.
EIS analysis is a useful approach to understand the influence of superhydrophobic surfaces on the corrosion resistance properties of LDHs. The general protective mechanism of S-LDHs is analyzed through combined results of contact angle measurements and corrosion resistance in long-term immersion in a corrosive solution.
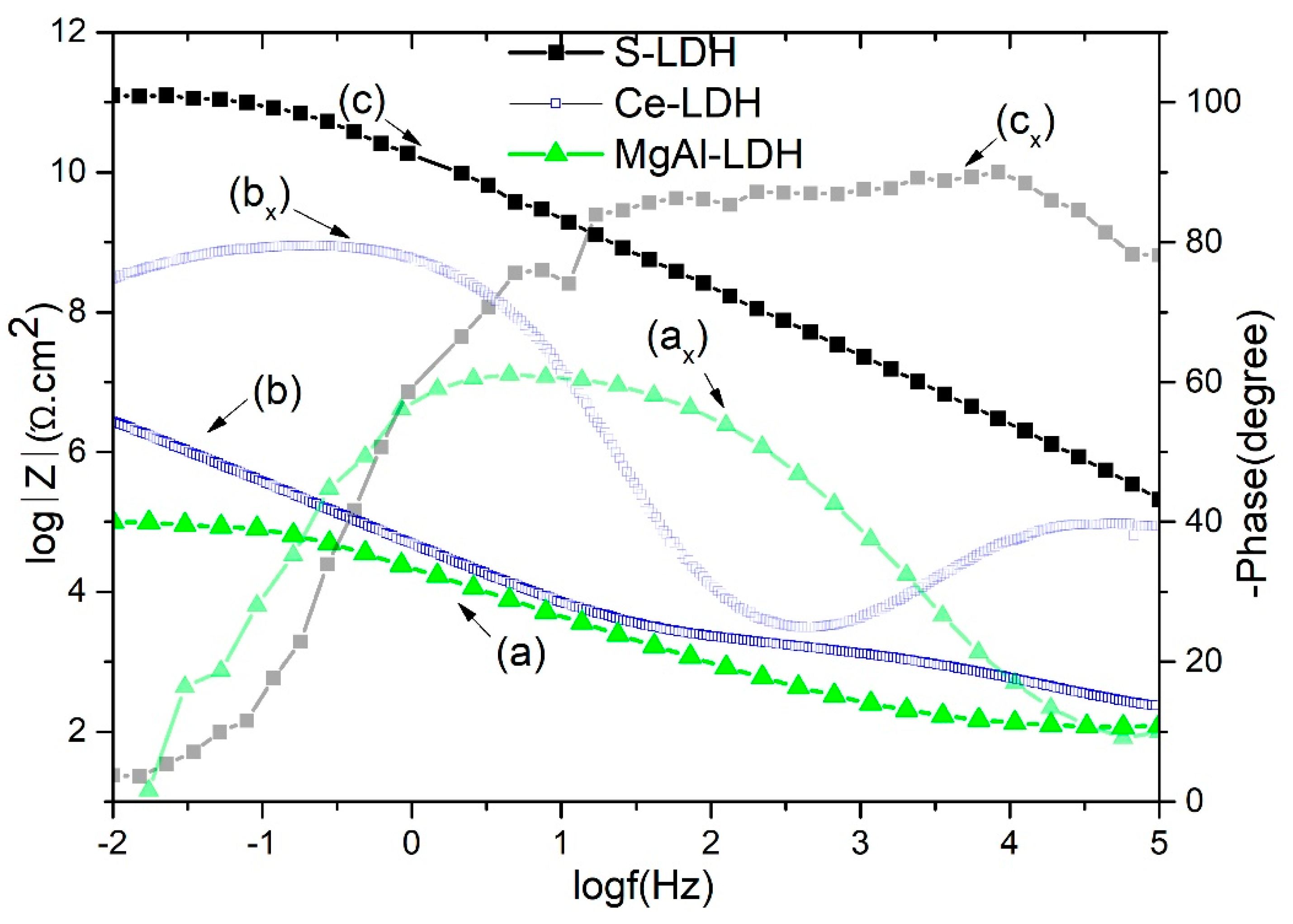
Figure 7 shows the bode plots of developed layered double hydroxide thin films against a 0.1 M NaCl solution. The impedance modulus at the low frequency of 0.01 Hz (|Z|
0.01) can be used to compare the corrosion resistance of film on contact with the 0.1 M NaCl solution. As can be seen, the impedance modulus is much higher for S-LDH compared to Ce-LDH and MgAl-LDH. It is worth noting that the low-frequency impedance modulus of stearate-modified Ce-LDH is nearly four orders higher than Ce-LDH and six orders higher than virgin MgAl-LDH. In our previous work, Ce-LDH corrosion resistance behavior, a possible protection mechanism, and long term EIS measurements are reported in detail [
17]. The improved corrosion resistance properties of cerium-modified LDHs could be due to its precipitation effect on the cathodic sites on contact with corrosive solutions. The higher impedance value of S-LDHs can be ascribed to air film on the superhydrophobic surface, low surface energy properties, the presence of cerium inhibitors inside the LDH galleries, and combined barrier effect of anodic and LDH layers. Furthermore, it is difficult to exchange stearate/carbonate anions with the surrounding chloride anions. The time constant (
Figure 7) in the high-medium frequency range corresponds to LDH outer porous network contribution, while in the medium frequency range it exhibits the LDH inner barrier layer contribution in corrosion resistance properties. The stearate-modified Ce-LDH has shown a 40° larger phase angle than Ce-LDH, while it is 70° higher than virgin MgAl-LDH, demonstrating the superior corrosion resistance of S-LDH.
The Bode plots of S-LDHs after various time intervals of immersion (max. 720 h) is shown in
Figure 8. S-LDHs demonstrate the impedance modulus of 10
10.4 (Ω cm
2) after 24 h of immersion in a 0.1 M NaCl solution, while the impedance modulus has shown a gradual decline in continuous contact with the corrosive solution. The |Z|
0.01 value of S-LDH reduced from 10
10.4 to about 10
8.9 Ωcm
2 after 720 h. The findings describe the higher stability of S-LDH with only a decline of 10
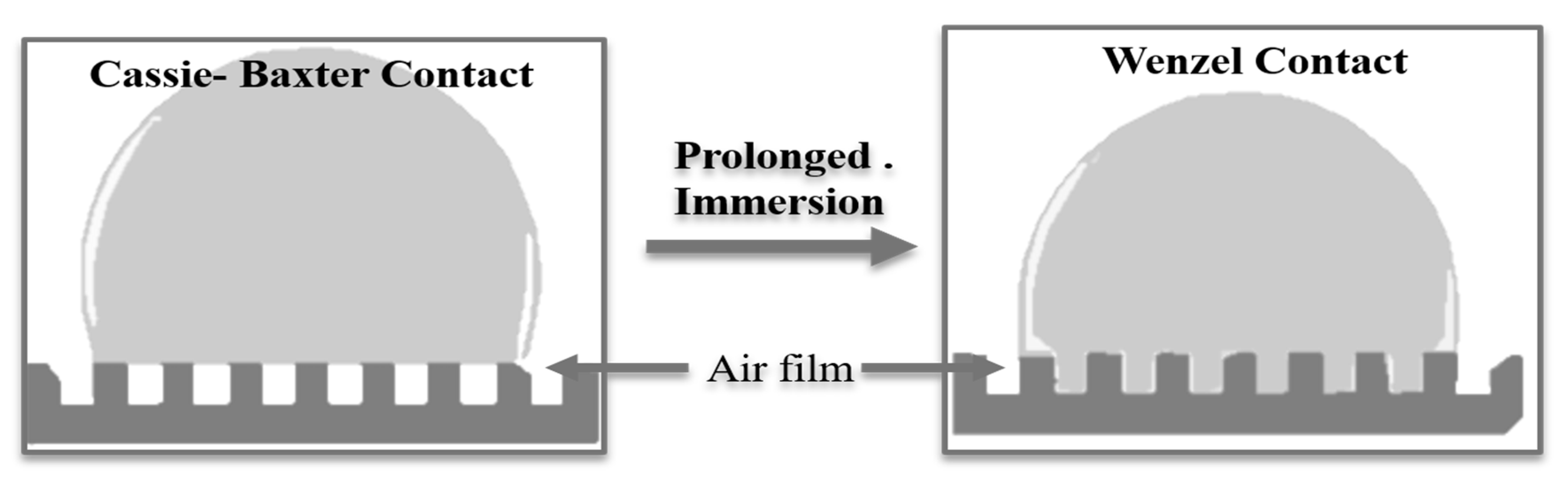
1.5 after a long-time immersion in a chloride’s solution. This verifies the low anion-exchange process with chlorides and improved barrier properties that protect the underlying substrate. This long-term stability of the superhydrophobic surface can be ascribed to the presence of stearate, which is stretched outside the surface and is highly efficient in long-term protection. Overall, different layers of LDH geometry provide a barrier against chloride attack to protect the underlying substrate. The bottom anodic layer is sealed by LDH precursors along with the precipitation of cerium hydroxide/oxides on it, further the final upper air film, and the dense bilayer LDH network sandwiched between them. Stearate-intercalated LDHs repelled the permeation of water molecules and chlorides, which is a key important parameter of that work where stearate introduction causes the lowering of surface energy and endorses superhydrophobic characteristics that enhance the active protection for LDHs; the presence of cerium in the LDH frame protects by its inhibition action through reducing the cathodic reaction rate on contact with corrosive media [
26,
27]. The strong passive and active protection were found to develop the compactness and integrity of LDHs, which is significant for delaying the corrosion process and is a powerful approach to enhancing anti-corrosion behavior. The Ce (III) addition on LDHs can be subjected to self-healing properties by the formation of Ce (OH)
3/CeO
2 on protective film degradation. It can be concluded that the stearate plays a role in preventing the attack of aggressive anions; however, the effect was not so conspicuous due to their structural characteristics. As a result, the defect sites were repaired, and corrosion was inhibited efficiently. Two different models are reported to describe the surface wetness in the case of LDH thin films, (a) the Cassie–Baxter model and (b) the Wenzel model, by which the contact angles and wetting natures of the surfaces can be explained. In the Cassie–Baxter model, the air-film on the surface of the film provides the interface for the liquid droplet and the coating film. On the other side, the Wenzel model liquid droplet penetrates the air pockets present in the gap of asperities. Given the susceptibility towards corrosion, air pockets act as an additional layer that hinders the contact of liquid media and solid coating film thus following the Cassie–Baxter model. On the horizontal surface, the chloride solution can remain spherical until the liquid droplet completely dissolves with time. However, on the tilted surface, the droplets will slip off and corrosion resistance properties can further be improved. The superhydrophobic Cassie–Baxter surface on long immersion in a chloride solution may change to Wenzel contact, and thus anticorrosive performance will decline with time, as shown in
Figure 9. This is in good accordance with the long-time corrosion measurement (
Figure 8).
Table 1 provides a comparison of the impedance modulus |Z|
0.01 that can be used to compare the corrosion resistance of film against corrosive solution. The value of |Z|
0.01 in this work is significantly higher compared to the values in the literature for other LDH-based coating systems [
15,
19,
28]. However, one must also consider that, (a) a mild electrolyte was used in the current study to monitor the small impedance changes, (b) crystallization time to synthesize LDHs varied in the literature work, that make influence on the film thickness and further on the corrosion resistance properties, (c) the EIS measurements were monitored for different time intervals in the literature studies, and it is difficult to make comparisons with the data.
The SEM images of S-LDHs and corresponding digital images of water contact angles (WCAs) after different intervals of immersion in the 0.1 M NaCl solution are shown in
Figure 10. It is clear that the LDH structure remains intact after 720 h of immersion, and besides of few LDH curvy plate distortions, no other dominant defects were observed. This is well correlated with the EIS long-term immersion results. Stearate intercalated LDHs repelled the permeation of water molecules and chlorides, which is a key important parameter to improve the corrosion resistance properties. The SEM images and gradual decline of impedance modulus are very consistent with the corresponding WCAs. On regular contact with the corrosive solution, there is a decline in the WCAs of the stearate-modified LDHs, but overall the system demonstrates outstanding stability after long term immersion.