Enrichment Cultures of Extreme Acidophiles with Biotechnological Potential
Abstract
1. Introduction
| Source | Main Minerals of Ore/Concentrate | T, °C | Microbial Population | Reference |
|---|---|---|---|---|
| Heap bioleaching | Pyrite, arsenopyrite | Gradual increase from ambient temperature to 60–80 °C | A. ferrooxidans, Leptospirillum spp., Sulfobacillus spp. Temperature increase led to the predominance of the genera Acidianus, Metallosphaera, Sulfolobus | [14] |
| Pyrrhotite, pyrite, sphalerite, pentlandite, violarite, chalcopyrite and graphite | Gradual increase from 0 to 30 and 90 °C | A. ferrooxidans, A. caldus and L. ferrooxidans, S. thermosulfidooxidans | [15] | |
| Pyrite, chalcocite, covellite, enargite | No data | Acidithiobacillus, Leptospirillum | [30] | |
| Pyrite, chalcopyrite, molybdenite, sphalerite, galena | No data | Acidithiobacillus ferrivorans, A. ferrooxidans, Rhodanobacter, Thiobacillus, Leptospirillum, Acidiphilium | [31] | |
| Pyrite, chalcocite, covellite, enargite | No data | Acidithiobacillus, Sulfobacillus, Acidiferrobacter | [20] | |
| Bioleach reactor | Pyrite | 42 | Leptospirillum ferriphilum, A. caldus, Ferroplasma acidiphilum, Sulfobacillus benefaciens | [32] |
| Pyrite, chalcopyrite, sphalerite | 45 | A. caldus, L. ferriphilum, Sulfobacillus sp., Ferroplasma sp. | [33] | |
| Pyrite, arsenopyrite, chalcopyrite | 45 | A. caldus, Sulfobacillus thermosulfidooxidans, “Sulfobacillus montserratensis” | [34] | |
| Pyrite, arsenopyrite | 40–50 | Sulfobacillus sp., A. caldus, L. ferriphilum, Ferroplasma sp., Acidiplasma sp. | [35] | |
| Pyrite, arsenopyrite | 40–50 | Acidithiobacillus, Leptospirillum, Sulfobacillus, archaea Ferroplasma, Acidiplasma, Cuniculiplasma, “Ca.Carboxiplasma ferriphilum” (A-plasma) | [36] | |
| Pyrite, arsenopyrite | 40–50 | Acidithiobacillus, Leptospirillum, Sulfobacillus, archaea Ferroplasma | [37] |
- To obtain enrichments of extremely acidophilic microorganisms used in the processes of bioleaching sulfide ores, using nutrient media containing ferrous sulfate, elemental sulfur and copper sulfide concentrate as nutrient substrates at wide temperature range 25–50 °C;
- To determine the microbial composition of the enrichment cultures using the metabarcoding of variable V3–V4 fragments of 16S rRNA genes and compare enrichments grown under different conditions;
- To study the dynamics of the oxidation of ferrous iron ions, sulfur, and sulfide minerals (pyrite and chalcopyrite) by the obtained enrichments in the temperature range of 25–50 °C to establish the effect of temperature on the activity of enrichments.
- Based on the data obtained, to make a conclusion about the possibility of using the obtained enrichment cultures in biotechnological processes.
2. Materials and Methods
2.1. Obtaining the Enrichments
2.2. Microbial Population Analysis
2.3. Study of Dynamics of Oxidation of Ferrous Iron Ions, Sulfur, and Sulfide Minerals (Pyrite and Chalcopyrite) by Obtained Enrichments
3. Results
3.1. Obtaining and Conducting Analysis of Enrichments
3.2. Study of Dynamics of Oxidation of Ferrous Iron, Sulfur, and Sulfide Minerals by Obtained Enrichments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahmoud, A.; Cezac, P.; Hoadley, A.F.A.; Contaminea, F.; D′Hugues, P. A review of sulfide minerals microbially assisted leaching in stirred tank reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Johnson, D.B.; Roberto, F.F. Evolution and current status of mineral bioprocessing technologies. In Biomining Technologies. Extracting and Recovering Metals from Ores and Wastes; Johnson, D.B., Bryan, C.G., Schlömann, M., Roberto, F.F., Eds.; Springer: Cham, Switzerland, 2023; pp. 1–13. [Google Scholar] [CrossRef]
- Tezyapar Kara, I.; Kremser, K.; Wagland, S.T.; Coulon, F. Bioleaching metal-bearing wastes and by-products for resource recovery: A review. Environ. Chem. Lett. 2023, 21, 3329–3350. [Google Scholar] [CrossRef]
- Dopson, M.; Okibe, N. Biomining microorganisms: Diversity and modus operandi. In Biomining Technologies. Extracting and Recovering Metals from Ores and Wastes; Johnson, D.B., Bryan, C.G., Schlömann, M., Roberto, F.F., Eds.; Springer: Cham, Switzerland, 2023; pp. 89–110. [Google Scholar] [CrossRef]
- Tonietti, L.; Esposito, M.; Cascone, M.; Barosa, B.; Fiscale, S.; Muscari Tomajoli, M.T.; Sbaffi, T.; Santomartino, R.; Covone, G.; Cordone, A.; et al. Unveiling the bioleaching versatility of Acidithiobacillus ferrooxidans. Microorganisms 2024, 12, 2407. [Google Scholar] [CrossRef]
- Aliyu, G.O.; Ezugworie, F.N.; Onwosi, C.O.; Nnamchi, C.I.; Ekwealor, C.C.; Igbokwe, V.C.; Sani, R.K. Multi-stress adaptive lifestyle of acidophiles enhances their robustness for biotechnological and environmental applications. Sci. Total Environ. 2024, 954, 176190. [Google Scholar] [CrossRef]
- Jones, S.; Santini, J.M. Mechanisms of bioleaching: Iron and sulfur oxidation by acidophilic microorganisms. Essays Biochem. 2023, 67, 685–699. [Google Scholar] [CrossRef]
- Dopson, M.; Holmes, D.S. Metal resistance in acidophilic microorganisms and its significance for biotechnologies. Appl. Microbiol. Biotechnol. 2014, 98, 8133–8144. [Google Scholar] [CrossRef]
- Wheaton, G.; Counts, J.; Mukherjee, A.; Kruh, J.; Kelly, R. The confluence of heavy metal biooxidation and heavy metal resistance: Implications for bioleaching by extreme thermoacidophiles. Minerals 2015, 5, 397–451. [Google Scholar] [CrossRef]
- Pivovarova, T.A.; Kondrat'eva, T.F.; Batrakov, S.G.; Esipov, S.E.; Sheichenko, V.I.; Bykova, S.A.; Lysenko, A.M.; Karavaiko, G.I. Phenotypic Features of Ferroplasma acidiphilum Strains YT and Y-2. Microbiology 2002, 71, 698–706. [Google Scholar] [CrossRef]
- Cárdenas, J.P.; Martínez, V.; Covarrubias, P.; Holmes, D.S.; Quatrini, R. Predicted CO/CO2 fixation in Ferroplasma spp. via a novel chimaeric pathway. Adv. Mat. Res. 2009, 71–73, 219–222. [Google Scholar] [CrossRef]
- Gonzalez-Toril, E.; Llobet-Brossa, E.; Casamayor, E.O.; Amann, R.; Amils, R. Microbial ecology of an extreme acidic environment, the Tinto River. Appl. Environ. Microbiol. 2003, 69, 4853–4865. [Google Scholar] [CrossRef] [PubMed]
- Druschel, G.K.; Baker, B.J.; Gihring, T.M.; Banfield, J.F. Acid mine drainage biogeochemistry at Iron Mountain, California. Geochem. Trans. 2004, 5, 13–32. [Google Scholar] [CrossRef]
- Logan, T.C.; Seal, T.; Brierley, J.A. Whole-ore heap biooxidation of sulfidic gold-bearing ores. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 113–138. [Google Scholar] [CrossRef]
- Riekkola-Vanhanen, M. Talvivaara Sotkamo mine—Bioleaching of a polymetallic nickel ore in subarctic climate. Nova Biotechnol. Chem. 2010, 10, 7–14. [Google Scholar] [CrossRef]
- Jiménez-Paredes, A.E.; Alfaro-Saldaña, E.F.; Hernández-Sánchez, A.; García-Meza, J.V. An autochthonous Acidithiobacillus ferrooxidans metapopulation exploited for two-step pyrite biooxidation improves Au/Ag particle release from mining waste. Mining 2021, 1, 335–350. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, H.-S.; Lee, C.-G.; Park, S.-J.; Choi, N.-C. A comparative study on bioleaching properties of various sulfide minerals using Acidiphilium cryptum. Appl. Sci. 2023, 13, 5997. [Google Scholar] [CrossRef]
- Koizhanova, A.; Kenzhaliyev, B.; Magomedov, D.; Kamalov, E.; Yerdenova, M.; Bakrayeva, A.; Abdyldayev, N. Study of factors affecting the copper ore leaching process. Chem. Eng. 2023, 7, 54. [Google Scholar] [CrossRef]
- Kumar, J.; Sharma, N.; Singh, S.P. Genome-resolved metagenomics inferred novel insights into the microbial community, metabolic pathways, and biomining potential of Malanjkhand acidic copper mine tailings. Environ. Sci. Pollut. Res. 2023, 30, 50864–50882. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, Q.; Li, Z. Isolation and characterization of a novel iron–ulfur oxidizing bacterium Acidithiobacillus Ferrooxidans YQ-N3 and its applicability in coal biodesulfurization. Minerals 2023, 13, 95. [Google Scholar] [CrossRef]
- Vardanyan, A.; Khachatryan, A.; Castro, L.; Willscher, S.; Gaydardzhiev, S.; Zhang, R.; Vardanyan, N. Bioleaching of sulfide minerals by Leptospirillum ferriphilum CC from polymetallic mine (Armenia). Minerals 2023, 13, 243. [Google Scholar] [CrossRef]
- Casas-Vargas, J.C.; Martínez-Bussenius, C.; Videla, Á.; Vera, M. Novel indigenous strains and communities with copper bioleaching potential from the Amolanas Mine, Chile. Minerals 2024, 14, 867. [Google Scholar] [CrossRef]
- Joulian, C.; Hubau, A.; Pino-Herrera, D.; Guezennec, A.-G. Bioleaching of polymetallic sulphidic mining residues: Influence of increasing solid concentration on microbial community dynamics and metal dissolution. Res. Microbiol. 2024, 175, 104112. [Google Scholar] [CrossRef]
- Vardanyan, A.; Zhang, R.; Khachatryan, A.; Melkonyan, Z.; Hovhannisyan, A.; Willscher, S.; Kamradt, A.; Jost, M.; Zhang, Y.; Wang, C.; et al. Extraction of copper from copper concentrate by indigenous association of iron-oxidizing bacteria. Separations 2024, 11, 124. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, W.; Yan, Z.; Shen, L.; Yu, R.; Wu, X.; Li, J.; Qiu, G.; Streit, W.; Liu, Y. The bio-desulfurization of cassiterite–polymetallic sulfide ores enhanced by a consortium of moderately thermophilic bacteria. Separations 2025, 12, 61. [Google Scholar] [CrossRef]
- Tupikina, O.V.; Minnaar, S.H.; Rautenbach, G.F.; Dew, D.W.; Harrison, S.T. Effect of inoculum size on the rates of whole ore colonisation of mesophilic, moderate thermophilic and thermophilic acidophiles. Hydrometallurgy 2014, 149, 244–251. [Google Scholar] [CrossRef]
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T.L. Bioleaching of arsenopyrite from Janggun mine tailings (South Korea) using an adapted mixed mesophilic culture. Hydrometallurgy 2018, 181, 21–28. [Google Scholar] [CrossRef]
- Borja, D.; Nguyen, K.A.; Silva, R.A.; Ngoma, E.; Petersen, J.; Harrison, S.T.L.; Park, J.H.; Kim, H. Continuous bioleaching of arsenopyrite from mine tailings using an adapted mesophilic microbial culture. Hydrometallurgy 2019, 187, 187–194. [Google Scholar] [CrossRef]
- Chen, W.; Yin, S.; Wang, L.; Znang, M. Re-inoculated bacteria driving microbial community functional response to bioleaching of low-grade copper sulfide ores: Potential to improve copper recovery. J. Sustain. Metall. 2023, 9, 1226–1238. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Ma, L.; Liang, Y.; Niu, J.; Gu, Y.; Zhang, X.; Hao, X.; Dong, W.; She, S.; et al. Microbial communities from different subsystems in biological heap leaching system play different roles in iron and sulfur metabolisms. Appl. Microbiol. Biotechnol. 2016, 100, 6871–6880. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Liang, Y.; Liu, X.; Yin, H. Metagenome-scale analysis yields insights into the structure and function of microbial communities in a copper bioleaching heap. BMC Genet. 2016, 17, 21. [Google Scholar] [CrossRef]
- Morin, D.H.R.; d’Hugues, P. Bioleaching of a Cobalt-Containing Pyrite in Stirred Reactors: A Case Study from Laboratory Scale to Industrial Application in Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 35–55. [Google Scholar] [CrossRef]
- Okibe, N.; Gericke, M.; Hallberg, K.B.; Johnson, D.B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation. Appl. Environ. Microbiol. 2003, 69, 1936–1943. [Google Scholar] [CrossRef]
- Dopson, M.; Lindstrom, E.B. Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite. Microb. Ecol. 2004, 48, 19–28. [Google Scholar] [CrossRef]
- van Hille, R.P.; van Wyk, N.; Froneman, T.; Harrison, S.T.L. Dynamic evolution of the microbial community in BIOX leaching tanks. Adv. Mater. Res. 2013, 825, 331–334. [Google Scholar] [CrossRef]
- Bulaev, A.; Kadnikov, V.; Elkina, Y.; Beletsky, A.; Melamud, V.; Ravin, N.; Mardanov, A. Shifts in the microbial populations of bioleach reactors are determined by carbon sources and temperature. Biology 2023, 12, 1411. [Google Scholar] [CrossRef] [PubMed]
- Bulaev, A.; Artykova, A.; Diubar, A.; Kolosoff, A.; Melamud, V.; Kolganova, T.; Beletsky, A.; Mardanov, A. Biooxidation of a pyrite-arsenopyrite concentrate under stressful conditions. Microorganisms 2024, 12, 2463. [Google Scholar] [CrossRef] [PubMed]
- Bulaev, A.; Melamud, V. Biox of color metals from washery refuses. Int. Res. J. 2018, 12, 63–78. (In Russian) [Google Scholar] [CrossRef]
- Valiyev, K.; Bugubaeva, A.; Nechaeva, A.; Artykova, A.; Melamud, V.; Stom, D.; Boduen, A.; Bulaev, A. The development of innovated complex process for treatment of old flotation tailings of copper-zinc sulfide ore. Molecules 2024, 29, 1550. [Google Scholar] [CrossRef]
- Melamud, V.S.; Pivovarova, T.A. Specific features of the growth of the type strain of Sulfobacillus thermosulfidooxidans in medium 9K. Appl. Biochem. Microbiol. 1998, 34, 309–315. [Google Scholar]
- Bulaev, A.; Melamud, V. Two-stage oxidative leaching of low-grade copper–zinc sulfide concentrate. Microorganisms 2022, 10, 1781. [Google Scholar] [CrossRef]
- Reznikov, A.A.; Mulikovskaya, E.P.; Sokolov, I.Y. Metody Analiza Prirodnykh Vod [Methods for Analysis of Natural Waters]; Nedra: Moscow, Russia, 1970; p. 488. (In Russian) [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, E1. [Google Scholar] [CrossRef]
- Das, G.K.; Acharya, S.; Anand, S.; Das, R.P. Jarosites: A review. Miner. Process. Extr. Metall. Rev. 1996, 16, 185–210. [Google Scholar] [CrossRef]
- Muravyov, M.I.; Bulaev, A.G. Two–step oxidation of a refractory gold–bearing sulfidic concentrate and the effect of organic nutrients on its biooxidation. Miner. Eng. 2013, 45, 108–114. [Google Scholar] [CrossRef]
- Bulaev, A.G. Effect of organic carbon source on pyrite biooxidation by moderately thermophilic acidophilic microorganisms. Microbiology 2020, 89, 301–308. [Google Scholar] [CrossRef]
- Holanda, R.; Hedrich, S.; Falagan, C.; Nancucheo, I.; Dall'Agnol, H.; Grail, B.M.; Johnson, D.B. Characteristics of Acidibacillus spp.: A novel genus of acidophilic iron-oxidising firmicutes. Adv. Mater. Res. 2015, 1130, 36–39. [Google Scholar] [CrossRef]
- Nancucheo, I.; Johnson, D.B. Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl. Environ. Microbiol. 2010, 76, 461–467. [Google Scholar] [CrossRef]
- Dopson, M.; Baker-Austin, C.; Hind, A.; Bowman, J.P.; Bond, P.L. Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl. Environ. Microbiol. 2004, 70, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Ballester, A.; Blazquez, M.L.; Gonzalez, F.; Munoz, J.A. New information on the chalcopyrite bioleaching mechanism at low and high temperature. Hydrometallurgy 2003, 71, 47–56. [Google Scholar] [CrossRef]
- Hedrich, S.; Joulian, C.; Graupner, T.; Schippers, A.; Guézennec, A.-G. Enhanced chalcopyrite dissolution in stirred tank reactors by temperature increase during bioleaching. Hydrometallurgy 2018, 179, 125–131. [Google Scholar] [CrossRef]
- Ríos, D.; Bellenberg, S.; Christel, S.; Lindblom, P.; Giroux, T.; Dopson, M. Potential of single and designed mixed cultures to enhance the bioleaching of chalcopyrite by oxidation-reduction potential control. Hydrometallurgy 2024, 24, 106245. [Google Scholar] [CrossRef]
- Christel, S.; Herold, M.; Bellenberg, S.; Buetti-Dinh, A.; El Hajjami, M.; Pivkin, I.V.; Sand, W.; Wilmes, P.; Poetsch, A.; Vera, M.; et al. Weak iron oxidation by Sulfobacillus thermosulfidooxidans maintains a favorable redox potential for chalcopyrite bioleaching. Front. Microbiol. 2018, 9, 3059. [Google Scholar] [CrossRef]

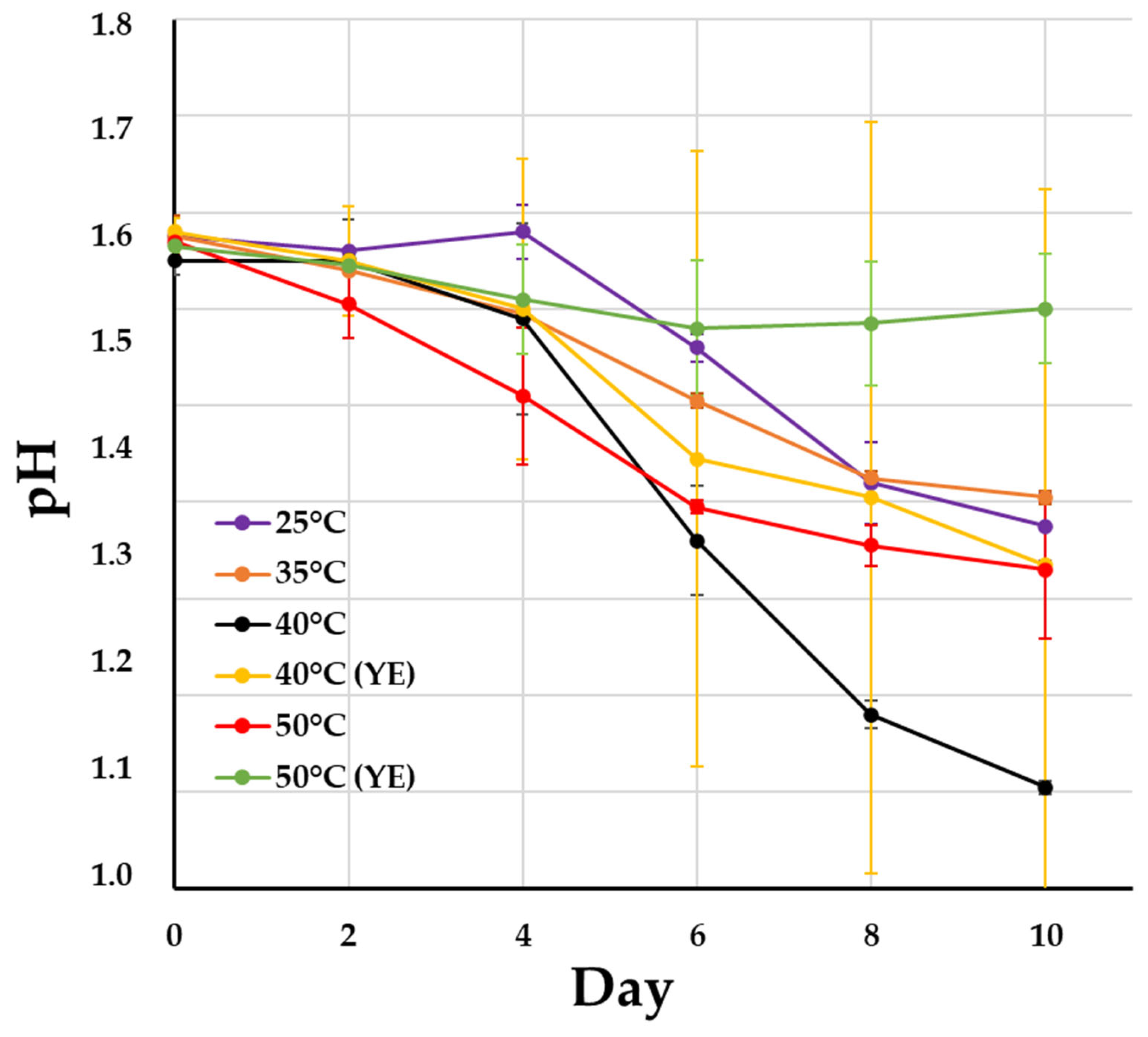
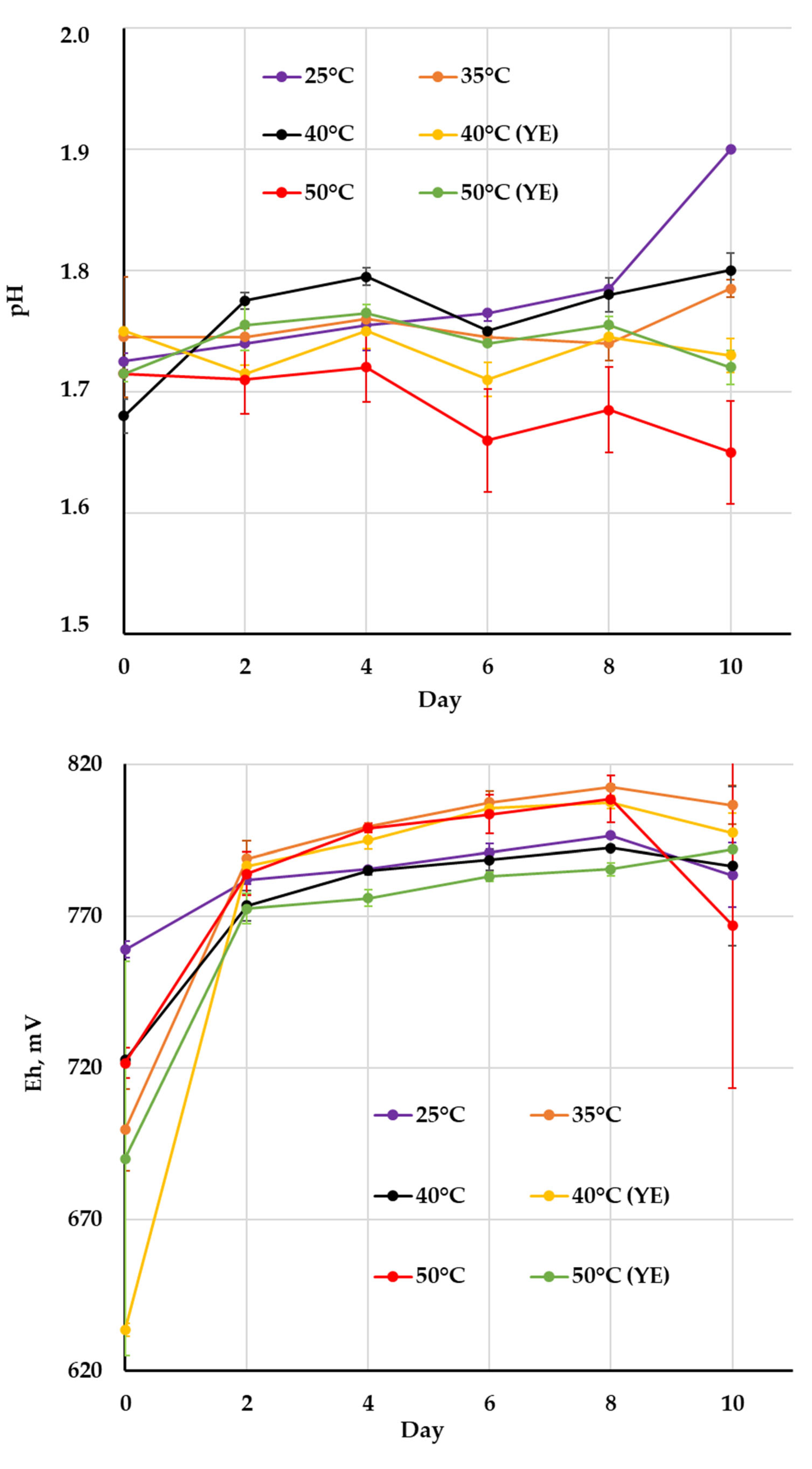


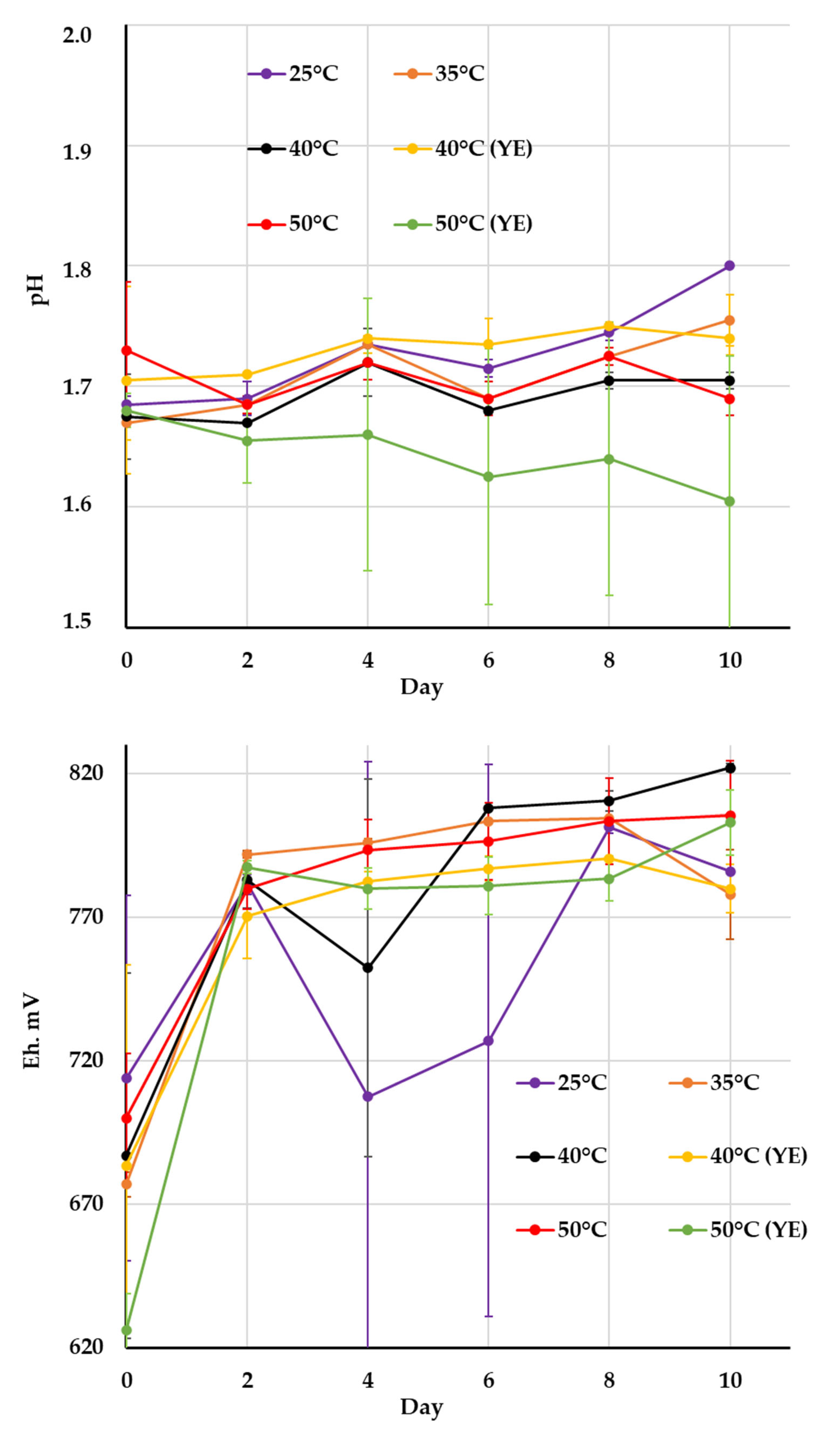
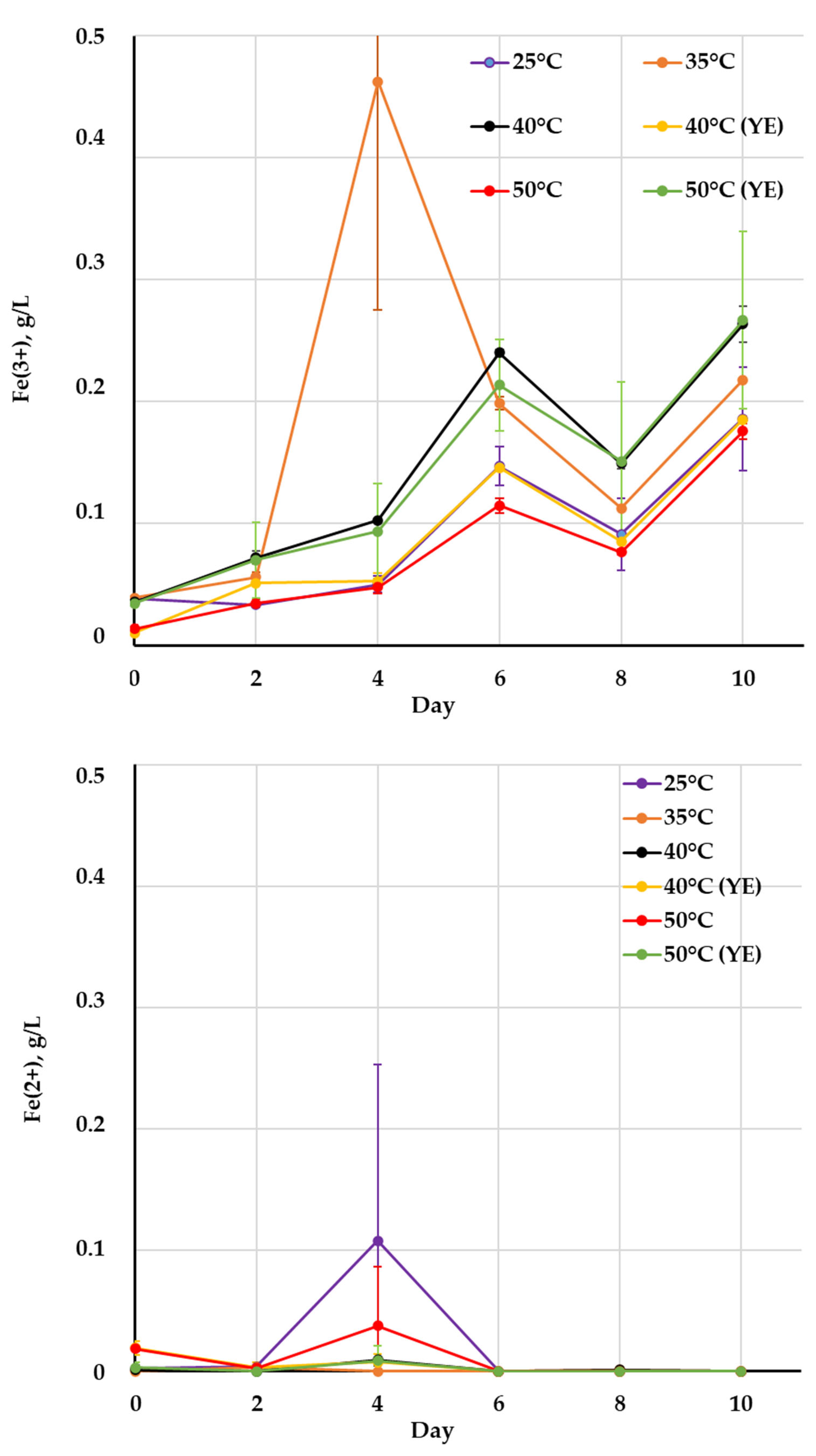



| Cultivation Conditions | pH | Eh, mV | Fe3+, g/L | Fe2+, g/L | Microbial Cell Number, ×107 cell/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T, °C | YE | Incubation, Days | Initial | Final | Initial | Final | Initial | Final | Initial | Final | Initial | Final |
| 25 | - | 20 | 2.05 | 1.16 | 325 | 872 | 0.28 | 2.8 | 1.96 | 0 | 0.2 | 42.3 |
| 35 | - | 20 | 2.05 | 1.54 | 325 | 858 | 0.28 | 1.4 | 1.96 | 0 | 0.2 | 26.6 |
| 40 | - | 30 | 2.05 | 1.73 | 325 | 771 | 0.28 | 0.56 | 1.96 | 0 | 0.2 | 1.2 |
| 40 * | - | 7 | 1.73 | 1.12 | 771 | 795 | 0.56 | 1.89 | 1.96 | 0 | 2.2 | 11.3 |
| 40 | + | 5 | 2.05 | 1.85 | 325 | 757 | 0.28 | 1.33 | 1.96 | 0 | 0.2 | 4.7 |
| 50 | - | 30 | 2.05 | 1.73 | 325 | 680 | 0.28 | 0.56 | 1.96 | 0.14 | 0.2 | 0.2 |
| 50 * | - | 7 | 1.73 | 1.55 | 680 | 728 | 0.28 | 0.84 | 1.96 | 0 | 1.2 | 1.8 |
| 50 | + | 5 | 2.05 | 1.57 | 325 | 803 | 0.28 | 1.19 | 1.96 | 0 | 0.2 | 7.8 |
| Genus | Enrichment | ||||||
|---|---|---|---|---|---|---|---|
| Inoculum | 25 °C | 35 °C | 40 °C | 40 °C + YE | 50 °C | 50 °C + YE | |
| Acidithiobacillus | 22.97 | 37.74 | 26.83 | 93.08 | 0.00 | 7.01 | 0.00 |
| Acidiferrobacter | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Acidiphilum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sulfobacillus | 4.31 | 12.71 | 6.21 | 5.44 | 39.09 | 2.04 | 94.59 |
| Leptospirillum | 5.74 | 42.88 | 39.15 | 0.00 | 56.89 | 0.00 | 0.01 |
| Acidibacillus | 0.83 | 6.52 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ferroplasma | 66.03 | 0.07 | 0.79 | 1.48 | 0.00 | 13.54 | 0.00 |
| Acidiplasma | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cuniculiplasma | 0.00 | 0.00 | 26.94 | 0.00 | 0.01 | 0.00 | 0.00 |
| A-plasma | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| E-plasma | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Acidophiles | 99.88 | 99.92 | 99.93 | 100.00 | 96.00 | 22.59 | 94.60 |
| Escherichia-Shigella | 0.06 | 0.02 | 0.02 | 0.00 | 1.23 | 0.00 | 3.93 |
| Sphingomonas | 0.01 | 0.00 | 0.01 | 0.00 | 0.10 | 0.19 | 0.54 |
| Faecalibacterium | 0.00 | 0.00 | 0.00 | 0.00 | 0.45 | 0.00 | 0.00 |
| Limnochordaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 11.79 | 0.00 |
| Halobacteroidaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 2.08 | 0.00 |
| Methylomicrobium | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 2.48 | 0.00 |
| Dehalococcoidia | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.41 | 0.00 |
| Anaerolineaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 3.45 | 0.06 |
| Aminicenantales | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.10 | 0.00 |
| Cyanobium | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.53 | 0.00 |
| Bathyarchaeia | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.63 | 0.00 |
| Methanosaeta | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.85 | 0.00 |
| Woesearchaeales | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 17.01 | 0.00 |
| Candidatus Aenigmarchaeum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 8.53 | 0.00 |
| Methanoregula | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.96 | 0.00 |
| Methanofastidiosales | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.55 | 0.00 |
| Other microbial groups | 0.05 | 0.04 | 0.04 | 0.00 | 2.18 | 11.85 | 0.87 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Genus | Properties | Reference | |||||
|---|---|---|---|---|---|---|---|
| Relation to Temperature | Topt (Tmin–Tmax), °C | pHopt (pHmin–pHmax) | Oxidized Inorganic Substrate | Carbon Nutrition | Consumed Organic Matter | ||
| Acidithiobacillus | Mesophiles, moderate thermophiles | 25–45 (5–52) | 2.0–2.3 (1.0–3.5) | Fe2+ and S | Autotrophs, mixotrophs | YE | [4] |
| Sulfobacillus | Thermotolerant microorganism | 38–39 (30–47) | 1.5 (0.8–2.2) | Fe2+ and S | Mixotroph | YE, glucose, fructose, arabinose, mannose, glycerin, mannitol, citric acid | |
| Leptospirillum | Thermotolerant microorganism | 30–37 (10–45) | 1.3–1.8 (no data) | Fe2+ | Autotroph | - | |
| ‘Acidibacillus’ | Mesophiles, moderate thermophiles | 30–43 (20–50) | 1.7–2.8 (no data) | Fe2+ and S | Heterotrophs | YE | |
| Ferroplasma | Thermotolerant microorganisms | 35 (15–45) | 1.7 (1.3–2.2) | Fe2+ | Mixotroph | YE | |
| Cuniculiplasma | Thermotolerant microorganism | 37–40 (10–48) | 1.0–1.2 (0.5–4.0) | - | Heterotroph | YE and meat extract, tryptone | |
| A-plasma | - | - | - | Presumably Fe2+ | Heterotroph | - | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiyev, K.; Yskak, A.; Latyuk, E.; Artykova, A.; Berik, R.; Chashkov, V.; Bulaev, A. Enrichment Cultures of Extreme Acidophiles with Biotechnological Potential. Mining 2025, 5, 49. https://doi.org/10.3390/mining5030049
Valiyev K, Yskak A, Latyuk E, Artykova A, Berik R, Chashkov V, Bulaev A. Enrichment Cultures of Extreme Acidophiles with Biotechnological Potential. Mining. 2025; 5(3):49. https://doi.org/10.3390/mining5030049
Chicago/Turabian StyleValiyev, Khussain, Aliya Yskak, Elena Latyuk, Alena Artykova, Rakhimbayev Berik, Vadim Chashkov, and Aleksandr Bulaev. 2025. "Enrichment Cultures of Extreme Acidophiles with Biotechnological Potential" Mining 5, no. 3: 49. https://doi.org/10.3390/mining5030049
APA StyleValiyev, K., Yskak, A., Latyuk, E., Artykova, A., Berik, R., Chashkov, V., & Bulaev, A. (2025). Enrichment Cultures of Extreme Acidophiles with Biotechnological Potential. Mining, 5(3), 49. https://doi.org/10.3390/mining5030049






