1. Borate Glass and Alkali Modification
Crystals and crystalline materials have a three-dimensional periodic structure with translational and point symmetries, therefore, their physical properties are anisotropic. Glasses and amorphous materials have a random structure with no translational symmetry and no point symmetry, therefore their macroscopic physical properties are isotropic. A glass is formed through a liquid-glass transition by rapid cooling from a supercooled liquid state into a nonequilibrium glassy state. Various kinds of organic and inorganic glasses are known among polymers, drugs, minerals, metals, semiconductors, etc. Among the inorganic glasses, oxide network glasses such as SiO2, B2O3, and P2O3, have attracted much attention for their widespread practical applications by the combination of hardness and transparency at room temperature. The network structure of oxide glasses is remarkably modified by alkali ions, and such a modification has been used to control the various physical properties of glasses such as the glass transition temperatures, elastic properties, etc.
Borate glass (B
2O
3) is one of the typical covalent network glasses. The network structure of the borate glass is formed by randomly connected planar BO
3 triangles and most of them form planar boroxol B
3O
6 rings [
1]. Alkali metals remarkably modify the network structure of a borate glass, and the variation in the microscopic structure and related physical properties as a function of the alkali content has been extensively studied. The alkali ions in a borate glass induce the change of the boron coordination number of a boron atom from three to four and non-bridging oxygens are created as alkali content increases. Due to these changes of the covalent bonds in the network structure, various kinds of structural groups are formed such as pentaborate, dipentaborate, diborate, triborate, metabolite, etc., depending the alkali metals and their contents [
2,
3]. A rich variation of the structural units causes remarkable changes in the various physical properties. For example, the Poisson’s ratio of cesium borate glasses shows a minima and maxima with the increasing cesium content [
4]. Such non-linear variations in the various physical quantities versus the alkali contents are called a “
borate anomaly”.
The medium range order (MRO) is the arrangement of the structural units, which controls various physical properties such as the elastic constants and density. The MRO is estimated by the first sharp diffraction peak (FSDP) in the static structure factor, S(Q), measured by the neutron and X-ray diffraction experiments. The peak width ∆Q
fsdp of FSDP corresponds to a structural correlation length of
Lfsdp = 2π/∆Q
fsdp, which defines the MRO. The peak value Q
fsdp corresponds to the real-space ordering with a periodicity of 2π/Q
fsdp [
5].
Figure 1a shows the static structure factor S(Q) of the single-alkali borate glasses, 0.22M
2O-0.78B
2O
3 (M = Li, Na, K, Rb, and Cs) measured by neutron scattering [
6]. The FSDP and the second peak are denoted by Q
1 and Q
2, respectively, in
Figure 1a. The MRO correlation length
Lfsdp = 2π/∆Q
fsdp was determined from the width of a peak at Q
1, i.e., ∆Q
1. The correlation between
Lfsdp and the ionic radius R
ion [
7] is shown in
Figure 1b. For the larger alkali ions,
Lfsdp is nearly proportional to
Rion. This relation indicates that a larger alkali ion occupies a void in the B-O network structure, while a smaller alkali ion M
+ locates near a BO
4− tetrahedron.
According to the study of the packing in alkali modified oxide glasses by Giri et al., ionic and covalent packings were discussed in terms of the relative volumes of the modified alkali cations compared to that of the networking forming oxygen [
8]. For modifying alkali ions (K, Rb, and Cs) having an ionic radius larger than oxygen, the packing is dominated by the modifier alkali ions, and it is called ionic packing. Therefore, the linear relation between
Lfsdp and the ionic radius
Rion nearly holds for the larger alkali ions. For the alkali ions (Li and Na) having an ionic radius smaller than oxygen, the packing is controlled by the oxygen covalent network, and it is called covalent packing. For the smaller alkali ions, the linear relation between
Lfsdp and the ionic radius
Rion does not hold.
By modification of the oxide glasses by two dissimilar alkali ions, the maximum electric resistivity or the expansion coefficient were observed [
9,
10,
11,
12]. Such an anomalous variation is called the mixed-alkali effect (MAE) caused by the coexistence of two dissimilar alkali ions. For the various transport properties, such as the ionic conductivity and viscosity, a remarkable deviation from linearity was observed when one alkali cation substitutes for another at the totally fixed content of the alkali metals. In the MAE, interactions between dissimilar cations reduce their mobility, and remarkably affect the dynamic properties. Such interactions were thought to be associated with network-mediated “pairs” of dissimilar alkali ions, which are energetically favored relative to pairs of similar alkalis [
13]. These non-linear behaviors are technologically important for developing low-loss electrical glasses. The non-transport physical properties, such as density, glass transition temperature, and elastic modulus, also reveal noticeable deviations from the linear variation of the alkali contents. However, the physical origins of the MAE on these non-transport properties are not yet clarified. This paper reviews the MAE of the borate glasses studied of the thermal analysis, neutron diffraction, ultrasonic method, Brillouin scattering, Raman scattering, and neutron inelastic scattering on the mixed-cesium lithium borate glasses,
x{(1−
y)Cs
2O-
yLi
2O}-(1−
x)B
2O
3. The glass transition temperature, heat capacity, elastic constants, internal Raman bands, and boson peaks show the deviation from the linear relation to lithium fraction. These various physical properties are discussed on the basis of the changes of coordination number of boron, creation of non-bridging oxygen, and dissimilar alkali ion pairs.
2. Glass Transition Temperatures
In various kinds of glasses, the liquid-glass transition temperature,
Tg, has been extensively studied. However, the understanding of the nature of the
Tg is still not enough. Kauzmann suggested the empirical relation,
Tg ~ 2/3
Tmelt, where
Tmelt is the melting temperature [
14]. Tanaka studied the correlation between the
Tg and the average coordination number,
z, per atom in covalent glasses. He found the empirical relation, ln
Tg ≈ 1.6 K ×
z + 2.3 K, and it holds well for chalcogenide glasses [
15]. Although there have been many studies to understand the nature of the glass transition, and to relate
Tg to the glass network connectivity, there is still no universal relationship satisfied by almost all kinds of glass formers [
16].
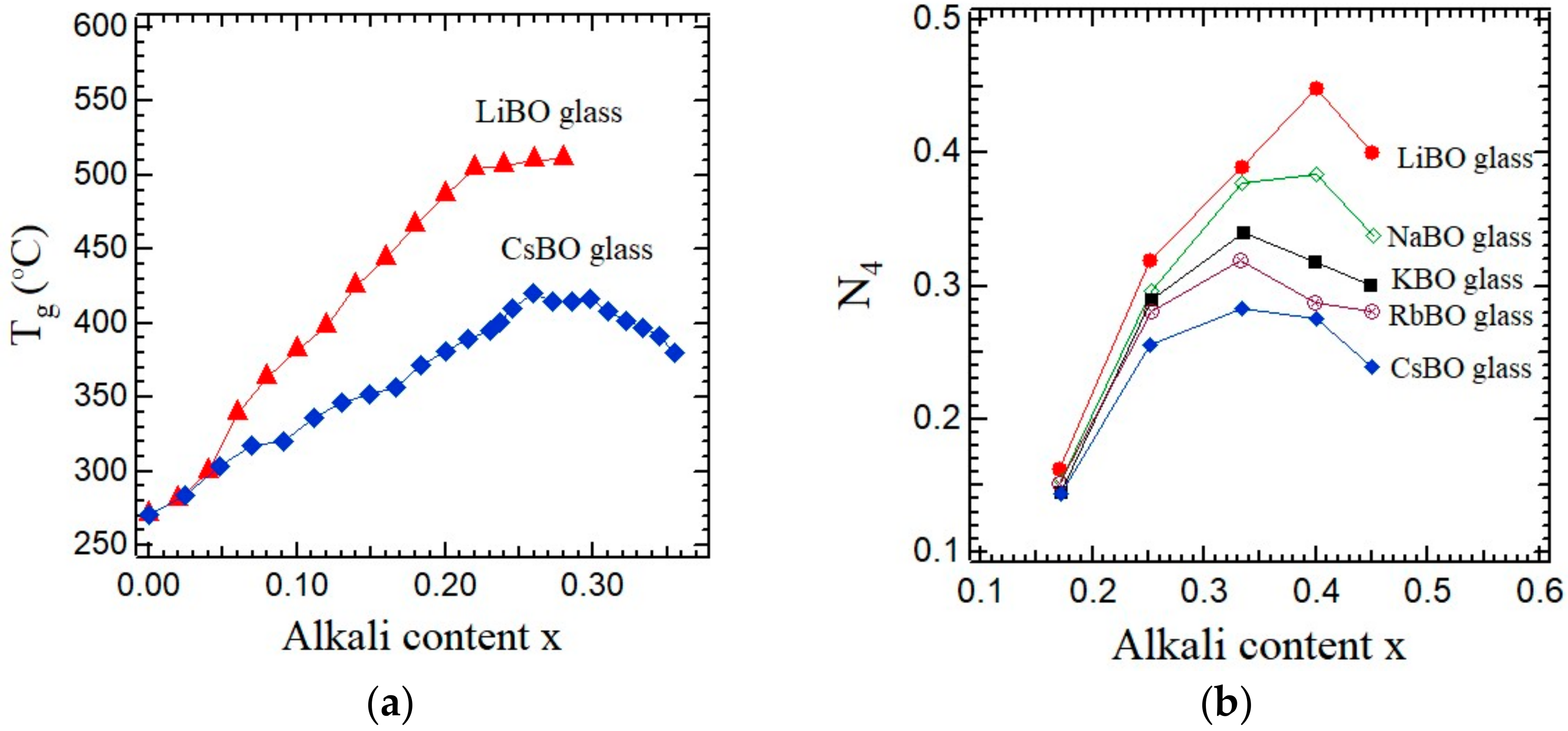
The
Tg of single-lithium and cesium borate glasses show remarkable alkali content dependences as shown in
Figure 2a [
4]. In the single-lithium borate glasses, the
Tg monotonically increases as the Li content
x increases. In the single-cesium borate glasses, the
Tg monotonically increases at first as the Cs content
x increases. The maximum of the
Tg appears at about
x = 0.28, then decreases with a further increase in
x. The difference in the alkali content dependences of the
Tg between the lithium and cesium borate glasses is closely related to the difference in their network structures. Regarding the lithium borate glasses, Kerner discussed the relation between the
Tg and the average coordination number of the glass former atoms [
17]. However, the relation for other alkali borate glasses was not discussed.
Zhong and Bray studied the alkali dependence of the number fractions of boron with three and four coordination numbers in the single-alkali borate glasses,
xM
2O-(1−
x)B
2O
3 (M = Li, Na, K, Rb, and Cs), using NMR [
18]. The coordination number of boron in the pure borate glass is three, while by the alkali modification, it changes to four and non-bridging oxygens are created. The number fractions of boron atoms with three and four coordination numbers are denoted by
N3 and
N4, respectively, where
N3 +
N4 = 1. In the single-alkali borate glasses, the correlation between the
Tg and
N4 was reported as shown in
Figure 2b [
18]. The variations of the
Tg and
N4 versus the alkali content
x in
Figure 2a,b are qualitatively in agreement. The variation of
N4 on the alkali content presumably arises from the competition between the two processes [
4]. One process is the formation of BØ
4− units with 4-coordinated boron, where Ø is a bridging oxygen and the positive alkali ion M
+ is adjacent to the negative BØ
4− unit to keep the local charge neutrality. As the alkali ion size decreases, this process is enhanced. In the lithium borate glass, this process is more active than that in the cesium borate glass. Another process is the formation of a negative nonbridging oxygen (NBO), O, on a BØ
2O
− unit. The positive alkali ion M
+ is adjacent to the negative O
−. As the alkali ion size increases, the negative Coulombic potential energy of the B-Ø–M
+ configuration may decrease. In the cesium borate glass, this second process is more active than that in the lithium borate glass. Based on a comparison of plots of the lithium and cesium borate glasses between
Figure 2a,b, the larger
N4 of the lithium borate glass is related to the higher
Tg of the lithium borate glass. These results indicate that the first process of
N4 is more effective for the increase in the
Tg.
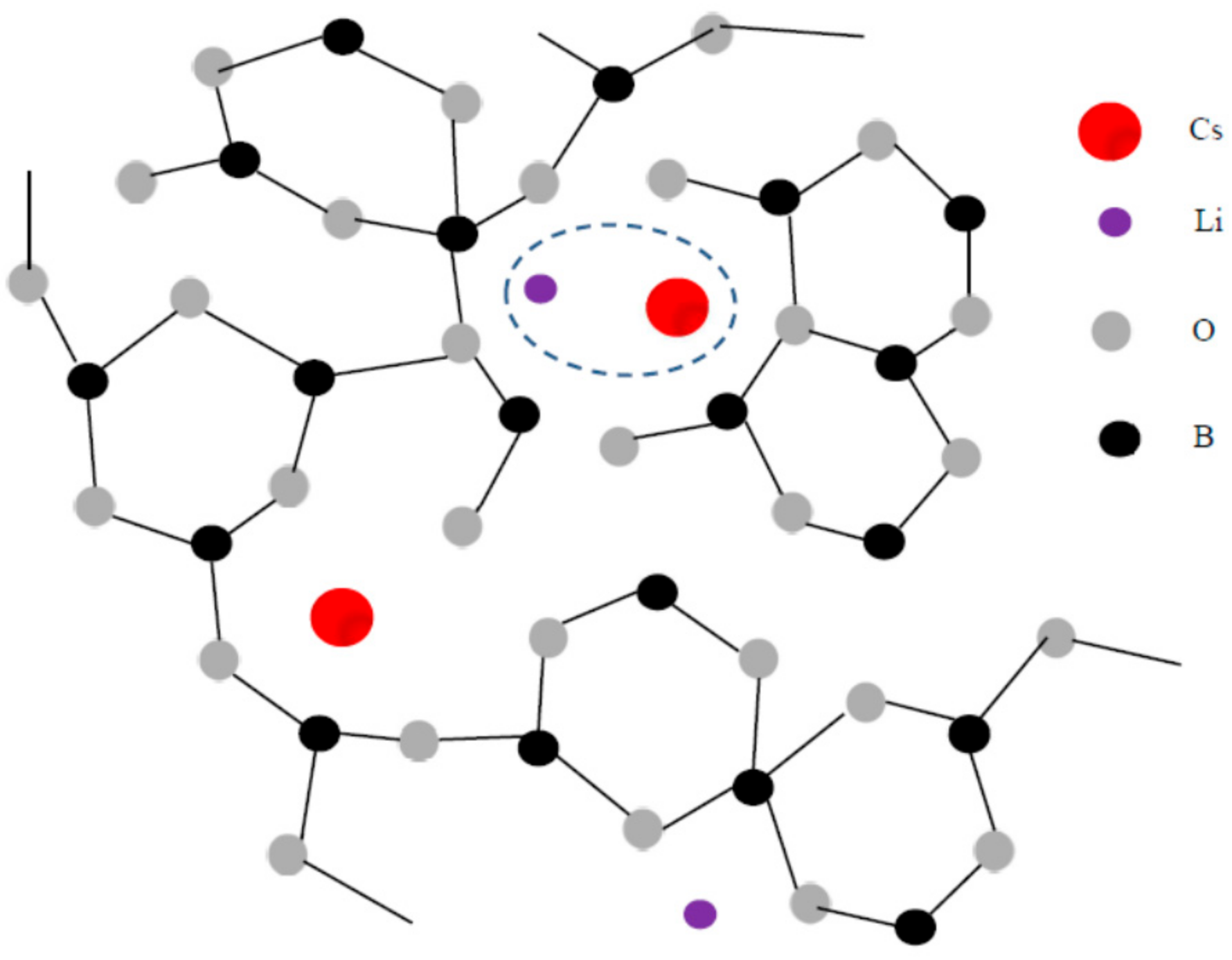
Next, we discuss the MAE on
Tg. A schematic illustration of a mixed-lithium cesium borate glass is shown in
Figure 3. As discussed in
Section 1. “Borate glass and alkali modification” using the FSDP of a static structure factor S(Q), a smaller Li
+ ion with the ionic radius of 0.60 A locates near the B atom, while a larger Cs
+ ion with the ionic radius of 1.69 A locates in a larger void of the B-O network. Zhong and Bray also studied the MAE on the
Tg by NMR [
18]. They found that the measured
N4 for the mixed-alkali borate glasses is less than the
N4 (average) value expected from a simple superposition calculated by the following equation:
In some cases,
N4 (average) is even less than either of the
N4 values for the relevant single-alkali borate glasses. Ingram [
19] made the following assumptions in an effort to explain the MAE: (1) in a mixed-alkali glass there is a preponderance of mixed-alkali pairs, e.g., (LiK)
2+ as compared to the alkali pairs, e.g., (LiLi)
2+; and (2) these mixed-alkali pairs are effectively immobilized.
Figure 4 shows the Li fraction
y dependence of the
Tg of the mixed-lithium cesium borate glasses, 0.28{(1−
y)Cs
2O-
yLi
2O}-0.72B
2O
3. The solid line shows the linear relation. A negative deviation from the linear dependence is clearly observed. The minimum glass transition temperature at about
y = 0.2 was observed for the Li fraction
y dependence of a fixed alkali content. By using the molecular dynamic simulations, Vegiri et al. reported that the abundance of the BØ
4− units decreases from the single-lithium to cesium glasses and exhibits a negative deviation from linearity in the mixed-cesium lithium glasses [
13]. The existence of a minimum at about
y = 0.2 suggests a decrease in
N4 or an increase of the non-bridging oxygen around this fraction by the MAE. A similar minimum of the
Tg for the constant alkali content was also reported in the mixed-cesium sodium borate glasses, 0.33{(1−
y)Cs
2O-
yNa
2O}-0.67B
2O
3, and a correlation was shown between
Tg and
N4 determined by the infrared absorption [
20]. A molecular dynamics investigation of mixed-alkali borate glasses reported that the abundance of BØ
4− units was found to decrease from Li to Cs and to exhibit a negative deviation from linearity in the mixed-cesium lithium borate glasses [
13]. The dissimilar (CsLi)
2+ pair may locate in a larger void, and it suppresses the formation of the BØ
4− units. The existence of a dissimilar pair can be the origin of the minimum
Tg at about
y = 0.2 in
Figure 4.
3. Excess Heat Capacity at Low Temperatures
Glasses reveal the universal features of their thermal properties at low temperatures. The heat capacity shows an excess part and the thermal conductivity has a plateau at around 10 K [
22,
23]. These anomalous behaviors are closely related to the phonon dispersion in the terahertz frequency (THz) range. The excess heat capacity has been observed as the deviation from the Debye
T3 law at low temperatures. The broad peak in a C
p/
T3 vs.
T plot is observed by this non-Debye excess heat capacity, where C
p is the heat capacity at a constant pressure. In the B
2O
3 glass, the peak of C
p/
T3 was observed at the peak temperature,
Tm = 5.8 K [
24]. The origin of a peak is the low-energy excess part of the vibrational density of states (VDoS) over the Debye model defined by g(E)/E
2, where g(E) is VDoS. The peak of g(E)/E
2 has also been observed in the THz range of the inelastic scattering spectra, which is called the boson peak (BP) [
23,
25]. The temperature dependences of the heat capacity of the single-alkali borate glasses, 0.22M
2O-0.78B
2O
3 (M = Li, Na, K, Rb, Cs), were reported between 2 K and 150 K by the relaxation method [
26]. With an increase in the ionic radius of the alkali ion, the
Tm of C
p/
T3 decreases. This fact is attributed to the enhancement of the contraction of the boron-oxygen network by the increase in the alkali charge density due to the decrease in the alkali ionic radius. It was found that the
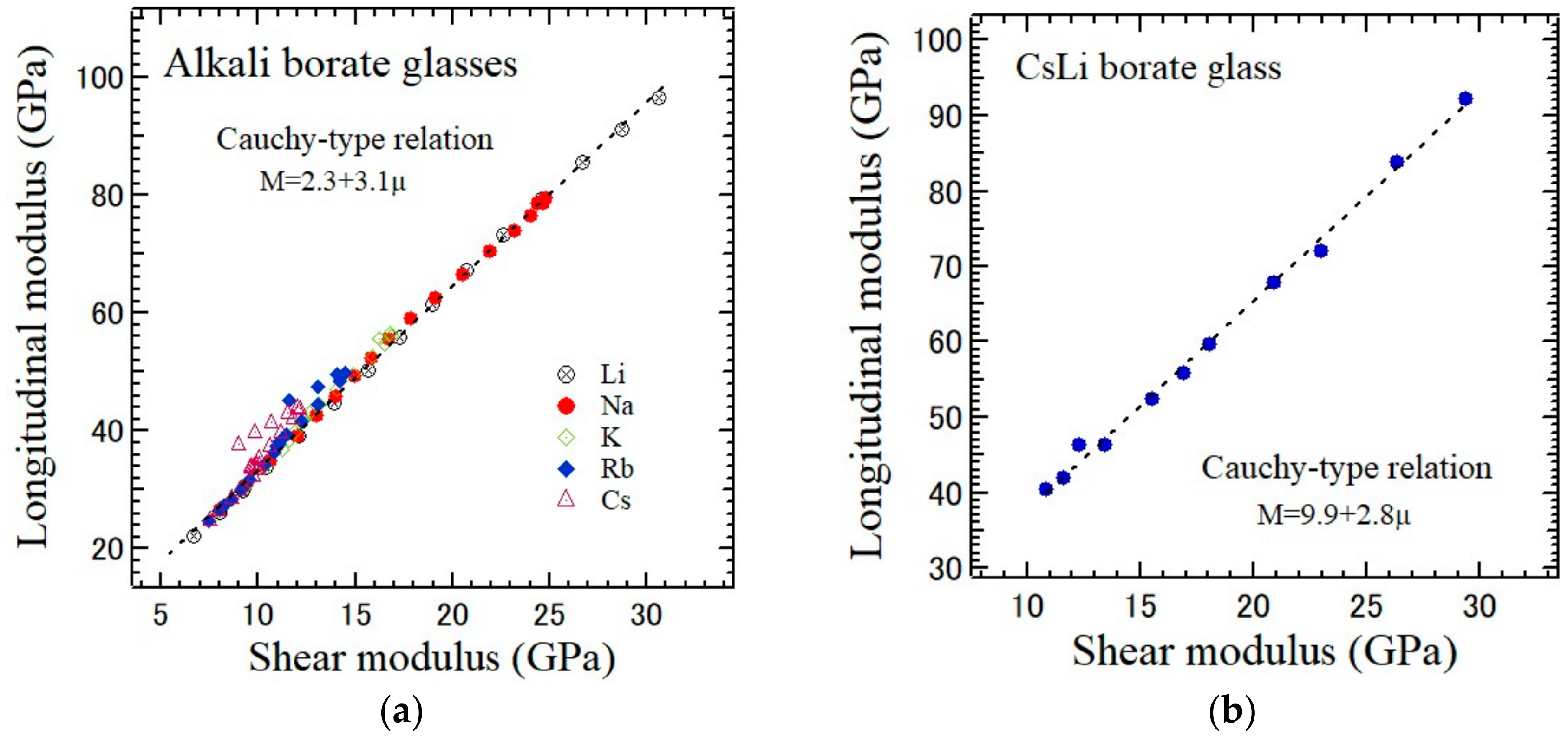
Tm is proportional to the shear modulus μ measured by an ultrasonic method.
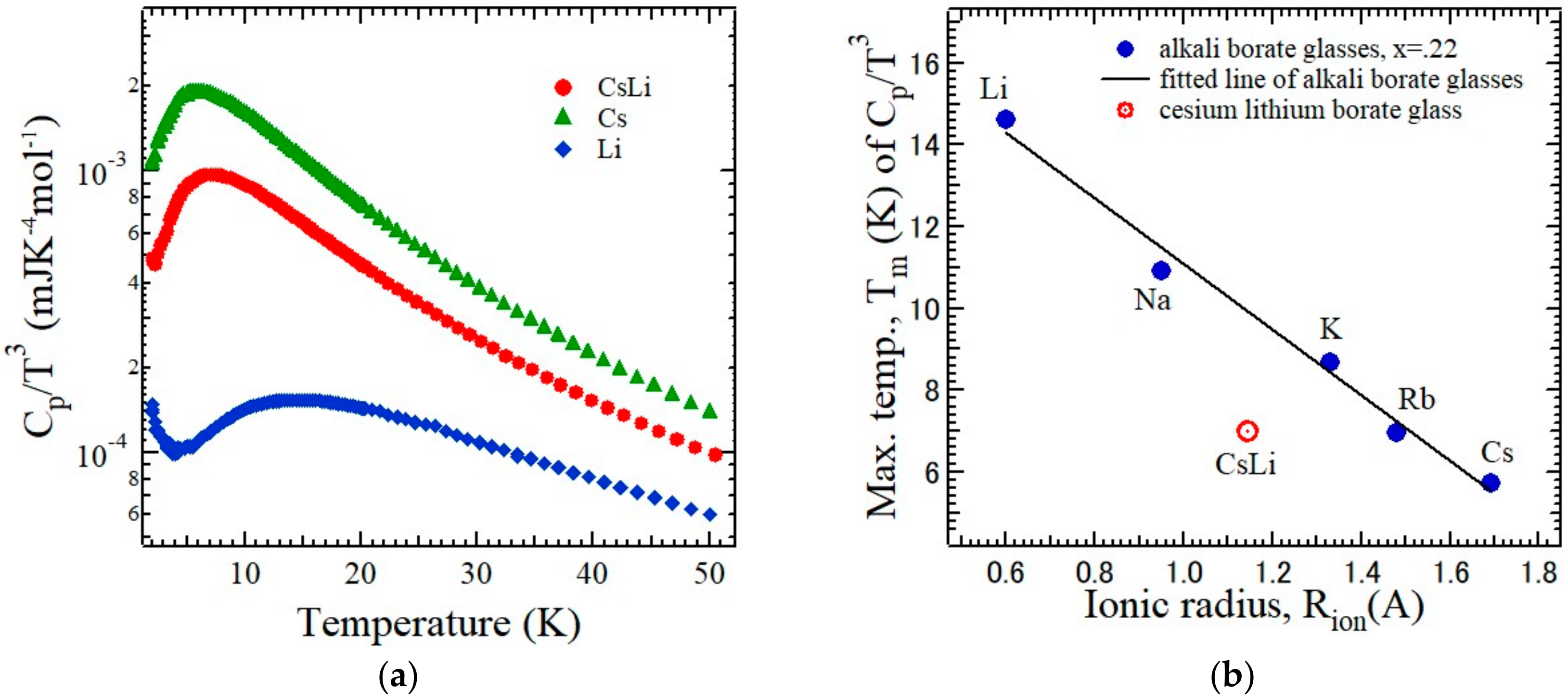
The temperature dependences of the heat capacity C
p of the mixed-alkali borate glasses, 0.11M
2O-0.11 M’
2O-0.78B
2O
3 (M, M’ = Li, K, and Cs), were measured using a relaxation calorimeter to investigate the MAE [
27]. In order to discuss the excess heat capacity, the temperature dependence of C
p/
T3 of a mixed-lithium cesium (LiCs) borate glass (M = Li, M’ = Cs) is plotted to investigate the deviation from the Debye
T3 law at low temperatures as shown in
Figure 5a, where the dependences of the single-cesium borate (M = M’ = Cs) and single-lithium borate (M = M’ = Li) glasses are also plotted for comparison [
26]. The broad peaks of C
p/
T3 were observed with the
Tm in the range 5–15 K for all three glasses. To clarify the MAE, the
Tm of the single-alkali and mixed-alkali borate glasses was plotted versus the ionic radius of alkali ion as shown in
Figure 5b, where the ionic radius of the mixed-alkali glass is the arithmetical mean of the radius of the two alkali ions. The significant negative deviation of
Tm of a mixed-cesium lithium borate glass from that of linearity of the single-alkali borate glasses between the alkali cation size and
Tm was clearly observed. When two dissimilar alkali species are co-doped, a different modification of the boron-oxygen network appears. The number of the BØ
4− units, N
4, in a mixed-alkali glass was lower than the value expected from that of a single-alkali borate glass. The result of molecular dynamic simulations predicted that the N
4 decreases from lithium to cesium in the mixed-cesium lithium glasses [
20]. The negative deviation of
Tm in the mixed-cesium lithium glass can be attributed to the decrease in the N
4 caused by the dissimilar alkali mixing. The existence of the (CsLi)
2+ dissimilar pair may be the origin of the negative deviation in
Figure 5b.
5. Vibrational Properties
Vibrational spectroscopy, such as Raman scattering and infrared (IR) spectroscopy, is a powerful tool to study the local atomic structure of condensed matters, especially glasses with random structures. When a center of symmetry exists, the exclusion principle holds, namely, the Raman active mode is IR inactive and the IR active mode is Raman inactive. However, glass has no center of symmetry due to its random structure. Generally, the modes higher than 200 cm
−1 are the internal mode of a molecule and the modes lower than 200 cm
−1 are the external modes reflecting the intermolecular interaction. In glasses the modes higher than 200 cm
−1 originate from groups of molecules and the structural units. While the modes lower than 200 cm
−1 originate from the vibrational modes of medium range order of the glass network structure, and the lowest frequency broad peak below 200 cm
−1 is called a boson peak. As discussed in
Section 3, a boson peak is the low-energy excess part of the vibrational density of states (VDoS). Typical Raman scattering spectra of the alkali borate glasses between 10~1700 cm
−1 are shown in
Figure 9 [
22,
34,
35,
36]. The remarkable variation of boson peaks below 200 cm
−1 was clearly observed. In addition the high frequency Raman bands above 200 cm
−1 also shows a noticeable variation, especially the middle frequency range between 700 and 850 cm
−1, where the modes of various structural units, such as the boroxol ring, pentaborate borate, metaborate groups, etc., were observed [
37].
5.1. Internal Raman Bands
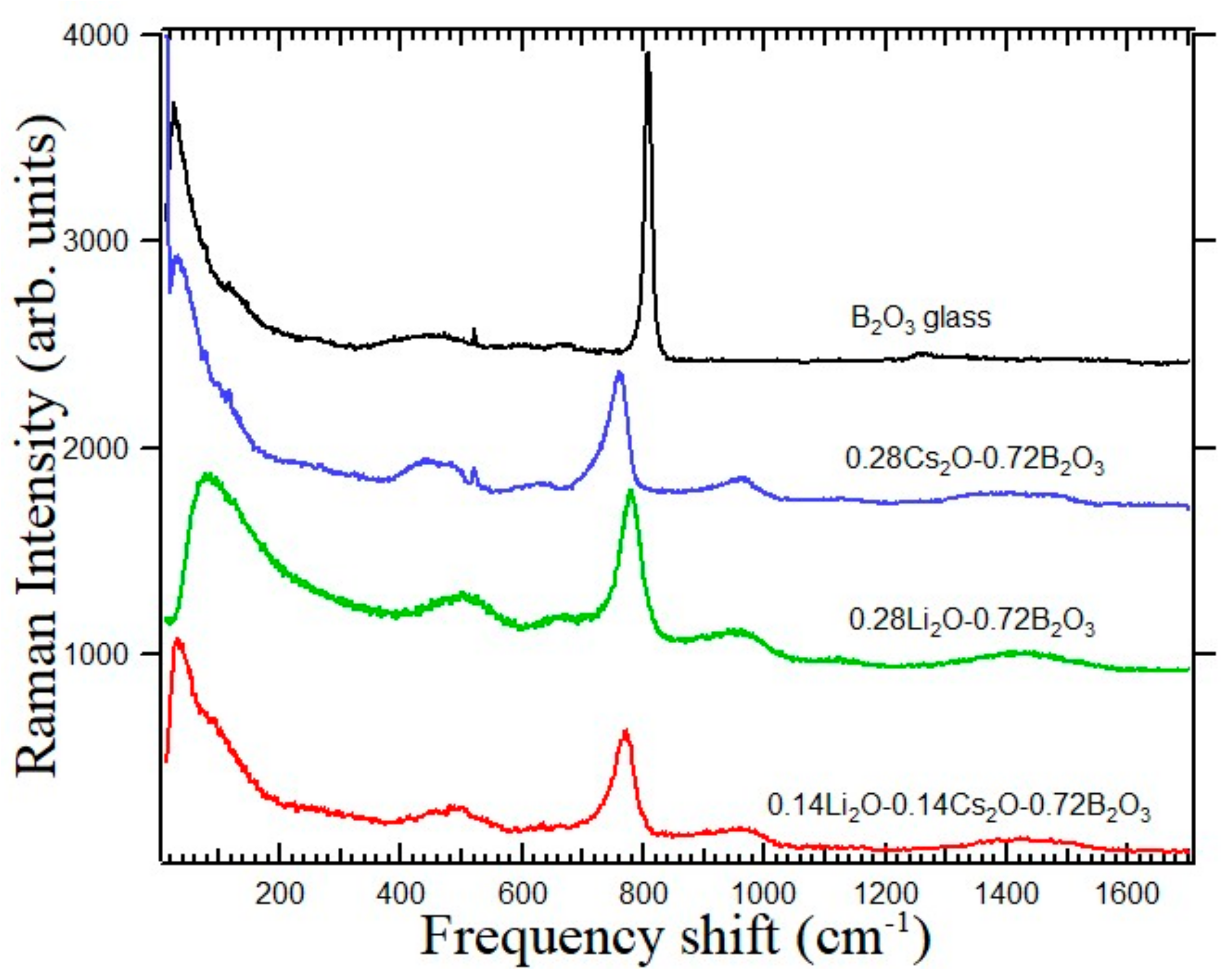
The intense and sharp peak at 808 cm
−1 of the B
2O
3 glass in
Figure 9 is the localized symmetric breathing mode of the B
3O
3 planer boroxol ring with the coordination number of boron is three. When the B
2O
3 glass is modified by alkali ions, the coordination number of boron changes to four. As shown in
Figure 9, for the 0.28Cs
2O-0.72B
2O
3 and 0.28Li
2O-0.72B
2O
3 glasses, the intense peak appears at 758 cm
−1 and 777 cm
−1, respectively [
34]. The 0.14Cs
2O-0.14Li
2O-0.72B
2O
3 glass shows the intense peak at 770 cm
−1. The Raman scattering spectra of the 0.28{(1−
y)Cs
2O-
yLi
2O}-0.72B
2O
3 glasses with
y = 0.2, 0.5, and 0.8 between 650 and 850 cm
−1 are shown in
Figure 10a [
22]. According to the analysis of the internal Raman bands, the average characteristic mode frequency of the pentaborate, dipentaborate, and chain type metaborate groups are 770, 755 and 730 cm
−1, respectively.
These band intensities were determined by a multi-peaks fitting. The intensities of these three characteristic modes as a function on the Li fraction are shown in
Figure 10b. As the Li fraction
y increases with a constant alkali content, the pentaborate groups increase, while the dipentaborate and chain type metaborate groups decrease. These dependences of the mode intensities are not linear with the Li fraction
y, and such nonlinear changes of the structural units can be caused by the MAE.
5.2. Boson Peaks
In
Figure 9, the boson peak spectrum of the B
2O
3 glass is well fitted by a log-normal function and its peak frequency is 26 cm
−1. According to the vibrational study by hyper-Raman scattering, the origin of the boson peak is attributed to the librational motions of two adjacent boroxole rings [
38]. As shown
Figure 9, the boson peak frequency of the 0.28Cs
2O-0.72B
2O
3 glass at 26 cm
−1 is nearly same as that of the B
2O
3 glass, while that of the 0.28Li
2O-0.72B
2O
3 glass at 72 cm
−1 is about three times of that of the B
2O
3 glass. The Cs ions with a large ionic size only slightly changes the boron-oxgen network structure by the low charge density. However, the Li ions with a small ionic size cause shrinking of the boron-oxygen network structure by the high charge density [
26,
39]. By application of high pressure, it was reported that the boson peak frequency significantly increases up to 68 cm
−1 at 4 GPa by shrinking of the boron-oxygen network structure [
40].
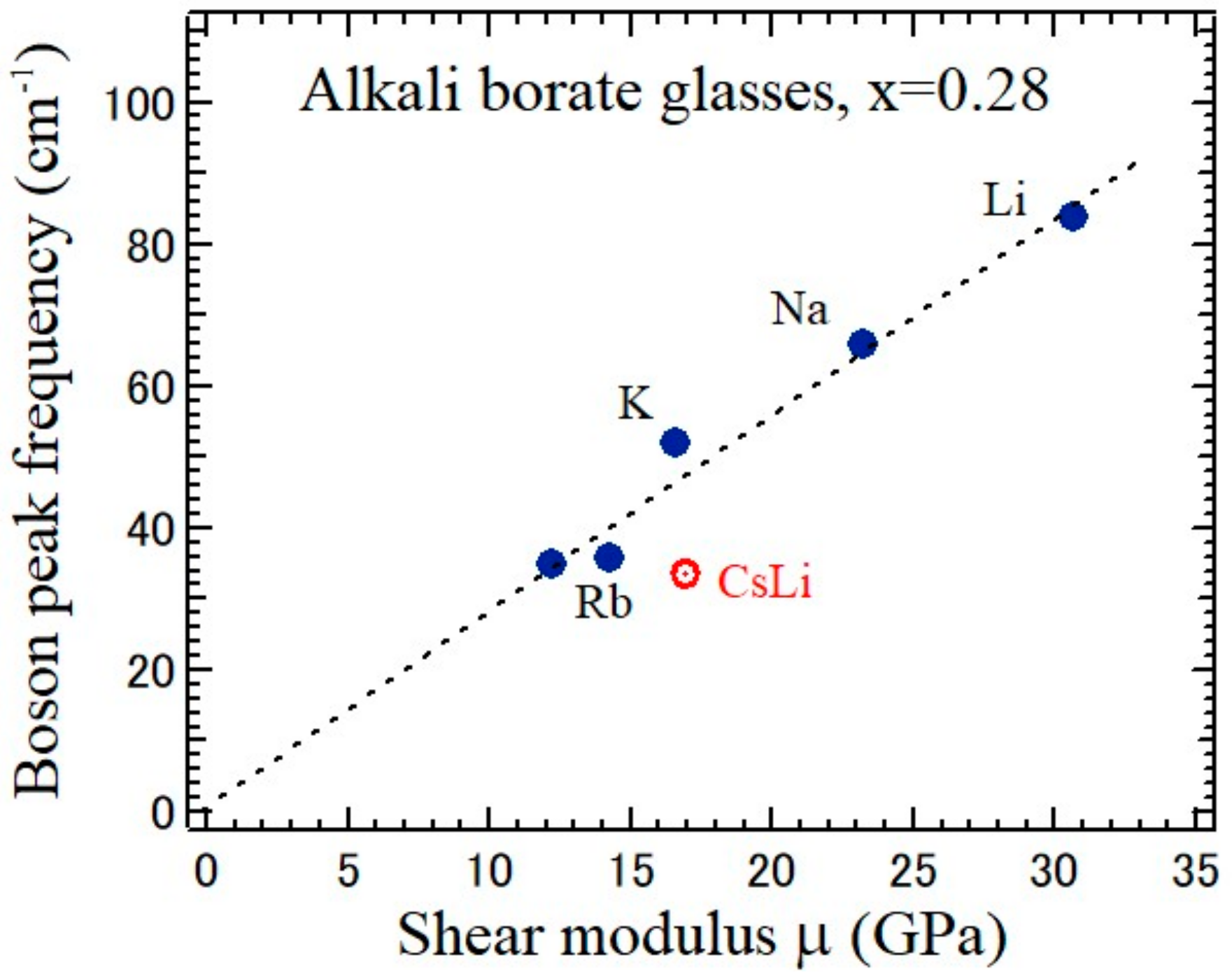
Figure 11 shows the relation between the boson peak frequency determined by Raman scattering versus the shear modulus determined by the ultrasonic method in the single-alkali borate glasses. The solid circles denote the values of the 0.28M
2O-0.72B
2O (M = Li, Na, K, Rb, and Cs) and the open circle denotes the value of the 0.14Cs
2O-0.14Li
2O-0.72B
2O
3 (CsLi) glasses [
4,
21]. The deviation of the plot of the mixed-cesium lithium borate glass (CsLi) from the linear relation of single-alkali borate glasses in
Figure 11 may suggest the MAE.
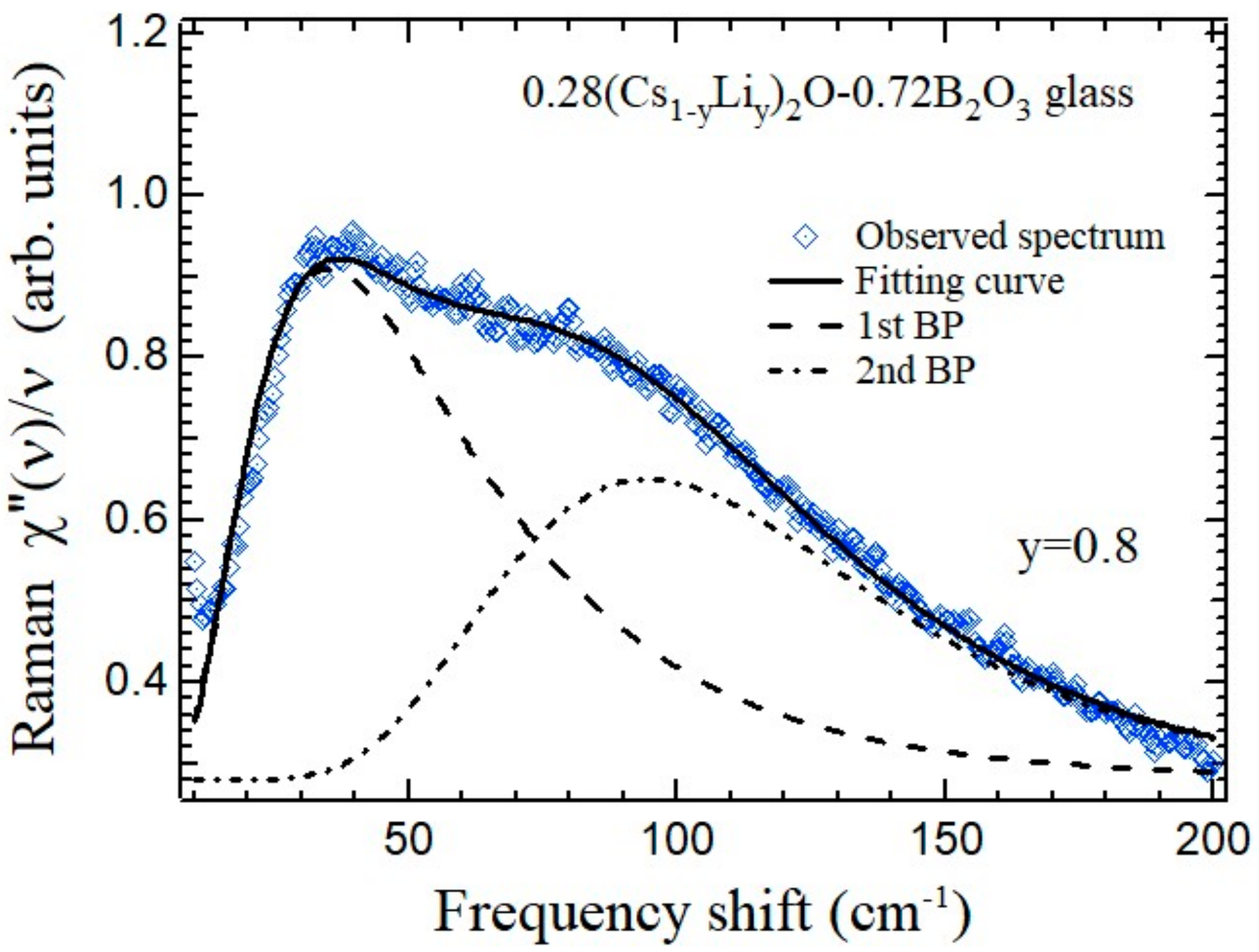
The boson peak spectra of the mixed-cesium lithium 0.28{(1−
y)Cs
2O-
yLi
2O}-0.72B
2O
3 glasses were studied by Raman scattering and splitting of the boson peak was observed [
21]. The results of fitting of the boson peak spectrum of the 0.28{0.2Cs
2O-0.8Li
2O}-0.72B
2O
3 glass (
y = 0.8) between 10 and 200 cm
−1 are shown in
Figure 12 [
21]. The boson peak of the single-alkali borate glasses is well fitted by a log-normal function. However, the boson peak of the mixed-alkali borate glasses has a distorted broad peak and such a single mode fitting is impossible. Therefore, the broad peak is fitted by two log-normal functions.
The fitted results indicate the splitting of a boson peak in the mixed-cesium lithium borate glasses. The origin of this splitting is probably related to two kinds of structural correlations related to the heterogeneous structure including the Cs-rich and Li-rich regions. The static structure factor, S
CsLi(Q), of the mixed-cesium lithium borate glasses cannot be reproduced by the average between S
Cs(Q) of the cesium borate and S
Li(Q) of the lithium borate glasses [
41]. This fact means that there is no phase separation into the cesium borate and lithium borate glasses. The detailed analysis of the static structure factor, S
CsLi(Q), of the mixed-cesium lithium borate glasses and the boson peak spectra, S
CsLi(E), measured by neutron scattering will be published elsewhere.
6. Summary
The modification of oxide glasses by dissimilar alkali ions causes a maximum in the electric resistivity or the expansion coefficient, and that is called the mixed-alkali effect (MAE). MAE is very important not only in basic sciences but also in technology, while its mechanism is not yet clarified. In order to give new insights into MAE, this paper reviews the experimental studies on the glass transition temperature, heat capacity, elastic moduli, and optical vibrations in the mixed-cesium lithium borate glasses, x{(1−y)Cs2O-yLi2O}-(1−x)B2O3. For the single-alkali borate glasses, xM2O(1−x)-B2O3 (M = Li, Na, K, Rb, and Cs), the glass transition temperature, Tg = 270 °C, of the pure borate glass monotonically increases as the alkali content x increases. However, for the mixed-cesium lithium borate glasses with a constant alkali content x, the Tg versus the lithium fraction y has a minimum. The excess heat capacity at low temperatures caused by the excess vibrational density of states, and it shows the nonlinear variation against the lithium fraction y. The lithium fraction dependences of elastic properties are analyzed about the longitudinal modulus, Poisson’s ratio, and Cauchy-type relation. In Raman scattering spectroscopy, the internal vibrational bands related to the boron-oxide structural groups shows a nonlinear variation versus the lithium fraction y. The boson peak of single-alkali borate glasses is a single peak, however, in the mixed-cesium lithium borate glasses the splitting of a boson peak occurs by MAE. These nonlinear behaviors on the thermal, elastic, and vibrational properties are discussed on the basis of the changes of glass network structure modified by dissimilar alkali ions. The possible origin of the MAE in the mixed borate glasses is the appearance of dissimilar alkali ion pairs with different ionic radii.